Abstract
Aim
Septal rebound stretch (SRSsept) reflects an inefficient deformation of the septum during systole and is a potential new echocardiographic tool to predict response to Cardiac Resynchronization Therapy (CRT). However, there are only limited data on the potential predictive value of SRSsept on echocardiographic response. We evaluated the predictive value of SRSsept on echocardiographic response to CRT in a large population.
Methods and results
A total of 138 consecutive patients with functional class II–IV heart failure who underwent CRT were studied. Echocardiography was performed at baseline and after a mean follow-up period of 22 ± 8 months. Echocardiographic response to CRT was defined as a reduction in LV end-systolic volume ≥ 15%. Receiver operating characteristic curve analysis was performed to define the optimal cut-off value for SRSsept. Multivariable analyses were performed to adjust for potential confounders.
Mean age was 68 ± 8 years (30% female). Mean baseline LV ejection fraction was 26 ± 7%, 51% had ischemic etiology. LBBB or LBBB like morphology was present in 95% of patients. Mean SRSsept was 4.4 ± 3.2%, 56% of patients had SRSsept ≥ 4%. Ninety six patients (70%) were echocardiographic responders. Baseline SRSsept was significantly higher in responders compared to non-responders (5.1 ± 3.2 vs 3.0 ± 2.7, P < 0.001). The optimal cut-off value for SRSsept to predict response to CRT was 4.0%. After both univariate (OR 3.74, 95% CI 1.72–8.10) and multivariate analyses (OR 3.71, 95% CI 1.49–9.2), baseline SRSsept > 4% independently predicted the response to CRT.
Conclusions
Baseline septal rebound stretch is independently associated with echocardiographic response to CRT.
Abbreviations: SRSSept, septal rebound stretch; CRT, cardiac resynchronization therapy; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; IVMD, inter-ventricular mechanical delay; LBBB, left bundle branch block; RBBB, right bundle branch block; NYHA, New York Heart Association
Keywords: Septal rebound stretch, Cardiac resynchronization therapy, Heart failure, Response, LV-dyssynchrony
Highlights
-
•
Septal rebound stretch was used to predict response to CRT in patient who underwent CRT-D implantation.
-
•
Echocardiographic response to CRT was defined as a reduction in LV end-systolic volume ≥ 15%.
-
•
Baseline SRSsept was significantly higher in echocardiographic responders compared to non-responders to CRT.
-
•
The optimal cut-off value for SRSsept was 4%.
-
•
Baseline septal rebound stretch was independently associated with echocardiographic response to CRT.
1. Introduction
Cardiac resynchronization therapy (CRT) has proven effectiveness in the treatment of severe heart failure, improving symptoms and quality of life as well as decreasing mortality in a majority of treated patients [1], [2], [3], [4], [5]. Unfortunately, CRT is ineffective in 30–40% of patients and in some cases it can even worsen symptoms [6]. Several echocardiography techniques have been used to aid in patient selection for CRT prior to implantation with promising results in observational single center trials. However, no ideal echocardiographic approach for the assessment of dyssynchrony has yet been found. The largest prospective trial (PROSPECT) [7], showed poor predictive value of several conventional and tissue Doppler-based echocardiographic methods. In recent years, 2D-speckle tracking echocardiography has been used to assess left ventricular (LV) dyssynchrony with inconsistent results [8], [9], [10], [11]. These time-based mechanical LV-dyssynchrony measurements did however not take into account the inefficient regional myocardial deformation during systole, which can be reverted by CRT. Stretching in the septum during systole can be echocardiographically quantified by septal rebound stretch (SRSsept) and is a novel measure for inefficient septal deformation. Although SRSsept has the potential to predict CRT response, only few studies have been performed [12], [13], [14]. Moreover, these studies were small sized and had methodological limitations. The aim of our current study was to assess the predictive value of SRSsept on echocardiographic response to CRT in a large study population.
