Abstract
Background
We hypothesized that among patients presenting with dyspnea on exertion (DOE), those who were found to have hyperdynamic left ventricle (i.e. LVEF ≥ 70%) on stress radionuclide myocardial perfusion imaging (RNMPI), are more likely to have features of diastolic dysfunction on transthoracic echocardiography.
Methods
Medical records of 1892 consecutive patients who presented between February 2011 and September 2012 with the chief complaint of DOE and were referred to stress RNMPI were reviewed. Among these, patients who had no evidence of reversible ischemia and had hyperdynamic left ventricle on perfusion imaging, were selected and their recent echocardiograms were reviewed for evidence of diastolic dysfunction. Logistic regression analysis was used to develop an equation to predict diastolic dysfunction with the ejection fraction as the predictor. A two-way analysis of variance model was used to detect differential patterns of ejection fraction across diastolic dysfunction and gender.
Results
A hyperdynamic left ventricle identified on stress RNMPI was found to be a significant predictor of diastolic dysfunction on echocardiography in logistic regression analysis (odds ratio = 1.24, 95% CI = 1.13–1.35, p < 0.0001). A hyperdynamic left ventricle on stress RNMPI has a specificity of 96.77% (CI 83.24–99.46%) and a positive predictor value of 97.83% (CI 88.43–99.64%) in identifying diastolic dysfunction.
Conclusion
In patients presenting with DOE who have no evidence of reversible ischemia on radionuclide stress testing but have hyperdynamic left ventricle, a search should be made for alternate cardiac etiology for this complaint such as diastolic dysfunction and heart failure with preserved ejection fraction.
Keywords: Hyperdynamic left ventricle, Stress radionuclide myocardial perfusion imaging, Diastolic dysfunction, Transthoracic echocardiography
1. Objective
Is hyperdynamic left ventricle (defined as left ventricular with an ejection fraction ≥ 70%) seen on stress RNMPI, in patients presenting with dyspnea on exertion, a marker of diastolic dysfunction on transthoracic echocardiography?
2. Introduction
Heart failure (HF) is a serious health problem especially in the United States, with an estimated incidence of 660,000 cases per year in this country, and a prevalence of more than 5 million (1). The incidence of this complex phenomenon is on the rise especially in the older population with HF being the most common discharge diagnosis among patients older than 65 years and also being the primary cause of re-admission within 60 days (1). As the population ages, HF is expected to become an even greater public health problem. A new clinical entity that is gaining prominence in recent times is heart failure with preserved ejection fraction (HFpEF). In fact, according to various studies, up to 50% of patients with clinical features suggestive of heart failure have normal or preserved ejection fraction [2], [3]. The ADHERE Database revealed that these patients have almost similar mortality, rates of hospitalization and impaired quality of life compared to patients who have heart failure with reduced ejection fraction [4], [5]. Most of these patients with HFpEF have abnormalities in diastolic function, i.e. diastolic dysfunction (DD), seen on transthoracic echocardiography which is characterized by features such as abnormal left ventricular relaxation and increased left ventricular chamber stiffness (6).
Patients presenting with dyspnea on exertion are often referred for stress RNMPI to evaluate for ischemic heart disease. A result citing normal perfusion and preserved left ventricular ejection fraction (LVEF) on stress RNMPI has the potential to minimize further evaluation for other cardiac causes of dyspnea on exertion such as diastolic dysfunction which might indicate HFpEF in appropriate clinical setting. Some of these patients with normal perfusion and preserved LVEF on stress RNMPI, in fact, have hyperdynamic left ventricles with ejection fractions more than or equal to 70%. We hypothesized that these patients with hyperdynamic left ventricles i.e. those with left ventricular ejection fraction (LVEF) greater than or equal to 70% on stress RNMPI are more likely to have evidence of diastolic dysfunction on transthoracic echocardiography.
3. Methods
Electronic medical records of 1892 consecutive patients who were referred for stress RNMPI at our center between February 2011 and September 2012, were reviewed.
Inclusion criteria
-
–
Patients presenting with the complaint of “dyspnea on exertion” or “shortness of breath on exertion.”
-
–
Patients should have undergone stress RNMPI for evaluation of this complaint.
-
–
Patients should have a normal perfusion i.e. no evidence of reversible ischemia and preserved left ventricular ejection fraction (defined as left ventricular ejection fraction ≥ 50%) on stress RNMPI. Patients with evidence of prior infarction but currently having no evidence of reversible ischemia and with preserved LVEF were included in the study.
-
–
Patients should have undergone an interpretable transthoracic echocardiography (TTE) within 4 months of the stress RNMPI study.
Exclusion criteria
-
–
Patients presenting with complaint/s other than or in addition to dyspnea or shortness of breath on exertion such as chest pain, or fatigue. Also patients who had undergone stress testing for other reasons such as pre-op evaluation, or postcoronary artery bypass grafting surgery. were also excluded from the study.
