Abstract
Objective
To compare the risk of incident hypertension between initiators of TNF-α inhibitors and initiators of non-biologic disease modifying anti-rheumatic drugs (hereafter referred to as non-biologics) in rheumatoid arthritis patients taking methotrexate monotherapy.
Methods
We conducted a cohort study using insurance claims data (2001–2012) from the US. We identified initiators of use of either TNF-α inhibitors or non-biologics. Subsequent exposure to these agents was measured monthly in a time-varying manner. The outcome of interest was incident hypertension, defined by a diagnosis and a prescription for an anti-hypertensive drug. Marginal structural models estimated hazard ratios adjusted for both baseline and time-varying confounders. To validate the primary analysis, we designed a verification analysis to evaluate a known association between leflunomide (a non-biologic disease modifying agent) and hypertension.
Results
We identified 4,822 initiations of TNF-α inhibitor use and 2,400 of non-biologic use. Crude incidence rates of hypertension per 1,000 person-years of follow-up were 36 (95%CI 32–41) for the TNF-α inhibitor group and 42 (95%CI 34–51) for the non-biologics group. The crude hazard ratio of TNF-α inhibitors versus non-biologics for the risk of incident hypertension was 0.85 (95%CI 0.67–1.1). After adjusting for both baseline and time-varying covariates using marginal structural models, the HR was 0.95 (95%CI 0.74–1.2). In the verification analysis, the adjusted HR of incident hypertension was 2.3 (95%CI 1.7–3.0) in leflunomide initiators compared with methotrexate initiators.
Conclusion
Treatment with TNF-α inhibitors was not associated with a reduced risk of incident hypertension compared with non-biologics in rheumatoid arthritis patients.
Keywords: Hypertension, TNF-α inhibitors, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis is an auto-immune disease that affects approximately 1.3 million adults in the United States.1 Pharmacologic treatment with non-biologic and biologic disease modifying anti-rheumatic drugs forms the mainstay of rheumatoid arthritis management. Non-biologic disease-modifying anti-rheumatic drugs, hereafter referred to in this context as non-biologic drugs, include agents, such as methotrexate, sulfasalazine, hydroxychloroquine, and leflunomide that halt disease progression by suppressing inflammation. In contrast, biologic disease-modifying anti-rheumatic drugs target specific components of the immune system, such as T cells, B cells, and cytokines (i.e., tumor necrosis factor (TNF)-α and interleukins) that play an important role in the pathogenesis of rheumatoid arthritis.
Hypertension is one of the most prevalent co-morbid conditions in the patients with rheumatoid arthritis. The prevalence of hypertension among rheumatoid arthritis patients is documented in the range of 30%–70% in prior studies.2–4 Systemic inflammation, reflected by increased c-reactive protein, is hypothesized to play a role in the development of hypertension among RA patients. High levels of c-reactive protein are associated with reduced production of nitric oxide in endothelial cells,5 which may result in increased blood pressure through vasoconstriction.4 Additionally, high amount of CRP may also up-regulate the expression of angiotensin type 2 receptors and may result in high blood pressure.6 Consistent with these proposed mechanisms, elevated levels of CRP are found to be associated with a greater risk of developing hypertension in a large prospective cohort study.7
If greater systemic inflammation is associated with higher blood pressure in rheumatoid arthritis patients, reducing inflammation through treatment with a disease-modifying anti-rheumatic drug may reduce the risk of hypertension. As evidence also suggests a correlation between increased BP and high levels TNF-α,8 biologic drugs acting through inhibition of TNF-α may have greater BP lowering effect compared with non-biologic anti-rheumatic drugs. Results from several small studies support the potential blood pressure-lowering effect of TNF-α inhibitors in rheumatoid arthritis patients.9–11 Yet, no study has compared the effect of TNF-α inhibitors with non-biologics on the development of incident hypertension in a population-based cohort of RA patients.
Managing the increased risk of cardiovascular diseases in rheumatoid arthritis patients remains a challenge for physicians. Since hypertension is a major risk factor for developing cardiovascular disease in patients with and without rheumatoid arthritis,12 it is important to examine any potential reduction in the risk of incident hypertension associated with TNF-α inhibitor treatment in rheumatoid arthritis patients. Therefore, the primary objective of this study was to compare the risk of incident hypertension associated with initiation of TNF-α inhibitors versus non-biologic disease-modifying anti-rheumatic drugs in rheumatoid arthritis patients. We hypothesized that initiation of TNF-α inhibitors would be associated with a lower risk of developing hypertension compared to initiation of non-biologic drugs in patients with rheumatoid arthritis.
