Abstract
The main objective of this study was to create a postnatal model for cardiac hypertrophy (CH), in order to explain the mechanisms that are present in childhood cardiac hypertrophy. Five days after implantation, intraperitoneal (IP) isoproterenol (ISO) was injected for 7 days to pregnant female mice. The fetuses were obtained at 15, 17 and 19 dpc from both groups, also newborns (NB), neonates (7–15 days) and young adults (6 weeks of age). Histopathological exams were done on the hearts. Immunohistochemistry and western blot demonstrated GATA4 and PCNA protein expression, qPCR real time the mRNA of adrenergic receptors (α-AR and β-AR), alpha and beta myosins (α-MHC, β-MHC) and GATA4. After the administration of ISO, there was no change in the number of offsprings. We observed significant structural changes in the size of the offspring hearts. Morphometric analysis revealed an increase in the size of the left ventricular wall and interventricular septum (IVS). Histopathological analysis demonstrated loss of cellular compaction and presence of left ventricular small fibrous foci after birth. Adrenergic receptors might be responsible for changing a physiological into a pathological hypertrophy. However GATA4 seemed to be the determining factor in the pathology. A new animal model was established for the study of pathologic CH in early postnatal stages.
Keywords: Fetal cardiac hypertrophy, Isoproterenol, GATA4, α-MHC, β-MHC
1. Introduction
Pathologic cardiac hypertrophy (CH) is a process usually studied in adults, where the differentiated post mitotic myocardium is capable of responding to different adverse stimuli, genetic as well as environmental, increasing its size. Since this process can develop cardiac insufficiency and culminate in sudden death [1], it's diagnosis is considered a marker of poor prognosis. However this disease is not exclusive of adulthood. Approximately 95% of cases of CH are detected between the ages of 15 and 25 years [2]. It is estimated that the frequency of CH in the fetal stage is around 7% and probably higher. About 11% of the fetuses die in uterus due to this disease [3]. Physiologic CH is a key process for heart development during the fetal period, it increases while proliferation decreases and then, it decreases after birth as maturity is achieved [4]. However, what mechanisms turn a physiological into a pathological cardiac hypertrophy during fetal or postnatal life still remains a question, probably due to the lack of experimental in vivo models.
Among the most utilized adult in vivo experimental models to induce hypertrophy is the use of ISO. It is a simple, easy to administer and reproducible method with low mortality [5], [6], [7] compared with other techniques of short duration, with high stress or those that require surgical procedures such as coronary artery ligature or transverse aortic constriction that imply a high risk of morbidity and mortality [6], [8].
ISO is a synthetic catecholamine with the addition of two methyl groups. It is structurally similar to adrenaline and is joined to β-adrenergic receptors (β-AR), producing a superior effect of up to ten times that of adrenaline itself [9], [10]. Different studies in adult murine models have demonstrated the flexibility of ISO. A single subcutaneous (SC) dose of ISO between 10 and 85 mg/kg in adult rats causes myocardial necrosis and fibrosis [11], [12], [13], [14], [15]. Low doses of ISO (0.3 to 6 mg/kg) during the 1st, 2nd and 3rd weeks induce necrosis in areas of the myocardium [16], [17], [18]. In turn, moderate doses (35 mg/kg) administered SC for 3 days induced dilated cardiomyopathy. Chronic treatment (100 mg/kg) > 2 weeks in adult rats produces diastolic dysfunction and causes decrease of fatty acids and glucose in the myocardium. Changes similar to those were observed in rat hearts after myocardial infarction of moderate severity [19], [20]. Cardiotoxicity after ISO administration with osmotic mini-pumps is able to cause CH after 7 days; however, 14 days after treatment and once the mini-pumps have been removed, the pathology reverses itself [21]. On the other hand, concentrations > 5 mg and up to 60 mg/kg/day for 7 days after IP administration, favors a pathological hypertrophic response, which affects the left ventricle (LV) in the adult rat [22], [23]. Hypertrophy produced by ISO is very similar to CH in humans, determined by microarray analysis of mRNA expression. Genes found in the ISO model have a higher correlation than CH generated by active exercise in 8-week-old adult mice [23]. Finally, there is only one study that evidences the use of ISO during pregnancy. However, results are contradictory as the authors observed that CH reverses itself a few weeks after birth [24]. These studies all demonstrate that the mechanisms and time of ISO administration are important to obtain the degree of myocardial damage desired.
ISO is a β-adrenergic stimulant and is able to cross the placental barrier like epinephrine [25], [26]. We hypothesized that prolonged IP administration of ISO in pregnant female mice is a stimulus capable of altering the myocyte physiology during fetal and neonatal stages, generating a pathologic CH with LV involvement, similar to experimental pathological models of hypertrophy generated in adulthood. For this reason our goal was to analyze the effect of IP administration of ISO in pregnant female mice for 7 days after the 5th day of implantation. We believe that the model proposed in this study not only raises new questions about the mechanisms of the evolution of this pathology, but can also be used to generate markers for early detection, as well as for evaluating the use of cell and/or regenerative therapy as treatment.
2. Methods
2.1. Mouse model for postnatal pathologic cardiac hypertrophy
To produce postnatal cardiac hypertrophy (p-CH), female Mus musculus Balb/C strain mice, previously paired with males of the same strain, were utilized. Observation of the vaginal plug was regarded as day 0 of gestation. From the fifth day postcoitus (dpc) shortly after the implantation of the embryo, pregnant female mice were injected IP with ISO 50 μg/kg/day (Sigma Aldrich, St. Louis, MO) in phosphate-buffered saline solution (PBS) for 7 days. The fetuses were obtained at (age groups) 15, 17 and 19 dpc from both groups, also newborns (NB), neonates (7–15 days) and young adults 6 weeks of age.
Another group was formed with pathologic CH mice induced in adult stage (CH-ad). The hypertrophy was induced in 6 week old mice injected IP with 50 mg/kg/day of ISO for 7 days. They were sacrificed 8 days after the end of ISO administration [27], [28].
A control group (CTR) for each group, was injected with PBS during the same time period as the experimental groups. All the animals were bred in the laboratory animal sources of the Hospital Infantil de México Federico Gómez, under the Mexican Official Norm NOM-062-ZOO-1999. They were kept in of 12 hour light/dark cycles.
2.2. Evaluation of the effects of ISO in pregnant female mice
To confirm the effects of ISO in the offspring of treated pregnant female mice, the maternal weight was recorded from the first day of gestation until the time of sacrifice. The number of reabsorptions by litter and the number of offsprings were recorded. After labor or cesarean delivery, maternal hearts were washed by perfusion with PBS and fixed in 3.5% paraformaldehyde. In both groups ISO and PBS, dissections of the heart chambers and transverse dissections were performed. Images were analyzed with a stereoscopic microscope (Olympus, Tokyo, Japan). Micrographs and measurements of the dissected hearts along the free walls of both ventricles were done using ImageJ 1.46 software (National Institutes of Health, Bethesda, MD).
2.3. Cardiac hemodynamics
Echocardiography was carried out at 6 weeks to supervise the cardiac hemodynamics with a VisualSonics (Toronto, Canada) Vevo 770 echocardiograph, with a linear transducer (30″) in B and M modes. M mode was used to measure the LV ejection fraction (LVEF) in five 6 week mice for of each group. Each mouse was anesthetized with inhaled isoflurane (2% for induction and 1.5% for maintenance) [29].
2.4. Morphometric analysis
After mice were sacrificed, five hearts were taken from each age group, from both study groups and washed by perfusion with PBS, stopped in diastole with KCl, and fixed in paraformaldehyde. The ratio of the heart weight with the body weight (HW/BW) was determined using the body weight in g and the heart weight in mg [28].
Morphometric analysis of the heart was done with micrographs taken on a clear field with a stereoscopic microscope using ImageJ 1.46 software. The following were determined: a) thickness of each ventricular wall and IVS; b) ventricular lumen; c) total surface of the heart, and d) left and right ventricular cavities.
2.5. Histological analysis
Evaluation of myocardial fibrosis and interstitial space index was done. Dissected hearts were dehydrated with alcohol (30–100%), made transparent with cedar oil and embedded in paraffin. Transverse dissections were done to obtain 5-μ-thick sections. Histological cuts were deparaffinized and rehydrated with alcohol until water. Nuclei were stained with hematoxylin and eosin. Three hearts from each age group were analyzed and micrographs were taken at 40 × using an optical microscope. Three sections of each heart were evaluated in the LV and IVS. At each level, three fields were selected. The analysis was carried out using UTHSCSA Image Tool v.3.0. The area of muscle tissue was determined and the remainder was considered to be interstitial space [7], [30].