-
–
Abnormal stress RNMPI including evidence of reversible ischemia (i.e. a positive stress test) or a reduced LVEF below 50%.
-
–
Patients who did not undergo a TTE within 4 months of the stress RNMPI study. Also patients with uninterpretable TTEs or TTEs with poor image quality were excluded from the study.
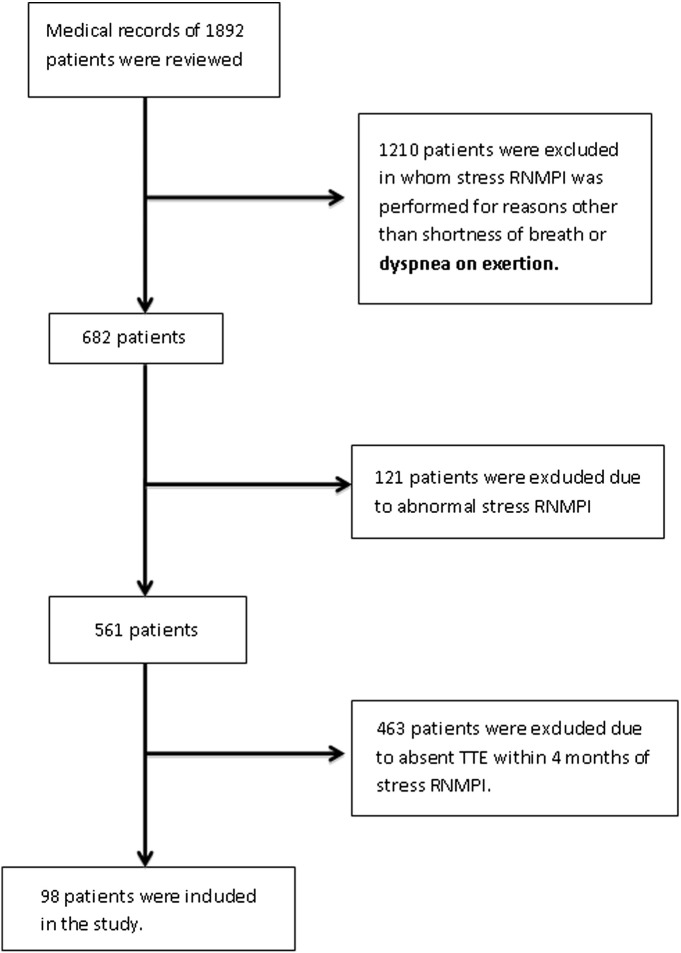
Based on the above inclusion and exclusion criteria a total of 98 patients were included in the study. The process by which 1892 patients yielded 98 subjects is outlined in Fig. 1.
Fig. 1.
Flowchart showing the process by which 1892 patients whose charts were reviewed yielded the final 98 patients who were included in the study.
4. Estimation of left ventricular ejection fraction on stress RNMPI
We shall provide a brief description of how stress RNMPI is performed at our center. The radioisotope used for myocardial perfusion imaging is Technetium Tc-99 m tetrofosmin (Myoview® kit for the preparation of Technetium-99 m tetrofosmin injection, GE Healthcare). This radioisotope is administered intravenously to obtain both resting and stress myocardial perfusion images. Data acquisition starts at around 15–30 min after exercise stress and 60–120 min after pharmacological stress. Myocardial imaging data was acquired with a dual headed gamma camera (Siemens® C-Cam) equipped with a low-energy, high-resolution collimator. A total of 64 images are obtained over a 180-degree orbit using a 90-degree angle between heads. Acquisitions are sometimes attenuation-corrected (based on the discretion of the technician) and gated for 16 frames/cardiac cycle. Total acquisition time is approximately 25 min.
Vendor specific (Cedars-Sinai® Quantitative Gated SPECT software) computer-enhanced edge detection methods were used to assess the left ventricular epicardial and endocardial margins during the entire cardiac cycle. The computer calculated stress global LVEF from the gated SPECT images using an automated algorithm.
5. Assessment of left ventricular diastolic function on transthoracic echocardiography
The assessment of the left ventricular diastolic function (i.e. presence or absence of diastolic dysfunction) was based on the echocardiographic criteria laid down by the American Society of Echocardiography (7). We have used four echocardiographic variables to assess the left ventricular diastolic function as outlined below. All the reference ranges used below to classify echocardiographic parameters of diastolic function of left ventricle are age specific keeping in view that the mean age of our study population was 69.1 years.
