Abstract
Introduction
Data on long term function of the Melody valve are scarce.
Patients and methods: single institution; results of percutaneous pulmonary valve implantation (PPVI) from 2006 to 2014. The function of the valved conduit was analyzed by Doppler echocardiography. Annual Chest X-ray after implant and permanent screening for events (e.g. Endocarditis).
Results
112 Melody valves were implanted in 111 patients; mean age 19.3 years (4.5–81.6). No pre-stenting of the RVOT was performed (n = 4) at first. In the next 107 patients pre-stenting was always performed. In 82 patients 1 pre-stent, 18 patients 2, in 6 patients 3 stents and 1 patient 4 stents were used. The Melody stent was dilated up to 24 mm (n = 4), 22 mm (n = 72), 20 mm (n = 28) and 18 mm (n = 6). When stenotic, the Doppler gradient reduced from 67.0 mm Hg (SD 13.9) to 18.9 mm Hg (SD 10.4) (p < 0.001); pulmonary regurgitation (PR) was reduced from median 3.5/4 (range 0–4/4) to none or trivial (p < 0.001). There was no significant change in RVOT peak velocity at 5 years (p = 0.122) nor PR (p = 0.835). Type 1 stent fractures were observed in 1/4 non-pre-stented patients and in 5/107 pre-stented (p < 0.05).
Endocarditis occurred in 8/112 valves; freedom of endocarditis was 85% at 5 years. In 2 patients early surgical replacement was necessary. Six were sterilized with antibiotic treatment; 2 patients required re-stenting and re-PPVI due to residual gradient.
Conclusion
Adequate pre-stenting of the RVOT before PPVI nearly abolishes or delays stent fracture. Cusp function is well preserved in mid-term follow-up; endocarditis is a threat.
Keywords: Melody valved stent, Percutaneous pulmonary valve implantation, Pre-stenting, Stent fracture, Endocarditis
1. Introduction
Percutaneous pulmonary valve implantation (PPVI) has widely been used throughout the world with broadening indications [1], [2]. The technique has been applied in over 7000 patients with good clinical and functional results, a very low mortality and a decreasing incidence of complications such as mediastinal bleeding after tearing the RVOT or conduit rupture, coronary artery occlusion due to coronary compression or elongation, perforation or exclusion of a pulmonary artery, dislocation or embolization of the valved stent, and tearing chordae of the tricuspid valve [3], [4], [5], [6]. In experienced hands the implantation procedure has become safe and predictable with good results in early follow-up. However, in order to determine the place of PPVI between the different treatment strategies, long term follow-up data must be available. This is important as PPVI must be compared with surgical series, and indications for PPVI are expected to shift from “symptomatic and curative” to “prognostic and preventive”. This study provides an interim analysis of PPVI with Melody valves in our center.
2. Patients and methods
This is a single institution interim analysis of an observational ongoing study of PPVI implants; our center drains patients with congenital heart disease of a population of about 4 million people. Since 2006 percutaneous pulmonary valve replacement is performed in our center with the Melody® valve (Medtronic Inc. Minneapolis, MN, USA). The Melody valve consists of a balloon-expandable Cheatham-Platinum CP stent frame (NuMED®, Hopkinton, NY, USA) with a trimmed bovine jugular valved vein sutured at the inside (Contegra®, Medtronic Inc. Minneapolis, MN, USA) [1], [2], [3], [4], [5], [6], [7]. The implantations were performed as previously described [8]. All implants were done under general anesthesia and prophylactic antibiotic treatment (before, 6 and 18 h after procedure). Access was either through the femoral or jugular vein. In all but the first 12 patients, balloon interrogation of the RVOT with simultaneous angiography of the coronary arteries was performed prior to stent implantation. In stenotic or calcified conduits a high pressure balloon was used (Mullins, NuMED, USA or Atlas Gold, Bard PV, US), in conduit free outflow tracts a semi-compliable low pressure balloon (Tyshak, NuMED, US) was used [8]. After discharge no medication was prescribed to the patients, specifically no anti-aggregation medication. Endocarditis profylaxis was recommended in all patients. As requested by the Belgian health insurance for this new technique, all patients are in strict follow-up after implantation. A clinical evaluation and an echocardiography is performed 1 month, 3, 6 and 12 months after valve implantation and thereafter on yearly base. Chest X-ray is performed at 6 and 12 months and thereafter annually to look for recompression or stent fractures. The database was screened for the event of endocarditis. Cusp dysfunction was assessed by means of transthoracic echocardiography based on gradient across the valve and regurgitation. All patient data was collected in a dedicated registry. Informed consent was obtained in all patients by either themselves or by their parents or legal guardian. Approval by the local ethics committee was granted prior to the start of the study.
2.1. Statistical analysis
For the statistical analysis SPSS version 20 (IBM) was used. Continuous data are expressed as mean and standard deviation or median and range. Proportions and frequencies are expressed in percentage. Paired data were analyzed by a paired t-test and Kruskal Wallis test. For survival analysis Kaplan Meier plots were used. Statistical significance was reached when the p-value was < 0.05.