2.6. Indirect immunofluorescence
To establish the expression levels of the GATA4 transcription factor and cell proliferation, immunofluorescence studies were done using an anti-GATA4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and proliferating cell nuclear antigen (PCNA, Dako, U.S.A.). Three hearts were used from each age group. Each trial was carried out in triplicate. Tissue slides were treated with citrate solution at a 15 lb/in2 pressure for antigen release. Nonspecific activity was treated with protein blocking solution (Biogenex, Fremont, CA). The primary antibody was visualized with anti-mouse Zenon Alexa Fluor 488 (Life Technologies, Grand Island, NY). After washing, nuclei were contrasted with Draq7 (Biostatus, Leicestershire, UK). Finally, Vectashield was used as mounting media (Vector Laboratories, Burlingame, CA). Histopathological cuts were observed in a confocal microscope (Zen 2009, Carl Zeiss, Dublin, CA). Micrographs were captured at 40 × and 15 fields were evaluated for each region (LV and IVS). ImageJ 1.46 program was used for quantification. Percentage of expression was calculated.
2.7. Western blot
Hearts were treated with lysis solution T-PER (Pierce, Rockford, IL). For total protein extraction, protein quantification was done according to the Bradford method (Bio-Rad, Hercules, CA). Each sample was diluted in protein loading buffer (30 μg) and polyacrylamide gel electrophoresis (10%) was carried out. Proteins were transferred to a PVDF membrane, previously treated with blocking solution (5% nonfat dry milk powder in TBS) for 1 h to prevent nonspecificity. Anti-GATA4 primary antibody was incubated in blocking buffer for 12 h at 4 °C. Horseradish-peroxidase secondary antibody was incubated at room temperature and detection was performed using a western blotting luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA). GAPDH protein (Santa Cruz Biotechnology, Santa Cruz, CA) was used to determine the levels of expression of each protein. Photographic film was used to develop the pictures. Experimental and standard bands were scanned and analyzed using the ImageJ 1.46 software. Experimental data were normalized.
2.8. qPCR real time
Fresh hearts from each group were dissected into three parts (RV, LV and IVS) after removal the atrium and large vessels. Total RNA of the LV was isolated using Trizol reagent (Life Technologies). cDNA was synthesized using 1 mg of total RNA. The reactions were carried out for each of the genes using an Mx30005Tm QPCR system with MxPro QPCR v.3.00 (Agilent Technologies, Santa Clara, CA) and SYBR Green (Life Technologies) as detection systems. The total volume used was 20 μl using 100 ng of cDNA. Amplification conditions were as follows: 10 min of denaturation at 95 °C followed by 40 cycles at 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s. The final elongation step consisted of 72 °C for 10 min. The primers are indicated in Supplemental Table 1. Negative control was performed with a PCR reaction that contained water. Each PCR reaction was done in triplicate.
The Livak method (also called ΔΔCt) was used for analyzing the relative quantification. Ribosomal RNA 18S gene was used for normalization and as a relative calibrator of the genetic expression for each of the genes in the different regions of the CTR and CH-ad groups [31].
2.9. Analysis of results
Data were expressed as mean ± SD. For comparing values between the two groups, Student t test was used; p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of isoproterenol in pregnant female mice
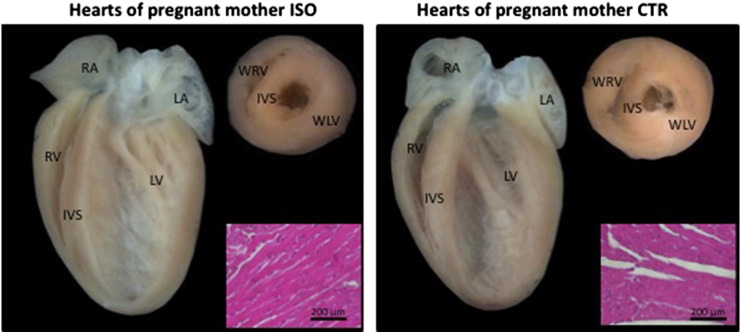
It is worth noting that ISO did not cause structural changes in female mice during gestation. Supplemental Fig. 1, micrograph showing similarity of hearts of pregnant females treated and control. A four-chamber cut was made parallel to the atrioventricular and transverse groove, taking as reference the ventricular base, without finding obvious morphological differences (p > 0.05). In regard to body weight and heart weight, we were not able to establish significant differences in the females from the two groups. The average offsprings and reabsorptions obtained in pregnant female mice treated with ISO were similar to the CTR group (Table 1).
Supplementary Fig. 1.
Impact of isoproterenol (ISO) on pregnant female mice.
Table 1.
Oligonucleotides used in this work (todos fueron diseñados para este trabajo, si no, hay que ponerles otra columna con la cita).
| Gene |
Primer |
|
|---|---|---|
| Sense | Antisense | |
| α-AR | GGCACAGAAGATGCTGACAA | CTGCCCCTTGGTGACATACT |
| β-AR | GGCATTGAGTGGACCTTCAT | TCGAGGCTTCTGGAAGTTGGT |
| α-MHC | GGCACAGAAGATGCTGACAA | CTGCCCCTTGGTGACATACT |
| β-MHC | GGCATTGAGTGGACCTTCAT | TCGAGGCTTCTGGAAGTTGGT |
| RNA 18S | GAGGTGAAATTCTTGGACCGG | TCTTGGCAAATGCTTTCGCT |
3.2. Heart analysis of the offsprings treated with isoproterenol during pregnancy
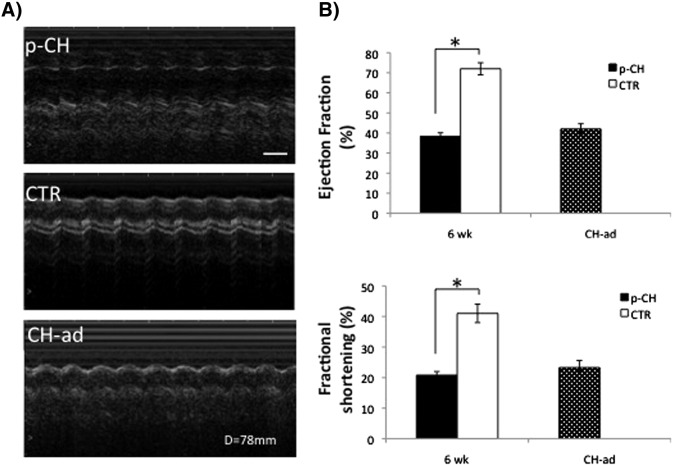
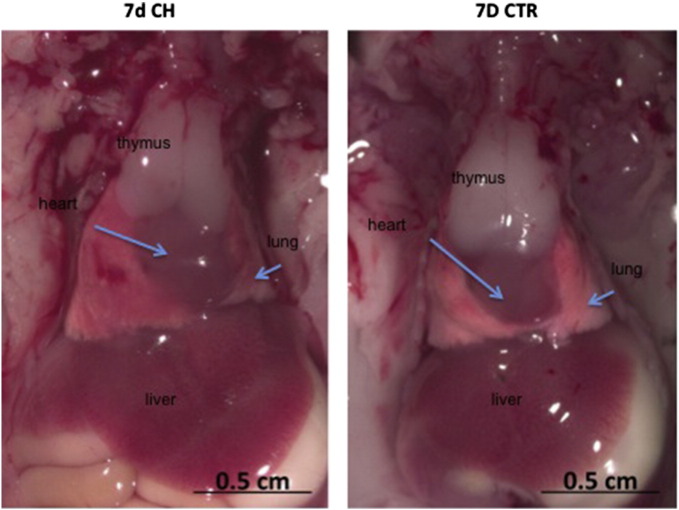
Prolonged administration (7 days) of ISO during development of the offspring, caused changes in their behavior; a sedentary behavior was evident in the mice shortly after birth and at 6 weeks, unlike offsprings of PBS treated mice, due to cardiac alterations (Supplementary video 1, Supplementary video 2, Supplementary video 3, Supplementary video 4). These results were supported by monitoring cardiac hemodynamics in 6 week mice treated with ISO and were associated with a moderate increase in LV pressure compared with 6 week CTR mice (6 weeks: p-CH = 119 ± 5 mm Hg vs. CTR = 92 ± 7 mm Hg, p < 0.05); the latter similar to that determined in CH-ad (95 ± 8 mm Hg, Fig. 1). An increase in the size of the thymus, lung and liver shortly after birth but not in body weight, without finding differences in growth between the p-CH and CTR groups is worth noting (Supplemental Fig. 2).