2. Methods
2.1. Selection of patients
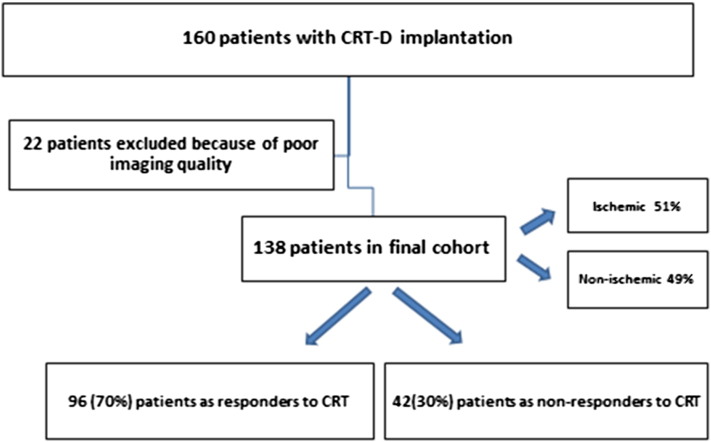
From January 2008 to December 2009 one-hundred sixty consecutive patients with chronic heart failure (New York Heart Association functional class II–IV), LVEF ≤ 35% and wide QRS complex ≥ 120 msec who were scheduled for CRT, were included in the present study. Patients with pre-existent pacemaker or ICD implantations were excluded in order to avoid chronic RV-pacing which may affect the assessment of SRSsept. Also patients with a recent myocardial infarction (< 3 months) or decompensated heart failure were excluded. Etiology was considered ischemic in the presence of significant coronary artery disease (≥ 50% stenosis in 1 or more of the major epicardial coronary arteries) and/or history of myocardial infarction or prior revascularization by PCI or CABG. 22 patients with poor echocardiographic window at baseline were excluded from the analysis (Fig. 1). All patients were on optimal medical therapy, including angiotensin-converting enzyme inhibitors and beta-blockers. This prospective registry was approved by the institutional board. Baseline characteristics included age, gender, etiology of heart failure, clinical history, medical therapy, NYHA functional class, ECG and procedural data were collected prospectively and analyzed retrospectively. Routine follow-up visits were scheduled at 2 months post-implant, and then every 6 months thereafter. The routine follow-up in some of our patients took place in referring hospitals. The clinical status of all survivals at the closure of the study (December 2013) was verified. Data on mortality and hospitalization were collected from reviewing our hospital records, referring hospitals and by contacting general practitioners.
Fig. 1.
Title: Flowchart of study population.
2.2. The study protocol and echocardiographic data acquisition
All patients underwent 2-dimensional echocardiography prior to biventricular ICD implantation and at follow-up in the second year after CRT implantation. The images were obtained on a Vivid 7 ultrasound machine (General Electric, Milwaukee, WI) using a 3.5 MHz transducer at a depth of 16 cm in the parasternal (long- and short axes) and apical (2 and 4 chambers) views. The images were stored in cine-loop format by well-trained echocardiographists and reviewed by an independent cardiologist who was not involved in the study. The left ventricular end-diastolic diameter (LVEDD), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic diameter (LVESD) and left ventricular end-systolic volume (LVESV) were measured and the LVEF was calculated using Simpson's technique [15].
2.3. LV-dyssynchrony measurement with septal rebound stretch (SRSsept)
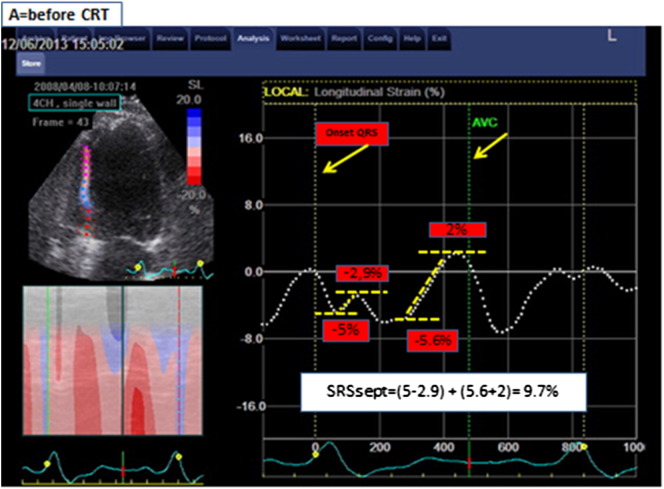
The analysis was performed using EchoPac version 7.0.1, General Electric. The acquisition of the 2D images was performed with at least 40 fps to allow for proper speckle tracking analysis off line. The analysis was performed by a blinded cardiologist to whom only gray-scale imaging of the septal wall and aortic flow recordings was available. Longitudinal speckle tracking technique was used to assess the deformation in the septal wall. The region of interest was set along the endocardial border from the base to the apex, excluding the apical cap, and adapted to match the wall thickness and checked visually and adjusted if necessary. Global wall deformation (i.e., calculated over the entire length of the wall) was used for analysis. SRSsept was defined as the cumulative amount of systolic stretch after prematurely terminated shortening in septum (Fig. 2). The effective septal shortening was defined as the end-systolic (i.e., at aortic valve closure) value of deformation.