3. Results
A total of 112 Melody valves were implanted in 111 patients between 2006 and 2014; mean age 19.3 years (range 4.5–81.6 years); 1 valve was explanted immediately because of coronary compression; the median follow-up in the remaining 111 valves was 2.4 years (range 31 days–6.9 years). The diagnosis was tetralogy of Fallot (n = 63, 56.3%), common arterial trunc (n = 7, 6.3%), status post Ross operation (n = 22, 19.6%), pulmonary atresia (n = 4, 3.6%), after Rastelli type repair (n = 11, 9.8%) and pulmonary stenosis (n = 4, 3.6%). The target zone consisted of a homograft in 63 patients (56.2%), a Contegra® graft (Medtronic) in 14 patients (12.5%), after a transannular patch in 29 patients (25.9%), native pulmonary valve in 4 patients (3.6%) and 2 had a Freestyle® graft (Medtronic) (1.7%). In the first 4 patients no pre-stenting of the RVOT was performed as recommended by the company and by the product label. In the next 108 implants pre-stenting was performed prior to or at the time of PPVI (off label use for all stents). A total of 140 pre-stents were implanted. During the time period, pre-stenting evolved significantly. As our experience grew, stents were implanted until the outflow tract became a rigid non-restrictive tube without relative motion or wringing. Initially, the outflow tract was dilated up till the nominal value of the surgical conduit; later all conduits were expanded up to 22 to 24 mm before deployment of the Melody valve, provided pulmonary trunc size and coronary proximity would allow so. In patients without a previous surgical conduit (typically after a transannular patch), initially no stent (n = 1) but thereafter a single open cell bare stent (Andrastent XXL® Andramed, Reutlingen, Germany) was used to provide an adequate landing zone (n = 32): we preferred a bare open cell stent to maximize grip of the stent onto the non-stenotic outflow tract. If the stent was felt to be insufficiently stable to withstand immediately the manipulations involved with the Melody implantation (n = 24), the stent was left for 2 months to allow adequate ingrowth [8]. In 3 such pre-stented patients the diameter of the landing zone was first reduced with a CP stent to about 24–25 mm prior to Melody implantation. In patients with a surgical conduit (n = 78), no pre-stent was used in the first 3 patients, but thereafter 1 stent was used for pre-stenting in 53 patients, 2 stents in 15 patients, 3 stents in 6 patients and in one patient up to 4 stents were implanted prior to the implantation of the valved stent. When a conduit fracture or tear was expected (calcified conduit, expansion beyond nominal size, pressure resistant lesion), long covered CP stents (up to 65 mm) were used to cover the target area from before the proximal anastomosis until beyond the distal anastomosis.
Overall different types of stents were used: covered (n = 61) and bare (n = 6) Cheatham Platinum (CP)® stents (NUMED, Hopkinton NY, US), Andrastent XXL® (n = 51) (Andramed, Reutlingen, Germany), Max LD Intrastent® (n = 17) (eV3 Europe, Paris, France), Palmaz Genesis® (n = 5) (Cordis, Johnson & Johnson Interventional Systems Co., Warren, NJ) (off label use for all stents). The valved stent was dilated up to 24 mm in 4 patients, to 22 mm in 74 patients, to 20 mm in 28 patients and in 6 younger children only up to 18 mm.
3.1. Procedural complications
Some major complications occurred early in the series before 2008, when experience of deploying large stents in the RVOT was limited. In patient no. 3 the pulmonary artery was perforated by the Ensemble delivery system, this was closed surgically. In patient no. 9 coronary compression occurred during PPVI with need for urgent surgical removal of the stent. Conduit rupture with extravasation occurred in patient no. 12 who was referred to surgery to control the bleeding; the ruptured homograft was oversewn and the Melody valve implanted by a transventricular approach. Conduit tears with mild and contained extravasation was observed in 3 patients during balloon interrogation or pre-stenting; the tear was excluded with a covered stent in 1 patient, and with the Melody valve in both other patients. All these complications were managed without significant residual problem, except in patient 9 where mild left ventricular septal dysfunction persisted. With added experience the technique evolved with respect to systematic balloon interrogation of the RVOT, minimizing the possibility of tearing the tricuspid chordae and coronary compression, usage of adapted guiding wires, better control of big sheaths and guide wires during positioning of the stents, preventive usage of long covered stents. No major complication occurred after 2008.
3.2. Hemodynamic result
In 49 (44.2%) patients PPVI was performed for predominantly RVOT obstruction (Doppler gradient > 50 mm Hg), 36 (32.4%) patients had important pulmonary valve regurgitation (PR > 3–4/4), and in 26 (23.4%) patients a mixed type of PS and PR was the indication for PPVI. Echocardiographic data are presented in Table 1.
Table 1.
