Abstract
Background
Calcified amorphous tumor (CAT) of the heart is a rare non-neoplastic intracavitary cardiac mass. Several case reports have been published but large series are lacking.
Objective
To determine clinical features, current management and outcomes of this rare disease.
Design
A systematic review of all articles reporting cases of CAT in order to perform a pooled analysis of its clinical features, management and outcomes.
Data sources
An electronic search of all English articles using PUBMED was performed. Further studies were identified by cross-referencing from relevant papers.
Inclusion criteria
We restricted inclusion to articles reporting cases of CAT in the English language literature published up to July 2014.
Data extraction
One author performed data extraction using predefined data fields.
Results
A total of 27 articles, reporting 42 cases of CAT were found and included in this review.
Conclusion
In this review, the most frequent presenting symptoms were dyspnea and embolic events. Mitral valve and annulus were the most frequent location of CAT. Surgery was most of the time required to confirm diagnosis, and was relatively safe. Overall outcome after surgical resection was good.
Keywords: Calcified amorphous tumor, Heart, Nonneoplastic, Mass, Review
Highlights
-
•
CAT is a rare form of non-neoplastic intracavitary cardiac mass.
-
•
The most frequent symptoms are dyspnea and embolic events.
-
•
The most frequent locations of CAT are mitral valve or annulus.
-
•
The most frequent associated conditions are valve and end-stage renal disease.
-
•
Overall outcome is good after surgical resection.
1. Introduction
Calcified amorphous tumor (CAT) of the heart is a rare non-neoplastic intracavitary cardiac mass, with microscopic features of calcification and amorphous fibrinous material. Since its first description in 1997 as a specific entity by Reynolds and colleagues [1], several case reports have been published. Still, large series are lacking and, to date, we have no clear overview of the scope of the disease. In this article, we aim to perform a systematic review of the literature and to build a registry of all published cases of CAT in order to determine its clinical features, current management and prognosis.
2. Methods
We performed a systematic search using PubMed, according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines, for articles reporting cases of CAT in the English language literature. Additional articles were identified by a manual search of references of relevant papers. Authors of articles were contacted, if needed, to obtain additional data that was of interest to our review. We included all articles published since the first report (May 1st, 1997) up to July 31st, 2014. The titles and abstract of the identified articles were screened to determine if they met inclusion criteria. Full text articles were then retrieved and reviewed. Reference lists of the retrieved articles were searched for relevant literature. Predetermined variables were first author, year of publication, title, journal, patient clinical informations (age, gender…), tumor size and location, presenting symptoms, associated conditions, treatment, follow-up and outcome.
3. Results
A total of 27 articles reporting 42 cases of CAT were found. Table A lists the clinical characteristics of the patients. Table B reports the pooled clinical data. The mean age at presentation was 54 years (range 16–85), with a female predominance (64%). CAT was detected in all cardiac chambers, but predominated on the mitral valve or annulus (36%), in the right atrium (21%) or the right ventricle (17%). Mean tumor size was 29 × 17 mm, ranging from 1.7 mm punctate lesion to very large masses (20 × 90mm) or even diffuse left ventricular (LV) infiltration.
Table A.
Clinical characteristics of published cases of calcified amorphous tumor.