METHODS
Study design and data source
We conducted a retrospective cohort study to evaluate the association between TNF-α inhibitors and the risk of incident hypertension, using claims data between January 2001 and December 2012 from two commercial US health plans - WellPoint and United HealthCare. These databases contain longitudinal claims information including medical diagnoses, procedures, hospitalizations, physician visits, and pharmacy dispensing on more than 40 million fully-insured subscribers across the United States. These databases have been utilized in previous published studies of rheumatoid arthritis patients.13–15 The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
Study population and eligibility criteria
The study population consisted of rheumatoid arthritis patients at least 18 years of age who were continuously enrolled in their health plans for at least 6 months before the first recorded rheumatoid arthritis diagnosis. Rheumatoid arthritis diagnosis was identified with a minimum of 2 ICD-9-CM diagnosis codes for rheumatoid arthritis (714.xx) at least 7 days but no more than 365 days apart combined with at least 1 filled prescription for a disease-modifying anti-rheumatic drug. This algorithm is reported to have a positive predictive value of 86.2% in a validation study.16
We further required the identified rheumatoid arthritis patients to meet the following inclusion criteria, 1) initiation of either TNF-α inhibitor initiators or non-biologic disease-modifying anti-rheumatic drugs; agents included in the TNF-α inhibitor class were adalimumab, certolizumab, etanercept, golimumab, and infliximab. Agents included in the non-biologic class were hydroxychloroquine, sulfasalazine, minocycline, gold compounds, azathioprine, cyclophosphamide, and penicillamine. Initiators of leflunomide and cyclosporine were not included in the non-biologic group since both agents are known to raise blood pressure.17,18 Since some disease-modifying anti-rheumatic drugs are administered in the outpatient setting such as infusions in physician offices, we used Healthcare Common Procedure Coding System J-codes found on outpatient services claims to comprehensively capture disease-modifying anti-rheumatic drug use in addition to pharmacy claims, 2) use of methotrexate monotherapy in the 6-month period prior to initiation of either a TNF-α inhibitor or another non-biologic drug to ensure similar rheumatoid arthritis activity between the groups,19 3) no prevalent use of any other disease-modifying anti-rheumatic drug (except for methotrexate) in the 6-month period prior to initiation of either a TNF-α inhibitor or another non-biologic drug to capture new users of these medications,20 and 4) absence of existing cardiovascular diseases, including myocardial infarction, angina, heart failure, cerebrovascular disease, other forms of chronic heart disease, hypertension, or use of any anti-hypertensive medications (listed in eAppendix 1) recorded any time prior to initiation of either a TNF-α inhibitor or another non-biologic drug.
Study enrollment using sequential cohorts approach
The above listed eligibility criteria were assessed during every month between July 2001 and November 2012 to create a sequence of 137 observational cohorts, each with an enrollment period of 1-month. In each of these monthly cohorts, eligible initiations of TNF-α inhibitor or non-biologic drug were identified. In the second step, the participants were pooled across all these cohorts and analyzed as a single pooled cohort. Of note, some of the patients may be eligible for inclusion in more than one monthly cohort conditional upon meeting all the eligibility criteria at a later time point in the study. Please refer to eAppendix Figure 1 for a schematic representation of the sequential cohort setup. This methodology is conceptually similar to design of a clinical trial and when combined with appropriate statistical analyses, it properly accounts for time-varying confounding as detailed in prior publications.21,22
Drug exposure measurement and follow-up
After enrollment into one of the two study groups based on the disease-modifying anti-rheumatic drug initiation (TNF-α inhibitors or non-biologic drugs), exposure to these agents was ascertained every month during follow-up as exposed or unexposed based on at least one day of prescription supply availability in a month for the respective classes. To account for imperfect adherence to drug classes under investigation during the study period, we restricted the analysis to months in which the participants adhered to the initiated disease-modifying anti-rheumatic drug treatment.22 Accordingly, in addition to stopping the follow-up at disenrollment from the health plan, administrative end point of the study (December 2012), or study outcome (hypertension), we also stopped the follow-up at the month in which patients changed their initiated treatment for non-clinically indicated reasons. A treatment change was defined as either discontinuation of the originally initiated disease-modifying anti-rheumatic drug (no prescription refill for two consecutive months for the respective class of drug after accounting for day supply of the most recent prescription) or initiation of a disease-modifying anti-rheumatic drug from another group (initiation of a TNF-α inhibitor in non-biologic drug group patients and vice versa) during follow-up. Treatment changes for clinically indicated reasons were not considered instances of non-compliance. Accordingly, we did not stop the follow-up at a treatment change when such a change was preceded by diagnosis of a suspected adverse event (heart failure, malignancy, or infections for the TNF-inhibitor group and hepatic events or infections for the non-biologic drug group).
Study outcome measurement
The outcome of hypertension was defined based on at least one diagnosis of hypertension (ICD-9-CM code 401.xx) and a new prescription for an antihypertensive agent (list provided in eAppendix 1). This algorithm of combining a diagnosis claim with a medication claim is reported to have 93% specificity in identifying patients with hypertension in administrative databases.23
Covariates
Two groups of covariates were considered: 1) time-fixed covariates that were measured only once in the study at the time of enrollment for every participant including gender, diagnosis of diabetes (ICD-9 code 250.xx), hyperlipidemia (ICD-9 code 272.xx), obesity (ICD-9 code 278.0x), smoking (ICD-9 codes: 305.1x, 649.0x, 989.84, V1582; procedure codes for smoking counseling: 99406, 99407, S9075, S9453; or prescriptions for varenicline, bupropion, and nicotine), a combined comorbidity score,24 use of anti-diabetic and lipid-lowering agents in the 6 months prior to initiating TNF-α inhibitors or non-biologics; and 2) time-varying covariates that were updated monthly post-initiation of either TNF-α inhibitors or non-biologic disease-modifying anti-rheumatic drugs including use of non-steroidal anti-inflammatory drugs (NSAIDs), injectable steroids, cumulative dose of oral steroids (in prednisone equivalent milligrams), methotrexate, leflunomide or cyclosporine, and other non-TNF biologics, number of hospitalizations, emergency room visits, office visits, and distinct generic drugs. Additionally, participant age was updated every year in the study.
