Abstract
Background
Ischemic postconditioning (IPostC), has been proposed as a useful approach to reduce infarct size in all species, but its clinical utility remains unclear.
Objective
To investigate the role played by the protocol used on the efficacy of IPostC in protecting the diseased human myocardium.
Methods
Myocardial atrial samples from patients were subjected to a 90 min ischemia/120 min reoxygenation followed by different IPostC protocols to investigate the role of the time of ischemia (30, 60, 90 and 120 s) and the number of cycles (1, 2, 3 and 4) with 60 and 120 s of total ischemic time. Muscles were also subjected to ischemic preconditioning (IPreC). The release of lactate dehydrogenase (LDH) and the measurement of tetrazolium bromide (MTT) were determined.
Results
IPostC increased the LDH and decreased the MTT values from those of control, independently of the duration of the conditioning ischemia. LDH and MTT values also worsened by augmenting the number of IPostC cycles whereas they were significantly improved by IPreC. However, analysis of individual results indicated that in approximately 1/3 of the cases IPostC exhibited some degree of protection especially in the presence of increased ischemic injury.
Conclusions
The present findings show that IPostC of the human myocardium may be influenced by the protocol used and also by the degree of the preceding ischemic injury. IPostC was beneficial in approximately 1/3 of the cases; however in the remaining cases it increased ischemic damage and, therefore, these results raise a word of caution on its broad clinical use.
Keywords: Ischemic postconditioning, Human myocardium, Ischemic injury, Right atrial appendage
1. Introduction
Infarct size is recognized as a major determinant of myocardial functional recovery and mortality after an acute myocardial infarction [1]. Hence limitation of infarct size is critical to improve survival and to prevent the development of heart failure. The most effective treatment to reduce infarct size is the re-opening of the culprit occluded coronary artery by coronary angioplasty or thrombolysis. Adjunctive treatments at reperfusion, such as β-blockers and angiotensin converting enzyme inhibitors, can ameliorate morbidity and mortality, although not via a reduction in infarct size [2]. Despite these improvements in treatment, mortality remains elevated in high risk patients [3] and the prevalence of heart failure is also increasing [4], which justifies the search for therapies that would effectively reduce infarct size.
It has been suggested that reperfusion injury accounts for 50% of the final size of a myocardial infarction [5] and that ischemic postconditioning (IPostC), a sequence of short reperfusion/ischemia episodes after a prolonged ischemic period, can be a useful approach to reduce infarct size. The first evidence of infarct size reduction associated with IPostC was reported by Zhao et al. [6] in a canine model. Afterwards, experimental studies in various models and species (dog, rabbit, mouse, rat and pig) have confirmed the beneficial action of IPostC [7], although other studies have reported no protection [8], [9], [10] or even a detrimental effect [11]. The reason for this discrepancy could be due to the use of different IPostC protocols, variations on the duration of the ischemic insult, and even the anesthetic regimen, and the animal species and type of strain utilized [12].
Staat et al. [13] performed the first prospective clinical trial on the efficacy of IPostC in patients with ST-segment elevation myocardial infarction (STEMI). In this study involving a small number of patients, they showed a reduction in myocardial infarct size, as estimated by the blood levels of creatine kinase. However, afterwards, small size randomized trials [14] and, more recently, larger randomized studies [15], [16], [17] have reported a lack of reproducibility of the IPostC response. The source of disagreement is not clear but it is possible that the lack of uniformity on the IPostC protocol between studies may play a role. Therefore, in order to determine the most effective IPostC protocol to protect the ischemic and reoxygenated human myocardium, we used a well characterized in vitro experimental model [18]. This model provides us a matchless opportunity to test cardioprotective strategies in the human myocardium and to investigate the underlying mechanisms in a safe, rapid and inexpensive manner.
2. Materials and methods
2.1. Patients
The study was approved by the local Ethics Committee and informed consent was obtained from all the participating patients. The right atrial appendage was obtained from patients undergoing elective cardiac surgery prior to cannulation of the heart and the establishment of cardiopulmonary bypass. Demographic data, presence of vascular risk factors and medical treatment received for each participant patient were recorded. A total of 150 patients (50 in each study) were sequentially recruited without the exclusion criteria.
2.2. Study groups
2.2.1. Study 1
2.2.1.1. Duration of the IPostC ischemic time
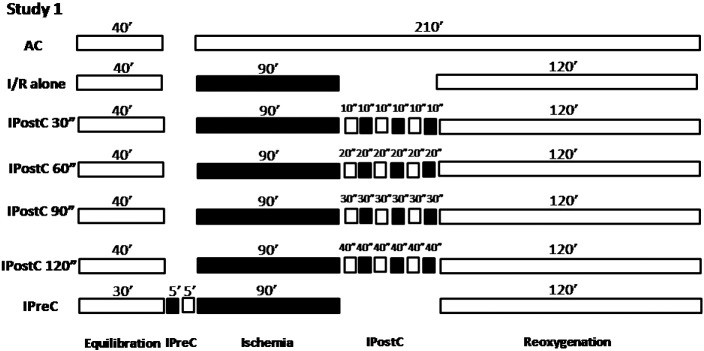
To study the role of the ischemia time in IPostC, the muscles obtained from the right atrial appendage were postconditioned with 30, 60, 90 and 120 s of total ischemia and divided into 3 cycles of 10, 20, 30 and 40 s, of ischemia/reoxygenation (I/R) respectively (n = 50), following 90 min of ischemia (Fig. 1).
Fig. 1.