| Report | Case no | Sex | Age | Tumor site | Presentation | Follow-up | Comorbidities/underlying disease | Size (mm) | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Reynolds et al. (1997) [1] | 1 | M | 16 | LA | Exercise intolerance, near-syncope | Residual calcifications in LA | Mediastinal RT and chemo for neuroblastoma at age 3 months | 45 × 23 × 3 | Surgical excision |
| 2 | M | 30 | LV | Near-syncope, chest pain, palpitations | Small residual calcified nodule in LV, 3 years 6 months | None | 30 × 12 × 10 | Surgical excision | |
| 3 | F | 33 | RV | Shortness of breath | Lost to follow-up | Recurrent PE | 20 × 23 | Surgical excision | |
| 4 | F | 34 | RV | Vertigo, orthopnea | Lost to follow-up | Systemic lupus-like illness | 35 × 25 × 15 | Surgical excision | |
| 5 | F | 48 | MV | CVA | NED 4 months | MR and TR, cleft mitral valve leaflet | 30 × 2 | Surgical excision | |
| 6 | F | 60 | LV, MV | CVA, retinal emboli | Alive, left jugular foramen tumor | MR and AR with enlarged LA | 15 × 15 × 15 | Surgical excision | |
| 7 | M | 65 | RV, TV | Shortness of breath | Died of non cardiac cause 30 days after diagnosis | CAD, recurrent PE | 33 × 27 × 12 | No surgery | |
| 8 | F | 67 | RA | Syncope | NED 18 years | CAD, CHF | 65 × 50 × 50 | Surgical excision | |
| 9 | M | 67 | LV | Syncope | NED 1 year 4 months | CAD, ESRD, tumoral calcinosis | 15 × 3 × 3 | Surgical excision | |
| 10 | F | 73 | RA, SVC | Dizziness, dyspnea on exertion | NED 1 year 5 months | Diverticulitis, partial resection, total parenteral nutrition | 20 × 90 | Surgical excision | |
| 11 | F | 75 | LV | “Funny sensation” in chest | NED 6 years 11 months | Diabetes mellitus | 20 × 20 × 20 | Surgical excision | |
| Chaowalit et al. (2005) [15] | 12 | F | 20 | RV | PE, dyspnea | N/A | Chest trauma 3 years earlier | 40 × 22 × 18 | Surgical excision |
| Lewin et al. (2006) [12] | 13 | F | 60 | RV | Syncope | Died 1 day after surgery | No prior cardiac history. | 40 × 30 × 25 | Surgical excision |
| Fealey et al. (2007) [14] | 14 | F | 20 | RV | PE, cough, shortness of breath, fatigue | Residual calcified RV nodule, recurrent PE 2 years after surgical resection, second resection | None | 40 × 35 × 25 | Surgical excision |
| Khulbey et al. (2008) [16] | 15 | M | 26 | RA | Prolonged fever and constitutional symptoms | NED 1 year | Atrial septal closure | 30 × 20 × 20 | Surgical excision |
| Inamdar et al. (2008) [17] | 16 | F | 85 | MA | Chronic fatigue | N/A | MAC, ESRD, HTN, DM | 23 × 9 | Surgical excision |
| Ho et al. (2008) [3] | 17 | M | 44 | LV and mitral chordal apparatus diffuse infiltration | Shortness of breath on exertion | N/A | Severe LV systolic dysfunction | Diffuse infiltration | Referred for heart transplantation |
| Gutierrez et al. (2008) [18] | 18 | M | 35 | RA | Septic shock | NED 2 months | ESRD (Alport syndrome), HTN | 20 × 15 × 14 | Surgical excision |
| Flynn et al. (2009) [19] | 19 | M | Young | RV | Syncope, PE, severe tricuspid regurgitation | N/A | N/A | 14 × 12 × 5 | Surgical excision and pulmonary TEA |
| Habib et al. (2010) [5] | 20 | F | 58 | MA, MV, diffuse LV infiltration | Ventricular tachycardia | Medical treatment, persistent VT | Ventricular tachycardia, SCD, mild mitral regurgitation | Diffuse infiltration | Medical treatment |
| Gupta et al. (2010) [20] | 21 | F | 40 | RA | Shortness of breath on exertion, cough, fatigue | NED 8 months | None | 30 × 20 × 15 | Surgical excision |
