Abstract
Phosphatidylserine on the dying cell surface helps identify apoptotic cells to phagocytes, which then engulf them. A candidate phagocyte receptor for phosphatidylserine was identified using phage display, but the phenotypes of knockout mice lacking this presumptive receptor, as well as the location of the protein within cells, cast doubt on the assignment of this protein as the phosphatidylserine receptor.
The genome project and sequence databases have generally illuminated the molecular basis of cellular functions, but sometimes this illumination can be an electronic will-o'-the-wisp. Over the past few months, a series of papers culminating in the description of a knockout mouse by Lengeling and co-workers in Journal of Biology [1] has strongly suggested that a gene sequence thought to illuminate the molecular basis of apoptotic cell clearance has in fact led us astray, and that the path to it will have to be retraced.
The problem
Apoptosis, or programmed cell death, is a normal physiologic process for orderly removal of effete cells. As a process, apoptosis fell below the notice of cell biologists for quite some time, in part because cells dying an apoptotic death in vivo vanish almost immediately from view. They vanish because they are promptly engulfed, either by a neighbor or by a professional phagocytic macrophage; within the confines of the resulting phagosome, the dying cell digests itself from the inside out while the engulfing cell digests it from the outside in. To orchestrate its disappearance, the suicidal cell must make its intentions known to its neighbors, triggering signaling pathways that activate the engulfment machinery of the phagocyte. Investigation of the molecules involved in apoptotic cell recognition is a growing and industrious scientific subfield of the larger apoptosis research enterprise, with a host of specific proteins identified and cloned. One salient feature of the proteins identified is that most are receptors or enzymes residing on the surface of the phagocytic cell, along with a burgeoning number of bridging molecules from the extracellular fluid (Figure 1). Almost none of the molecules identified is a feature of the apoptotic cell surface. One molecule that universally distinguishes apoptotic cells is known, however, although it has the disadvantageous property that it cannot be cloned: it is the phospholipid phosphatidylserine (PS), which newly appears on the surface of cells undergoing apoptosis.
Figure 1.
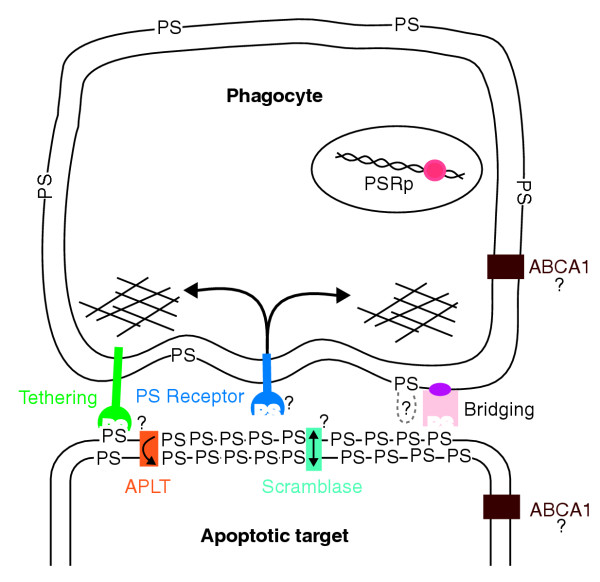
Phosphatidylserine (PS) is a central player in the recognition and engulfment of apoptotic cells. PS may be recognized by a variety of tethering receptors (shown as a single entity in green) and bridging molecules (shown as a single entity in pink) that help tether the apoptotic target to the phagocyte. The PS receptor signals to a pathway that leads to engulfment, for example by rearranging elements of the cytoskeleton (shown as cross-hatching). The proteins that correspond to the PS receptor, the aminophospholipid translocase (APLT), and the scramblase are unknown, as are the functions of ABCA1 and the PS exposed on the surface of the phagocyte. PSRp denotes the protein encoded by the psr gene, which is found within the nucleus.
