Abstract
Objective
The recurrence and progression of ameloblastoma are unpredictable. Therefore, we examined the influence of clinical factors on recurrence time and analyzed the clinical factors associated with early recurrence and cancerization. We then developed a staging system to predict early recurrence and cancerization.
Methods
All of the primary craniofacial ameloblastoma patients treated in Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine were recorded. There were 87 recurrent cases used to create a staging system and tested in a Cox regression analysis for risk factors associated with early recurrence or cancerization following surgery.
Results
There were 890 craniofacial ameloblastoma patients, and 72 cases had recurrence. There were also 15 cases with cancerous recurrence. The overall recurrence rate was 9.78%, and the cancer rate was 1.69%. The primary cases were classified into the following 3 stages based on clinicopathological features: stage I, the maximum tumor diameter ≤6 cm; stage II, the maximum diameter of tumor >6 cm or tumor invasion to the maxilla sinus/orbital floor/soft tissue; and stage III, tumor invasion of the skull base or metastasis into regional lymph nodes. When the method of surgery was controlled by partial correlation, the staging had significance with recurrence time (P=0.004). The Cox analysis showed the tumor stage was correlated with recurrence time (P=0.027) and cancerization time (P=0.002). However, the surgical method did not influence the recurrence time when adjusted for cofounding variables.
Conclusions
Tumor larger than 6 cm and invasion to soft tissues or adjacent anatomical structures are associated with early recurrence. This staging system can be used to predict the risk factors of early recurrence and cancerization in ameloblastoma patients.
Keywords: Recurrence, ameloblastoma, stage, ameloblastic carcinoma, cancerization
Introduction
Ameloblastoma is one of the common benign neoplasms unique to the craniofacial region (1-3). The tumor is odontogenic in origin and is described as a locally aggressive tumor. Several recurrent cases have demonstrated that there are malignant changes in pre-existing ameloblastoma, which are termed secondary type ameloblastic carcinoma. Although ameloblastoma is a common odontogenic tumor, the incidence of these tumors is very low and the reported numbers are insufficient to permit clinical research studies (1,4). Furthermore, the incidence of ameloblastic carcinomas derived from ameloblastomas is too limited for analysis (5,6). The treatment of ameloblastoma is based on numerous retrospective studies, and there is currently no meta-analysis in the literature to guide the therapy and management of these patients in a universally acceptable manner.
The tumor is essentially benign in nature, but recurrent cases can present as ameloblastic carcinomas or malignant ameloblastomas (6,7). The functional and aesthetic preservation of the craniofacial region is important for an adequate quality of life in these patients (8). Current evidence suggests that enucleation is not an accepted method of therapy due to the high recurrence rates specifically for solid or multicystic variants (7) with tumor extension beyond clinically appreciable margins (9). The cystic or unilocular ameloblastomas are the only variants appropriate for curettage and chemical cauterization with Carnoy’s solution because the other subtypes require aggressive resections (10). The overall recurrence rate of ameloblastoma with current methods of treatment is approximately 10% (7), and recurrent cases are malignant (11). This result is relatively high for a benign tumor. Unfortunately, we are not currently able to predict either recurrences or the development of malignant ameloblastoma (11,12).
We have identified the relation between several clinical factors of early recurrence and cancerization through experience. In this study, we report and analyze the recurrent and cancerous cases of ameloblastoma using both Cox regression and the Kaplan-Meier method to identify the clinical factors associated with early recurrence and cancerization.
Materials and methods
Patients
This study examined all patients diagnosed with primary ameloblastoma in the Department of Oral Maxillofacial Head & Neck Oncology, Shanghai Ninth People’s Hospital from 2001 to 2014. There were 87 recurrent cases, including 15 cancerous recurrences. Only the patients who developed recurrence after the first surgery were included in the study, and the patients are termed recurrent ameloblastoma cases in this manuscript. This retrospective study was approved by the Institutional Review Board of Shanghai Ninth People’s Hospital.
Follow-up and data analysis
The initial surgical treatment included aggressive curettage and partial or segmental resections. The treatment planning involved thorough consultations with the tumor board of the institute. The patient follow-up for cases with tumors in the Department of Oral Maxillofacial Head & Neck Oncology, Shanghai Ninth People’s Hospital involved examinations every 3 to 6 months. During the follow-up, the patients were clinically examined and followed by orthopantomogram & computed tomography of the head and neck. If recurrence was suspected, an incision biopsy was performed to confirm the recurrence.