| Vaideeswar et al. (2010) [13] | 22 | M | 56 | RA | Progressive shortness of breath, PE, blurring of vision | NED 2 years | N/A | N/A | Surgical excision and pulmonary TEA |
| 23 | M | 35 | RA | Exertional dyspnea, dizziness on walking, PE | Died 7 days after surgery | N/A | 32 × 23 × 13 | Surgical excision and pulmonary TEA | |
| Kubota et al. (2010) [8] | 24 | F | 64 | LV, MA | Incidental | NED 3 years | ESRD, DM | 3 × 27 | Surgical excision |
| 25 | M | 44 | LV, PM | Incidental | NED 3 years | ESRD | 6 × 28 | Surgical excision | |
| Greaney et al. (2011) [21] | 26 | F | 69 | LV, MV | Left-sided heart failure, stroke | NED 3 months | Severe COPD | 20 | Surgical excision |
| Ananthakrishna et al. (2011) [22] | 27 | F | 45 | LV | Breathlessness | NED 4 months | Rheumatic heart disease | 40 × 35 × 20 | Surgical excision, mitral and aortic valve replacement |
| Vlasseros et al. (2011) [2] | 28 | F | 65 | LV, MV | Visual loss (central retinal artery occlusion) | NED 8 months | DM, HTN | 26 × 17 × 5 | Surgical excision and MVR |
| Lin et al. (2011) [23] | 29 | F | 74 | LA | Incidental | NED 6 months | None | 14 × 27 | Surgical excision |
| De Sousa et al. (2011) [24] | 30 | M | 17 | TV | Cardiomegaly | NED 3 months | Ebstein anomaly | 15 × 15 × 13 | Surgical excision and TV valvuloplasty |
| Fujiwara et al. (2012) [7] | 31 | M | 58 | MA | Incidental | N/A | ESRD, MAC | N/A | Surgical excision |
| 32 | M | 65 | MA | Incidental | N/A | ESRD, MAC | 7 × 2 | Surgical excision | |
| Nishigawa et al. (2012) [25] | 33 | F | 78 | LA | Incidental | N/A | MAC | 1.7 | Surgical excision |
| Nazli et al. (2013) [4] | 34 | F | 54 | LV | Central retinal artery occlusion | NED 1 year | Hypothyroidism | 38 × 25 | Surgical excision |
| Yamamoto et al. (2013) [26] | 35 | F | 82 | MA | Progressive heart failure | N/A | N/A | 37 × 4 | Surgical excision |
| Kawata et al. (2013) [9] | 36 | M | 59 | MA | Incidental | N/A | DM, ESRD, MAC | 28 × 6 | Surgical excision |
| Rehman et al. (2014) [27] | 37 | F | 62 | RV | Severe exertional dyspnea, chronic pulmonary embolism | N/A | Atrial septal defect | 30 × 20 | Surgical excision |
| Mohamedali et al. (2014) [10] | 38 | F | 69 | MA | Dyspnea, epigastric pain | N/A | ESRD, MAC | 7 × 3 × 3 | Surgical excision |
| Choi et al. (2014) [28] | 39 | F | 57 | RA | Dyspnea, fever, cough | NED 1 year | N/A | 19 × 13 × 8 | Surgical excision |
| Hussain et al. (2014) [29] | 40 | F | 80 | LV | Near-syncope | N/A | HTN, dyslipidemia, CAD, lung adenocarcinoma | 50 | Surgical excision |
| 41 | F | 69 | MV | Palpitations | N/A | HTN, DM, dyslipidemia | 10 × 10 | Surgical excision and MV valvuloplasty | |
| 42 | F | 60 | RA | Dyspnea | N/A | Breast cancer 10 years earlier | 16 × 23 | Surgical excision |
AR: aortic regurgitation, CAD: coronary artery disease, COPD: chronic obstructive pulmonary disease, CVA: cerebrovascular accident, DM: diabetes mellitus, ESRD: end-stage renal disease, F: female, HTN: hypertension, LA: left atrium, LV: left ventricle, M: male, MA: mitral annulus, MAC: mitral annular calcification, MR: mitral regurgitation, MV: mitral valve, N/A: not available, NED: no evidence of disease, PE: pulmonary embolism, PM: papillary muscle, RA: right atrium, RT: radiotherapy, RV: right ventricle, SCD: sudden cardiac death, TEA: thromboendarterectomy, TR: tricuspid regurgitation, TV: tricuspid valve, VT: ventricular tachycardia.
Table B.
Demographic and clinical data of the population. (n = 42).