Experimental protocol for Study 1. All groups were equilibrated for 30–40 min at 37 °C in aerobic conditions. Muscles (n = 50) were subjected to 90 min of ischemia followed by 120 min of ischemia/reoxygenation (I/R). Some muscles did not undergo further treatment (I/R alone),others were postconditioned (IPostC) by 30, 60, 90 and 120 s of total ischemia divided in 3 cycles of 10, 20, 30 and 40 s or preconditioned (IPreC) with 5 min of ischemia and 5 min of reoxygenation while others were maintained under aerobic conditions (AC) for the entire experimental period.
2.2.2. Study 2
2.2.2.1. Number of IPostC cycles
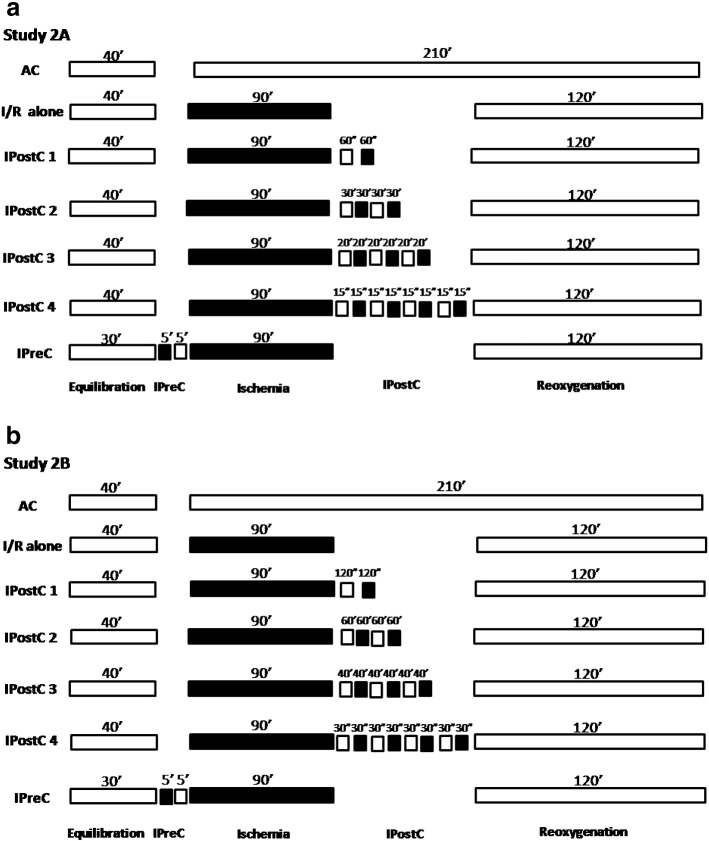
To investigate the role of the number of cycles in IPostC, the muscles obtained from the right atrial appendage of another 100 patients were postconditioned by 1, 2, 3 and 4 cycles of I/R for a total of 60 (Study 2A; n = 50) and 120 s (Study 2B; n = 50) of ischemia after 90 min of ischemia (Fig. 2a & b).
Fig. 2.
Experimental protocol for Study 2A. All groups were equilibrated for 30–40 min at 37 °C in aerobic conditions. Muscles (n = 50/study) were subjected to 90 min of ischemia followed by 120 min of ischemia/reoxygenation (I/R). Some muscles did not undergo further treatment (I/R alone), others were postconditioned (IPostC) with 1, 2, 3 and 4 cycles of ischemia/reoxygenation for a total ischemic time of 60 s or preconditioned (IPreC) with 5 min of ischemia and 5 min of reoxygenation while others were maintained under aerobic conditions (AC) for the entire experimental period.
In all the studies the IPostC stimulus was applied 30 s after the 90 min ischemic time, a period that has been shown to be necessary to induce protection [19].
2.3. Experimental preparation
The right atrial appendages were collected in buffer Krebs Henseleit Hepes (KHH) containing: 118 mM NaCl, 4.8 mM KCl, 27.2 mM NaHCO3, 1.2 mM MgCl2, 1.0 mM KH2PO4, 1.25 mM CaCl2, 10 mM glucose, 20 mM HEPES and pH 7.4 at 4–5 °C. Briefly, the appendage was mounted onto a ground glass plate with the epicardial surface facing down and then sliced using surgical skin graft blades (Swann-Morton, UK) to a thickness of between 300–500 μm. The right atrial appendage and the slide were always kept moist throughout the procedure. The muscles (weight 30–50 mg) were then transferred to an Erlenmeyer (Trallero and Schlee, Barcelona, Spain) containing 10 ml of oxygenated buffer KHH (pH 7.4) and placed into a shaking water bath maintained at 37 °C. For the induction of simulated ischemia, the buffer KHH was bubbled with 95% N2–5% CO2 (pH 6.8–7.0) and d-glucose (Sigma, St. Louis, MO) was removed and substituted with 2-deoxy-d-glucose (Sigma, St. Louis, MO).
After sectioning the right atrial appendage, the muscles were equilibrated for 30–40 min. In all studies simulated ischemia was induced for a period of 90 min followed by 120 min of reoxygenation and then randomly allocated to the various protocols of IPostC (see below). Some muscles were aerobically incubated for an identical time period and others were subjected to ischemic preconditioning (IPreC), induced by a 5 min ischemia followed by a 5 min reoxygenation prior to the 90 min ischemia, a protocol shown to elicit optimal protection in this preparation [18].