Although the mechanistic details of PS appearance on the apoptotic cell surface have not been completely resolved, the basic outlines are clear (Figure 1). PS is normally sequestered in the inner leaflet of the plasma membrane bilayer by an active transporter, the aminophospholipid translocase (APLT). In apoptotic cells this enzyme is down-regulated, and an enzyme activity called scramblase is up-regulated; scramblase acts to randomize the lipids between the two leaflets of the plasma membrane, bringing PS to the surface [2]. This rearrangement is universal, occurring in vivo in a wide variety of cell types [3,4] and in both vertebrate and invertebrate organisms [5]. The ready conclusion is that PS has something to do with apoptotic cells identifying themselves to phagocytes, a conclusion borne out by the finding that masking PS on the apoptotic cell surface with the PS-binding protein annexin V blocks phagocytosis [6]. Moreover, many of the known bridging and receptor molecules bind to PS with varying degrees of specificity (Figure 1), including serum-derived protein S [7], milk fat globule protein MFG-E8 [8], and the scavenger receptors such as SR-BI [9]. Nevertheless, because the inhibition of engulfment of apoptotic cells by vesicles of PS is stereospecific [10], in the absence of bridging molecules there must be a receptor on phagocytes that directly and specifically recognizes PS itself.
Identifying the phosphatidylserine receptor
The first experimental evidence for the existence of a PS receptor came from Fadok and co-workers [11]. Their approach began with the production of monoclonal antibodies against 'stimulated' macrophages whose recognition of apoptotic cells is inhibited by PS vesicles in a stereospecific fashion [10], in contrast to 'unstimulated' macrophages, whose uptake of PS-expressing target cells is insensitive to PS vesicles (even though it is sensitive to annexin V) [6]. One monoclonal antibody, mAb 217, was selected because it bound preferentially to unfixed stimulated macrophages, and this binding was inhibited by PS vesicles. The antigenic target of mAb 217 would thus appear to have the hallmarks of a PS receptor: it is on the cell surface, it recognizes PS, and, as the authors went on to show, mAb 217 blocks engulfment of apoptotic cells [11].
What exactly might this receptor do? This question was examined in more detail in a later paper from the same laboratory [12], where an experimental system was established that allowed the binding step of the uptake of an apoptotic cell to be distinguished from the engulfment step. Bound cells or particles not expressing PS were engulfed only upon addition of PS vesicles, but coating target cells with PS vesicles did not result in significant binding to macrophages. The authors concluded that the PS receptor did not bind PS-expressing targets tightly (tethering), but that low-affinity binding could nonetheless stimulate engulfment. Curiously, it was reported that addition of mAb 217 would itself induce uptake of previously bound bystander cells, in contrast to the earlier observation that pre-incubation with the antibody prevented uptake of subsequently added apoptotic cells [11], suggesting perhaps that signal transduction induced by occupancy of the PS receptor is transient.
The next step was to identify the molecule corresponding to the PS receptor. On western blots, mAb 217 reacted with a protein of an apparent molecular weight of 70 kDa. Treatment of cellular extracts with a mixture of four O-glycosidases reduced the size of the protein to roughly 50 kDa, suggesting that the target of the antibody is glycosylated and is thus presumably a cell-surface protein. When a phage display library expressing proteins from mouse macrophages was panned with mAb 217, half of the one dozen phages sequenced contained identical sequences, from a protein in the sequence databases with a theoretical molecular weight of 47 kDa [11]. Information from the databases suggested that the gene encoding the protein was highly expressed in heart, skeletal muscle, and kidney, with lower levels of expression in many tissues. Sequence analysis suggested that the protein contained one potential transmembrane domain, although this hydrophobic region was interrupted by an aspartic acid residue in mammals and two glutamic acid residues in the Caenorhabditis elegans (nematode) homolog. Two pieces of evidence linked this gene to the engulfment of apoptotic cells. One was that expression of the mammalian gene, denoted psr, or its nematode homolog PSR-1 [13], in lymphoid cells conferred an inefficient capability to bind apoptotic targets, and perhaps to engulf them as well, notable in view of the conclusion (mentioned above) that the PS receptor does not contribute to the tethering step of engulfment [12]. The second piece of evidence was that transfecting cells with small-interfering RNA corresponding to this gene, so as to decrease expression, reduced both binding of mAb 217 to transfected cells and phagocytosis of apoptotic targets by transfected cells; whether binding of apoptotic targets by transfected cells was affected was not reported.