Statistical analysis
In order to account for the time-varying selection bias introduced by artificial censoring at deviation from the initiated disease-modifying anti-rheumatic drug in our study, we used time-varying stabilized inverse probability treatment weights.22,25 These weights were calculated monthly during the follow-up as a ratio of two subject-specific conditional probabilities. The numerator (the stabilizing factor) was the probability of receiving the disease-modifying anti-rheumatic treatment actually received in a month conditional on time-fixed covariates (measured at baseline) and past treatment history, while the denominator was the predicted probability of receiving the disease-modifying anti-rheumatic treatment actually received in a month conditional on time-fixed covariates (measured at baseline), time-varying covariates (measured in the prior month), and past treatment history. Since in this study we compared two active treatments, the numerator and denominator for inverse probability treatment weights were computed as a product of two probabilities derived from two nested logistic regression models. The first model estimated the monthly probability of receiving either of the two disease-modifying anti-rheumatic treatments (TNF-α inhibitors or non-biologic treatment) and the second model estimated the monthly probability of receiving TNF-α inhibitors among those who received either of the two disease-modifying anti-rheumatictreatments. Please refer to eAppendix 2 for technical details of inverse probability treatment weight calculations. After calculation, the weights were truncated at 1st and 99thpercentile to prevent unduly influence by outliers with very large weights and to improve precision.22,26
After truncation of the inverse probability treatment weights, a weighted pooled logistic regression model was specified to derive estimates for the effect of TNF-α inhibitors compared with non-biologic disease-modifying anti-rheumatic drugs on the risk of incident hypertension. This model adjusted for only time-fixed covariates since time-varying covariates were accounted for while constructing inverse probability treatment weights. Under the assumption of no unmeasured confounding, parameters of this weighted pooled logistic model estimate hazard ratios (HR) of a marginal structural model.22 Since some of the patients may be eligible for inclusion in more than one monthly cohorts conditional upon meeting all the eligibility criteria, we used a robust variance estimator to estimate conservative 95% confidence intervals (CI).27
In order to evaluate the cumulative effect of TNF-α inhibitor use on the risk of incident hypertension, we further specified a dose-response marginal structural model with restricted cubic splines for continuous cumulative months of treatment with TNF-α inhibitors with 3 knots (at 12, 24, and 36 months). For this dose-response model, we calculated standardized monthly survival probabilities for up to 3-years of follow-up and produced inverse probability-weighted survival curves. In addition to calculating adjusted HRs as described above, survival difference between the two groups at 3-years of follow-up was reported for this dose-response marginal structural model along with 95% CI, which were derived using nonparametric bootstrapping with 500 samples. All the analyses were conducted using SAS 9.3 (SAS institute, Cary, NC).
Sensitivity analyses
To evaluate the robustness of our findings, we conducted two sensitivity analyses. In the first sensitivity analysis, we restricted analysis to patients using NSAIDs or steroids prior to their study enrolment date. This sensitivity analysis was designed to ensure restriction of the study cohort to a homogenous group of patients with higher disease activity at baseline. In the second sensitivity analysis, we restricted the TNF-inhibitor initiator group to only infliximab users to facilitate the comparison of our study with a prior study from Klarenbeek et al., who documented a reduction in BP in users of infliximab compared to users of non-biologic disease-modifying anti-rheumatic drugs.10
Verification (positive control) analysis
To validate the results of observational studies evaluating an unknown association, some authors have suggested the use of a verification analysis that demonstrates the presence of known effects of a drug in the data set being studied.28 Following this suggestion, we designed a verification analysis to evaluate a known association between leflunomide and hypertension17,29 in our dataset using exactly the same methodology (sequential cohorts) and analysis techniques (marginal structural models) that were used for the primary analysis. eAppendix 3 provides the details on the study design and analytic methods for this verification analysis.
RESULTS
Cohort selection
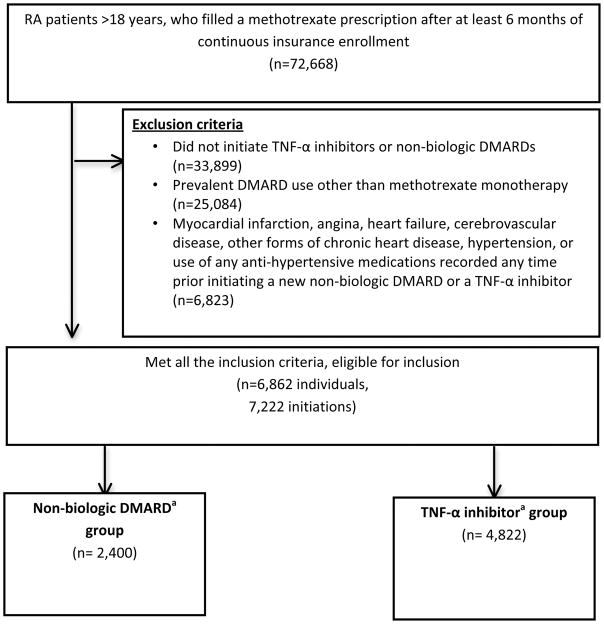
We identified a total of 72,668 patients with rheumatoid arthritis who had at least 6 months of continuous insurance enrollment prior to their first recorded rheumatoid arthritis diagnosis in our databases. Of these, 6,862 individuals met all the inclusion criteria, who accounted for 7,222 eligible initiations for the disease-modifying anti-rheumatic drugs of interest (Figure 1). The TNF-α inhibitor group included 4,822 eligible initiations, while the non-biologic drug group included 2,400 eligible initiations. Mean (± Standard deviation) follow-up time was 19 (± 18) months for the TNF-α inhibitor group, while 12 (± 13) months for the non-biologic drug group (Median (interquartile range) 13 (6–26 months) and 6 (4–14 months), respectively).