| Variables | Values |
|---|---|
| Male gender | 15 (36) |
| Age, years | 54 ± 18 |
| CAT localization | |
| Mitral valve or annulus | 15 (36) |
| Right atrium | 9 (21) |
| Right ventricle | 7 (17) |
| Left ventricle | 6 (14) |
| Left atrium | 3 (7) |
| Tricuspid valve or annulus | 2 (5) |
| Tumor size, mm | 29 ± 16 × 17 ± 11 |
| Clinical presentation (multiple symptoms possible) | |
| Dyspnea | 19 (45) |
| Syncope | 9 (21) |
| Pulmonary embolism | 8 (19) |
| Incidental | 7 (17) |
| Systemic embolism | 5 (12) |
| Chest pain | 2 (5) |
| Other | 9 (21) |
| Follow-up | |
| No evidence of disease, months | 12 [2-216] |
| Residual disease | 3 (14) |
| Recurrence | 1 (5) |
| No data available (lost to follow-up or unavailable data) | 21 (50) |
| Outcome | |
| Death (CAT or surgery related) | 2 (5) |
| Associated conditions | |
| Valve disease | 13 (31) |
| End stage renal disease | 9 (21) |
| Mitral annulus calcification | 6 (14) |
| Diabetes mellitus | 6 (14) |
| Coronary artery disease | 5 (12) |
| Hypertension | 4 (10) |
| Congestive heart failure | 2 (5) |
| Treatment | |
| Surgical excision | 39 (93) |
| Other | 3 (7) |
Values are given as mean ± SD, no. (%), or median [range].
The most frequent presenting symptom was dyspnea (45%) followed by syncope (21%). Pulmonary or systemic embolization was reported in 31% of the cases. CAT was discovered incidentally in 17% of the patients.
The most frequently associated conditions were valve disease (31%) concomitant with MAC (14%), end-stage renal disease (ESRD) (21%), diabetes (14%), and coronary artery disease (12%).
Surgery was performed in most of the reported cases (93%), with a vast majority of favorable outcomes. Nonetheless, two patients died postoperatively (5%). Medical treatment was favored in 2 cases presenting diffuse calcium infiltration of the LV. Recurrence occurred in 1 case and residual calcifications were observed in 14% on follow-up imaging studies. The mean follow-up without evidence of disease was 12 months.
4. Discussion
This review, encompassing all published cases of CAT allows giving a scope of this rare disease. However, there might have been some bias. Indeed, the condition is inherently underreported, since suspected but unoperated and thus unconfirmed cases were probably, were probably most of the time not reported.
Pathophysiologic hypotheses involving an organized thrombus origin favored by hypercoagulability [1] and/or phosphocalcic metabolism abnormalities [2] have been raised, but the pathogenesis of CAT remains poorly understood. Our review does not support the hypothesis of hypercoagulability.
Although endomyocardial biopsy was used for diagnosis in one case of diffuse LV infiltration [3], ruling out other cardiac masses or neoplasms requires surgery for histological confirmation in most of the cases, as clinical presentation of patients with cardiac masses tends to be similar. Current imaging cardiac techniques do not specifically differentiate cardiac CAT from other masses, but some features may contribute to establish the diagnosis [4]. On echocardiography, CAT usually appears as a calcified endocavitarian mass that may be located in any cardiac chamber, on any valves or on valvular annuli (Fig. 1). Size can vary from small punctate lesions to very large masses. Diffuse LV myocardial infiltrations have been reported [3], [5] but these forms probably constitute a distinct type of disease.
Fig. 1.
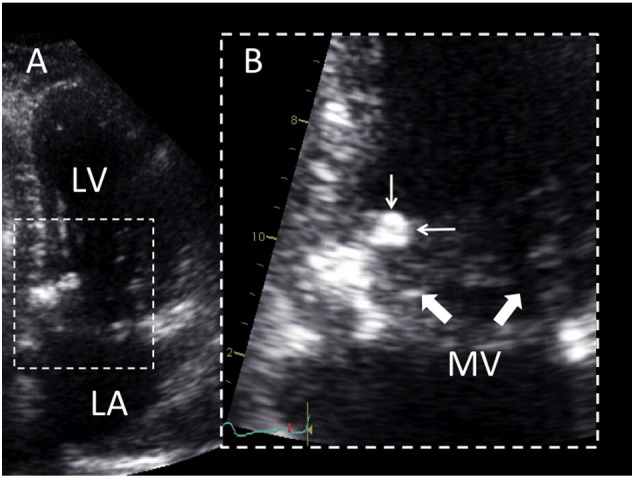
2D echocardiography of CAT (personal unpublished data).
Echocardiographic four chamber view (Panel A) showing a mobile mass of 7 × 9 mm, attached on the ventricular side of the mitral annulus, close to the posterior commissure. Zoom view (Panel B) allows characterizing the relationship of the mass (small arrows) with the mitral leaflets (large arrows). LV, left ventricle; LA, left atrium.