Fig. 2.
Title: Measurement of SRSsept.
Legend: Example of septal rebound stretch (SRSsept) measurement. Global longitudinal deformation measured over the entire length of the septum is represented by the dash white lines. Negative slope of the deformation curve indicates shortening; positive slope indicates stretching. Systolic stretch that occurs after initial shortening defines systolic rebound stretch. If more than 1 episode of stretch occurs, the absolute amount of stretch is summed to calculate systolic rebound stretch. In this patient with high amount of SRSsept, systolic shortening is interrupted early during systole, resulting in prominent systolic stretching. Note that stretching and shortening occurring after AVC (aortic valve closure) are ignored for rebound stretch measurement.
Furthermore, we used the inter-ventricular mechanical delay (IVMD) to assess the interventricular mechanical dyssynchrony as it has been reported by the previous study [7] as a predictor of response to CRT, however, with low sensitivity and specificity.
The off-line analyses were performed on digitally stored images by an independent observer (A.G.) blinded to the clinical and other echocardiographic information. All SRSsept measures were additionally performed by another independent, well trained, and experienced cardiologist (P.P.D.). Furthermore, these measurements were repeated by the first observer, in order to assess inter-observer and intra-observer variability. Inter-observer and intra-observer variability were expressed as standard error of measurement (SEM).
2.4. Echocardiographic response to CRT
Response to CRT was defined as a reduction of ≥ 15% in LVESV compared to baseline echocardiographic measurement, at the second year of echocardiographic follow up [7], [11].
2.5. Statistical analysis
Continuous variables are expressed as mean ± SD and a non-parametric Mann–Whitney U-test was used to analyze differences between groups. Categorical variables are presented as number and percentages and the chi-squared test was used to analyze differences between groups. Paired observations (observations of the same variable at different time points) were analyzed using the nonparametric Wilcoxon test. Logistic regression analysis was performed to identify whether SRSsept and IVMD predicted echocardiographic response. In the multivariate logistic regression analyses baseline SRSsept as continuous variable and as dichotomized variable defined by the optimal cut-off value, as well as IVMD were analyzed separately after adjustment for clinical variables (age, gender, LVEF, QRS width, LBBB/RBBB, ischemic/non-ischemic cardiomyopathy). Receiver operating characteristic curve analysis was performed to find the optimal cut-off value for baseline SRSsept to predict echocardiographic response. In subsequent analyses, SRSsept was analyzed as a continuous variable and as a dichotomous variable as defined by the optimal cut-off value. P-values < 0.05 were considered statistically significant in all analyses. Statistical analysis was performed using SPSS statistical software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
3. Results
3.1. Baseline characteristics
Of the 160 patients initially included, 22 patients were excluded due to poor 2D image quality. Therefore, 138 patients were eventually analyzed. The baseline characteristics of study population according to echocardiographic response are summarized in Table 1. Mean age was 68 ± 8 years with 70% male gender. 59% had functional class 3 heart failure. Mean QRS duration was 164 ± 23 msec with 95% LBBB or LBBB like morphology and mean EF was 26 ± 7% with 51% ischemic etiology. Echocardiographic follow-up was performed at 22 ± 8 months. According to the predefined criterion of a reduction in LVESV ≥ 15%, 96 (70%) patients were classified as responders to CRT and 42 (30%) as non-responders. 30 of 42 (71%) non-responders had an ischemic cardiomyopathy. All patients had optimized medical therapy, including angiotensin-converting enzyme or angiotensin-receptor antagonist (86%), beta-blockers (85%), diuretics (80%) and spironolactone (44%) at maximally tolerated dosages.