Figure 1.
Sample derivation flow-chart
Abbreviations: DMARDs- disease modifying anti-rheumatic drugs, TNF- tumor necrosis factor
aNon-biologic DMARDs include hydroxychloroquine, sulfasalazine, minocycline, azathioprine, cyclophosphamide, gold compounds, and penicillamine.
bTNF-α inhibitors include adalimumab, certolizumab, etanercept, golimumab, and infliximab
Patient characteristics
Characteristics of our study participants were presented at baseline as well as at 6-months, and at 12-months during the follow-up among those who remain uncensored at 6 and 12 months in the study to understand potential reasons for differential censoring over time between the two groups (Table 1).
Table 1.
Patient characteristics of non-biologic disease-modifying anti-rheumatic drug (DMARD) and TNF-α inhibitor groups
| Baseline | Patients remaining in the cohort 6 months post-index | Patients remaining in the cohort 12 months post-index | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Non-biologic DMARD | TNF-α inhibitor | Non-biologic DMARD | TNF-α inhibitor | Non-biologic DMARD | TNF-α inhibitor | |
| Total n | 2400 | 4822 | 1195 | 3571 | 655 | 2439 |
| Proportion relative to baseline | 100% | 100% | 50% | 74% | 27% | 51% |
| Time-fixed covariates* | ||||||
| Female | 1899 (79) | 3515 (73) | 943 (79) | 2579 (72) | 515 (79) | 1771 (73) |
| Diabetes | 127 (5) | 226 (5) | 58 (5) | 159 (4) | 38 (6) | 103 (4) |
| Hyperlipidemia | 538 (22) | 1096 (23) | 268 (22) | 844 (24) | 153 (23) | 580 (24) |
| Obesity | 114 (5) | 207 (4) | 49 (4) | 153 (4) | 26 (4) | 92 (4) |
| Smoking | 166 (7) | 354 (7) | 77 (6) | 253 (7) | 34 (5) | 157 (6) |
| Combined comorbidity score (mean(SD)) | 0.5 (0.9) | 0.4 (0.8) | 0.5 (0.9) | 0.4 (0.8) | 0.5 (0.9) | 0.4 (0.8) |
| Lipid lowering agents | 162 (7) | 318 (7) | 85 (7) | 247 (7) | 48 (7) | 180 (7) |
| Diabetes medications | 57 (2) | 102 (2) | 28 (2) | 76 (2) | 18 (3) | 46 (2) |
| Time-varying covariates | ||||||
| Age, in years (mean(SD)) | 48 (12) | 46 (11) | 49 (12) | 46 (11) | 50 (11) | 46 (11) |
| Rheumatoid arthritis- related drug usea | ||||||
| NSAIDs | 1163 (48) | 2724 (56) | 538 (45) | 1648 (46) | 294 (45) | 1044 (43) |
| Injectable steroid use | 614 (26) | 1372 (28) | 251 (21) | 870 (24) | 91 (14) | 440 (18) |
| Oral steroid use | 1254 (52) | 2766 (57) | 570 (48) | 1648 (46) | 262 (40) | 825 (34) |
| Cumulative dose of oral steroids among users, in mg prednisone equivalents (mean(SD)) | 861 (1439) | 893 (1206) | 899 (1256) | 699 (725) | 809 (1401) | 653 (662) |
| Leflunomide or cyclosporineb | - | - | 37 (3) | 62 (2) | 33 (5) | 72 (3) |
| Non-TNF biologicsb | - | - | 9 (1) | 64 (2) | 8 (1) | 42 (2) |
| Health care utilization* | ||||||
| Any hospitalization | 115 (5) | 259 (5.4) | 57 (5) | 147 (4) | 46 (7) | 104 (4) |
| Any emergency room visit | 230 (10) | 498 (10) | 107 (9) | 301 (8) | 44 (7) | 194 (8) |
| Number of office visits (mean(SD)) | 5 (3) | 6 (3) | 5 (3) | 5 (3) | 4 (3) | 5 (3) |
| Number of distinct generic prescriptions filled (mean(SD)) | 6 (4) | 7 (4) | 7 (4) | 7 (4) | 7 (4) | 6 (4) |
Abbreviations: DMARDs- disease modifying anti-rheumatic drugs, NSAID- non steroidal anti-inflammatory drug, SD- standard deviation, TNF- tumor necrosis factor.
Numbers in the table are n(%) unless indicated otherwise.
Time fixed-covariates were measured at the index date for everyone. Rheumatoid arthritis related drug use and healthcare utilization variables were reported for 6 months pre-index for Baseline columns, months 1 through 6 post-index for next two columns, and months 7 through 12 post-index for last two columns.
Users of leflunomide or cyclosporine or non-TNF biologics during the pre-index period were not included in the study. Use of these agents was controlled for as time-varying covariates if patients included in our study initiated these agents post-index.
Both groups had similar distribution of the comorbid conditions and concurrent medication use at baseline. However, the non-biologic disease-modifying anti-rheumatic drug group had a higher proportion of females (79% vs 73%). During the follow-up, the non-biologic group was censored more frequently compared to the TNF-α inhibitor group (50% vs 26% censored at 6 months, and 73% vs 49% censored at 12 months). However, among those who remained uncensored at 6 and 12 months, the distribution of the time-fixed covariates was observed to be very similar compared with the distribution at baseline.