Because of the calcifications, CAT can be mistaken for osteosarcoma, calcified myxoma or vegetations on imaging studies but the histopathologic appearance of the former is straightforward and entirely benign as it is composed of nodular calcium deposits surrounded by an amorphous hyalinized material [6] (Fig. 2). Clinical characteristics of the patient are also helpful, as patients frequently present concomitant conditions such as pre-existing valve disease (MAC for instance), end-stage renal disease (ESRD), or other cardiovascular risk factors. MAC-related CAT seems to constitute a subgroup of CAT, and is usually associated with ESRD [7], [8], [9], [10]. Kubota and colleagues proposed the descriptive term of “swinging calcified amorphous tumor” for mobile lesions arising from a MAC [8]. This subgroup of CAT seems to carry a high embolic risk, and rapid growth characteristics. Because of its mobile characteristics, one might postulate that CAT may causally contribute to the occurrence of stroke. However, the presence of MAC in itself is associated with an increased risk of stroke, independently of traditional risk factors [11]. In the current registry, CAT was associated with ESRD in 21% and with MAC in 14% of the patients.
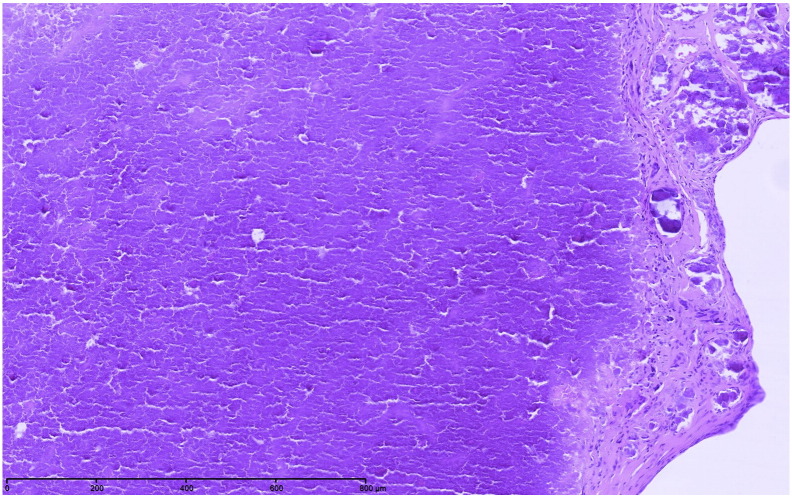
Fig. 2.
CAT microscopic image (personal unpublished data).
Microscopic findings showing heterogeneous calcium deposits with surrounding amorphous eosinophilic and fibrinous material (hematoxilin and eosin, original × 100).
The discovery of CAT may be incidental, but most of the time symptoms are related to embolization or obstruction, depending on its size and location. The most common presenting symptoms in this registry were dyspnea, embolic event and syncope. An embolic event is a frequent presenting condition and mobile lesions definitely indicate a higher embolic risk [8]. However, traditional cardiovascular risk factors are highly prevalent, which may contribute to the high prevalence of cerebrovascular events at presentation. Less frequently, atypical chest pain or ventricular arrhythmia may occur but the etiological role of the CAT is unclear [5]. The growth rate of CAT is largely unknown, but may be relatively fast, especially in MAC-related CAT. Indeed, tumors growing very rapidly in a time span of 6 weeks to 1 year have been reported [8], [9].
Surgical resection remains the diagnostic and therapeutic standard for pedunculated lesions, although it carries some procedural risk: 2 patients (5%) died during the perioperative period [12], [13]. Recurrence after surgery seems infrequent as only 1 patient presented recurrent disease 2 years after surgical resection [14].
5. Conclusions
Our systematic review, encompassing all published cases of CAT provides more insights into the clinical characteristics of this poorly known disease. Patients with CAT can present a large variety of symptoms, most of the time related to embolization or obstruction depending on the size and the location of the mass, but may also be discovered incidentally. CAT can arise from any cardiac chamber or valve, but in this review mitral valve and annulus appear to be its most frequent location and its size can vary from small punctate lesions to large pedunculated masses. Surgery is most of the time required to confirm diagnosis, and appears relatively safe. MAC-related CATs seem to constitute a subtype of this benign tumor, exhibiting specific characteristics including a rapid growth and a high embolic risk.