Echocardiographic data PPVI.
| Indication for PPVI | Before implant | SD | After implant | SD | p-Valuesb | 3 years after implant | SD | p-Valueb | 5 years after implant | SD | p-Valueb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stenosis PS | n = 48 | Mean | Mean | Mean | Mean | |||||||
| RVOT gradient (mm Hg) | 67.0 | 13.9 | 18.9 | 10.4 | < 0.001 | 27.4 | 13.0 | 0.330 | 30.6 | 9.6 | 0.220 | |
| RVOT Vmax (cm/s) | 3.5 | 0.7 | 1.7 | 0.85 | < 0.001 | 2.5 | 0.5 | 0.163 | 2.7 | 0.4 | 0.122 | |
| Regurgitation PR | n = 36 | |||||||||||
| Median | Range | Median | Range | Median | Range | Median | Range | |||||
| PR (grade at 4) | 3.5 | 0–4 | 0.25 | 0–1 | < 0.001 | 0.5 | 0–1 | 0.729 | 0.5 | 0–2 | 0.835 | |
| Mixed PS and PR | n = 26 | |||||||||||
| Mean | Mean | Mean | Mean | |||||||||
| RVOT gradient (mm Hg) | 52.5 | 11.1 | 20.9 | 8.5 | < 0.001 | 31.3 | 19.9 | 0.824 | 38.0 | 18 | 0.689 | |
| RVOT Vmax (cm/s) | 3.3 | 0.9 | 2.0 | 0.75 | < 0.001 | 2.6 | 0.9 | 0.103 | 3.0 | 0.9 | 0.417 | |
| Median | Range | Median | Range | Median | Range | Median | Range | |||||
| PR (grade at 4)a | 2 | 0–3 | 0 | 0–2 | < 0.001 | 1 | 0–1 | 0.979 | 1 | 0–1 | 0.505 | |
Pulmonary regurgitation is expressed in grade: 0 = none, 1 = slight, 2 = mild, 3 = moderate and 4 = severe.
Statistical difference with baseline condition (before implant).
The homografts (n = 63) measured at implantation 20.9 ± 2.9 mm (range 12–27 mm) but had shrunken down to 16.6 ± 2.8 mm (10–21 mm); after Melody implantation the lumen was expanded up to 21.3 ± 1.5 mm (range 18–24 mm) (p < 0.01); the homograft was augmented 0.42 ± 2.7 mm (range − 4 to + 10 mm) from implantation until post-Melody (p = 0.14). Similarly the Contegra® grafts (n = 14) measured 19.8 ± 3.6 mm at implantation (range 12–22 mm), and the lumen was expanded from 15.7 ± 3.5 mm (range 10–22 mm) up to 21.7 ± 1.0 mm (range 20–24 mm) (p < 0.001); the Contegra was augmented 1.9 ± 3.5 mm (range − 2 to + 10 mm) from implantation until post-Melody (p = 0.51).
In patients with a significant stenosis, the Doppler gradient across the RVOT significantly decreased from mean 67.0 ± 13.9 mm Hg to 18.9 ± 10.4 mm Hg (p < 0.001). During follow-up, no significant increase of RVOT gradient was observed at 3 years (26.7 ± 13.7 mm Hg, p = 0.330) and at 5 years (30.7 ± 8.6 mm Hg, p = 0.220) (Fig. 1).
Fig. 1.
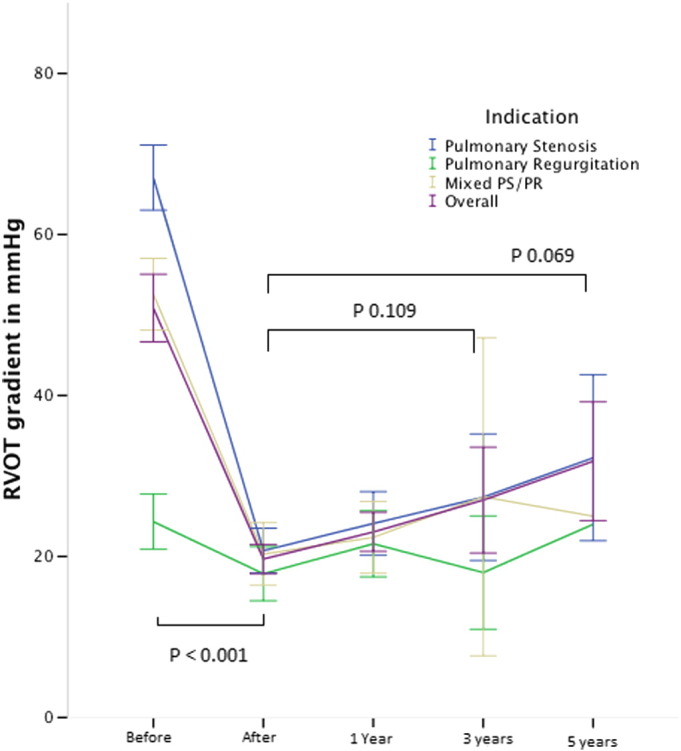
Evolution of peak Doppler RVOT gradient expressed in mm Hg before, immediately after implant and during follow-up. Indication for PPVI: (blue) pulmonary stenosis, (green) pulmonary regurgitation, (brown) mixed PS/PR and (purple) overall the 3 groups. Error bars express the 95% confidence interval. Valves at risk: pre: 112; post 111; 1 year: 90; 3 years: 36; 5 years: 10.