Table 1.
Baseline characteristics of study population according to echocardiographic response to CRT.
| All patients N = 138 | Non-responders N = 42 (30%) | Responders N = 96(70%) | P-value | |
|---|---|---|---|---|
| Age (years) | 68 ± 8 | 70 ± 9 | 67 ± 8 | 0.05 |
| Male gender | 70% | 69% | 70% | 0.93 |
| Ischemic | 51% | 71% | 43% | 0.002 |
| Non-ischemic | 49% | 29% | 57% | |
| Atrial fibrillation | 23% | 24% | 23% | 0.90 |
| Sinus rhythm | 77% | 76% | 77% | |
| LBBB or LBBB like morphology | 95% | 93% | 97% | 0.37 |
| RBBB | 5% | 7% | 3% | |
| QRS duration (ms) | 164 ± 23 | 159 ± 20 | 167 ± 23 | 0.05 |
| NYHA functional class | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.6 ± 0.5 | 0.83 |
| LVEF (%) | 26 ± 7 | 25 ± 6 | 26 ± 7 | 0.52 |
| LV end-diastolic volume (ml) | 147 ± 56 | 150 ± 54 | 146 ± 57 | 0.47 |
| LV end-systolic volume (ml) | 109 ± 46 | 111 ± 43 | 108 ± 48 | 0.47 |
| Systolic blood pressure (mmHg) | 123 ± 18 | 121 ± 19 | 124 ± 17 | 0.20 |
| Diastolic blood pressure (mmHg) | 73 ± 11 | 72 ± 10 | 73 ± 11 | 0.36 |
| Medication use | ||||
| Diuretics | 80% | 91% | 75% | 0.04 |
| Beta-blocker | 85% | 83% | 85% | 0.75 |
| Ace-inhibitors or AT II | 86% | 85% | 87% | 0.69 |
| Spironolacton | 44% | 55% | 40% | 0.10 |
| SRSsept (%) | 4.4 ± 3.2 | 3.0 ± 2.7 | 5.1 ± 3.2 | < 0.001 |
| IVMD (ms) | 43.4 ± 25.5 | 35.9 ± 21.6 | 47.2 ± 26.7 | 0.045 |
| Position of LV-lead | 0.814 | |||
| Posterior/postero-lateral | 67% | 64% | 69% | |
| Lateral | 22% | 21% | 22% | |
| Midcardiac vein | 4% | 5% | 4% | |
| Epicardial | 7% | 10% | 5% |
Abbreviations: LVEF = left ventricular ejection fraction, LVEDD = left ventricular end diastolic diameter, LVESD = left ventricular systolic diameter, LVEDV = left ventricular end diastolic volume, LVESV = left ventricular end systolic volume. SRSsept = systolic rebound stretch in septum. IVMD = inter ventricular mechanical delay.
3.2. Echocardiographic analysis
LV-dyssynchrony indices were analyzed at baseline and at mean follow-up of 22 ± 8 months. During analysis of SRSsept, 828 segments were evaluated and only 3% of segments were excluded from analysis due to non-valid tracking. The inter- and intra-observer variability for SRSsept were assessed in 20 randomly selected patients. The standard error of measurement (SEM) for the inter-observer variability was 0.67. The SEM for the intra-rater variability was 1.04.
3.3. Effects of CRT on echocardiographic parameters
During the follow-up, responders showed a significant reduction in end-systolic and diastolic LV volumes compared with non-responders (LVESV in responders reduced from 108 ± 48 ml to 60 ± 33, P < 0.001 and in non-responders from 111 ml ± 43 to 120 ml ± 41, P = 0.1). Furthermore, a significant improvement in LVEF was noted in responders compared with non-responders (from 26% ± 7 to 45% ± 10, P < 0.001 and from 25% ± 6 to 26% ± 6, P = 0.25 respectively).