However, the natural history of the disease, its embolic potential as well as its best management strategy remain largely unknown. Therefore, there is a need for collecting the clinical data and building up a registry of all the patients presenting with echocardiographic features suggestive of CAT, in order better characterize the natural history of the disease, its outcome and the best therapeutic approach.
Competing interests
All authors declare that they have no conflicts of interest relevant to this manuscript.
Contributor Information
Quentin de Hemptinne, Email: quentindh@gmail.com.
Didier de Cannière, Email: didier_decanniere@stpierre-bru.be.
Jean-Luc Vandenbossche, Email: jlvdboss@ulb.ac.be.
Philippe Unger, Email: punger@ulb.ac.be.
References
- 1.Reynolds C., Tazelaar H.D., Edwards W.D. Calcified amorphous tumor of the heart (cardiac CAT) Hum Pathol. 1997;28:601–606. doi: 10.1016/s0046-8177(97)90083-6. [DOI] [PubMed] [Google Scholar]
- 2.Vlasseros I., Katsi V., Tousoulis D., Tsiachris D., Bousiotou A., Souretis G. Visual loss due to cardiac calcified amorphous tumor: a case report and brief review of the literature. Int J Cardiol. 2011;152:e56–e57. doi: 10.1016/j.ijcard.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Ho H.H., Min J.K., Lin F., Wong S.C., Bergman G. Images in cardiovascular medicine. Calcified amorphous tumor of the heart. Circulation. 2008;117:e171–e172. doi: 10.1161/CIRCULATIONAHA.107.730838. [DOI] [PubMed] [Google Scholar]
- 4.Nazli Y., Colak N., Atar I.A., Alpay M.F., Haltas H., Eryonucu B. Sudden unilateral vision loss arising from calcified amorphous tumor of the left ventricle. Tex Heart Inst J. 2013;40:453–458. [PMC free article] [PubMed] [Google Scholar]
- 5.Habib A., Friedman P.A., Cooper L.T., Suleiman M., Asirvatham S.J. Cardiac calcified amorphous tumor in a patient presenting for ventricular tachycardia ablation: intracardiac echocardiogram diagnosis and management. J Interv Card Electrophysiol. 2010;29:175–178. doi: 10.1007/s10840-009-9418-3. [DOI] [PubMed] [Google Scholar]
- 6.Miller D.V., Tazelaar H.D. Cardiovascular pseudoneoplasms. Arch Pathol Lab Med. 2010;134:362–368. doi: 10.5858/134.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara M., Watanabe H., Iino T., Kobukai Y., Ishibashi K., Yamamoto H. Two cases of calcified amorphous tumor mimicking mitral valve vegetation. Circulation. 2012;125:e432–e434. doi: 10.1161/CIRCULATIONAHA.111.072793. [DOI] [PubMed] [Google Scholar]
- 8.Kubota H., Fujioka Y., Yoshino H., Koji H., Yoshihara K., Tonari K. Cardiac swinging calcified amorphous tumors in end-stage renal failure patients. Ann Thorac Surg. 2010;90:1692–1694. doi: 10.1016/j.athoracsur.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 9.Kawata T., Konishi H., Amano A., Daida H. Wavering calcified amorphous tumour of the heart in a haemodialysis patient. Interact Cardiovasc Thorac Surg. 2013;16:219–220. doi: 10.1093/icvts/ivs430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamedali B., Tatooles A., Zelinger A. Calcified amorphous tumor of the left ventricular outflow tract. Ann Thorac Surg. 2014;97:1053–1055. doi: 10.1016/j.athoracsur.2013.06.115. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin E.J., Plehn J.F., D'Agostino R.B., Belanger A.J., Comai K., Fuller D.L. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–379. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 12.Lewin M., Nazarian S., Marine J.E., Yuh D.D., Argani P., Halushka M.K. Fatal outcome of a calcified amorphous tumor of the heart (cardiac CAT) Cardiovasc Pathol. 2006;15:299–302. doi: 10.1016/j.carpath.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Vaideeswar P., Karunamurthy A., Patwardhan A.M., Hira P., Raut A.R. Cardiac calcified amorphous tumor. J Card Surg. 2010;25:32–35. doi: 10.1111/j.1540-8191.