Pulmonary regurgitation was significantly reduced in all patients from median 3.5/4 (range 0–4/4) to none or trivial (p < 0.001). Pulmonary regurgitation showed no significant change at 3 years (0.5/4, range 0–1, p = 0.729) and at 5 years after implant (0.5/4, range 0–2, p = 0.835) (Fig. 2).
Fig. 2.
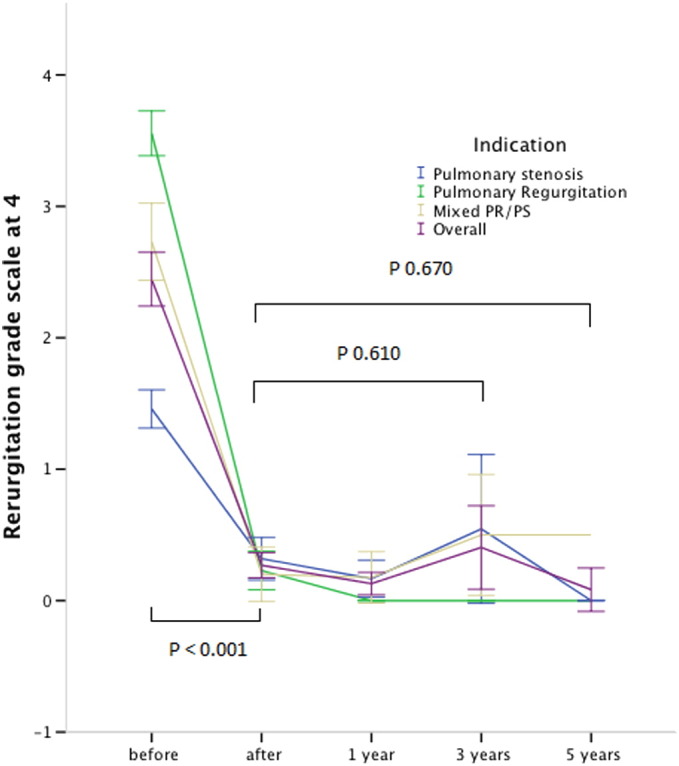
Evolution of pulmonary regurgitation before, immediately after implant and during follow-up. Indication for PPVI: (blue) pulmonary stenosis, (green) pulmonary regurgitation, (brown) mixed PS/PR and (purple) overall the 3 groups. Error Bars express the 95% confidence interval. Valves at risk: pre: 112; post 111; 1 year: 90; 3 years: 36; 5 years: 10.
3.3. Follow-up
Three patients died during follow-up, all for Melody unrelated reasons (ventricular arrhythmia previously existent from a dysfunctional left ventricle, convulsions, old age 85 years with primary diagnosis of critical pulmonary stenosis); for all survival analyses they are withdrawn non-censored. Freedom from valve related re-intervention was 96% 3 years after implant and 89% 5 years after implant (Fig. 3).
Fig. 3.
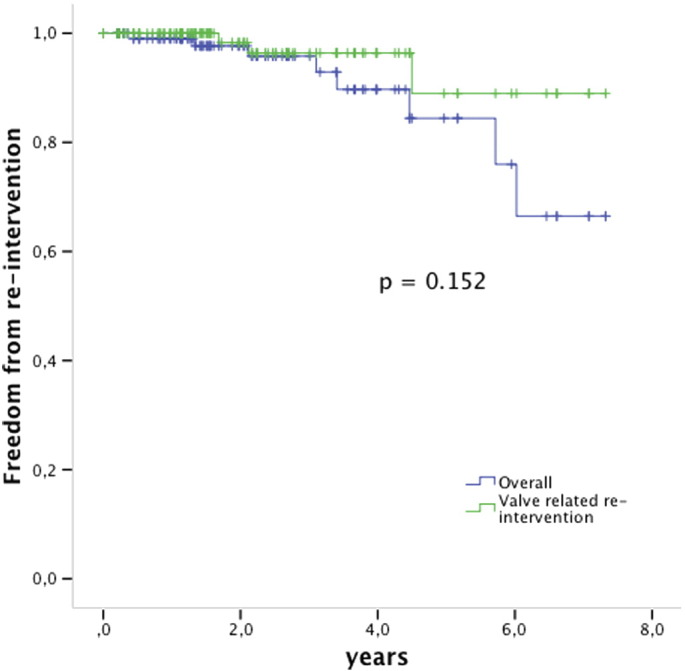
Kaplan Meier survival analysis free from re-intervention. The green line shows the valve related freedom from re-intervention, the blue line is the overall freedom from re-intervention including e.g. balloon dilation for somatic growth. Valves at risk: time 0: 112; 2 years: 55; 4 years: 20; 6 years: 8.
3.4. Stent explantation
Four valved stents were explanted after successful implantation. In 2 patients the Melody valve was implanted in a restrictive tube; because of a persisting gradient across the RVOT and inability to dilate, the RVOT was reconstructed with a homograft after respectively 45 and 47 months. Two Melody stents were resected in the acute phase of endocarditis at 16 and 38 months after implantation.