2.4. Measurement of tissue injury and viability
Tissue injury was determined by measuring the leakage of lactate dehydrogenase (LDH) into the incubation medium during the 120 min of reoxygenation. An advanced kinetic, based on the formation of NAD + l-lactate which is directly proportional to the amount of LDH activity used. The absorbance was measured at a 340-nm wavelength with a MultiSkan FC spectrometer and the results, obtained after subtraction of the aerobic control values, were expressed as AU/g wet wt.
Tissue viability was assessed by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO) to a blue formazan product in the muscles at the end of the 120 min reoxygenation period. The absorbance of the formazan formed was measured at a 550-nm wavelength with the MultiSkan FC spectrometer and the results, obtained after subtraction of the aerobic control values, were expressed as AU/g wet wt.
2.5. Statistical analysis
Continuous variables were expressed as means ± S.E.M. and compared using ANOVA followed by Dunnett's test; all IPostC protocols were compared with I/R alone group. Linear regression and logistic binary regression were also used to compare the effect of co-morbid conditions and medical treatments. A uni-variate analysis was performed to study the effect of the concomitant cardiac pathologies, the associated co-morbid conditions and the medical treatments received by the muscle donors on IPostC. Statistical analyses were performed using the SPSS 20 and GraphPad Prisma 6. A p < 0.05 was considered statistically significant.
3. Results
Table 1. summarizes the demographic data, the associated clinical co-morbid conditions and the type of heart disease suffered by the donors of the right atrial appendages. Fig. 3, Fig. 4, Fig. 5 show the mean values of LDH leakage and MTT for all the groups in studies 1, 2A and 2B, respectively. They show that in all the studies the LDH and MTT mean values for the I/R alone groups (controls) were similar. They also demonstrate that IPreC was beneficial in the three studies, with significant reduction in LDH leakage and improvement in MTT when compared to I/R alone.