PS receptor knockouts
What happens when the psr gene is deleted? Wang and colleagues [13] examined this question in C. elegans, and found that, in their words, "in a time course analysis of cell corpses during development, in almost all embryonic stages, more cell corpses were observed in psr-1 embryos than in wild type embryos... On average, cell corpses of psr-1 embryos persisted for 55% longer than those of wild type animals." In these studies, expression of the protein recognized by mAb 217 was not compared in wild-type and knockout animals.
The first report of the effects of deletion of the psr gene in mammals came from the laboratory of Richard Flavell [14]. These investigators concluded that the protein encoded by the gene is required for clearance of apoptotic cells in the mouse. They observed lung developmental abnormalities and occasional brain hyperplasia, which were "associated with increased numbers of nonphagocytosed apoptotic cells". They also adoptively transferred fetal liver cells from knockout mice into lethally irradiated hosts and found that fewer of the stimulated macrophages recovered from these animals were able to engulf apoptotic cells compared with wild-type controls. Reactivity with mAb 217 was not compared between cells from knockout and wild-type animals.
The second report of a knockout of this gene in mice appeared earlier this year [15], and this study noted severe developmental defects in definitive erythropoietic and T-lymphopoietic lineages. Reduced numbers of macrophages and apoptotic cells were observed in fetal livers of knockout versus wild-type animals; the fraction of apoptotic cells that were not phagocytosed was higher in the knockouts. Once again, reactivity with mAb 217 was not compared between cells from knockout and wild-type animals.
In contrast to these studies, in the third report of a knockout of this gene by Lengeling and colleagues [1], which appears in these pages, reactivity with mAb 217 was compared between cells from knockout and wild-type animals, and no difference was observed; in each case, staining was restricted to the plasma membrane of fetal-liver-derived macrophages. This finding was foreshadowed by recent reports that the product of the psr gene is actually a nuclear protein, as judged from localization of green fluorescent protein (GFP) constructs as well as immunolocalization with antibodies directed specifically against the protein encoded by the psr gene [16,17]. Sequence analysis also indicates the presence of nuclear localization sequences in the encoded protein. Western blot analysis by Lengeling and colleagues, using a commercial antibody generated against a peptide present in the protein encoded by the psr gene, indicated that this protein does disappear from the knockout mouse. Lengeling and colleagues also document that deletion of this gene causes "perinatal lethality, growth retardation and a delay in the terminal differentiation of kidney, intestine, liver, and lungs during embryogenesis." On the other hand, in a variety of assays, no defect was observed in vivo or in vitro in the clearance of apoptotic cells in the knockout mouse.
Where do we stand?
The simplest interpretation of these studies is that the psr gene does not encode a PS receptor; rather, the gene appears to encode a nuclear protein that plays a role in development and differentiation, perhaps as a regulatory protein related to the iron-oxidase family of proteins [16]. The experimental link between the PS receptor and the presumptive psr gene is mAb 217 binding to an epitope in phage display. This methodology has the potential to identify weak cross-reacting epitopes, and the Lengeling study has shown that mAb 217 does weakly cross-react with a peptide within the protein encoded by the psr gene. This cross-reactivity could explain the reactivity of mAb 217 with recombinant PSR protein expressed in bacteria [13], although it is not clear whether it can also explain the results using RNA interference (RNAi) [12]. If the cloned gene does indeed encode a critical regulator of hematopoietic differentiation, it is perhaps not surprising that defects were observed in macrophage function in worms and mammals. If this interpretation is correct, this gene should no longer be the subject of concentrated attention from those who study the clearance of apoptotic cells. More importantly, mAb 217 remains as an important foundation for renewed attempts to identify the genuine PS receptor.
In the meantime, the product of the psr gene will live on in the databases identified as encoding a PS receptor. In doing so, it joins a rogue's gallery of functions without molecules and molecules without functions that are linked to PS (Figure 1). In a very similar story, there is a protein identified in the databases as the phospholipid scramblase that is not that protein [18], and another identified as an aminophospholipid translocase [19] that is probably not the one responsible for transport and sequestration of PS at the plasma membrane. At the same time, there is a protein, known as ced-7 in nematodes [20] or ABCA1 in mammals [21] that is required for engulfment of apoptotic cells and that seems to be involved in phospholipid movements [22] but whose function is unclear. Finally, PS itself poses some puzzles, since engulfment seems to require its exposure not only on the apoptotic target, but at lower levels on the phagocyte surface as well [23]. Why this should be the case is a mystery. All in all, PS seems to have an involved secret life whose molecular outlines are frustratingly well hidden.