3.5. Re-intervention
Re-dilation was performed in 3 patients for somatic growth and none for recompression. Redo PPVI was performed in a patient who presented with progressive obstruction and at that point undiagnosed endocarditis; he was first treated with a bare stent to relieve the stenosis, and when cultures turned positive, IV antibiotics were given for 6 weeks. He received a second Melody valve 10 months later. Another patient had a residual gradient after endocarditis; he was re-stented 2 months after the endocarditis, and a new valved stent was implanted 3 months later (not included in this series). Both patients are doing clinically well with now 14 and 4 months of additional follow-up.
3.6. Stent recompression or fracture
During the follow-up stent fractures were observed in 1/4 (25%) of the non-pre-stented patients and in 5/107 (4, 6%) of the pre-stented group (p < 0.05) (Fig. 3). Mild stent recompression was observed in 3 patients and was seen mostly at the anterior site close to the sternum, without hemodynamic compromise in any of those patients. However, one of these patients developed endocarditis. All observed stent fractures were type I according to Nordmeyer et al., no type II or III fractures were observed [9], [10] (Fig. 4).
Fig. 4.
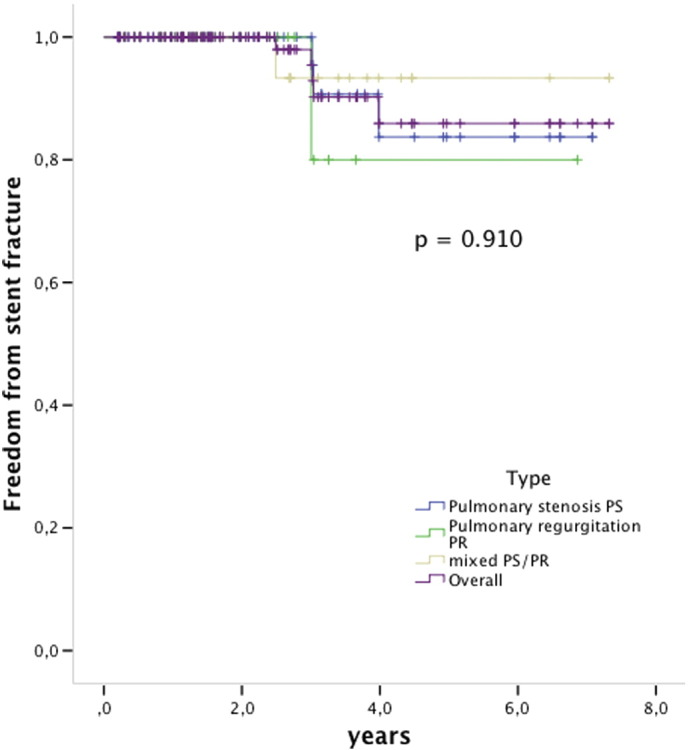
Kaplan Meier survival free from stent fractures. The indication for PPVI was (blue) pulmonary stenosis, (green) pulmonary regurgitation, (brown) mixed PS/PR and ](purple) overall the 3 groups. Log rank p = 0.910. Valves at risk: time 0: 112; 2 years: 57; 4 years: 18; 6 years: 9.
3.7. Endocarditis
In 8 patients endocarditis was diagnosed based on the Duke's criteria as “definite” in 6, and “possible” in 2, median 16.0 months after implant (range 13.4–44.4 months). Freedom from endocarditis was 84.9% at 5 years (Fig. 5). In the patients with endocarditis the original conduit was a homograft (n = 5), native or patch (n = 2), or a Contegra® graft (n = 1); none of these patients had experienced endocarditis previously. The bacteria were Corynebacterium pseudo diphtheriticum (n = 1), HACEK (n = 3), Staphylococcus aureus (n = 1), Streptococcus viridans (n = 2) and Streptococcus sanguinis (n = 1). The most likely point of bacterial entrance was the dental mucosa after inadequate or no endocarditis prophylaxis before dental care or oral wounds in four patients, surinfected tinea pedis in one patient and poor personal hygiene and nail biting in one patient; endocarditis in one patient was classified cryptogenic.
Fig. 5.
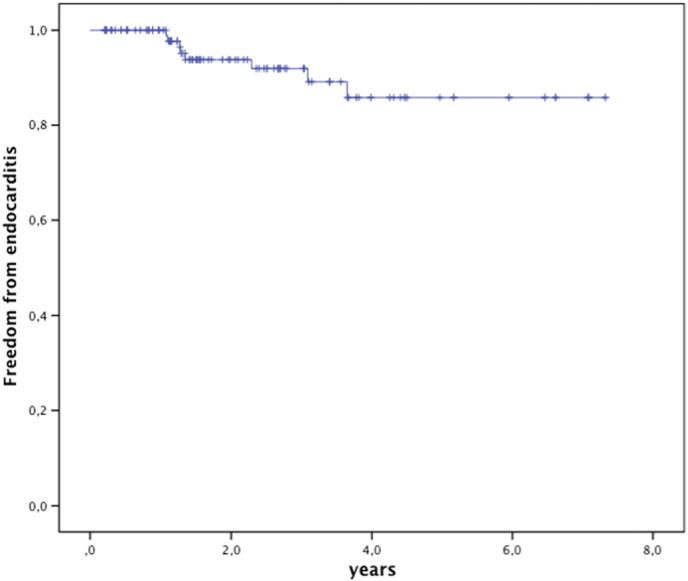
Kaplan Meier survival free from endocarditis. Valves at risk: time 0: 112; 2 years: 59; 4 years: 26; 6 years: 11.