Table 1.
Demographic data.
| Study 1 | Study 2A | Study 2B | |
|---|---|---|---|
| Sex (F/M) | 31/19 | 18/32 | 15/35 |
| Age | 65 ± 3 | 70 ± 10 | 63 ± 14 |
| Obesity | 26 (52%) | 29 (58%) | 24 (48%) |
| Diabetes | 12 (52%) | 28 (56%) | 13 (26%) |
| Insulin-dependent | 4 (8%) | 8 (16%) | 6 (12%) |
| Non insulin-dependent | 7 (14%) | 20(40%) | 7 (14%) |
| Dyslipidemia | 21 (42%) | 37 (74%) | 30 (60%) |
| Hypertension | 30 (60%) | 36 (72%) | 37(74%) |
| CAD | 18 (36%) | 22 (44%) | 15 (30%) |
| AAA | 3 (26%) | 8 (16%) | 12 (24%) |
| Congenital disease | 6 (12%) | 1 (2%) | 12 (24%) |
| LVEF | |||
| > 40% | 49 (100%) | 42 (84%) | 43 (86%) |
| < 40% | 0 (0%) | 8 (16%) | 7 (14%) |
| AF | |||
| Paroxistic | 4 (8%) | 5 (10%) | 1 (2%) |
| Permanent | 13 (26%) | 4 (8%) | 6 (12%) |
| AVD | 30 (60%) | 32 (64%) | 28 (56%) |
| MVD | 13 (26%) | 4 (8%) | 14 (28%) |
| TVD | 7 (14%) | 2 (45%) | 2 (4%) |
AAA, ascending aortic aneurism; AF, atrial fibrillation; AVD, arterial vascular disease; CAD, coronary artery disease; LVEF, left ventricle ejection fraction; MVD, mitral valve disease and TVD, tricuspid valve disease for all the groups (n = 50/study).
Fig. 3.
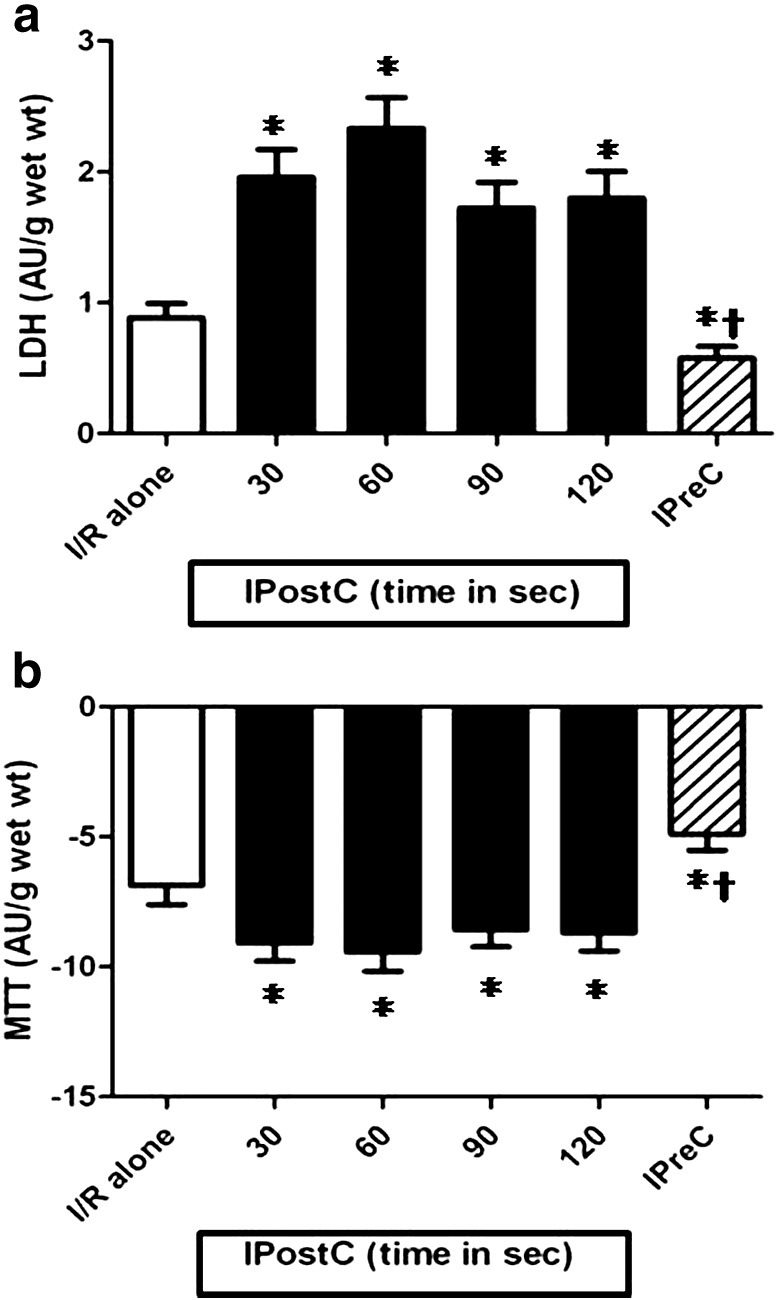
The effect of the ischemic time (30,60, 90 and 120 s) in IPost on the (a) LDH leakage and (b) MTT reduction of muscles (n = 50/group) subjected to 90 min of ischemia followed by 120 min of reoxygenation. The resulst were obtained after substraction of the aerobic control values. *p < 0.05 vs I/R alone group and †p < 0.05 vs all IPostC groups.
Fig. 4.
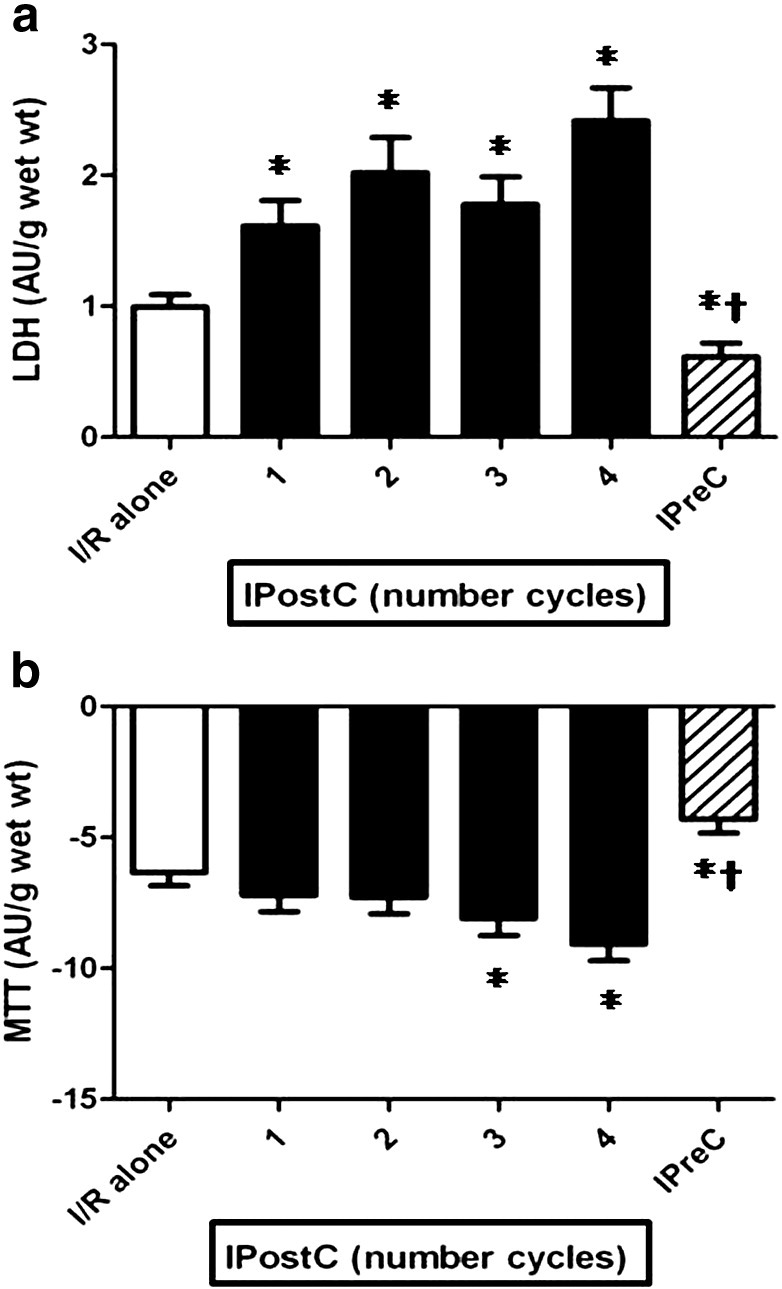
The effect of the numbers of cycles (1, 2, 3 and 4 cycles) used in IPostC with a total ischemia of 60 s on the (A) LDH leakage and (B) MTT reduction of muscles (n = 50/group) subjected to 90 min of ischemia followed by 120 min of reoxygenation. The results were obtained after substraction of the aerobic control values. *p < 0.05 vs I/R alone group and †p < 0.05 vs all IPostC groups.
Fig. 5.