Some interesting contrasts were observed in the distribution of time-varying covariates during follow-up. Use of rheumatoid arthritis-related medications for chronic pain such as NSAIDs and oral steroids was found to be higher in the TNF-α inhibitor group at baseline. However, during the follow-up a reversal of this trend was seen with lower prevalence of use of these agents in the TNF-α inhibitor group at 6 and 12 months compared to the non-biologic group.
Risk of incident hypertension
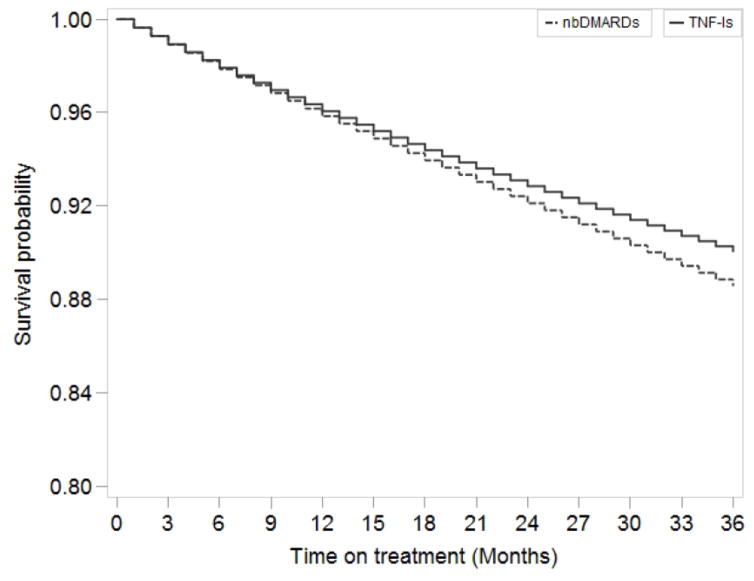
We observed a total of 370 incident hypertension cases during follow-up, 273 in the TNF-α inhibitor group and 97 in the non-biologic disease-modifying anti-rheumatic drugs group (Table 2). Crude incidence rates per 1,000 person-years were 36 (95% CI 32–41) for the TNF-α inhibitor group and 42 (95% CI 34–51) for the non-biologic drugs group. The crude HR for TNF-α inhibitors compared to non-biologic disease-modifying anti-rheumatic drugs on the risk of incident hypertension was 0.85 (95% CI 0.67–1.1). The HR adjusted for only time-fixed covariates was 0.92 (95% CI 0.73–1.2). After adjustment with both time-fixed and time-varying covariates using marginal structural models, the HR of incident hypertension was 0.95 (95% CI 0.74–1.2). In the dose response marginal structural model, the adjusted HR for hypertension after 3-years of continuous treatment with TNF-α inhibitors versus non-biologic disease-modifying anti-rheumatic drugs was 0.96 (95% CI 0.63–1.4). The difference in the absolute risk of hypertension (the survival difference) between continuous use of TNF-α inhibitors and non-biologic disease-modifying anti-rheumatic drugs was 1.5% (95% CI (−2.3%) to 4.8%) at 3-years of follow-up (Figure 2). Findings from both sensitivity analyses were qualitatively similar to our main results (Table 3).
Table 2.
Incidence rates and relative risks of hypertension in initiators of TNF-alpha inhibitors compared to the initiators of non-biologic disease-modifying anti-rheumatic drugs (DMARDs) for rheumatoid arthritis
| nbDMARDs | TNF-α inhibitors | |
|---|---|---|
| Hypertension Events | 97 | 273 |
| Follow-up (person-years) | 2,338 | 7,580 |
| Crude incidence rates/1000 person-years (95% CI) | 42 (34–51) | 36 (32–41) |
| Unadjusted HR | 1 | 0.85 (0.67–1.1) |
| Adjusted HR for only time-fixed covariatesa | 1 | 0.92 (0.73–1.2) |
| Adjusted HR for time-fixed and time-varying covariatesb using IPTWc | 1 | 0.95 (0.74–1.2) |
Abbreviations: CI- Confidence interval, HR- Hazard ratio, IPTW- Inverse probability treatment weights, TNF- tumor necrosis factor.
Time-fixed covariates (measured at initiation of either TNF-inhibitor of non-biologic DMARDs): gender, diabetes, hyperlipidemia, lipid-lowering agent use, anti-diabetic medication use, obesity, smoking, combined comorbidity score.
Time-varying covariates (updated monthly post-initiation of either TNF-inhibitor of non-biologic DMARDs): use of non-steroidal anti-inflammatory drugs, injectable steroids, cumulative dose of oral steroids, methotrexate use, leflunomide or cyclosporine use, other non-TNF biologic use, hospitalizations, emergency room visits, office visits, and number of distinct drugs, and age (updated every year in the study).
Weights were truncated at the 1st percentile (0.63) and 99th percentile (1.71). Mean (SD) of the weights were 0.99 (0.16) after truncation.
Figure 2.