Due to a progressive and symptomatic gradient, early explantation of the Melody valve and surgical homograft placement were necessary in 2 patients at 9 and 25 days after presentation. In the 6 remaining patients the conduit was sterilized with antibiotic treatment; 4 patients had still adequate valve function (gradient < 25 mm Hg, PR < 2/4), but 2 patients had a significantly increased gradient, which led to redo PPVI. Valve replacement was therefore required in 4/8 of the cases with endocarditis.
4. Discussion
The place of PPVI in the management of patients with RVOT dysfunction still needs to be determined. Initially the Melody valve was marketed “to extend the survival of surgical conduits” with an intended lifetime of 2 years (label Melody). However, early results suggest that PPVI with the Melody stent may have a more ambitious outlook. Our study confirms the good results of Melody valved stents in the right ventricular outflow tract during implantation and early follow-up [11]; moreover, good conduit function is maintained during medium term follow-up. Good mid-term function of the Melody conduit depends on good function of the different components of the prosthesis: the stent, the tissue conduit and the cusps.
4.1. Stent function
Stent failure may consist of recompression or fracture of the stent wires, resulting in infolding or collapse. External forces can act on the stent of the Melody: the valved stent frequently gets sandwiched between hard and dynamic structures as the sternum on one side and the high pressure aorta on the other side. Calcifications from previous grafts may act as a leverage to transmit and concentrate the external forces; the rhythmic motion of the heart beat will induce cyclic stress, that if of sufficient amplitude, will harden the metal and induce metal fatigue, and eventually may lead to fractures after sufficient cycles [12]. Recompression or collapse of the stent with hemodynamic compromise in the early experience occurred as frequently as 25% after 3 years [9]. Most fractures in the reported series occurred within 6 months [3], [9], [10], [11], [12], [13], [14]. It was hypothesized that the effect of these forces could be neutralized with adequate pre-stenting [10]. Pre-stenting adds stiffness to the scaffold, thereby reducing the stress amplitude of the metal to a level where fractures do not occur at all, or occur only after a much larger number of cycles (Basquin law) [12]. Theoretically pre-stenting may have some disadvantages: if insufficiently fixed, crossing strands may impose fretting, leverage effect may concentrate forces, and if different metals are used, electrolysis may occur. Lack of knowledge of the latter has withheld official instances and companies to recommend, or even allow complex pre-stenting [10]. In this series with pre-stenting we have observed in medium term follow-up only minimal fractures, no collapse and in few minimal recompression of the Melody stent without hemodynamic compromise. There was no need for re-stenting, nor valve-in-valve treatment [10], [15]. With PPVI, gradient relief is predominantly obtained by the pre-stenting, while the valved stent is predominantly used to abolish the regurgitation. The use of multiple overlapping stents and the use of simple X-ray (F & P) may have precluded the detection of small fractures of the Melody stent; however Doppler echocardiography excluded a clinically relevant change of valve function, which is the final goal of this treatment.
In 3 children a re-dilation was performed for somatic growth: a gradient reoccurrence in growing children can be expected. This underlines the value of stenting/dilating all outflow tracts to the maximum achievable diameter at any age.
4.2. Hammock effect
Blood or tissue may accumulate around the struts of the stent between the RVOT wall and the outside of the bovine valve tissue: this complication is no longer observed after modification of the Melody valve in 2003, when additional stitches were added over the full length and circumference of the trimmed bovine venous graft onto the CP stent [15]. The hammock complication still may occur in endocarditis where pus and vegetations may accumulate between the stent and tissue, which may lead to flow obstruction [16], [17].
4.3. Cusp failure
The cusps of the bovine jugular venous valve appear to be resistant to wear and tear; this is the experience of the Contegra® conduit with long lasting cusp function; the most frequent mode of Contegra® conduit failure is stenosis at distal anastomosis and endocarditis [18], [19], [20]. In our series with up to 7 years of follow-up, no significant obstruction or regurgitation at the level of the valve was observed. The pre-stenting resulting in a large, not-restrictive conduit with low energy turbulence and low acceleration across the valve, may have contributed to the longevity of the Melody cusps. This aspect needs to be evaluated when comparing with other series.
4.4. Endocarditis
Endocarditis now appears to be the most important threat to the Melody valve. Endocarditis of the Melody valve has been reported in many, if not all series [14], [15], [16], [17], [18], [19], [20], [21]. The incidence in our population was 3.0% per patient year after the first year, which is comparable with other reported series. Two out of 8 patients presented with a fast progressive obstruction, requiring early surgical replacement. Similar presentations have been reported where very urgent intervention was life-saving [1], [2], [17], [22]. The remaining 6 patients in our series were sterilized after a prolonged (6 weeks) cure of IV antibiotics. In 4 patients, the cusp function after treatment had not significantly changed from the pre-endocarditis evaluation. It seems that endocarditis of the Melody valve can be cured, however with significant morbidity (prolonged IV antibiotics) and needs for valve replacement in many. More details regarding endocarditis in this patient population were recently published [23].