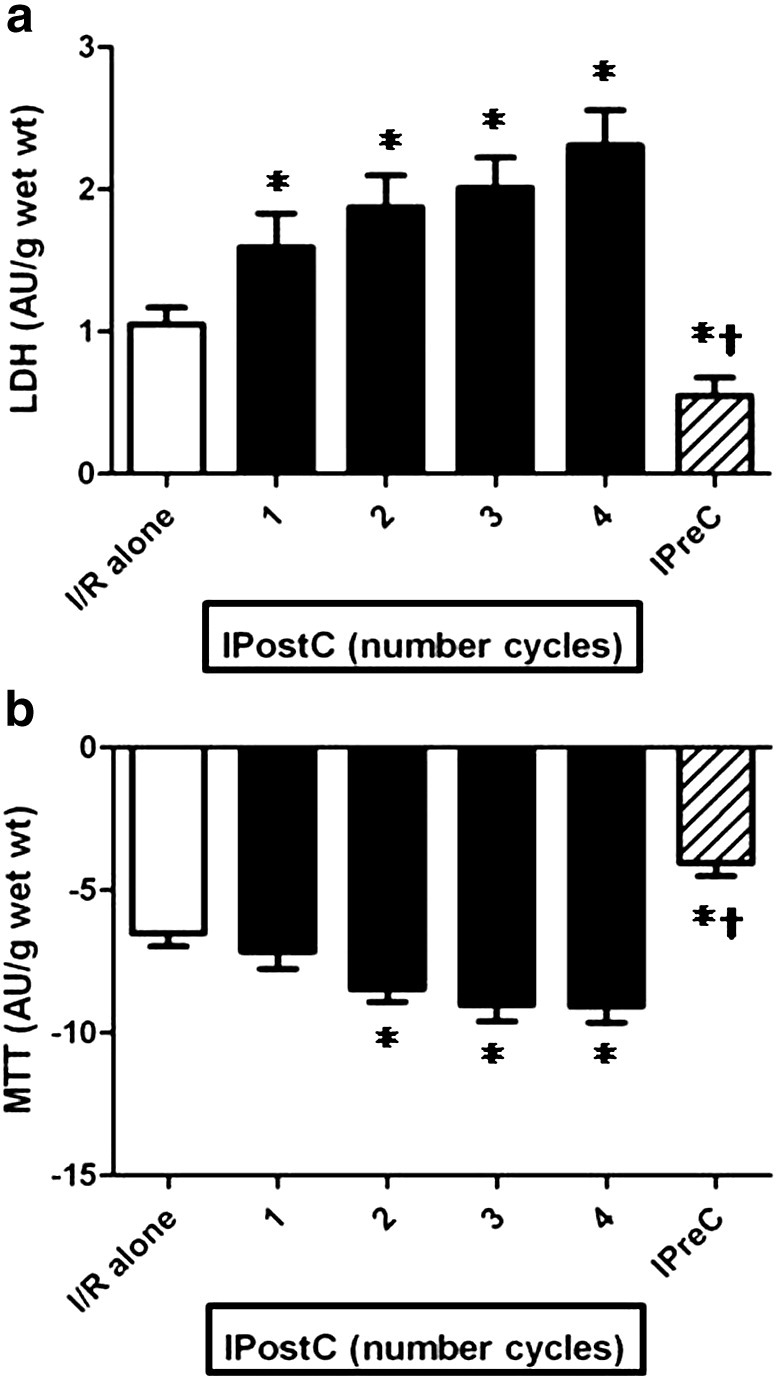
The effect of the numbers of cycles (1, 2, 3 and 4 cycles) in IPostC with a total ischemia of 120 s on the (A) LDH leakage and (B) MTT reduction of muscles (n = 50/group) subjected to 90 min of ischemia followed by 120 min of reoxygenation. The result were obtained after substraction of the aerobic control values. *p < 0.05 vs I/R alone group and †p < 0.05 vs all IPostC groups.
3.1. Effect of the IPostC ischemic time
Fig. 3a & b show that all the investigated ischemic time durations for IPostC were detrimental to a similar degree (Study 1), with a significant increase in LDH leakage and decrease in the MTT mean values in all the groups compared to I/R alone.
3.2. Effect of the number of IPostC cycles
Fig. 4a & b show that the number of IPostC cycles with a total of 60 s of ischemia (Study 2A) was also detrimental (mean values for LDH increased and MTT decreased) compared to I/R alone; 1 cycle was the least deleterious and 4 cycles were the most harmful. As shown in Fig. 5a & b, an identical pattern was observed with 120 s of total ischemia (Study 2B), with a clear tendency to augment myocardial damage when the number of cycles increased.
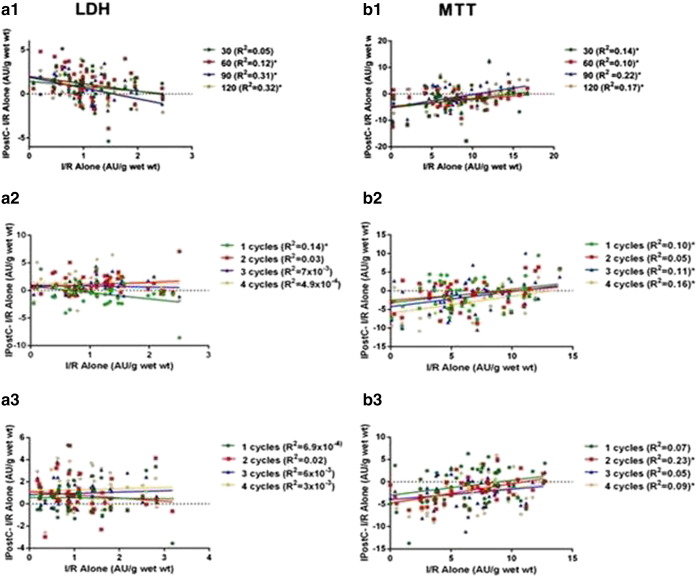
Analysis of the individual results for all the studies (see Fig. 6) revealed that, in some of the studies, IPostC exhibited a weak but statistically significant relationship for LDH and for MTT when the difference between IPostC and its corresponding I/R alone value on one side was compared with I/R alone on the other, suggesting that IPostC is more beneficial with severe degrees of ischemic injury. Thus, even though the mean values for LDH and MTT globally revealed a detrimental effect in all three studies, approximately in 1/3 of the cases IPostC exhibited some degree of benefit.