3.4. Effects of CRT on clinical outcome
Clinical outcome was assessed after a mean follow-up of 57 ± 12 months. During the follow-up 32 combined clinical events occurred. 20 patients died 11 / 42(26.2%) in non-responders group and 9 / 96(9.4%) in responders (P = 0.010). 20 patients were hospitalized due to worsening of heart failure (11 in non-responders group and 9 in responders, P = 0.010). Eight patients who died were also hospitalized due to heart failure during the follow-up. In the entire population a significant improvement in clinical status was noted, with a reduction in NYHA functional class from 2.6 ± 0.5 to 1.78 ± 0.71 (P < 0.001). The NYHA class during follow up in the responder group was 1.7 ± 0.7 and in the non-responder group was 2.0 ± 0.6 (P = 0.03).
3.5. Baseline SRSsept and response to CRT
The cumulative amount of systolic stretch after prematurely terminated shortening in septum could be quantified in 97% of patients. As displayed in Table 1, the baseline value of SRSsept in non-responders and responders was significantly different (3.0% ± 2.7 vs 5.1% ± 3.2 respectively, P < 0.001). Receiver operating characteristic curve analysis was performed to define the optimal cut-off value for SRSsept to predict the echocardiographic response. The area under the curve for SRSsept was 0.70 (CI 0.59–0.80), and the optimal cut-off value to predict response to CRT was > 4%, yielding a sensitivity and specificity of, respectively, 66% and 66% (Fig. 3). When patients divided in 2 groups according to SRSsept ≥ 4% versus < 4%, 56% of patients had SRSsept ≥ 4%. Furthermore, SRSsept < 4% was more common in male gender and in patients with ischemic cardiomyopathy. SRSsept > 4% was more common in patients with wider QRS duration (Table 2). In univariate and multivariate analyses, SRSsept ≥ 4% independently predicted the response to CRT (Table 3). In univariate analysis, the QRS duration and non-ischemic etiology independently predicted the response to CRT.
Fig. 3.
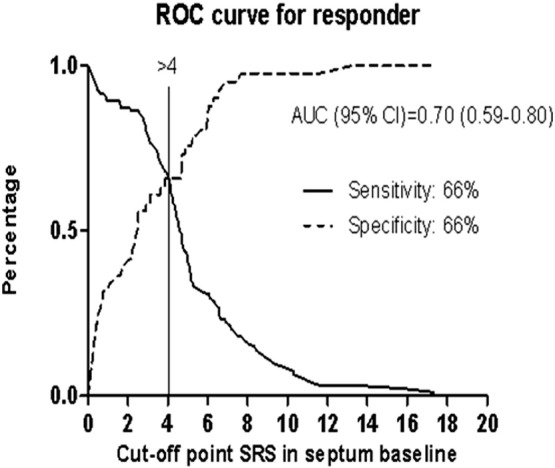
Title: ROC curve for responder.
Legend: Receiver operating characteristic curves for SRSsept with reverse remodeling as outcome. AUC = area under the curve.
Table 2.
Baseline characteristics of study population according to SRSsept ≥ 4% versus < 4%.
| All patients N = 138 | SRSsept < 4% N = 59 (44%) | SRSsept ≥ 4% N = 76 (56%) | P-value | |
|---|---|---|---|---|
| Age (years) | 68 ± 8 | 67 ± 9 | 69 ± 8 | 0.226 |
| Male gender | 70% | 80% | 62% | 0.026 |
| Ischemic | 51% | 63% | 42% | 0.018 |
| Non-ischemic | 49% | 37% | 58% | |
| Atrial fibrillation | 23% | 27% | 20% | 0.312 |
| Sinus rhythm | 77% | 73% | 80% | |
| LBBB or LBBB like morphology | 95% | 93% | 97% | 0.236 |
| RBBB | 5% | 7% | 3% | |
| QRS duration (ms) | 165 ± 23 | 156 ± 23 | 171 ± 20 | < 0.001 |
| NYHA functional class | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.6 ± 0.5 | 0.885 |
| LVEF (%) | 26 ± 7 | 27 ± 7 | 25 ± 7 | 0.219 |
| LV end-diastolic volume (ml) | 147 ± 56 | 146 ± 56 | 148 ± 57 | 0.912 |
| LV end-systolic volume (ml) | 109 ± 47 | 107 ± 46 | 110 ± 47 | 0.776 |
| Systolic blood pressure (mmHg) | 123 ± 18 | 125 ± 19 | 122 ± 17 | 0.422 |
| Diastolic blood pressure (mmHg) | 73 ± 11 | 72 ± 12 | 73 ± 10 | 0.455 |
All abbreviations were explained in Table 1.