Preventing endocarditis is important for long term survival of the valved stent. In this series we did not observe reactivation of a previous endocarditis. Endocarditis did not occur within the first year of implantation, making implantation-related infection or colonization of an early thrombus very unlikely. No conduit is immune to endocarditis, but some tissues may be more vulnerable to infection. Preventive strategies must be explored such as patient education, avoidance of entry ports for bacteria, antibiotics in case of a predictable high level bacteremia and avoidance of high energy turbulence by adequate pre-stenting of the RVOT. The possible role of micro-thrombi, blood stasis in and around the conduit, surface characteristics and cusp motion and redundancy should be also investigated.
4.5. Comparison with surgical conduits
The valved stent compares favorably on re-intervention with other conduits such as the homograft and Contegra conduit [18], [19], [20], [21], [22], [23], [24], [25]. The surgical alternative with valved conduit implantation requires longer hospital stay, is not free of either early or late complications, and requires repeated re-interventions of increasing complexity and risk. A recent report from Vergales et al. shows that in the USA the total cost of PPVI is less than surgical valve replacement and that it would require a very dramatic short term survival of the PPVI to be less competitive; this study reflects the US financial data but cannot be extrapolated to the European situation [26]. [27]
4.6. Limitations
Since implants only started in 2006 in our center these data reflect on a limited series with medium term follow-up. Stent integrity was assessed by chest X-ray and not with rotational fluoroscopy; this is partly due to the fact that many X-rays are performed at referring centers without access to rotational fluoroscopy. We did not perform a controlled 2-arm study to prove the efficacy of pre-stenting on the incidence of stent fractures: reducing the stress amplitude by adding more material is a basic engineering principle when avoiding metal fatigue, and we expected objections from the ethical board against a non-prestented study group with such expensive treatment and with potential risk.
5. Conclusion
Adequate pre-stenting of the RVOT before percutaneous valve replacement offers good stent support which nearly abolishes or significantly delays stent fracture or recompression in medium term follow-up. Cusp function is good in mid-term follow-up; its relationship with widely opened conduits with a low gradient and turbulence needs to be addressed. The Melody valve is vulnerable for endocarditis, avoidance requires further research.
Disclosures
MG is proctor for Medtronic® and NUMED®.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
This research was sponsored in part by an unrestricted Eddy Merckx Research grant and Sporta MonVentoux 2013.
References
- 1.Bonhoeffer P., Boudjemline Y., Saliba Z., Hausse A.O., Aggoun Y., Bonnet D. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation. 2000;102(7):813–816. doi: 10.1161/01.cir.102.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Bonhoeffer P., Boudjemline Y., Saliba Z., Merckx J., Aggoun Y., Bonnet D. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356(9239):1403–1405. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 3.McElhinney D.B., Cheatham J.P., Jones T.K., Lock J.E., Vincent J.A., Zahn E.M. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circ Cardiovasc Interv. 2011;4(6):602–614. doi: 10.1161/CIRCINTERVENTIONS.111.965616. [DOI] [PubMed] [Google Scholar]
- 4.Morray B.H., McElhinney D.B., Cheatham J.P., Zahn E.M., Berman D.P., Sullivan P.M. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013;6(5):535–542. doi: 10.1161/CIRCINTERVENTIONS.113.000202. [DOI] [PubMed] [Google Scholar]
- 5.Mauri L., Frigiola A., Butera G. Emergency surgery for extrinsic coronary compression after percutaneous pulmonary valve implantation. Cardiol Young. 2013;23(3):463–465. doi: 10.1017/S1047951112001187. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong A.K., Balzer D.T., Cabalka A.K., Gray R.G., Javois A.J., Moore J.W. One-year follow-up of the Melody transcatheter pulmonary valve multicenter post-approval study. JACC Cardiovasc Interv. 2014;7(11):1254–1262. doi: 10.1016/j.jcin.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Bonhoeffer P., Boudjemline Y., Qureshi S.A., Le Bidois J., Iserin L., Acar P. Percutaneous insertion of the pulmonary valve. J Am Coll Cardiol. 2002;39(10):1664–1669. doi: 10.1016/s0735-1097(02)01822-3. [DOI] [PubMed] [Google Scholar]
- 8.Boshoff D.E., Cools B.L., Heying R., Troost E., Kefer J., Budts W. Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv. 2013;81(6):987–995. doi: 10.1002/ccd.24594. [DOI] [PubMed] [Google Scholar]