Fig. 6.
Correlation between I/R alone and the difference between IPostC and I/R alone for all the LDH and MTT values in Study 1(a1 & b1) and 2A(a2 & b2) and 2B(a3 & b3); n = 50/study. *p < 0.05.
A uni-variate analysis did not show a significant beneficial neither detrimental effect of the concomitant cardiac pathologies, the associated co-morbid conditions and the medical treatments received by the muscle donors on the effect of IPostC (data not shown).
4. Discussion
This is the first study to investigate the most effective IPostC protocol in the human myocardium. This information cannot be obtained from in vivo studies and we took advantage of a well characterized in vitro model to carry out the investigation. The results demonstrate that IPostC can be harmful to the human myocardium, although in approximately 1/3 of the cases exhibited some degree of benefit. The effect of IPostC is independent on the duration of the postconditioning ischemic times used but it shows a tendency to augment myocardial damage when the number of cycles was increased. These findings are of clinical importance and they warrant further discussion.
A previous review of the literature Zhou C et al. [20], and also the most recent randomized clinical trials [15], [16], [17], show a lack of uniformity on the IPostC protocol used, with different interval times used for the brief periods of ischemia and also reperfusion. The majority of these studies used 3 or 4 cycles of ischemia/reperfusion that in the present investigations we found to be more detrimental than 1 or 2 cycles. Indeed, this heterogeneity without definition as to which is the most effective IPostC protocol makes difficult the comparison among studies and the understanding on the real clinical utility of the intervention. The NHLBI Workshop that resulted in the formation of the CAESAR (NIH Cardioprotection Consortium), stated: “Only treatments providing consistent and robust benefit in pre-clinical studies involving different models and laboratories should be considered. Although this may seem an obvious pre-requisite, the failure to take this factor into consideration has led to a large number of negative clinical trials.” As a result, this consortium has created a network of research laboratories, which are using a variety of clinically relevant pre-clinical animal myocardial infarction models, to test the efficacy of novel therapeutic agents to ensure that they confer consistent and robust cardioprotection before entering the clinical arena [21], [22]. The present studies were performed in line with this vision and the findings suggest that IPostC may be harmful to patients with cardiac diseases and that, therefore, no further clinical trials should be carried out until issues like the most effective IPostC protocol and the role of the degree of preceding ischemic injury are fully elucidated in pre-clinical investigations.
A number of potential explanations for the failure of IPostC to induce cardioprotection have been put forward [23], [24]. One could be the existing variability in age and sex, the associated comorbidities and the treatment with different medications [25]. Animal studies have shown that the beneficial properties of IPostC are lost with advanced age [26], [27] and that the effect is smaller in females [28]. Also, a meta-analysis from randomized controls trials has suggested that the beneficial effect of IPostC is more pronounced in young and male patients [20], although other investigators have reported a more pronounced protection in women [29]. In the present studies we did not demonstrate a relationship of IPostC with age, sex or any of the other clinical conditions and medical treatments that have been considered to be confounding factors [30].
The duration of acute myocardial ischemia is a major determinant of the final myocardial infarction size and has been proposed as another factor that could influence IPostC. On the one hand, a pre-clinical study has reported that IPostC can be detrimental with short periods of ischemia [11] but so far there is no evidence that IPostC may be beneficial with longer ischemic times. However, on the other hand, it is possible to argue that with severe ischemia there could be little salvageable myocardium left, so that the protective effect of any intervention might be too small to measure. In the context of an acute coronary syndrome, and due to the uncertainty over the beginning of the ischemia and the degree of collateral flow and severity of the ischemic insult, it would be difficult to clarify this point. In our studies the period of ischemia was fixed (90 min) and collateral flow was not a confounding factor and, therefore, the issue of the severity of the ischemic insult should not be a relevant factor to explain the lack of efficacy of IPostC. Yet, in this setting, the response to ischemia greatly differed among patients and the relationships seen between the degree of ischemic myocardial damage and postconditioning (Fig. 6) suggest that IPostC tends to be more beneficial with the more severe degrees of ischemic injury. A complete clarification of this issue would require additional studies.
In contrast to the results on IPostC, our studies have consistently shown benefit by IPreC, thus suggesting that the mechanism of protection of the two interventions could be mediated by different mechanisms. However, a number of laboratories have shown that IPostC and IPreC may share some common pathways [31], [32] albeit they may exhibit some differences in the mechanism and level of protection [9], [33]. If IPostC and IPreC possess some distinctive mechanisms of protection, it could be argued that the combined application of the two interventions could induce a more efficient protection. Nonetheless, the validity of this argument could be challenged, since it has been reported in the skeletal muscle of rats subjected to ischemia and reperfusion that the combination of IPreC and IPostC reduced glycogen depletion to a degree similar to that of IPreC alone whilst IPostC alone did not show protection [34]. Other investigators have observed a loss of protection in the human right atrial myocardium with the co-application of IPreC and IPostC [35]. Differences in the protocol and the animal model used may explain the discrepancy between these results but, certainly, this is an area of great clinical interest that would require further investigations.