A retrospective chart was prepared for all the important initial clinical, pathological, and demographic factors from the documented records of the 87 patients. The factors responsible for early recurrence and cancerization were grouped to create a staging system which was tested using Cox regression and Kaplan-Meier methods by adjusting for the following factors: age, gender, method of initial therapy (resection or curettage), radiological finding (unicystic or multicystic), and histological types [World Health Organization (WHO)]. The statistical analysis was performed with SPSS software (Version 17.0; SPSS Inc., Chicago, IL, USA).
Results
Descriptive statistics
There were 890 craniofacial ameloblastoma patients treated during the study period, and 87 patients were recurrent cases. There were 17 patients with tumors in the maxilla, 70 tumors in the mandible, and 4 involving the skull base. The surgical records of these patients revealed that 49 patients were treated by aggressive curettage, and 38 patients were resected with margin. The recurrence rate was 9.78%, and the cancerous rate was 1.69%.
As shown in Figure 1, the patient ages ranged from 13 to 72 years, and the median age was 35.0 years. The study cohort consisted of 54 male and 33 female patients, and there was no significant gender predilection.
1.
Histogram of age. SD, standard deviation.
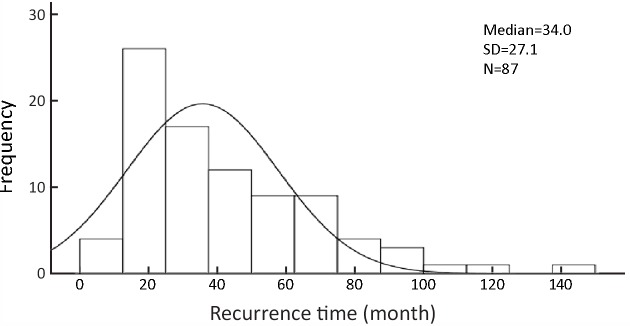
The recurrence time from the time of first surgery to diagnosis of recurrence was evaluated. The median time of recurrence after the initial surgery was 34.0 months. The data in Figure 2 illustrate the time interval of recurrence.
2.
Histogram of recurrence time. SD, standard deviation.
Survival
The following three stages were used according to the primary tumor before initial surgery: stage I, the maximum tumor diameter ≤6 cm; stage II, the maximum diameter of tumor >6 cm or tumor invasion to the maxilla sinus/orbital floor/soft tissue; and stage III, tumor invaded the skull base or metastasis into regional lymph nodes.
The relation between recurrence time and initial stage of presentation according to preoperative records for the 87 patients was analyzed with the correlation method. The results showed that our method of staging had a significant relation with recurrence time (P=0.008). Additionally, a partial correlate analysis was used to avoid errors associated with the surgical method. This analysis showed that despite the method of surgery (curettage or bone resection) there was a significant correlation between the time of recurrence in the cohort and tumor stage (P=0.004) (Table 1).
1.
Correlation of recurrence time and stage grouping after method of initial surgery was controlled
| Control variables | Correlation | P (two-tailed) | df |
| a, cells contain zero-order (Pearson) correlations; df, degree of freedom. | |||
| Nonea | −0.283 | 0.008 | 85 |
| First operation (Curettage or bone resection) | −0.310 | 0.004 | 84 |
Table 2 shows the demographic details and the clinical and radiological observations for the cohort. The one-way analysis of variance (ANOVA) and Chi-square test were used to identify the risk factors associated with recurrence time and cancerization, respectively. The one-way ANOVA analysis showed that only tumor stage was correlated with recurrence time using our staging system. We also found that patient age, site, surgical method, and tumor stage were correlated with cancerization.
2.