Fig. 1.
Ventricular hemodynamics. A) Echocardiographic M-mode tracings from 6 week treated mice during gestation and control same age compared to cardiac hypertrophic adult mice (CH-ad). The 6 week mice showed a pronounced increase in left ventricular dimensions equal to the hypertrophy of stage adult. B) Left ventricular function estimated from fractional shortening measured at the end of the study periods (6 weeks) was significantly better as compared with 6 W CTR.
Supplementary Fig. 2.
Analysis of size of the thymus, lung, heart and liver.
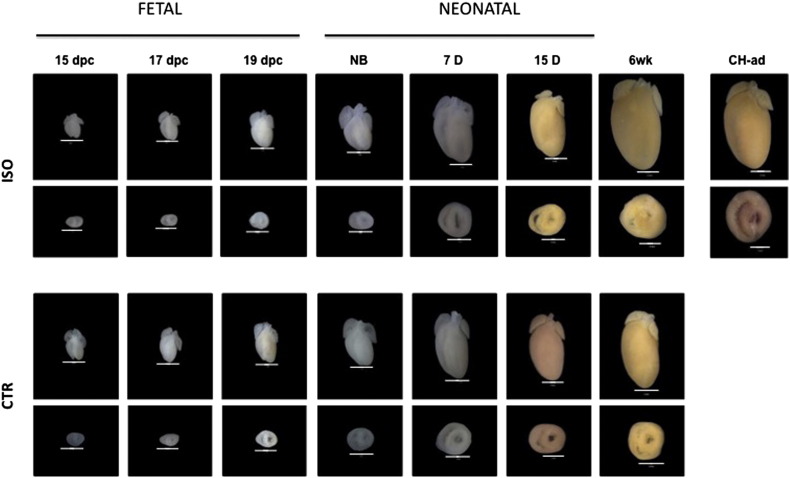
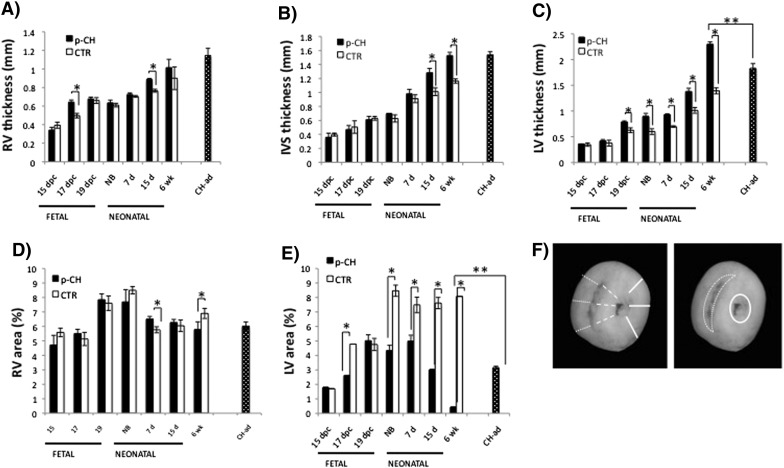
In turn, hearts from the p-CH group during the different stages of the study were visibly larger than in the CTR group (Supplemental Fig. 3). Therefore, in the p-CH group the significant increase in the size of the heart was notable (HW/BW) especially after birth (Supplemental Table 2). It's worth noting that morphometric analysis of the offspring hearts of mice treated with ISO during their pregnancy, revealed that the thickness of the right ventricular wall in the fetus hardly changed in relation to the CTR group (Fig. 2A, B). In regard to the IVS, we found significant differences only between the age groups of 15 days and 6 weeks (15 days p-CH = 1.28 ± 0.06 vs. CTR = 1.01 ± 0.05; 6 weeks p-CH = 1.52 ± 0.04 vs. CTR = 1.16 ± 0.03, p < 0.05) (Fig. 2B). Meanwhile in the LV of the p-CH group, a significant increase in thickness was evident in the fetus of 19 dpc up to offsprings of 6 weeks (19 dpc: p-CH = 0.78 ± 0.02 vs. CTR = 0.62 ± 0.04; NB: p-CH = 0.89 ± 0.05 vs. CTR = 0.59 ± 0.05; 7 days: p-CH = 0.92 ± 0.01 vs. CTR = 0.69 ± 0.01; 15 d: p-CH = 1.38 ± 0.06 vs. 1.01 ± 0.05; 6 weeks: p-CH = 2.29 ± 0.04 vs. CTR = 1.39 ± 0.05, p < 0.05) (Fig. 2C), In addition, a reduction of > 50% in the diameter of the ventricular lumen was evident in the LV of the offspring treated with ISO during pregnancy (NB: p-CH = 4.34 ± 0.35 vs. CTR = 8.46 ± 0.38; 7 d: p-CH = 4.99 ± 0.39 vs. CTR = 7.49 ± 0.53; 15 d: p-CH = 3.013 ± 0.03 vs. CTR = 7.61 ± 0.38; 6 weeks: p-CH = 0.43 ± 0.005 vs. CTR = 8.079 ± 0.001, p < 0.05) (Fig. 2E).
Supplementary Fig. 3.
Evolution of ISO-induced cardiac hypertrophy during pregnancy.
Fig. 2.
Evolution of morphological changes in response to the administration of isoproterenol during gestation. A–C) Gradual increase of free wall thickness of the ventricular and interventricluar septum. D–E) Lumen area. F) Volume. Right ventricle = fine dots; interventricular septum = coarse dots; left ventricle = continuous line. Significant differences *p < 0.05 compared with control.
With the aim of validating hypertrophy we compared the 6 week mice treated with ISO with a model of induced hypertrophy during the adult stage. Anatomically we observed little variation, first we found that the heart size in relation to body size in the p-CH 6 week mice was significantly higher than in the CH-ad mice (6 weeks: p-CH = 0.009 ± 0.0004 vs. CH-ad = 0.008 ± 0.0002, p < 0.05). Also significant differences were found in the thickness of the LV of the p-CH 6 week mice than the induced hypertrophy in adult stage (6 weeks: p-CH = 2.29 ± 0.04 vs. CH-ad = 1.82 ± 0.09, p > 0.05), while it's cavity decreased more considerably in comparison with the CH-ad (6 weeks: p-CH = 0.43 ± 0.005 vs. PCH-ad = 3.16 ± 0.08, p < 0.05) (Fig. 2C, D).
3.3. Histopathological changes
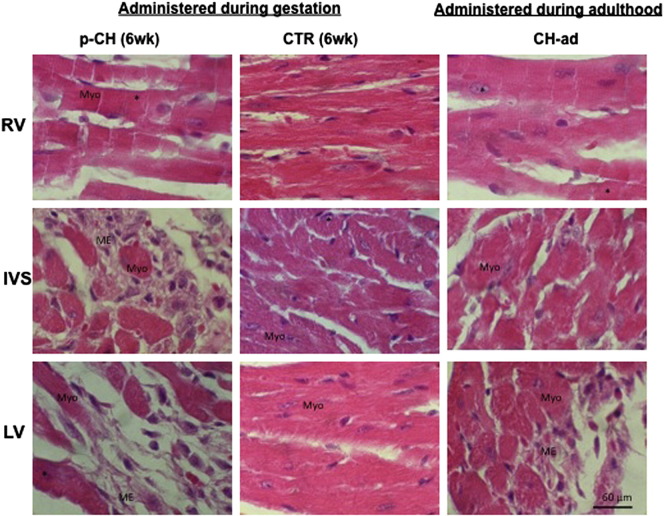
In the offsprings treated with ISO, the most significant changes of the IVS as well as of the LV in fetal and neonatal stage were the loss of the parallel alignment of the fibers (myofibrillar disorganization). In the majority of the myocytes, the presence of enlarged nuclei and excessive cytoplasmic vacuoles was also evident, similar to reports of hypertrophy in the adult stage, induced with ISO (Fig. 3). After the offspring birth, the presence of some fibrotic foci was evident in the LV and IVS; however, in the 6 week mice multiple fibrotic lesions were located mainly towards the ventricular lumen, characterized by accumulation of myofibroblasts with an excess of extracellular matrix secondary to myofibrillar degeneration (Fig. 3). This histological evidence was clearly reflected in the increase of the interstitial space index (Table 2). In contrast, in CTR group of the same ages, myofibrillar structure was normal without collagen accumulation.