A potential limitation of our studies is the use of atrial tissue as that may not fully represent the response of ventricular myocardium. However, we [36] and others [37] have observed that the right atrial and left ventricular myocardium have similar tolerance to ischemia and comparable response to protective interventions as IPreC. Another limitation could be that they were performed in in vitro isolated myocardial tissue and, therefore, caution must be taken when extrapolating the present results to clinical conditions. Nonetheless, the laboratory model used in this study provides us a matchless opportunity to test and refine protective interventions in the human myocardium in a safe, rapid and inexpensive manner before they are considered for clinical use.
5. Conclusions
The present findings have important implications and raise a note of caution on the clinical utility of IPostC to reduce myocardial injury following a period of ischemia. They have demonstrated that although IPostC induces some degree of benefit in 1/3 of the cases it can be harmful. The effect of IPostC may depend to certain extent on the protocol used and on the degree of injury of the preceding ischemia. Therefore, it is advisable that these issues are fully clarified before further clinical trials are carried out.
Funding source
The current study was supported by Instituto de Salud Carlos III (FIS) [grant number 12/00119].
Conflict of interest
None.
Acknowledgments
We are grateful to the Department of Cardiac Surgery at the University Hospital Vall d'Hebron (Barcelona) for the harvesting of the right atrial appendages and to Dr. Xavier Vidal for the help with the statistical analyses.
References
- 1.Gersh B.J., Stone G.W., White H.D., Holmes D.R., Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA. 2005;293(8):979–986. doi: 10.1001/jama.293.8.979. [DOI] [PubMed] [Google Scholar]
- 2.Thibault H., Angoulvant D., Bergerot C., Ovize M. Postconditioning the human heart. Hert Metab. 2007;37:19–22. [Google Scholar]
- 3.Fox K.A., Carruthers K.F., Dunbar D.R., Graham C., Manning J.R., de aedt H. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK–Belgian Study) Eur. Heart J. 2010;31(22):2755–2764. doi: 10.1093/eurheartj/ehq326. [DOI] [PubMed] [Google Scholar]
- 4.Jhund P.S., McMurray J.J. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation. 2008;118(20):2019–2021. doi: 10.1161/CIRCULATIONAHA.108.813493. [DOI] [PubMed] [Google Scholar]
- 5.Prasad A., Stone G.W., Holmes D.R., Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the “dark side” of reperfusion. Circulation. 2009;120(21):2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Z.Q., Corvera J.S., Halkos M.E., Kerendi F., Wang N.P., Guyton R.A. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 7.Skyschally A., van Caster P., Iliodromitis E.K., Schulz R., Kremastinos D.T., Heusch G. Ischemic postconditioning: experimental models and protocol algorithms. Basic. Res. Cardiol. 2009;104(5):469–483. doi: 10.1007/s00395-009-0040-4. [DOI] [PubMed] [Google Scholar]
- 8.Vinten-Johansen J., Zhao Z.Q., Zatta A.J., Kin H., Halkos M.E., Kerendi F. Postconditioning-A new link in nature's armor against myocardial ischemia-reperfusion injury. Basic. Res. Cardiol. 2005;100(4):295–310. doi: 10.1007/s00395-005-0523-x. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz L.M., Lagranha C.J. Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia–reperfusion injury in pigs. Am. J. Physiol. Heart Circ. Physiol. 2006;290(3):H1011–H1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 10.Tang X.L., Sato H., Tiwari S., Dawn B., Bi Q., Li Q. Cardioprotection by postconditioning in conscious rats is limited to coronary occlusions < 45 min. Am. J. Physiol. Heart Circ. Physiol. 2006;291(5):H2308–H2317. doi: 10.1152/ajpheart.00479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manintveld O.C., Te Lintel Hekkert M., van den Bos E.J., Suurenbroek G.M., Dekkers D.H., Verdouw P.D. Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am. J. Physiol. Heart Circ. Physiol. 2007;292(3):H1551–H1560. doi: 10.1152/ajpheart.00151.2006. [DOI] [PubMed] [Google Scholar]
- 12.Darling C.E., Solari P.B., Smith C.S., Furman M.I., Przyklenk K. Postconditioning’ the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic. Res. Cardiol. 2007;102(3):274–278. doi: 10.1007/s00395-007-0643-6. [DOI] [PubMed] [Google Scholar]
- 13.Staat P., Rioufol G., Piot C., Cottin Y., Cung T.T., L'Huillier I. Postconditioning the human heart. Circulation. 2005;112(14):2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 14.Schevchuck A., Laskey W.K. Ischemic conditioning as an adjunct to percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2013;6(4):484–492. doi: 10.1161/CIRCINTERVENTIONS.113.000146. [DOI] [PubMed] [Google Scholar]