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 14.Fealey M.E., Edwards W.D., Reynolds C.A., Pellikka P.A., Dearani J.A. Recurrent cardiac calcific amorphous tumor: the CAT had a kitten. Cardiovasc Pathol. 2007;16:115–118. doi: 10.1016/j.carpath.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Chaowalit N., Dearani J.A., Edwards W.D., Pellikka P.A. Calcified right ventricular mass and pulmonary embolism in a previously healthy young woman. J Am Soc Echocardiogr. 2005;18:275–277. doi: 10.1016/j.echo.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Khulbey S., Ramana K.V., Kumar S., Dikshit V. Amorphous cardiac tumor of right atrium late after atrial septal defect closure. Indian J Thorac Cardiovasc Surg. 2008;24:129–131. [Google Scholar]
- 17.Inamdar V., Wanat F.E., Nanda N.C., Pothineni K.R., Burri M.V., Kimmler S. Amorphous calcific tumor of the mitral annulus echocardiographically mimicking a vegetation. Echocardiography. 2008;25:537–539. doi: 10.1111/j.1540-8175.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez-Barrios A., Muriel-Cueto P., Lancho-Novillo C., Sancho-Jaldón M. Calcified amorphous tumor of the heart. Rev Esp Cardiol. 2008;61:892–893. [PubMed] [Google Scholar]
- 19.Flynn A., Mukherjee G. Calcified amorphous tumor of the heart. Indian J Pathol Microbiol. 2009;52:444–446. doi: 10.4103/0377-4929.55026. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R., Hote M., Ray R. Calcified amorphous tumor of the heart in an adult female: a case report. J Med Case Rep. 2010;4:278. doi: 10.1186/1752-1947-4-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greaney L., Chaubey S., Pomplun S., St Joseph E., Monaghan M., Wendler O. Calcified amorphous tumour of the heart: presentation of a rare case operated using minimal access cardiac surgery. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.02.2011.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananthakrishna R., Nanjappa M.C., Kamalapurkar G., Bhat P., Panneerselvam A., Chander N. Cardiac tumour in a patient with rheumatic heart disease. BMJ Case Rep. 2011 doi: 10.1136/bcr.04.2011.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y.-C., Tsai Y.-T., Tsai C.-S. Calcified amorphous tumor of left atrium. J Thorac Cardiovasc Surg. 2011;142:1575–1576. doi: 10.1016/j.jtcvs.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 24.de Sousa J.S., Tanamati C., Marcial M.B., Stolf N.A.G. Calcified amorphous tumor of the heart: case report. Rev Bras Cir Cardiovasc. 2011;26:500–503. doi: 10.5935/1678-9741.20110031. [DOI] [PubMed] [Google Scholar]
- 25.Nishigawa K., Takiuchi H., Kubo Y., Masaki H., Tanemoto K. Calcified amorphous tumor: three-dimensional transesophageal echocardiography. Asian Cardiovasc Thorac Ann. 2012;20:355. doi: 10.1177/0218492311423071. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M., Nishimori H., Wariishi S., Fukutomi T., Kond N., Kihara K. A cardiac calcified amorphous tumor stuck in the aortic valve that mimicked a chameleon's tongue: report of a case. Surg Today. 2014;44:1751–1753. doi: 10.1007/s00595-013-0698-y. [DOI] [PubMed] [Google Scholar]
- 27.Rehman A., Heng E.E., Cheema F.H. Calcified amorphous tumour of right ventricle. Lancet. 2014;383:815. doi: 10.1016/S0140-6736(13)60997-6. [DOI] [PubMed] [Google Scholar]
- 28.Choi E.K., Ro J.Y., Ayala A.G. Calcified amorphous tumor of the heart: case report and review of the literature. Methodist Debakey Cardiovasc J. 2014;10:38–40. doi: 10.14797/mdcj-10-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain N., Rahman N., Rehman A. Calcified amorphous tumors (CATs) of the heart. Cardiovasc Pathol. 2014 doi: 10.1016/j.carpath.2014.07.003. [DOI] [PubMed] [Google Scholar]