Demographic details and univariate analysis of various clinical and radiological observations to recurrence time and cancerous cases
| Variables | Recurrence [n (%)] (N=87) | Mean recurrence time (month) | P (one-way ANOVA) | Cancerization [n (%)] (N=15) | P (Chi-square test) |
| ANOVA, analysis of variance. | |||||
| Age (year) | 0.570 | 0.015 | |||
| ≤30 | 28 (32.18) | 37.54 | 3 (20.00) | ||
| 31−50 | 38 (43.68) | 42.95 | 4 (26.67) | ||
| >50 | 21 (24.14) | 45.48 | 8 (53.33) | ||
| Gender | 0.369 | 0.062 | |||
| Male | 54 (62.07) | 39.76 | 13 (86.67) | ||
| Female | 33 (37.93) | 45.18 | 2 (13.33) | ||
| Site | 0.174 | <0.001 | |||
| Maxilla | 17 (19.54) | 33.76 | 9 (60.00) | ||
| Mandible | 70 (80.46) | 43.77 | 6 (40.00) | ||
| Radiology | 0.786 | 0.748 | |||
| Unicystic | 26 (29.89) | 43.04 | 5 (33.33) | ||
| Multicystic | 61 (70.11) | 41.30 | 10 (66.67) | ||
| Initial surgical method | 0.138 | 0.048 | |||
| Curettage | 49 (56.32) | 39.84 | 5 (33.33) | ||
| Bone resection | 38 (43.68) | 44.37 | 10 (66.67) | ||
| Pathology | 0.244 | 0.432 | |||
| Unicystic | 14 (16.09) | 39.21 | 2 (13.33) | ||
| Desmoplastic | 10 (11.49) | 52.10 | 0 | ||
| Peripheral | 6 (6.90) | 57.50 | 1 (6.67) | ||
| Solid/multicystic | 57 (65.52) | 39.00 | 12 (80.00) | ||
| Stage | 0.028 | <0.001 | |||
| I | 61 (70.11) | 46.64 | 2 (13.33) | ||
| II | 18 (20.69) | 33.11 | 11 (73.33) | ||
| III | 8 (9.20) | 24.63 | 2 (13.33) | ||
The Cox analysis confirmed that tumor stage was correlated with recurrence time. Table 3 illustrates the Cox regression using the type of initial surgery method, site, pathological classification, radiological features (unicystic or multicystic), age group, and gender analyses with our staging system. Interestingly, the histological type of tumor did not influence the outcome. The method of surgery had no influence on patient prognosis. Table 4 shows the Cox analysis of these factors in cancerous cases. The results revealed that tumor stage was significantly correlated with cancerization.
3.
Cox analysis for factors associated with recurrence time in the cohort
| Variables | P | HR | 95% CI for HR | |
| Lower | Upper | |||
| HR, hazard ratio; 95% CI, 95% confidence interval; *, Group 1: ≤30 years old, Group 2: 31–50 years old, Group 3: >50 years old. | ||||
| Age group* | 0.304 | |||
| Group 2:Group 1 | 0.209 | 0.683 | 0.377 | 1.238 |
| Group 3:Group 1 | 0.157 | 0.635 | 0.338 | 1.192 |
| Gender | ||||
| Female:male | 0.108 | 0.664 | 0.403 | 1.094 |
| Site | ||||
| Mandible:maxilla | 0.754 | 0.872 | 0.370 | 2.056 |
| Radiology | ||||
| Multicystic:unicystic | 0.314 | 1.447 | 0.704 | 2.975 |
| Initial surgical method | ||||
| Resection:curettage | 0.195 | 0.714 | 0.429 | 1.188 |
| Pathology | 0.269 | |||
| Desmoplastic:unicystic | 0.298 | 0.552 | 0.180 | 1.689 |
| Peripheral:unicystic | 0.107 | 0.390 | 0.124 | 1.226 |
| Multicystic/solid:unicystic | 0.665 | 0.831 | 0.360 | 1.918 |
| Tumor stage | 0.027 | |||
| Stage II:stage I | 0.015 | 2.130 | 1.156 | 3.923 |
| Stage III:stage I | 0.130 | 2.446 | 0.767 | 7.794 |
4.