Fig. 3.
Isoproterenol treatment induces cardiac hypertrophy and fibrosis. A) Hematoxylin–eosin staining of representative images of 6 week offspring mice treated during gestation with isoproterenol or PBS vs hypertrophy induced in adult stage. Fibrosis (*) were evident in both models with more damage in those treated during pregnancy and in the left ventricle.
Table 2.
Comparison HW/BW of offspring of pregnant mice treated with isoproterenol.
| 15 dpc | 17 dpc | 19 dpc | NB | 7 D | 15 D | 6 W-AD | CH-AD | |
|---|---|---|---|---|---|---|---|---|
| ISO | 0.017 ± 0.001 (1.7%)⁎ |
0.012 ± 0.0017 (1.2%)⁎ |
0.014 ± 0.0019 (1.4%)⁎ |
0.015 ± 0.0007 (1.4%)⁎ |
0.016 ± 0.0006 (1.6%)⁎ |
0.007 ± 0.0002 (0.6%)⁎ |
0.009 ± 0.0004 (0.9%)⁎ |
0.008 ± 0.0002 (0.8%)⁎ |
| CTR | 0.014 ± 0.001 (1.4%) |
0.0101 ± 0.000 (1.01%) |
0.012 ± 0.001 (1.4%) |
0.014 ± 0.0014 (1.6%) |
0.011 ± 0.0008 (1.1%) |
0.003 ± 0.0001 (0.4%) |
0.005 ± 0.0002 (0.62%) |
Cardiac hypertrophy was determined by the ratio of the heart weight (mg) to body weight (g). The values are the mean ± SME (n = 5 animals/age/group). All comparisons were performed using the Student's t test.
Indicates significant differences between groups (P < 0.05).
3.4. Proliferative activity of the left ventricle
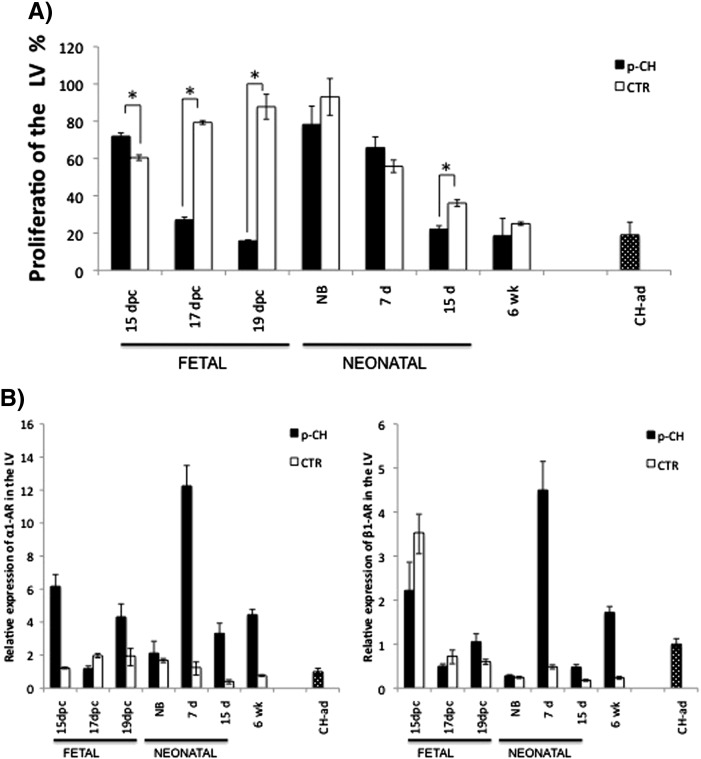
In the LV of offspring mice treated with ISO, a greater loss of proliferation was observed during the fetal stage compared to the CTR group (17 dpc: p-CH = 20.07% vs. CTR = 79.32%; 19 dpc: p-CH = 15.8% vs. CTR = 87.7%; p < 0.05). During the neonatal period, we found that the proliferative activity seemed to recover without reaching normal values (NB: p-CH = 78.2% vs. CTR = 93.02%, 7 days p-CH = 5.7% vs. CTR = 55.8%; 15 days: p-CH = 21.9% vs. CTR = 36.8%; Fig. 4A).
Fig. 4.
A) Mitotic activity of the left ventricle. Percentage of nuclei positive for proliferating cell nuclear antigen (PCNA). B–C) Relative expression of α- and β-aderenergic receptors in the left ventricle. Expression was quantified by extracting the total RNA of LV of age groups that received isoproterenol during gestation; qPCR real-time was used and normalized to the expression with the cardiac hypertrophy induced adult stage and 18S RNA by the 2Δ Ct method.
3.5. mRNA expression for adrenergic receptors
In the LV of offsprings treated with ISO we observed a considerable increase in the expression of the α1-AR messenger in fetal stage (six times more than adults) that decreased in NB; however, the level of expression recovered at 7 days (> 11 times). In the following ages analyzed (15 days and 6 weeks), the expression obtained was greater than that of CH-ad (Fig. 4B). With respect to the CTR group, long-term mRNA expression was always lower compared to the pathologic CH-ad group. Although in some fetal and neonatal stages the expression increased (1 to 1.9), but it did not surpass the expression observed in the adult stage hypertrophy (Fig. 4B).
In terms of the mRNA of β1-AR, only in three age groups of the six analyzed, was there an expression greater than that of CH-ad group (15 dpc: p-CH ≥ 1. 2 times more; 7 days: p-CH ≥ 3.5 times more and 6 weeks: p-CH ≥ 0.5 times more, p < 0.05). In the CTR, at 15 dpc the β1-AR increased its expression double than that of the pathologic CH-ad. In the rest of the age group analyzed, determined expression values of β1-AR were lower than the hypertrophy in adult stage (Fig. 4C).
3.6. GATA4 in the left ventricle
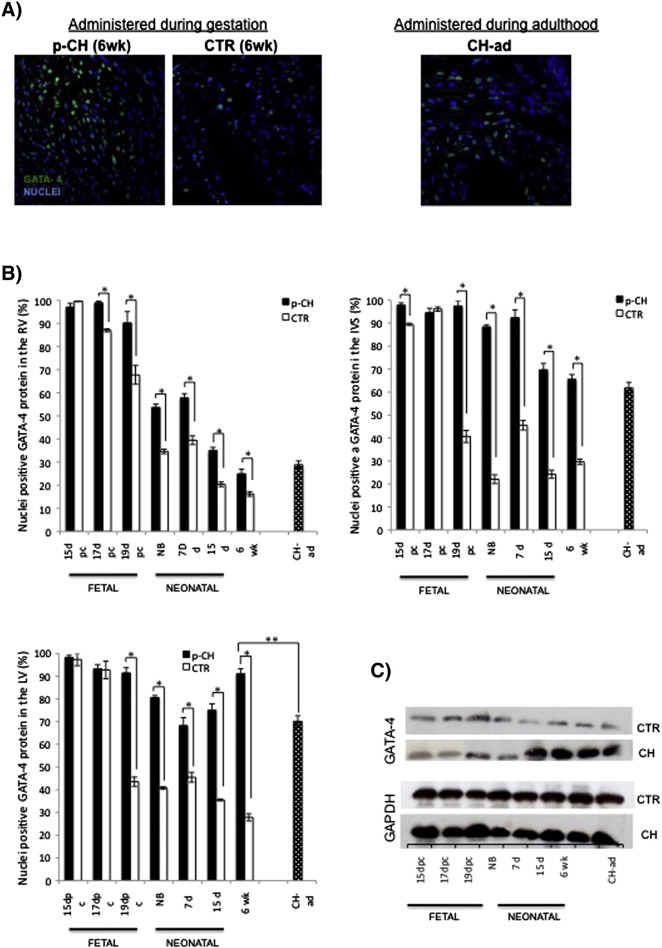
The total percentage of positive nuclei to GATA4 found in the LV of offsprings treated with ISO was always greater than the percentage obtained in the CTR group. However, significant differences were found between in the 19 dpc to 6 weeks (19 dpc: p-CH = 91.4% vs. CTR = 43.5%; NB: p-CH = 80.60% vs. CTR = 40.7%; 7 days: p-CH = 68.2 vs. CTR = 45.5%, 15 days: p-CH = 70.06% vs. CTR = 35.5%; 6 weeks: p-CH = 91.1% vs. CTR = 27.8%). GATA4 decreased up to 50% in the CTR group throughout the study. Also, a significant difference was evident between p-CH of 6 weeks vs CH-ad (6 weeks: p-CH = 91.17 vs. CH-ad = 70.14, p < 0.05) (Fig. 5A and B). These data were supported by Western blot, where a gradual increase was clearly observed in the GATA4 protein in the LV of mice treated with ISO during the neonatal and adult stage in comparison to the CTR group (Fig. 5C).