- 9.Nordmeyer J., Khambadkone S., Coats L., Schievano S., Lurz P., Parenzan G. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115(11):1392–1397. doi: 10.1161/CIRCULATIONAHA.106.674259. [DOI] [PubMed] [Google Scholar]
- 10.Nordmeyer J., Lurz P., Khambadkone S., Schievano S., Jones A., McElhinney D.B. Pre-stenting with a bare metal stent before percutaneous pulmonary valve implantation: acute and 1-year outcomes. Heart. 2011;97(2):118–123. doi: 10.1136/hrt.2010.198382. [DOI] [PubMed] [Google Scholar]
- 11.Vezmar M., Chaturvedi R., Lee K.J., Almeida C., Manlhiot C., McCrindle B.W. Percutaneous pulmonary valve implantation in the young 2-year follow-up. JACC Cardiovasc Interv. 2010;3(4):439–448. doi: 10.1016/j.jcin.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Ashbey M.S.H., Cebon D. 2nd ed. Elsevier; Canada: 2009. Materials: Science, Processing and Design. [Google Scholar]
- 13.Schievano S., Petrini L., Migliavacca F., Coats L., Nordmeyer J., Lurz P. Finite element analysis of stent deployment: understanding stent fracture in percutaneous pulmonary valve implantation. J Interv Cardiol. 2007;20(6):546–554. doi: 10.1111/j.1540-8183.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 14.McElhinney D.B., Hellenbrand W.E., Zahn E.M., Jones T.K., Cheatham J.P., Lock J.E. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507–516. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khambadkone S., Coats L., Taylor A., Boudjemline Y., Derrick G., Tsang V. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112(8):1189–1197. doi: 10.1161/CIRCULATIONAHA.104.523266. [DOI] [PubMed] [Google Scholar]
- 16.Patel M., Iserin L., Bonnet D., Boudjemline Y. Atypical malignant late infective endocarditis of Melody valve. J Thorac Cardiovasc Surg. 2012;143(4):e32–e35. doi: 10.1016/j.jtcvs.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Bhat D.P., Forbes T.J., Aggarwal S. A case of life-threatening Staphylococcus aureus endocarditis involving percutaneous transcatheter prosthetic pulmonary valve. Congenit Heart Dis. 2013;8(6):E161–E164. doi: 10.1111/chd.12013. [DOI] [PubMed] [Google Scholar]
- 18.Urso S., Rega F., Meuris B., Gewillig M., Eyskens B., Daenen W. The Contegra conduit in the right ventricular outflow tract is an independent risk factor for graft replacement. Eur J Cardiothorac Surg. 2011;40(3):603–609. doi: 10.1016/j.ejcts.2010.11.081. [DOI] [PubMed] [Google Scholar]
- 19.Boethig D., Schreiber C., Hazekamp M., Blanz U., Pretre R., Asfour B. Risk factors for distal Contegra stenosis: results of a prospective European multicentre study. Thorac Cardiovasc Surg. 2012;60(3):195–204. doi: 10.1055/s-0031-1298062. [DOI] [PubMed] [Google Scholar]
- 20.Breymann T., Blanz U., Wojtalik M.A., Daenen W., Hetzer R., Sarris G. European Contegra multicentre study: 7-year results after 165 valved bovine jugular vein graft implantations. Thorac Cardiovasc Surg. 2009;57(5):257–269. doi: 10.1055/s-0029-1185513. [DOI] [PubMed] [Google Scholar]
- 21.McElhinney D.B., Benson L.N., Eicken A., Kreutzer J., Padera R.F., Zahn E.M. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circ Cardiovasc Interv. 2013;6(3):292–300. doi: 10.1161/CIRCINTERVENTIONS.112.000087. [DOI] [PubMed] [Google Scholar]
- 22.Malekzadeh-Milani S., Ladouceur M., Patel M., Boughenou F.M., Iserin L., Bonnet D. Incidence and predictors of Melody valve endocarditis: a prospective study. Arch Cardiovasc Dis. 2015;108(2):97–106. doi: 10.1016/j.acvd.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Van Dijck I., Budts W., Cools B., Eyskens B., Boshoff D.E., Heying R. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart. 2015 doi: 10.1136/heartjnl-2014-306761. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Kalfa D., Feier H., Loundou A., Fraisse A., Mace L., Metras D. Cryopreserved homograft in the Ross procedure: outcomes and prognostic factors. J Heart Valve Dis. 2011;20(5):571–581. [PubMed] [Google Scholar]
- 25.Kalfa D.M., Loundou A., Nouaille de Gorce Y., Fraisse A., Metras D.R., Mace l. Pulmonary position cryopreserved homograft in non-Ross patients: how to improve the results? European journal of cardio-thoracic surgery: Off J Euro Assoc Cardiothorac Surg. 2012;42(6):981–987. doi: 10.1093/ejcts/ezs248. [DOI] [PubMed] [Google Scholar]
- 26.Vergales J.E., Wanchek T., Novicoff W., Kron I.L., Lim D.S. Cost-analysis of percutaneous pulmonary valve implantation compared to surgical pulmonary valve replacement. Catheter Cardiovasc Interv. 2013;82(7):1147–1153. doi: 10.1002/ccd.25128. [DOI] [PubMed] [Google Scholar]
- 27.Neyt M., Vinck I., Gewillig M., Van Brabandt H. Percutaneous pulmonary and aortic valve insertion in Belgium: going for conditional reimbursement or waiting for further evidence? Int J Technol Assess Health Care. 2009;25(3):281–289. doi: 10.1017/S0266462309990080. [DOI] [PubMed] [Google Scholar]