Table 3.
Uni- and multivariate predictors of response to CRT by logistic regression analysis.
| Univariate analysis |
Multivariate analysis‡ |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Age | 0.96 | 0.92–1.01 | 0.082 | |||
| Male gender | 1.04 | 0.47–2.27 | 0.930 | |||
| Non-ischemic etiology | 3.35 | 1.53–7.33 | 0.002 | |||
| QRS duration (ms) | 1.02 | 1.00–1.04 | 0.049 | |||
| LBBB versus RBBB | 2.32 | 0.45–12.0 | 0.317 | |||
| LVEF (%) | 1.03 | 0.97–1.08 | 0.349 | |||
| SRSsept % | 1.31 | 1.12–1.54 | 0.001 | 1.38 | 1.13–1.70 | 0.002 |
| SRSsept (≥ 4% vs < 4%) | 3.74 | 1.72–8.10 | 0.001 | 3.71 | 1.49–9.22 | 0.005 |
| IVMD | 1.02 | 1.00–1.04 | 0.033 | 1.01 | 0.98–1.03 | 0.582 |
All abbreviations were explained in Table 1.
Predictors analyzed separately after adjustment for age, gender, QRS duration, EF at baseline, LBBB/RBBB and ischemic/non-ischemic etiology.
4. Discussion
In this study with the largest study population until now, we demonstrated a strong and independent association between baseline SRSsept and echocardiographic response to CRT. These findings are in line with previous small studies, and possibly this will be an important new echocardiographic tool in predicting response to CRT.
Speckle tracking imaging technique provides information on deformation of certain segments of the myocardial wall. The timing of maximum deformation of a certain segment of the myocardial wall has been used to assess the LV-dyssynchrony. This method, also known as time-based index, has been studied to predict the response to CRT. These studies demonstrated, however, conflicting results regarding the value of time-based radial and longitudinal dyssynchrony indices in prediction of CRT response [8], [9], [11], [16], [17], [18], [19], [20]. In the EchoCRT trial [21], studying potential benefit of CRT in patients with chronic heart failure and narrow QRS, several echocardiographic techniques including radial strain (time-based index) did not predict mortality after either CRT or no CRT. One of the plausible reasons that these time-based indices failed to predict reverse remodeling after CRT is that these indices look only to the timing of maximum deformation of a certain segment and do not provide information about stretching (push away) of another segment at the same time [14].
It is important to stress that septal rebound stretch (SRSsept) measurement, performed by longitudinal speckle tracking analyses, does not look at the timing of deformation. However, SRSsept is based on the amount of stretch in the septal wall after the initial contraction during systole [12]. SRSsept selectively measures the amount of systolic stretch that occurs after early shortening and disregards the systolic pre-stretching and post-systolic shortening, both are inefficient deformations associated with the delayed activated segments. SRSsept assesses the amount of dyssynchrony-related wasted energy that can be recruited by CRT. Conversion of SRSsept into shortening is one of the primary working mechanisms through which CRT improves ventricular function [12]. The value of baseline SRSsept in predicting long-term prognosis, improvement in LV remodeling and neurohormonal activation after implantation of CRT device has been recently demonstrated [13]. Furthermore, SRSsept rather than time delay indices provided significant incremental value over clinical characteristics in prediction of CRT response [14]. In the current study baseline SRSsept independently predicted the echocardiographic response to CRT. Our study, as far as we can ascertain, is the largest study with 138 included patients with echocardiographic follow up in all patients at a mean of 22 ± 8 months and provides further evidence for the predictive value of SRSsept in response to CRT. Definition of response to CRT is still a matter of debate. In the present study, we assessed LV reverse remodeling as response to CRT after a mean 2-year follow-up. Previous study (REVERSE study) showed that the maximal amount of functional and LV remodeling improvements was reached at 2 years following CRT and these improvements sustained in 5-year follow-up [22]. Therefore, we used the available echocardiograms at 2 years following the implantation to define the echocardiographic response to CRT. The most optimal cut-off value of SRSsept for predicting response to CRT can be questioned. A previous study [13] used a baseline SRSsept of ≥ 4.7% in predicting survival without heart transplantation or assist device. However, they did not report sensitivity and specificity of the SRSsept. In our study the area under the curve for SRSsept was 0.70 and the optimal cut-off value to predict response to CRT was 4% with both a sensitivity and specificity of 66%. We acknowledge that sensitivity and specificity are not high and it may be a limitation for SRSsept as dyssynchrony index.