Inverse probability weighted survival curves for the effect of TNF-α inhibitors vs non-biologic DMARDs on the risk of incident hypertension, dose response marginal structural model*
Abbreviations: nbDMARDs- non biologic disease modifying anti-rheumatic drugs, TNFi- tumor necrosis factor-α inhibitors.
aCumulative treatment with TNF-α inhibitors modeled as restricted cubic splines. Model adjusted for, 1) time-fixed covariates (measured at initiation of either TNF-α inhibitor of non-biologic DMARDs): gender, diabetes, hyperlipidemia, lipid-lowering agent use, anti-diabetic medication use, obesity, smoking, combined comorbidity score, 2) time-varying covariates (updated monthly post-initiation of either TNF-α inhibitor of non-biologic DMARDs): use of NSAIDs, injectable steroids, cumulative dose of oral steroids, methotrexate use, leflunomide or cyclosporine use, other non-TNF biologic use, hospitalizations, emergency room visits, office visits, and number of distinct drugs, and age (updated every year in the study) using marginal structural models after inverse probability treatment weighting. Weights were truncated at the 1st percentile (0.51) and 99th percentile (1.99). Mean (SD) of the weights were 0.98 (0.19) after truncation.
Table 3.
Results from sensitivity analyses for the risk of hypertension in initiators of TNF-alpha inhibitors compared to the initiators of non-biologic disease-modifying anti-rheumatic drugs for rheumatoid arthritis
| Analysis | IPTWa adjusted HR (95% CI) |
|---|---|
| Main analysis | 0.95 (0.74–1.2) |
| Sensitivity analysis 1- Restricted to patients using NSAIDs or steroids prior to their study enrolment date | 0.85 (0.64–1.1) |
| Sensitivity analysis 2- TNF-inhibitor initiator group restricted to infliximab users only | 0.83 (0.60–1.2) |
Abbreviations: CI- Confidence interval, DMARDs- disease modifying anti-rheumatic drugs, HR- Hazard ratio, IPTW- Inverse probability treatment weights, NSAIDs- non-steroidal anti-inflammatory drugs TNF- tumor necrosis factor.
IPTW adjusted for time-fixed covariates (measured at initiation of either TNF-inhibitor of non-biologic DMARDs): gender, diabetes, hyperlipidemia, lipid-lowering agent use, anti-diabetic medication use, obesity, smoking, combined comorbidity score, as well as time-varying covariates (updated monthly post-initiation of either TNF-inhibitor of non-biologic DMARDs): use of NSAIDs, injectable steroids, cumulative dose of oral steroids, methotrexate use, leflunomide or cyclosporine use, other non-TNF biologic use, hospitalizations, emergency room visits, office visits, and number of distinct drugs, and age (updated every year in the study). Weights were truncated at the 1st percentile and 99th percentile.
Verification (positive control) analysis
In the verification analysis, 57 incident hypertension events in the leflunomide group (out of 768 total initiations) and 493 incident hypertension events in the methotrexate group (out of 11,652 total initiations) were observed. The crude incidence rates of hypertension per 1,000 person-years were 77 (95% CI 59–100) for leflunomide and 35 (95% CI 32–39) for methotrexate initiators. The crude HR was 2.2 (95% CI 1.7–2.9) in leflunomide initiators versus methotrexate. After adjusting for both time-fixed and time-varying covariates using marginal structural models, the HR of incident hypertension was 2.3 (95% CI 1.7–3.0) in leflunomide initiators. eAppendices 4 & 5 describes the patient characteristics and results from statistical models for this verification analysis, respectively.
DISCUSSION
In this large observational study of patients with rheumatoid arthritis on methotrexate monotherapy, we did not observe a reduced risk of incident hypertension associated with initiation of TNF-α inhibitors compared with non-biologic disease-modifying anti-rheumatic drugs after carefully controlling for multiple time-fixed and time-varying confounders. Further, no dose-response association between cumulative use of TNF-α inhibitors and the risk of incident hypertension was observed.
The finding of no risk reduction in hypertension after TNF-α inhibitor treatment observed in this study is in contrast with the a priori hypothesis based on observations from earlier studies.9–11 There are several factors that may explain this discrepancy. First, two of the three earlier studies measured reduction in blood pressure as the outcome within short follow-up times in small groups of patients, 16 patients for two weeks11 and 23 patients for up to 12 weeks9, after treatment with TNF-α inhibitors. It is possible that reductions observed immediately following TNF-α inhibitor treatment in these studies may not persist over long-term and hence TNF-α inhibitors may not confer any benefit in preventing hypertension. In a long-term follow-up study, Klarenbeek et al. observed reductions in systolic blood pressure up to 1 year after infliximab treatment in 128 patients, however the extent of reduction was modest (3–5 mmhg compared with non-biologic disease-modifying anti-rheumatic drugs).10 Second, the focus of our study was on development of incident hypertension and therefore we excluded patients with pre-existing hypertension or patients using anti-hypertensive medications from our study. None of the three prior studies employed these exclusion criteria, presumably to preserve power because nearly a third of the included patients had pre-existing hypertension in these studies. Therefore, it is possible that our findings may not be directly comparable to the prior studies due to differences in this important aspect of the study populations.