Fig. 5.
Analysis of GATA4 protein expression. A) Percentage of nuclei positive to GATA4 protein in the left ventricle. Comparison between treated subject and control, significant at p < 0.05 (*); and comparison between hypertrophy 6 week mice vs hypertrophy adult stage, significant at p < 0.001 (**), B) Immunofluorescence against GATA-4 (green). Representative images of 6 week mice offspring treated with ISO during gestation or PBS vs hypertrophy induced adult stage. DraQ7-stained nuclei (blue). C) Determination of Gata-4 protein for Western blot.
3.7. Analyses of α and β myosin in the LV
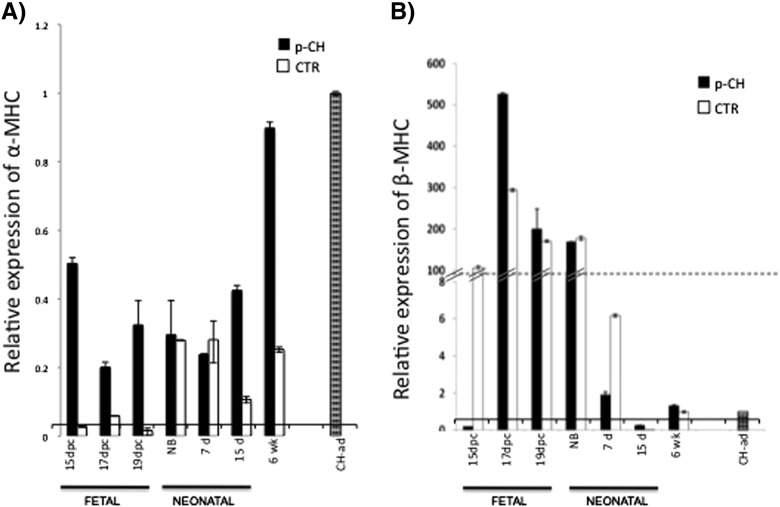
In all the ages analyzed from both groups, a decrease in α-MHC messenger expression was evident, including the 6 week mice. However α-MHC expression from offsprings treated with ISO was generally greater than in the CTR group (Fig. 6A). In contrast, β-MHC expression increased in all the analyzed age groups treated with ISO, despite the fact that the relative expression determined in the p-CH 6 week group was 0.3 times greater than in the CH-ad. An upregulate of β-MHC was evident in offspring of female mice treated with ISO, but these expression increased as the age increases (p < 0.05) (Fig. 6B).
Fig. 6.
Relative expression of α-MHC and β-MHC in the left ventricle. Expression was quantified by extracting the total RNA of LV of fetuses, neonatal and 6 weeks that received isoproterenol during gestation. qPCR real-time was used and normalized to the expression of the cardiac hypertrophy induced in adult stage and 18S RNA by the 2Δ Ct method.
4. Discussion
Cardiac hypertrophy can be of genetic or multifactorial etiology, such as metabolic diseases [32]. The disease's natural history varies, which makes its diagnosis difficult, especially in children [33]. In the majority of patients it can remain asymptomatic for a long period or in a compensatory stage and later on, start slowly degenerating before suffering sudden death [34]. Nonetheless, its mechanisms have not been studied much [35] during intra uterine life. In addition, the lack of in vivo experimental models has led to the use of in vitro models from primary rat or mice myocyte cultures in the neonatal stage along with its limitations [36], [37].
Using IP ISO during gestation for 7 days in pregnant female mice, we managed to induce cardiac hypertrophy in the offspring. Our study shows for the first time some of the key molecular mechanisms that affect the balance of physiological hypertrophy during fetal and neonatal stages, and that can contribute to pathological hypertrophy. It is important to state that progressive hypertrophy, non-reversible in the offspring treated with ISO during gestation, was not caused by maternal alterations, neither did the offspring pathology alter the physiology of the pregnant female mice. This event will raise new questions on maternal–fetal relationships. Results were contrary to the pioneer study published by Iwasaki [24], where the use of ISO during gestation, administered SC for 5 days in pregnant female rats causes a moderate growth of the LV in the hearts of the offspring, out of proportion to the IVS and intra- and intercellular disarrangement 4 weeks after birth. The physiology is progressively reverted as the age group the offspring increases.
It should be noted that the significant increase in the heart weight/body weight ratio and morphometric measurements, where an increase in ventricular wall thickness and reduction in the ventricular lumen was observed, are coherent with the anatomical changes described in concentric LV hypertrophy in both, adult mice and rats treated with ISO [27], [38]. These results do not correspond to what some authors suggest in regard to the disproportionate thickening of the septum, which is a common effect in human hearts during development, and is then lost during pre- and postnatal growth [39].
Histologically we found three basic processes that have repercussion in the heart after IP administration of ISO during gestation, when inducing postnatal hypertrophy. The first process was the loss of cell proliferation, followed by the loss of cell compaction and fibrotic foci. In regard to the proliferation during the fetal stage, we attribute that the loss of proliferation was due to the negative effect that the ISO agonist has over the β-adrenergic receptors, as suggested by in vitro culture studies [40]. We assume that the presence of ISO during the embryonic stage, allows a fraction of β-adrenergic receptors coupled to G proteins, to remain active during the fetal stage in the absence of the drug. This would allow the activation of non-classical routes that participate in cell proliferation, apoptosis and cell growth processes: activation of phosphodiesterase 4 (PDE4), that blocks cyclic AMP production [41]; activation of the PI3K route (phosphatidyl-inositol 3-kinase), or expression of transcription factor NF-κB (kappa beta nuclear factor) and the activation of Akt/PKB [42]; or expression of proinflammatory cytokines (IL-1 β, IL-6, IL-18 e IL-13) [43], or increase of vascular endothelial growth factor (VEGF); or cardiac remodeling proteins that increase the collagenase activity (collagen, fibronectin and metalloproteinases of the extracellular matrix [MMP]); or also signaling route activation of Ras/Raf/MEK/ERK (kinases regulated by extracellular signals) and MAPK (kinase proteins activated by mitogens) [44], not yet explored in this study. On the other hand the possibility exists that the negative effect of ISO over the β-adrenergic receptors in the embryonic stage, allows the expression of α-adrenergic receptors, which could modulate the progression of the disease. Since the results of qPCR real time showed that mRNA α-adrenergic receptors are the most abundant during the progression of the disease, the results seen in this study are congruent with the cardiac pathologies described in transgenic mice when there is overexpression of some of the subtypes of α1-AR [45], [46], [47], [48]. Therefore, we believe that the analysis of receptor expressions in intrauterine life could be the first step in determining heart failure.
In our study we demonstrated that IP administration of ISO also affects cell compaction after birth. Even though we did not analyze the changes in the expression of cell adhesion proteins like camp, or of the extracellular matrix, like collagen or fibronectin, the increase of interstitial space shows damage to the LV, possibly due to the loss of cell junction. Even without evaluating cell death in the different ages of the study, it was possible to localize some fibrotic foci in the LV of mice treated with ISO during gestation, from day 7 up to 6 weeks. These data support the results observed in fibroblast cultures where ISO is able to regulate the myofibroblast function responsible for collagen synthesis [49]. We propose that the observed ventricular growth was due to the increase in size of the myocytes and not to proliferation. In the future we are considering the evaluation of collagen types l and III, since studies by Marijianoski et al. suggest that the exchange of type III collagen for type I is essential for normal heart development. During fetal and neonatal stages the greater portion of collagen is type III (favoring elasticity) which, little by little, is substituted by type I collagen, responsible for providing rigidity to the microenvironment of the myocyte [50].