Acknowledgments
Acknowledgements
We thank Margaret Halleck for her spirited assistance in the generation of Figure 1.
References
- Böse J, Gruber AD, Helming L, Schiebe S, Wegener I, Hafner M, Beales M, Köntgen F, Lengeling A. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J Biol. 2004;3:15. doi: 10.1186/jbiol10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eijnde SM, Boshart L, Reutelingsperger CPM, De Zeeuw CI, Vermeij-Keers C. Phosphatidylserine is exposed by apoptotic cells in mouse embryos in vivo. Eur J Morphol. 1997;35:54–55. [Google Scholar]
- Van den Eijnde SM, Boshart L, Reutelingsperger CPM, De Zeeuw CI, Vermeij-Keers C. Phosphatidylserine plasma membrane asymmetry in vivo: a pancellular phenomenon which alters during apoptosis. Cell Death Differ. 1997;4:311–316. doi: 10.1038/sj.cdd.4400241. [DOI] [PubMed] [Google Scholar]
- Van den Eijnde SM, Boshart L, Baehrecke EH, De Zeeuw CI, Reutelingsperger CPM, Vermeij-Keers C. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis. 1998;3:9–16. doi: 10.1023/A:1009650917818. [DOI] [PubMed] [Google Scholar]
- Krahling S, Callahan MK, Williamson P, Schlegel RA. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6:183–189. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu HJ, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nature Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi A, Kawasaki Y, Ikemoto M, Arai H, Nakanishi Y. Role of class B scavenger receptor type I in phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells. J Biol Chem. 1999;274:5901–5908. doi: 10.1074/jbc.274.9.5901. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu YC, Fadok VA, Lee MC, Gengyo-Ando K, Cheng LC, Ledwich D, Hsu PK, Chen JY, Chou BK, et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science. 2003;302:1563–1566. doi: 10.1126/science.1087641. [DOI] [PubMed] [Google Scholar]
- Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302:1560–1563. doi: 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Masuko S, Noda M, Inayoshi A, Sanui T, Harada M, Sasazuki T, Fukui Y. Defective fetal liver erythropoiesis and T lymphopoiesis in mice lacking the phosphatidylserine receptor. Blood. 2004;103:3362–3364. doi: 10.1182/blood-2003-09-3245. [DOI] [PubMed] [Google Scholar]
- Cikala M, Alexandrova O, David CN, Proschel M, Stiening B, Cramer P, Bottger A. The phosphatidylserine receptor from Hydra is a nuclear protein with potential Fe(II)-dependent oxygenase activity. BMC Cell Biol. 2004;5:26. doi: 10.1186/1471-2121-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui P, Qin B, Liu N, Pan G, Pei D. Nuclear localization of the phosphatidylserine receptor protein via multiple nuclear localization signals. Exp Cell Res. 2004;293:154–163. doi: 10.1016/j.yexcr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Zhou QS, Zhao J, Wiedmer T, Sims PJ. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood. 2002;99:4030–4038. doi: 10.1182/blood-2001-12-0271. [DOI] [PubMed] [Google Scholar]
- Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- Wu YC, Horvitz HR. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell. 1998;93:951–960. doi: 10.1016/S0092-8674(00)81201-5. [DOI] [PubMed] [Google Scholar]
- Luciani MF, Chimini G. The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 1996;15:226–235. [PMC free article] [PubMed] [Google Scholar]
- Fielding PE, Nagao K, Hakamata H, Chimini G, Fielding CJ. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry. 2000;39:14113–14120. doi: 10.1021/bi0004192. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Williamson P, Schlegel RA. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 2000;7:645–653. doi: 10.1038/sj.cdd.4400690. [DOI] [PubMed] [Google Scholar]