Despite of the lack of reduction in the risk of hypertension, an important risk factor for cardiovascular disease, observed in this study, TNF-α inhibitors have been found to reduce the risk of cardiovascular diseases in patients with rheumatoid arthritis in prior studies.15,30–32 Our results suggest that the overall cardiovascular disease risk reduction conferred by TNF-α inhibitors may be mediated by their beneficial effects on carduivascular risk factors other than hypertension. These effects may include reduction in diabetes risk by improvement in insulin resistance33,34 or control of atherosclerotic process through improved control of inflammation.35,36
Our study has several strengths. We carefully designed our study in rheumatoid arthritis patients on methotrexate monotherapy only to ensure inclusion of patients who are homogenous in terms of their disease progression and compared the risk of hypertension between new users of TNF-α inhibitors and of non-biologic disease-modifying anti-rheumatic drugs in order to minimize confounding by indication.19 To address confounding due to factors that may affect the association between TNF-α inhibitors and hypertension in a time-varying manner, we used advanced statistical techniques of inverse probability treatment weights and marginal structural models. These methods have been shown to appropriately account for confounding due to factors that change with time in a longitudinal follow-up studies.25 In this particular study, comparison of steroids and NSAID use between the two study groups at baseline and after 12 months of follow-up (Table 1) indicated that the prevalence of these factors changed with time in both study groups in a manner that was suggestive of influence by the disease-modifying anti-rheumatic drug treatment (lower use of NSAIDs in the TNF-α inhibitor group at 12 months may be due to better control of disease activity). In the current study, the magnitude of this time-varying confounding was not large enough to alter the inference as evidence by similar HRs with largely overlapping confidence intervals observed in models that did and did not account for this confounding (Table 2). However, failure to appropriately account for time-varying confounding can potentially lead to invalid inferences in circumstance where the magnitude of this confounding is greater. To evaluate the robustness of our main results, we conducted three additional sensitivity analyses, which demonstrated consistent findings. To validate our null findings for the association between TNF-α inhibitors and hypertension, we demonstrated a known association between leflunomide and hypertension in a pre-specified verification analysis using the same study design, analysis technique, dataset and outcome definition that was used for the original analysis.
There are some limitations to our study. First, as there were no data available on rheumatoid arthritis disease activity measures such as the Disease Activity Score measured in 28 joints (DAS28) or the Rheumatoid Arthritis Disease Activity Index (RADAI) in the study database, our study may suffer from residual confounding by disease activity, as disease activity likely plays a major role in treatment selection and it may also be an independent risk factor for the outcome. To address this limitation, we accounted for several time-varying measured confounders as proxies, including steroid use and NSAID use, which are likely to be associated with the unmeasured disease activity. This study may further be subject to residual confounding by factors not captured in the claims data such as body weight, physical activity, alcohol consumption and dietary habits. Next, as we defined rheumatoid arthritis, hypertension, and other covariates based on diagnosis codes and/or prescription, misclassification bias is possible. To minimize this bias, we used validated algorithms to identify rheumatoid arthritis 16 and hypertension.23 However, since the algorithm that we used to define the outcome of hypertension requires that patients fill at least one prescription for an antihypertensive medication, the rates presented in this study do not include untreated hypertension and therefore may be an underestimation of the incidence of hypertension. For comparison, an incidence rate of 48.6 hypertension cases per 1000 person-years is reported in population-based cohorts (Atherosclerosis Risk in Communities and Multi-Ethnic Study of Atherosclerosis);37 which is higher than the rate seen in the current cohort (37.3 per 1000 person-years). However, the under-ascertainment of hypertension is unlikely to be differential between the two treatment groups in this study and therefore not expected to introduce a systematic bias.
In conclusion, treatment with TNF-α inhibitors was not associated with a reduced risk of incident hypertension compared with non-biologic disease-modifying anti-rheumatic drugs in rheumatoid arthritis patients. Future research identifying the mechanisms of the reduced cardiovascular disease risk with TNF-α inhibitor treatment may be helpful in targeting specific subgroups of patients for maximizing the cardiovascular benefits of TNF-α inhibitors.
Supplementary Material
Acknowledgments
Funding: This study was funded from internal sources of the Division of Pharamcoepidemiology and Pharmacoeconomics.
We acknowledge the contributions of Jun Liu, MD, MSc (Division of Pharmacoepidemiology and Pharamcoeconomics, Brigham and Women’s Hospital) in data management and cleaning.
Footnotes
Conflict of interest/Financial disclosures:
Dr. Solomon is supported by the NIH grants K24 AR055989, P60 AR047782, and R01 AR056215. He receives research grants from Amgen and Lilly. He serves in unpaid roles on studies sponsored by Pfizer, Novartis, Lilly, and Bristol Myers Squibb. He also receives royalties from UpToDate.com.
Dr. Kim is supported by the NIH grant K23 AR059677. She received research support from Pfizer and Lilly.
Dr. Liao is supported by the NIH grant K08 AR060257 and the Harold and Duval Bowen Fund.
Dr. Schneeweiss is principal investigator of the Harvard-Brigham Drug Safety and Risk Management Research Center funded by the US Food and Drug Administration. His work is partially funded by grants/contracts from PCORI, FDA, and NHLBI. Schneeweiss is consultant to WHISCON, LLC and to Aetion, Inc. of which he also owns shares. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis, and Boehringer-Ingelheim unrelated to the topic of this study.
Dr. Danaei and Dr. Desai have no relevant disclosures.