- 15.Hahn J.Y., Song Y.B., Kim E.K., Yu C.W., Bae J.W., Chung W.Y. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128(17):1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- 16.Limalanathan S., Andersen G.O., Klow N.E., Abdelnoor M., Hoffmann P., Eritsland J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. J. Am. Heart Assoc. 2014;3(2) doi: 10.1161/JAHA.113.000679. e000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roubille F., Mewton N., Elbaz M., Roth O., Prunier F., Cung T.T. No post-conditioning in the human heart with thrombolysis in myocardial infarction flow 2–3 on admission. Eur. Heart J. 2014;35(25):1675–1682. doi: 10.1093/eurheartj/ehu054. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J.G., Ghosh S., Ockleford C.D., Galinanes M. Characterization of an in vitro model for the study of the short and prolonged effects of myocardial ischaemia and reperfusion in man. Clin. Sci. (Lond) 2000;99(5):443–453. [PubMed] [Google Scholar]
- 19.Philipp S., Downey J.M., Cohen M.V. Postconditioning must be initited in less than 1 minute following reperfusion and is dependent on adenosine receptor and PI3-kinase. Circulation. 2004;110(Suppl 111):804–806. [Google Scholar]
- 20.Zhou C., Yao Y., Zheng Z., Gong J., Wang W., Hu S. Stenting technique, gender, and age are associated with cardioprotection by ischaemic postconditioning in primary coronary intervention: a systematic review of 10 randomized trials. Eur. Heart J. 2012;33(24):3070–3077. doi: 10.1093/eurheartj/ehs265. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz Longacre L., Kloner R.A., Arai A.E., Baines C.P., Bolli R., Braunwald E. New horizons in cardioprotection: recommendations from the 2010 National Heart Lung, and Blood Institute Workshop, Circulation. 2011;124(10):1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloner R.A., Schwartz Longacre L. State of the science of cardioprotection: challenges and opportunities—proceedings of the 2010 NHLBI Workshop on Cardioprotection. J. Cardiovasc. Pharmacol. Ther. 2011;16(3-4):223–232. doi: 10.1177/1074248411402501. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 24.Ovize M., Thibault H., Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ. Res. 2013;113(4):439–450. doi: 10.1161/CIRCRESAHA.113.300764. [DOI] [PubMed] [Google Scholar]
- 25.Ferdinandy P., Hausenloy D.J., Heusch G., Baxter G.F., Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol. Rev. 2014;66(4):1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 26.Boengler K., Schulz R., Heusch G. Loss of cardioprotection with ageing. Cardiovasc. Res. 2009;83(2):247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 27.Przyklenk K., Maynard M., Darling C.E., Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J. Am. Coll. Cardiol. 2008;51(14):1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 28.Penna C., Tullio F., Merlino A., Moro F., Raimondo S., Rastaldo R. Postconditioning cardioprotection against infarct size and post-ischemic systolic dysfunction is influenced by gender. Basic. Res. Cardiol. 2009;104(4):390–402. doi: 10.1007/s00395-008-0762-8. [DOI] [PubMed] [Google Scholar]
- 29.Yetgin T., Magro M., Manintveld O.C., Nauta S.T., Cheng J.M., den Uil C.A. Impact of multiple balloon inflations during primary percutaneous coronary intervention on infarct size and long-term clinical outcomes in ST-segment elevation myocardial infarction: real-world postconditioning. Basic. Res. Cardiol. 2014;109(2):403–412. doi: 10.1007/s00395-014-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heusch G. Reduction of infarct size by ischaemic post-conditioning in humans: fact or fiction? Eur. Heart J. 2012;33(1):13–15. doi: 10.1093/eurheartj/ehr341. [DOI] [PubMed] [Google Scholar]
- 31.Vinten-Johansen J., Yellon D.M., Opie L.H. Postconditioning: a simple, clinically applicable procedure to improve revascularization in acute myocardial infarction. Circulation. 2005;112(14):2085–2088. doi: 10.1161/CIRCULATIONAHA.105.569798. [DOI] [PubMed] [Google Scholar]
- 32.Tsang A., Hausenloy D.J., Mocanu M.M., Yellon D.M. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase–Akt pathway. Circ. Res. 2004;95(3):230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 33.Iliodromitis E.K., Georgiadis M., Cohen M.V., Downey J.M., Bofilis E., Kremastinos D.T. Protection from post-conditioning depends on the number of short ischemic insults in anesthetized pigs. Basic. Res. Cardiol. 2006;101(6):502–507. doi: 10.1007/s00395-006-0606-3. [DOI] [PubMed] [Google Scholar]
- 34.Lintz J.A., Dalio M.B., Joviliano E.E., Piccinato C.E. Ischemic pre and postconditioning in skeletal muscle injury produced by ischemia and reperfusion in rats. Acta Cir. Bras. 2013;28(6):441–446. doi: 10.1590/s0102-86502013000600007. [DOI] [PubMed] [Google Scholar]
- 35.Roleder T., Golba K.S., Kunecki M., Malinowski M., Biernat J., Smolka G. The co-application of hypoxic preconditioning and postconditioning abolishes their own protective effect on systolic function in human myocardium. Cardiol. J. 2013;20(5):472–477. doi: 10.5603/CJ.2013.0131. [DOI] [PubMed] [Google Scholar]
- 36.Linares-Palomino J., Husainy M.A., Lai V.K., Dickenson J.M., Galinanes M. Selective blockade of protein kinase B protects the rat and human myocardium against ischaemic injury. J. Physiol. 2010;588(Pt 12):2173–2191. doi: 10.1113/jphysiol.2010.190462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speechly-Dick M.E., Grover G.J., Yellon D.M. Does ischemic preconditioning in the human involve protein kinase C and the ATP-dependent K + channel? Studies of contractile function after simulated ischemia in an atrial in vitro model. Circ. Res. 1995;77(5):1030–1035. doi: 10.1161/01.res.77.5.1030. [DOI] [PubMed] [Google Scholar]