During hypertrophy in the adult stage the reinduction of embryo-fetal stage genes is suggested, among which we can highlight GATA4 to be one of the predominant markers. Its expression is dependent on AR activation and PKA or PKC signaling mechanisms [21]. For this reason we decided to evaluate the changes in the expression of the protein of GATA4. It should be highlighted that in this study we evidenced that the percentage of nuclei positive to GATA4 is not reduced in the LV or in the IVS when mice are treated with ISO during gestation, compared with CTR. These results are in accordance with the protein increase observed in Western blot. They suggest that GATA4 plays an important role in the evolution of the disease postnatally. Note that GATA4 could be considered a marker of poor prognosis, it would be appropriate to determine it in the first week of life, especially in cases where echocardiography revealed an impaired LV.
Changes in the expression profile of α-MHC and β-MHC are indicative of structural changes in the sarcomere. In our study they were key elements in determining the effect of ISO on gestation. Sadoshima and Izumo documented that the substitution of α-MHC for β-MHC expression is a typical event in CH in the adult heart [51]. Waspe et al. reported that β-MHC is highly expressed in murine models during the fetal stage and decreases suddenly after birth, whereas α-MHC represents 40% in the first week of life and only 5%, 3 weeks later [52]. In this regard, our results demonstrate that mRNA expression of β-MHC in the LV does not change when offspring are treated with ISO during gestation, although after birth a reduction in the expression of the isoform was evident. We clearly observed that the expression of the α-MHC messenger increases in mice treated with ISO during gestation as the pathology progresses. α-MHC expression was possibly regulated by the expression of the GATA-4 transcription factor, as various studies have demonstrated that the GATA box (59-AGATAA-39) is present in the promoter regions of α-MHC [53] and cardiac troponin C [54].
We believe that this model will allow us to confirm the proposed in vitro regulation mechanisms, using primary cultures of myocytes from the neonatal stage. It will also be useful in the design of alternative therapies that encourage regeneration or prevent cell damage. It also raises new questions about the activation mechanisms of adrenergic receptors and/or how the mechanisms are regulated once the stimuli have activated the pathways. Among the possible candidates, we mention angiotensin II [55] or neuregulin [56], [57], [58]. Both are associated with neuro-humoral stimuli including changes of electric flow dependent on the membrane potential that at this time are not yet determined. Furthermore, it could be that these systems are regulated by reactive oxygen species capable of regulating the AR, as has been shown in cultured myocytes in adult and neonatal mice [59].
5. Conclusion
With the IP administration of ISO in this study, we demonstrate for the first time in an in vivo model, some molecular mechanisms that are altered in heart development. Adrenergic receptors might be responsible for directing a physiological into a pathological hypertrophy. However GATA4 seemed to be the determining factor in the pathology. This model can be used for early detection of biomarkers or to create regenerative therapies to reestablish the cardiac structure, avoiding ischemia and therefore, sudden death in the pediatric age.
The following are the supplementary data related to this article.
Table 1. Oligonucleotides used in this work.
Table 2. Comparison HW/BW of offspring of pregnant mice treated with isoproterenol.
Newborn mouse treated with PBS during development.
Newborn mouse treated with isoproterenol during development.
6 week mouse treated with isoproterenol during development.
6 week mouse treated with PBS during development.
Conflict of interest
None of the authors has any potential financial conflict of interest related to this work.
Akcnowledgments
This work was supported by the Hospital Infantil de México Federico Gómez as part of HIM/2011/006 SSA 946 federal grant. A. Del Olmo-Turrubiarte from Experimental Biology Program, Universidad Autónoma Metropolitana was supported by CoNaCyT MSc (233310)/PhD (39036) grants. We thank Lucía Lima from the Laboratorio de Investigación en Biología del Desarrollo y Teratogénesis Experimental, Hospital Infantil de México Federico Gómez, for the technical support on histology.
References
- 1.Levy D., Labib S.B., Anderson K.M., Christiansen J.C., Kannel W.B., Castelli W.P. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. Mar 1990;81(3):815–820. doi: 10.1161/01.cir.81.3.815. [PubMed PMID: 2137733. Epub 1990/03/01. eng] [DOI] [PubMed] [Google Scholar]
- 2.Kavey R.E. Left ventricular hypertrophy in hypertensive children and adolescents: predictors and prevalence. Curr Hypertens Rep. Oct 2013;15(5):453–457. doi: 10.1007/s11906-013-0370-3. [PubMed PMID: 23893038] [DOI] [PubMed] [Google Scholar]
- 3.Mongiovi M., Fesslova V., Fazio G., Barbaro G., Pipitone S. Diagnosis and prognosis of fetal cardiomyopathies: a review. Curr Pharm Des. 2010;16(26):2929–2934. doi: 10.2174/138161210793176428. [PubMed PMID: 20632954] [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Huang S., Sah V.P., Ross J., Jr., Brown J.H., Han J. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. Jan 23 1998;273(4):2161–2168. doi: 10.1074/jbc.273.4.2161. [PubMed PMID: 9442057. Epub 1998/01/27. eng] [DOI] [PubMed] [Google Scholar]
- 5.Yan L., L-NW, Yu-Hui X., Hong Z.L., Feng G.X., Ya-Jun Z. Arginine inhibits isoproterenol-induced cardiac hypertrophy through nitric oxide and polyamine pathways. Basic Clin Pharmacol Toxicol. 2008;103:124–130. doi: 10.1111/j.1742-7843.2008.00261.x. [DOI] [PubMed] [Google Scholar]
- 6.Akasu T., Ito M., Nakano T., Schneider C.R., Simmons M.A., Tanaka T. Myosin light chain kinase occurs in bullfrog sympathetic neurons and may modulate voltage-dependent potassium currents. Neuron. Dec 1993;11(6):1133–1145. doi: 10.1016/0896-6273(93)90226-h. [PubMed PMID: 7903859. Epub 1993/12/01. eng] [DOI] [PubMed] [Google Scholar]
- 7.Brooks W.W., Conrad C.H. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med. Aug 2009;59(4):339–343. [PubMed PMID: 19712573. Pubmed Central PMCID: 2779208. Epub 2009/08/29. eng] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P., Goyal M., Agarwal J.L. Effect of l-arginine on electrocardiographic changes induced by hypercholesterolemia and isoproterenol in rabbits. Indian Pacing Electrophysiol J. 2009;9(1):45–52. [PubMed PMID: 19165358. Pubmed Central PMCID: 2615061. Epub 2009/01/24. eng] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockman H.A., Koch W.J., Lefkowitz R.J. Seven-transmembrane-spanning receptors and heart function. Nature. Jan 10 2002;415(6868):206–212. doi: 10.1038/415206a. [PubMed PMID: 11805844. Epub 2002/01/24. eng] [DOI] [PubMed] [Google Scholar]