References
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–72. [PubMed] [Google Scholar]
- 3.Chung CP, Giles JT, Petri M, Szklo M, Post W, Blumenthal RS, Gelber AC, Ouyang P, Jenny NS, Bathon JM. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41(4):535–44. doi: 10.1016/j.semarthrit.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46(9):1477–82. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 6.Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, Li RK, Mickle DA, Verma S. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003;107(13):1783–90. doi: 10.1161/01.CIR.0000061916.95736.E5. [DOI] [PubMed] [Google Scholar]
- 7.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290(22):2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 8.Kim KI, Lee JH, Chang HJ, Cho YS, Youn TJ, Chung WY, Chae IH, Choi DJ, Park KU, Kim CH. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J. 2008;72(2):293–8. doi: 10.1253/circj.72.293. [DOI] [PubMed] [Google Scholar]
- 9.Sandoo A, Panoulas VF, Toms TE, Smith JP, Stavropoulos-Kalinoglou A, Metsios GS, Gasparyan AY, Carroll D, Veldhuijzen van Zanten JJ, Kitas GD. Anti-TNFalpha therapy may lead to blood pressure reductions through improved endothelium-dependent microvascular function in patients with rheumatoid arthritis. J Hum Hypertens. 2011;25(11):699–702. doi: 10.1038/jhh.2011.36. [DOI] [PubMed] [Google Scholar]
- 10.Klarenbeek NB, van der Kooij SM, Huizinga TJ, Goekoop-Ruiterman YP, Hulsmans HM, van Krugten MV, Speyer I, de Vries-Bouwstra JK, Kerstens PJ, Huizinga TW, Dijkmans BA, Allaart CF. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: a post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69(7):1342–5. doi: 10.1136/ard.2009.124180. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T. Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens. 2013 doi: 10.1038/jhh.2013.80. [DOI] [PubMed] [Google Scholar]
- 12.Kremers HM, Crowson CS, Therneau TM, Roger VL, Gabriel SE. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58(8):2268–74. doi: 10.1002/art.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SY, Schneeweiss S, Liu J, Daniel GW, Chang C-L, Garneau K, Solomon DH. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12(4):R154. doi: 10.1186/ar3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai RJ, Eddings W, Liao KP, Solomon DH, Kim SC. Disease modifying anti-rheumatic drug use and the risk of incident hyperlipidemia in patients with early rheumatoid arthritis: A retrospective cohort study. Arthritis Care Res (Hoboken) 2014 doi: 10.1002/acr.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon DH, Curtis JR, Saag KG, Lii J, Chen L, Harrold LR, Herrinton LJ, Graham DJ, Kowal MK, Kuriya B, Liu L, Griffin MR, Lewis JD, Rassen JA. Cardiovascular risk in rheumatoid arthritis: comparing TNF-alpha blockade with nonbiologic DMARDs. Am J Med. 2013;126(8):730e9–730 e17. doi: 10.1016/j.amjmed.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, Solomon DH. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozman B, Praprotnik S, Logar D, Tomsic M, Hojnik M, Kos-Golja M, Accetto R, Dolenc P. Leflunomide and hypertension. Ann Rheum Dis. 2002;61(6):567–9. doi: 10.1136/ard.61.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert N, Wong GW, Wright JM. Effect of cyclosporine on blood pressure. Cochrane Database Syst Rev. 2010;(1):CD007893. doi: 10.1002/14651858.CD007893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon DH, Lunt M, Schneeweiss S. The risk of infection associated with tumor necrosis factor alpha antagonists: making sense of epidemiologic evidence. Arthritis Rheum. 2008;58(4):919–28. doi: 10.1002/art.23396. [DOI] [PubMed] [Google Scholar]
- 20.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American Journal of Epidemiology. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 21.Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Stampfer MJ, Willett WC, Manson JE, Robins JM. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology (Cambridge, Mass) 2008;19(6):766. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danaei G, Rodriguez LA, Cantero OF, Logan R, Hernan MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22(1):70–96. doi: 10.1177/0962280211403603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullano MF, Kamat S, Willey VJ, Barlas S, Watson DJ, Brenneman SK. Agreement between administrative claims and the medical record in identifying patients with a diagnosis of hypertension. Med Care. 2006;44(5):486–90. doi: 10.1097/01.mlr.0000207482.02503.55. [DOI] [PubMed] [Google Scholar]
- 24.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. Journal of Clinical Epidemiology. 2011;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernán M, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 28.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309(3):241–2. doi: 10.1001/jama.2012.96867. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed on 03/2014];Arava Drug Label. Available on http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020905s020lbl.pdf.
- 30.Bili A, Tang X, Pranesh S, Bozaite R, Morris SJ, Antohe JL, Kirchner HL, Wasko MC. Tumor necrosis factor alpha inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66(3):355–63. doi: 10.1002/acr.22166. [DOI] [PubMed] [Google Scholar]
- 31.Ljung L, Askling J, Rantapaa-Dahlqvist S, Jacobsson L. The risk of acute coronary syndrome in rheumatoid arthritis in relation to tumour necrosis factor inhibitors and the risk in the general population: a national cohort study. Arthritis Res Ther. 2014;16(3):R127. doi: 10.1186/ar4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63(4):522–9. doi: 10.1002/acr.20371. [DOI] [PubMed] [Google Scholar]
- 33.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305(24):2525–31. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 34.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clinical rheumatology. 2007;26(9):1495–1498. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 35.Bilsborough W, Keen H, Taylor A, O’Driscoll GJ, Arnolda L, Green DJ. Anti-tumour necrosis factor-alpha therapy over conventional therapy improves endothelial function in adults with rheumatoid arthritis. Rheumatology international. 2006;26(12):1125–1131. doi: 10.1007/s00296-006-0147-y. [DOI] [PubMed] [Google Scholar]
- 36.Dixon WG, Symmons DPM. What effects might anti-TNF treatment be expected to have on cardiovascular morbidity and mortality in rheumatoid arthritis? A review of the role of TNF in cardiovascular pathophysiology. Annals of the rheumatic diseases. 2007;66(9):1132. doi: 10.1136/ard.2006.063867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR. Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension. 2010;55(3):776–784. doi: 10.1161/HYPERTENSIONAHA.109.143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.