4.1. Clinical application of SRSsept
Non-response is an important and unresolved issue in CRT. The costs and potential procedure-related complications of CRT underline the importance of identifying CRT nonresponders. Although current patient selection guidelines for CRT utilize QRS width as a surrogate for dyssynchrony, the results of our study support the additional value of SRSsept. Based on our study, a cut-off value of SRSsept ≥ 4% can be used, but future studies should confirm our findings.
5. Limitations
The present study is a retrospective analysis of prospective registry of a large single-center cohort of consecutive patients treated with CRT. We realize that retrospective analysis is inferior to prospective investigations with prespecified endpoints and cut-off values. The data however was collected systematically and longitudinally by independent physicians. Furthermore, echocardiograms were analyzed and validated by independent cardiologists. Although measurement variability might have been negatively influenced by the inclusion of patients with atrial fibrillation, the current study population most closely resembles daily practice. Strain measurement is sensitive to acute change in load and the calculations for SRSsept require measurements of timing of aortic valve closure to define end-systole. However, these measurements were at different times potentially different loading conditions and heart rates from which the LV images were acquired. Thus, the marking of the end-systolic phase may add a source of potential error to the measurements of SRSsept. Non-response to CRT is probably not solely due to insufficient patient selection. Suboptimal LV-lead placement must also be considered. However, there were no differences between responders and non-responders regarding the location of LV-lead in our study population. Also unfavorable pacemaker settings can contribute to non-response to CRT. Unfortunately, we were not able to perform echo-guided optimization after device implantation.
6. Conclusion
We demonstrated that stretch-based assessment of LV-dyssynchrony measured with SRSsept was able to predict echocardiographic response to CRT. These findings indicate that baseline SRSsept > 4% helps identifying potential CRT responders.
Author contributions
Concept/design: AG, PPD, and AE.
Data collection: AG.
Data analysis/interpretation: AG, PPD, and JPO.
Drafting article: AG, PPD, JPO, AE, and AA.
Statistics: AG.
Critical revision of article: all authors.
Approval of article: all authors.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
Funding: none.