Cox analysis for factors associated with cancerization time in the cohort
| Variables | P | HR | 95% CI for HR | |
| Lower | Upper | |||
| HR, hazard ratio; 95% CI, 95% confidence interval; *, Group 1: ≤30 years old, Group 2: 31–50 years old, Group 3: >50 years old. | ||||
| Age group* | 0.454 | |||
| Group 2:Group 1 | 0.338 | 0.420 | 0.071 | 2.474 |
| Group 3:Group 1 | 0.801 | 1.298 | 0.170 | 9.879 |
| Gender | ||||
| Female:male | 0.050 | 0.137 | 0.019 | 0.999 |
| Site | ||||
| Mandible:maxilla | 0.476 | 0.566 | 0.118 | 2.709 |
| Radiology | ||||
| Multicystic:unicystic | 0.502 | 1.967 | 0.272 | 14.216 |
| Initial surgical method | ||||
| Resection:curettage | 0.958 | 1.047 | 0.191 | 5.735 |
| Pathology | 0.254 | |||
| Desmoplastic:unicystic | 0.982 | 0.000 | 0.000 | 0.000 |
| Peripheral:unicystic | 0.393 | 0.261 | 0.012 | 5.689 |
| Multicystic/solid:unicystic | 0.486 | 2.832 | 0.151 | 53.074 |
| Tumor stage | 0.002 | |||
| Stage II:stage I | 0.000 | 25.783 | 4.203 | 158.175 |
| Stage III:stage I | 0.038 | 19.246 | 1.178 | 314.553 |
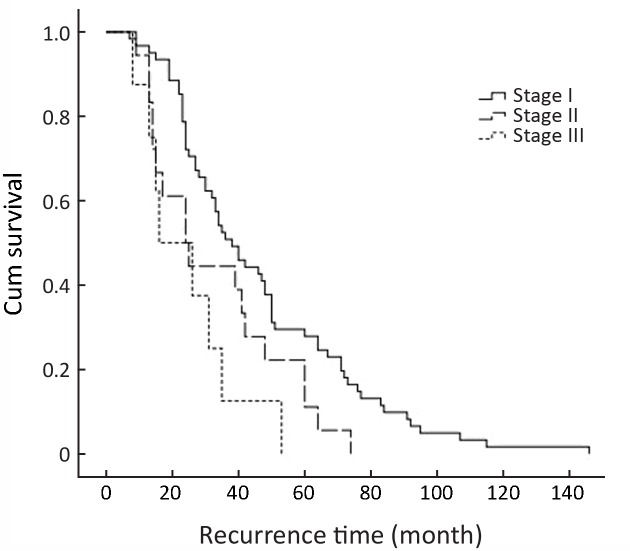
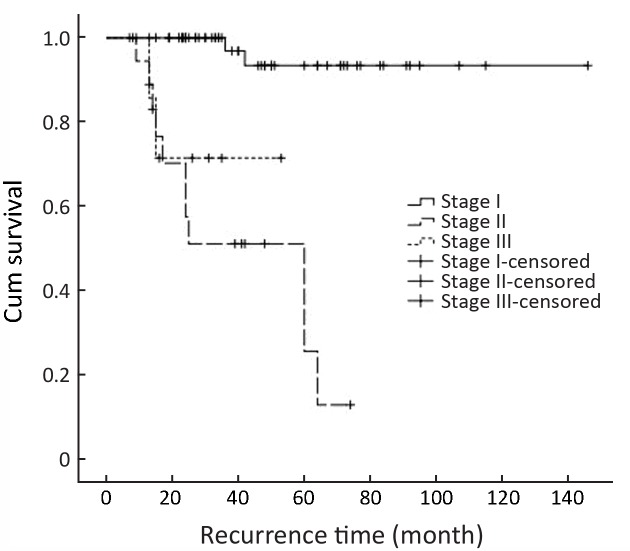
Figure 3 shows the Kaplan-Meier curves for recurrence time according to the stage in the cohort. Tumor stage could be a predictor of recurrence time (P=0.006). Figure 4 shows the Kaplan-Meier curves for recurrence time according to the stage in cancerous cases, which indicates a significant relation between tumor stage and recurrence time in cancerous cases (P<0.001).
3.
The Kaplan-Meier curves for recurrence time according to the stage in the cohort (P=0.006).
4.
The Kaplan-Meier curves for recurrence time according to the stage in cancerous cases (P<0.001).
Discussion
Ameloblastoma is a benign odontogenic tumor of epithelial origin and shows an aggressive course locally with bone destruction or expansion and resorption of teeth, which grows slowly and presents as either unicystic or multicystic solid tumor. The extent of surgery for this tumor is debatable, and treatments range from conservative management by simple curettage (10) to radical resection with to 1.5 to 2.0 cm (13) margins. Curettage or enucleations showed 55%–90% recurrences in solid and multilocular tumors. Thus, the current evidence recommends radical management in such cases (14,15). The method of surgery is debated in cystic variants, and curettage is discouraged by some authors (9) but advocated by others who consider functional preservation important (16). The current evidence from the review by McClary et al. (17), who analyzed numerous clinical and retrospective studies, recommends that curettage is acceptable only in unicystic ameloblastomas of the anterior mandible. Therefore, resection is the choice for posterior tumors and solid multicystic tumors.