- 10.Li H., Xie Y.H., Yang Q., Wang S.W., Zhang B.L., Wang J.B. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One. 2012;7(11):e48872. doi: 10.1371/journal.pone.0048872. [PubMed PMID: 23139821. Pubmed Central PMCID: 3490947. Epub 2012/11/10. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalitha G., Poornima P., Archanah A., Padma V.V. Protective effect of neferine against isoproterenol-induced cardiac toxicity. Cardiovasc Toxicol. 2013;13:168–179. doi: 10.1007/s12012-012-9196-5. [PubMed PMID: 23274852. Epub 2013/01/01. Eng] [DOI] [PubMed] [Google Scholar]
- 12.Meszaros J., Levai G. Ultrastructural and electrophysiological alterations during the development of catecholamine-induced cardiac hypertrophy and failure. Acta Biol Hung. 1990;41(4):289–307. [PubMed PMID: 2151869] [PubMed] [Google Scholar]
- 13.Rona G., Zsoter T., Chappel C., Gaudry R. Myocardial lesions, circulatory and electrocardiographic changes produced by isoproterenol in the dog. Rev Can Biol. Apr 1959;18(1):83–94. [PubMed PMID: 13646243] [PubMed] [Google Scholar]
- 14.Kahn D.S., Rona G., Chappel C.I. Isoproterenol-induced cardiac necrosis. Ann N Y Acad Sci. Jan 31 1969;156(1):285–293. doi: 10.1111/j.1749-6632.1969.tb16735.x. [PubMed PMID: 5291138] [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro D.A., Buttros J.B., Oshima C.T., Bergamaschi C.T., Campos R.R. Ascorbic acid prevents acute myocardial infarction induced by isoproterenol in rats: role of inducible nitric oxide synthase production. J Mol Histol. Apr 2009;40(2):99–105. doi: 10.1007/s10735-009-9218-1. [PubMed PMID: 19466570] [DOI] [PubMed] [Google Scholar]
- 16.Rona G., Chappel C.I., Gaudry R. Effect of dietary sodium and potassium content on myocardial necrosis elicited by isoproterenol. Lab Invest. Sep–Oct 1961;10:893–897. [PubMed PMID: 13743099] [PubMed] [Google Scholar]
- 17.Meszaros J., Khananshvili D., Hart G. Mechanisms underlying delayed afterdepolarizations in hypertrophied left ventricular myocytes of rats. Am J Physiol Heart Circ Physiol. Aug 2001;281(2):H903–H914. doi: 10.1152/ajpheart.2001.281.2.H903. [PubMed PMID: 11454597] [DOI] [PubMed] [Google Scholar]
- 18.Ocaranza M.P., Diaz-Araya G., Chiong M., Munoz D., Riveros J.P., Ebensperger R. Isoproterenol and angiotensin I-converting enzyme in lung, left ventricle, and plasma during myocardial hypertrophy and fibrosis. J Cardiovasc Pharmacol. Aug 2002;40(2):246–254. doi: 10.1097/00005344-200208000-00010. [PubMed PMID: 12131554] [DOI] [PubMed] [Google Scholar]
- 19.Heather L.C., Catchpole A.F., Stuckey D.J., Cole M.A., Carr C.A., Clarke K. Isoproterenol induces in vivo functional and metabolic abnormalities: similar to those found in the infarcted rat heart. J Physiol Pharmacol. Sep 2009;60(3):31–39. [PubMed PMID: 19826179. Epub 2009/10/15. eng] [PubMed] [Google Scholar]
- 20.Roy S.J., Mainzen Prince P.S. Protective effects of sinapic acid on cardiac hypertrophy, dyslipidaemia and altered electrocardiogram in isoproterenol-induced myocardial infarcted rats. Eur J Pharmacol. Nov 23 2012;699(1–3):213–218. doi: 10.1016/j.ejphar.2012.11.012. [PubMed PMID: 23178800. Epub 2012/11/28. Eng] [DOI] [PubMed] [Google Scholar]
- 21.Saadane N., Alpert L., Chalifour L.E. Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br J Pharmacol. Jul 1999;127(5):1165–1176. doi: 10.1038/sj.bjp.0702676. [PubMed PMID: 10455263. Pubmed Central PMCID: 1566134] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha H.N., Hong G.R., Kim Y.W., Kim J.Y., Dan J.M., Park S.Y. Deficiency of iNOS does not prevent isoproterenol-induced cardiac hypertrophy in mice. Korean J Physiol Pharmacol. Jun 2009;13(3):153–159. doi: 10.4196/kjpp.2009.13.3.153. [PubMed PMID: 19885031. Pubmed Central PMCID: 2766730. Epub 2009/11/04. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taglieri D.M., Monasky M.M., Knezevic I., Sheehan K.A., Lei M., Wang X. Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A. J Mol Cell Cardiol. Dec 2011;51(6):988–996. doi: 10.1016/j.yjmcc.2011.09.016. [PubMed PMID: 21971074. Pubmed Central PMCID: 3208757. Epub 2011/10/06. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki T., Takino Y., Suzuki T. Effects of isoproterenol on the developing heart in rats. Jpn Circ J. Jan 1990;54(1):109–116. doi: 10.1253/jcj.54.109. [PubMed PMID: 2139705] [DOI] [PubMed] [Google Scholar]
- 25.Burggren Warren W., Keller Bradley B. In: Development of Cardiovascular Systems: Molecules to Organisms. Burggren Warren W., Keller Bradley B., editors. Cambridge University Press; 1997. [Google Scholar]
- 26.Trend S.G., Bruce N.W. Resistance of the rat embryo to elevated maternal epinephrine concentrations. Am J Obstet Gynecol. Feb 1989;160(2):498–501. doi: 10.1016/0002-9378(89)90480-8. [PubMed PMID: 2916639. Epub 1989/02/01. eng] [DOI] [PubMed] [Google Scholar]
- 27.Kralova E., Mokran T., Murin J., Stankovicova T. Electrocardiography in two models of isoproterenol-induced left ventricular remodeling. Physiol Res. 2008;57(Suppl. 2):S83–S89. doi: 10.33549/physiolres.931556. [PubMed PMID: 18373388. Epub 2008/04/01. eng] [DOI] [PubMed] [Google Scholar]
- 28.Wolfram J.A., Liner A., Richardson S.L., Zhu X., Smith M.A., Hoit B.D. The role of E2F1 in the development of hypertrophic cardiomyopathy. Int J Clin Exp Pathol. Jun 20 2011;4(5):521–525. [PubMed PMID: 21738823. Pubmed Central PMCID: 3127073. Epub 2011/07/09. eng] [PMC free article] [PubMed] [Google Scholar]
- 29.Umar S., Nadadur R., Iorga A., Amjedi M., Matori H., Eghbali M. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J Appl Physiol. Oct 15 2012;113(8):1253–1259. doi: 10.1152/japplphysiol.00549.2012. [PubMed PMID: 22923507. Pubmed Central PMCID: 3472485] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukowski R., Rybalkin S.D., Loga F., Leiss V., Beavo J.A., Hofmann F. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci U S A. Mar 23 2010;107(12):5646–5651. doi: 10.1073/pnas.1001360107. PubMed PMID: 20212138. Epub 2010/03/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. Dec 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [PubMed PMID: 11846609. Epub 2002/02/16. eng] [DOI] [PubMed] [Google Scholar]
- 32.Elliott P., Andersson B., Arbustini E., Bilinska Z., Cecchi F., Charron P. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. Jan 2008;29(2):270–276. doi: 10.1093/eurheartj/ehm342. [PubMed PMID: 17916581] [DOI] [PubMed] [Google Scholar]
- 33.Lim S.H., Ra T.Y., Kim W.Y. Interface observation in Au/Ni/p-GaN studied by HREM and energy-filtering TEM. J Electron Microsc (Tokyo) 2003;52(5):459–464. doi: 10.1093/jmicro/52.5.459. [PubMed PMID: 14700077] [DOI] [PubMed] [Google Scholar]
- 34.Maron B.J., Ommen S.R., Semsarian C., Spirito P., Olivotto I., Maron M.S. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. Jul 8 2014;64(1):83–99. doi: 10.1016/j.jacc.2014.05.003. [PubMed PMID: 24998133] [DOI] [PubMed] [Google Scholar]
- 35.Llapur Milian J.R., Gónzalez Sánchez Raquel, Betancourt Perez Acelia, Rubio Olivares Doris Yisell. Left ventricular hypertrophy and cardiovascular risk factors present in hypertensive children and adolescents. Rev Cubana Pediatr. 2009;81(2) [Google Scholar]
- 36.Weber M.J., Dikeman M.E., Jaeger J.R., Unruh J.A., Murray L., Houser T.A. Effects of feeding a single or sequence of beta-adrenergic agonists on cull cow meat quality. Meat Sci. Feb 2013;93(2):275–281. doi: 10.1016/j.meatsci.2012.09.004. [PubMed PMID: 23031269. Epub 2012/10/04. eng] [DOI] [PubMed] [Google Scholar]