References
- 1.Nelson G.S., Berger R.D., Fetics B.J., Talbot M., Spinelli J.C., Hare J.M. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–3059. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 2.Abraham W.T., Fisher W.G., Smith A.L., Delurgio D.B., Leon A.R., Loh E., MIRACLE Study Group Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 3.St John Sutton M.G., Plappert T., Abraham W.T., Smith A.L., DeLurgio D.B., Leon A.R. Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study GROUP. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 4.Cleland J.G., Daubert J.C., Erdmann E., Freemantle N., Gras D., Kappenberger L. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 5.McAlister F.A., Ezekowitz J., Hooton N., Vandermeer B., Spooner C., Dryden D.M. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–2514. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 6.Epstein A.E., DiMarco J.P., Ellenbogen K.A., Estes N.A., 3rd, Freedman R.A., Gettes L.S., American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) American Association for Thoracic Surgery. Society of Thoracic Surgeons ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American Association for thoracic surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Bax J.J., Gorcsan J., III Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933–1943. doi: 10.1016/j.jacc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Suffoletto M.S., Dohi K., Cannesson M., Saba S., Gorcsan J., III Novel speckle-tracking radial strain from routine black- and white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–968. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 9.Gorscan J., III, Tanabe M., Bleeker G.B., Suffoletto M.S., Thomas N.C., Saba S. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol. 2007;50:1476–1483. doi: 10.1016/j.jacc.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Chung E.S., Leon A.R., Tavazzi L., Sun J.P., Nihoyannopoulos P., Merlino J. Results of the predictors of response to CRT trial. Circulation. 2008;117:2608–2618. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 11.Delgado V., Ypenburg C., van Bommel R.J., Tops L.F., Mollema S.A., Marsan N.A. Assessment of left ventricular dyssynchrony by speckle tracking imaging. J Am Coll Cardiol. 2008;51:1944–1952. doi: 10.1016/j.jacc.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 12.De Boeck B.W., Teske A.J., Meine M., Leenders G.E., Cramer M.J., Prinzen F.W. Septal rebound stretch reflects the functional substrate to cardiac resynchronization therapy and predicts volumetric and neurohormonal response. Eur J Heart Fail. 2009;11:863–871. doi: 10.1093/eurjhf/hfp107. [DOI] [PubMed] [Google Scholar]
- 13.Leenders G.E., De Boeck B.W., Teske A.J., Meine M., Bogaard M.D., Prinzen F.W. Septal rebound stretch is a strong predictor of outcome after cardiac resynchronization therapy. J Card Fail. 2012;18:404–412. doi: 10.1016/j.cardfail.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Chan Y.H., Wu L.S., Kuo C.T., Wang C.L., Yeh Y.H., Ho W.J. Incremental value of inefficient deformation indices for predicting response to cardiac resynchronization therapy. J Am Soc Echocardiogr. 2013;26:307–315. doi: 10.1016/j.echo.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A., American Society of Echocardiography's Nomenclature and Standards Committee. Task Force on Chamber Quantification. American College of Cardiology Echocardiography Committee. American Heart Association European Association of Echocardiography, European Society of Cardiology. Eur J Echocardiogr. 2006;7:79–108. [Google Scholar]
- 16.Miyazaki C., Redfield M.M., Powell B.D., Lin G.M., Herges R.M., Hodge D.O. Dyssynchrony indices to predict response to cardiac resynchronization therapy: a comprehensive prospective single-center study. Circ Heart. 2010;3:565–573. doi: 10.1161/CIRCHEARTFAILURE.108.848085. [DOI] [PubMed] [Google Scholar]
- 17.Lim P., Buakhamsri A., Popovic Z.B., Greenberg N.L., Patel D., Thomas J.D. Longitudinal strain delay index by speckle tracking imaging. A new marker of response to cardiac resynchronization therapy. Circulation. 2008;118:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.750190. [DOI] [PubMed] [Google Scholar]
- 18.Tatsumi K., Tanaka H., Matsumoto K., Kaneko A., Tsuji T., Ryo K. Relation between strain dyssynchrony index determined by comprehensive assessment using speckle tracking imaging and long-term outcome after cardiac resynchronization therapy for patients with heart failure. Am J Cardiol. 2012;109:1187–1193. doi: 10.1016/j.amjcard.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 19.De Boeck B.W., Meine M., Leenders G.E., Teske A.J., van Wessel H., Kirkels J.H. Practical and conceptual limitations of tissue Doppler imaging to predict reverse remodelling in cardiac resynchronization therapy. Eur J Heart Fail. 2008;10:281–290. doi: 10.1016/j.ejheart.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Beshai J.F., Grimmen R.A., Nagueh S.F., Baker J.H., 2nd, Beau S.L., Greenberg S.M. RethinQ Study Investigators. Cardiac resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–2471. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 21.Ruschitzka F., Abraham W.T., Singh J.P., Bax J.J., Borer J.S., Brugada J., EchoCRT Study Group Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 22.Linde C., Gold M.R., Abraham W.T., St John Sutton M., Ghio S., Cerkvenik J. Long-term impact of cardiac of cardiac resynchronization therapy in mild heart failure: 5-year results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Eur Heart. 2013;34:2592–2599. doi: 10.1093/eurheartj/eht160. [DOI] [PubMed] [Google Scholar]