The treatment decision is often made on an individual case basis with thorough consideration of the procedure morbidity. It is also important to consider possible recurrence, and a consultation of patient’s expectations is needed. Shanghai Ninth People’s Hospital manages unicystic ameloblastoma with aggressive curettage. However, solid and multi-locular tumors are resected with adequate margins. The decision is based on each case after thorough consultation with the patient to discuss their expectations and consideration of tumor board recommendations.
The volume of patients provides us a unique opportunity to chart recurrent tumors, and we found that the method of initial therapy was correlated with early recurrence. Although our initial analysis did not show any association for the method of surgery and recurrence time (P=0.417), we observed that the majority of unicystic ameloblastomas received conservative surgery whereas solid and multicystic tumors received radical resections.
The patients were grouped according to the staging system described in the study to identify indicators of early recurrence. This system of classification showed there was a significant relation between tumor stage and recurrence time (Table 1). Additionally, when the method of initial surgery was controlled, we found that there was a relation between tumor stage and recurrence rate (Table 2). The number of stage III patients was limited, so they were pooled with malignant variants. The stage I and stage III patients showed a significant difference in recurrence time; 70.11% (61) recurred in stage I whereas 20.69% (18) recurred in stage II. The stage III patients were segregated based on the literature, and our findings support the reported aggressiveness and practical surgical challenges involved with tumors located in the skull base.
The Kaplan-Meier method and multivariate Cox regression were utilized to validate our grouping method. The Kaplan-Meier curved showed that there are significant differences in recurrence times for the groups. The stage grouping system was significantly associated with early recurrence (except for stage III) after adjusting for other important variables such as histological type, subtype (unicystic or multicystic on radiographic evaluation), and surgery method.
The review by McClary et al. (17) discussed the importance of soft tissue involvement for recurrence. After the tumor spreads into the soft tissues, the predictability of complete excision decreases, and these are the practical problems for patients in stage II of our study. The stage II patients showed early recurrences relative to stage I patients.
Fregnani et al. (18) examined the clinicopathological and histological findings to predict the recurrence of 121 patients treated in a single institute from 1953 to 2003. The results suggested that the presence of radiological multi-locular lesions of ruptured basal cortical bone and histologically follicular tumors had poor outcome (18). Abdel-Aziz et al. (19) observed that a statistically significant decrease in recurrence-free survival was associated with stromal CD10 expression (P<0.001 by Log-rank test) and Ki67 labelling index (P<0.001 by Log-rank test) in 22 reported ameloblastoma cases. There are currently multiple immunohistological diagnostic methods advocated to predict the recurrence in ameloblastoma (19-21). However, the clinical features and treatment method are often not elaborated in these studies. The overall patient outcome depends on treatment method as evident in literature, and the size and involvement of adjacent anatomical structures increase the tumor load. These findings indicate the invasiveness of the tumor on the clinical scale and pathological scale. Thus, the stage II patients in our current study recurred early with respect to previously published data.
Conclusions
We conclude that tumors larger than 6 cm and involvement of soft tissues or adjacent anatomical structures are associated with early recurrence irrespective of method of surgery in this single centre retrospective study. Our method of staging can be utilized for staging craniofacial ameloblastoma. Additional studies are required to further validate the proposed system using primary tumors to predict recurrences.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81672745, 81671009) and projects of the Shanghai Natural Science Foundation (No. 15ZR1424600) and Shanghai Summit & Plateau Disciplines.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Tong Ji, Email: caowei561521@hotmail.com.
Wei Cao, Email: jitong70@hotmail.com.