- 37.Decker R.S., Rines A.K., Nakamura S., Naik T.J., Wassertsrom J.A., Ardehali H. Phosphorylation of contractile proteins in response to alpha- and beta-adrenergic stimulation in neonatal cardiomyocytes. Transl Res. Jan 2010;155(1):27–34. doi: 10.1016/j.trsl.2009.09.007. [PubMed PMID: 20004359. Pubmed Central PMCID: 3307141. Epub 2009/12/17. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiswal A., Kumar S., Enjamoori R., Seth S., Dinda A.K., Maulik S.K. Peripheral benzodiazepine receptor ligand Ro5-4864 inhibits isoprenaline-induced cardiac hypertrophy in rats. Eur J Pharmacol. Oct 10 2010;644(1–3):146–153. doi: 10.1016/j.ejphar.2010.06.058. [PubMed PMID: 20621082. Epub 2010/07/14. eng] [DOI] [PubMed] [Google Scholar]
- 39.Maron B.J., Edwards J.E., Epstein S.E. Disproportionate ventricular thickening in patients with systemic hypertension. Chest. Apr 1978;73(4):466–470. doi: 10.1378/chest.73.4.466. [PubMed PMID: 630963. Epub 1978/04/01. eng] [DOI] [PubMed] [Google Scholar]
- 40.Grisanti L.A., Talarico J.A., Carter R.L., Yu J.E., Repas A.A., Radcliffe S.W. Beta-adrenergic receptor-mediated transactivation of epidermal growth factor receptor decreases cardiomyocyte apoptosis through differential subcellular activation of ERK1/2 and Akt. J Mol Cell Cardiol. Jul 2014;72:39–51. doi: 10.1016/j.yjmcc.2014.02.009. [PubMed PMID: 24566221. Pubmed Central PMCID: 4037368] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S., Li Y., Kim S., Fu Q., Parikh D., Sridhar B. Phosphodiesterases coordinate cAMP propagation induced by two stimulatory G protein-coupled receptors in hearts. Proc Natl Acad Sci U S A. Apr 24 2012;109(17):6578–6583. doi: 10.1073/pnas.1117862109. [PubMed PMID: 22493261. Pubmed Central PMCID: 3340097] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Q., Kim S., Soto D., De Arcangelis V., DiPilato L., Liu S. A long lasting beta1 adrenergic receptor stimulation of cAMP/protein kinase A (PKA) signal in cardiac myocytes. J Biol Chem. May 23 2014;289(21):14771–14781. doi: 10.1074/jbc.M113.542589. [PubMed PMID: 24713698. Pubmed Central PMCID: 4031532] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanely Mainzen Prince P., Rajakumar S., Dhanasekar K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol. Oct 1 2011;668(1–2):233–240. doi: 10.1016/j.ejphar.2011.06.053. [PubMed PMID: 21763302] [DOI] [PubMed] [Google Scholar]
- 44.Vidal M., Wieland T., Lohse M.J., Lorenz K. Beta-adrenergic receptor stimulation causes cardiac hypertrophy via a Gbetagamma/Erk-dependent pathway. Cardiovasc Res. Nov 1 2012;96(2):255–264. doi: 10.1093/cvr/cvs249. [PubMed PMID: 22843704] [DOI] [PubMed] [Google Scholar]
- 45.Lin F., Owens W.A., Chen S., Stevens M.E., Kesteven S., Arthur J.F. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. Aug 17 2001;89(4):343–350. doi: 10.1161/hh1601.095912. [PubMed PMID: 11509451] [DOI] [PubMed] [Google Scholar]
- 46.Akhter S.A., Milano C.A., Shotwell K.F., Cho M.C., Rockman H.A., Lefkowitz R.J. Transgenic mice with cardiac overexpression of alpha1B-adrenergic receptors. In vivo alpha1-adrenergic receptor-mediated regulation of beta-adrenergic signaling. J Biol Chem. Aug 22 1997;272(34):21253–21259. doi: 10.1074/jbc.272.34.21253. [PubMed PMID: 9261135] [DOI] [PubMed] [Google Scholar]
- 47.Grupp I.L., Lorenz J.N., Walsh R.A., Boivin G.P., Rindt H. Overexpression of alpha1B-adrenergic receptor induces left ventricular dysfunction in the absence of hypertrophy. Am J Physiol. Oct 1998;275(4 Pt 2):H1338–H1350. doi: 10.1152/ajpheart.1998.275.4.H1338. [PubMed PMID: 9746484] [DOI] [PubMed] [Google Scholar]
- 48.Lemire I., Ducharme A., Tardif J.C., Poulin F., Jones L.R., Allen B.G. Cardiac-directed overexpression of wild-type alpha1B-adrenergic receptor induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. Aug 2001;281(2):H931–H938. doi: 10.1152/ajpheart.2001.281.2.H931. [PubMed PMID: 11454600] [DOI] [PubMed] [Google Scholar]
- 49.Benjamin I.J., Jalil J.E., Tan L.B., Cho K., Weber K.T., Clark W.A. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ Res. Sep 1989;65(3):657–670. doi: 10.1161/01.res.65.3.657. [PubMed PMID: 2527639] [DOI] [PubMed] [Google Scholar]
- 50.Marijianowski M.M., van der Loos C.M., Mohrschladt M.F., Becker A.E. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol. Apr 1994;23(5):1204–1208. doi: 10.1016/0735-1097(94)90612-2. [PubMed PMID: 8144790. Epub 1994/04/01. eng] [DOI] [PubMed] [Google Scholar]
- 51.Sadoshima J., Izumo S. Tyrosine kinases mediation of c-fos expression by cell swelling in cardiac myocytes. Heart Vessels. 1997;(Suppl. 12):194–197. [PubMed PMID: 9476581. Epub 1997/01/01. eng] [PubMed] [Google Scholar]
- 52.Waspe L.E., Ordahl C.P., Simpson P.C. The cardiac beta-myosin heavy chain isogene is induced selectively in alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes. J Clin Invest. Apr 1990;85(4):1206–1214. doi: 10.1172/JCI114554. [PubMed PMID: 2156896. Pubmed Central PMCID: 296553. Epub 1990/04/01. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molkentin J.D., Kalvakolanu D.V., Markham B.E. Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene. Mol Cell Biol. Jul 1994;14(7):4947–4957. doi: 10.1128/mcb.14.7.4947. [PubMed PMID: 8007990. Pubmed Central PMCID: 358867] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ip H.S., Wilson D.B., Heikinheimo M., Tang Z., Ting C.N., Simon M.C. The GATA-4 transcription factor transactivates the cardiac muscle-specific troponin C promoter-enhancer in nonmuscle cells. Mol Cell Biol. Nov 1994;14(11):7517–7526. doi: 10.1128/mcb.14.11.7517. [PubMed PMID: 7935467. Pubmed Central PMCID: 359288] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeyaraj D., Ashwath M., Rosenbaum D.S. Pathophysiology and clinical implications of cardiac memory. Pacing Clin Electrophysiol. Mar 2010;33(3):346–352. doi: 10.1111/j.1540-8159.2009.02630.x. [PubMed PMID: 20025710. Pubmed Central PMCID: 2865579. Epub 2009/12/23. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y.Y., Sawyer D.R., Baliga R.R., Opel D.J., Han X., Marchionni M.A. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. Apr 24 1998;273(17):10261–10269. doi: 10.1074/jbc.273.17.10261. [PubMed PMID: 9553078. Epub 1998/05/30. eng] [DOI] [PubMed] [Google Scholar]
- 57.Xu Y., Li X., Zhou M. Neuregulin-1/ErbB signaling: a druggable target for treating heart failure. Curr Opin Pharmacol. Apr 2009;9(2):214–219. doi: 10.1016/j.coph.2008.11.004. [PubMed PMID: 19070544. Epub 2008/12/17. eng] [DOI] [PubMed] [Google Scholar]
- 58.Rohrbach S., Yan X., Weinberg E.O., Hasan F., Bartunek J., Marchionni M.A. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. Jul 27 1999;100(4):407–412. doi: 10.1161/01.cir.100.4.407. [PubMed PMID: 10421602. Epub 1999/07/27. eng] [DOI] [PubMed] [Google Scholar]
- 59.Amin J.K., Xiao L., Pimental D.R., Pagano P.J., Singh K., Sawyer D.B. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. Jan 2001;33((1):131–139. doi: 10.1006/jmcc.2000.1285. [PubMed PMID: 11133229] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Oligonucleotides used in this work.
Table 2. Comparison HW/BW of offspring of pregnant mice treated with isoproterenol.
Newborn mouse treated with PBS during development.
Newborn mouse treated with isoproterenol during development.
6 week mouse treated with isoproterenol during development.
6 week mouse treated with PBS during development.