References
- 1.Simon EN, Merkx MA, Vuhahula E, et al. A 4-year prospective study on epidemiology and clinicopathological presentation of odontogenic tumors in Tanzania. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:598–602. doi: 10.1016/j.tripleo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Jing W, Xuan M, Lin Y, et al. Odontogenic tumours: a retrospective study of 1642 cases in a Chinese population. Int J Oral Maxillofac Surg. 2007;36:20–5. doi: 10.1016/j.ijom.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes AM, Duarte EC, Pimenta FJ, et al. Odontogenic tumors: a study of 340 cases in a Brazilian population. J Oral Pathol Med. 2005;34:583–7. doi: 10.1111/j.1600-0714.2005.00357.x. [DOI] [PubMed] [Google Scholar]
- 4.Shear M, Singh S. Age-standardized incidence rates of ameloblastoma and dentigerous cyst on the Witwatersrand, South Africa. Community Dent Oral Epidemiol. 1978;6:195–9. doi: 10.1111/j.1600-0528.1978.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 5.Siar CH, Lau SH, Ng KH. Ameloblastoma of the jaws: a retrospective analysis of 340 cases in a Malaysian population. J Oral Maxillofac Surg. 2012;70:608–15. doi: 10.1016/j.joms.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Rizzitelli A, Smoll NR, Chae MP, et al. Incidence and overall survival of malignant ameloblastoma. PLoS One. 2015;10:e0117789. doi: 10.1371/journal.pone.0117789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogrel MA, Montes DM. Is there a role for enucleation in the management of ameloblastoma? Int J Oral Maxillofac Surg. 2009;38:807–12. doi: 10.1016/j.ijom.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Haq J, Siddiqui S, McGurk M. Argument for the conservative management of mandibular ameloblastomas. Br J Oral Maxillofac Surg. 2016;54:1001–5. doi: 10.1016/j.bjoms.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Ghandhi D, Ayoub AF, Pogrel MA, et al. Ameloblastoma: a surgeon’s dilemma. J Oral Maxillofac Surg. 2006;64:1010–4. doi: 10.1016/j.joms.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Lee PK, Samman N, Ng IO. Unicystic ameloblastoma — use of Carnoy’s solution after enucleation. Int J Oral Maxillofac Surg. 2004;33:263–7. doi: 10.1006/ijom.2003.0496. [DOI] [PubMed] [Google Scholar]
- 11.Almeida Rde A, Andrade ES, Barbalho JC, et al. Recurrence rate following treatment for primary multicystic ameloblastoma: systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:359–67. doi: 10.1016/j.ijom.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Bansal S, Desai RS, Shirsat P, et al. The occurrence and pattern of ameloblastoma in children and adolescents: an Indian institutional study of 41 years and review of the literature. Int J Oral Maxillofac Surg. 2015;44:725–31. doi: 10.1016/j.ijom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Hong J, Yun PY, Chung IH, et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg. 2007;36:283–8. doi: 10.1016/j.ijom.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Sampson DE, Pogrel MA. Management of mandibular ameloblastoma: the clinical basis for a treatment algorithm. J Oral Maxillofac Surg. 1999;57:1074–7. doi: 10.1016/s0278-2391(99)90328-2. [DOI] [PubMed] [Google Scholar]
- 15.Sammartino G, Zarrelli C, Urciuolo V, et al. Effectiveness of a new decisional algorithm in managing mandibular ameloblastomas: a 10-years experience. Br J Oral Maxillofac Surg. 2007;45:306–10. doi: 10.1016/j.bjoms.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Huang IY, Lai ST, Chen CH, et al. Surgical management of ameloblastoma in children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:478–85. doi: 10.1016/j.tripleo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 17.McClary AC, West RB, McClary AC, et al. Ameloblastoma: a clinical review and trends in management. Eur Arch Otorhinolaryngol. 2016;273:1649–61. doi: 10.1007/s00405-015-3631-8. [DOI] [PubMed] [Google Scholar]
- 18.Fregnani ER, da Cruz Perez DE, de Almeida OP, et al. Clinicopathological study and treatment outcomes of 121 cases of ameloblastomas. Int J Oral Maxillofac Surg. 2010;39:145–9. doi: 10.1016/j.ijom.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Aziz A, Amin MM. EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: possible role in local recurrence. Diagn Pathol. 2012;7:14. doi: 10.1186/1746-1596-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurppa KJ, Catón J, Morgan PR, et al. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol. 2014;232:492–8. doi: 10.1002/path.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sah P, Menon A, Kamath A, et al. Role of immunomarkers in the clinicopathological analysis of unicystic ameloblastoma. Dis Markers. 2013;35:481–8. doi: 10.1155/2013/517834. [DOI] [PMC free article] [PubMed] [Google Scholar]