-
•
Mitral inflow velocity patterns — the primary measurements of the mitral inflow used were the peak velocity of early rapid filling (E-wave) and peak velocity of late diastolic filling due to atrial contraction (A-wave), the E/A ratio, deceleration time (DT) of early filling velocity and the isovolumic relaxation time (IVRT). Mitral E/A ratio of < 0.8, DT > 200 ms and IVRT ≥ 100 ms were chosen as cut offs to indicate the presence of echocardiographic evidence of diastolic dysfunction (except in patients with pseudonormalized mitral inflow filling pattern with evidence of diastolic dysfunction based on other echocardiographic variables).
-
•
Pulmonary vein flow velocity patterns — measurements of pulmonary venous waveforms included peak systolic (S) velocity, peak anterograde diastolic (D) velocity, the S/D ratio and the peak atrial flow reversal velocity in late diastole. The analysis of pulmonary vein flow velocities was based on evidence of diastolic dysfunction on other echocardiographic variables with S > D indicative of impaired relaxation and S < D of restrictive filling. Other supporting evidence of diastolic dysfunction was increased peak atrial flow reversal velocity (> 30 cm/s) and increased duration of peak atrial flow reversal waveform (especially if the duration of pulmonary vein atrial flow reversal waveform was longer than mitral inflow A waveform).
-
•
Tissue Doppler mitral annular velocities — three distinct longitudinal mitral annulus velocities were recorded: systolic (S′), early diastolic (E′) and late diastolic (A′) velocities. A decreased early diastolic mitral annulus velocity (E′ < 8 cm/s at the septal annulus) and an increased early rapid mitral inflow velocity (E) to early diastolic mitral annular velocity (E′) ratio (E/Ez′) were considered to indicate diastolic dysfunction.
-
•
Color M-mode flow propagation velocity — the mitral-to-apical flow propagation was measured using the slope method [7], [8]. Slowing of flow propagation velocity (< 50 cm/s) was considered to indicate impaired myocardial relaxation or diastolic dysfunction.
Patients were categorized as having diastolic dysfunction if they had at least 2 of the above echocardiographic variables showing supporting evidence. The same cardiologist assessed the echocardiograms of all the included patients.
6. Statistical analysis
A two-way ANOVA model was used to detect differential patterns of ejection fraction across diastolic dysfunction and gender. Logistic regression analysis was used to develop an equation to predict diastolic dysfunction with the ejection fraction as the predictor. Sensitivity and specificity were calculated for a series of diastolic dysfunction probabilities, and a receiver–operator characteristic (ROC) curve was constructed. A cutoff value for probability of diastolic dysfunction was chosen based on optimum sensitivity and specificity. The ejection fraction corresponding to this probability was calculated. In addition, the logistic equation was used to calculate the probability of diastolic dysfunction corresponding to an ejection fraction of ≥ 70.
7. Results
A total of 98 subjects were included in this analysis (Table 1).
Table 1.
Table showing the mean age and gender division of the 98 subjects included in the study along with the type of stress test done, ejection fractions on stress RNMPI and incidence of diastolic dysfunction on transthoracic echocardiography.
(RNMPI — radionuclide myocardial perfusion imaging).
| N | % | |
|---|---|---|
| Total subjects | 98 | |
| Gender | ||
| Male | 42 | 42.9 |
| Female | 56 | 57.1 |
| Age | ||
| Mean (SD) | 69.1 (11.1) | |
| Range | 45–91 | |
| Stress test type | ||
| Dobutamine stress test | 6 | 6.1 |
| Regadenoson stress test | 89 | 90.8 |
| Exercise stress test | 3 | 3.1 |
| Patient type | ||
| Inpatient | 2 | 2.0 |
| Outpatient | 96 | 98.0 |
| Ejection fraction on RNMPI (dichotomous) | ||
| < 70 | 52 | 53.1 |
| ≥ 70 | 46 | 46.9 |
| Ejection fraction on RNMPI (continuous) | ||
| Mean (SD) | 68.2 (11.0) | |
| Range | 50–97 | |
| Diastolic dysfunction on transthoracic echocardiography | ||
| Yes | 67 | 68.4 |
| No | 31 | 31.6 |
Ejection fraction was found to be a significant predictor of diastolic dysfunction in logistic regression analyses (odds ratio = 1.24, 95% CI = 1.13–1.35, p < 0.0001). The optimal sensitivity and specificity was detected at a probability of diastolic dysfunction of 0.78, sensitivity = 71.6%, specificity = 96.8%, which corresponded to an ejection fraction of 66. Using an ejection fraction of 70, the probability of diastolic dysfunction was 0.87. The area under the ROC curve = 0.873 (Fig. 2). A hyperdynamic left ventricle i.e. with an ejection fraction ≥ 70%, identified on stress RNMPI, is strongly associated with diastolic dysfunction on transthoracic echocardiography. A hyperdynamic left ventricle on stress RNMPI has the specificity of 96.77% (CI 83.24–99.46%) and a positive predictive value of 97.83% (CI 88.43–99.64%) in identifying diastolic dysfunction.
Fig. 2.
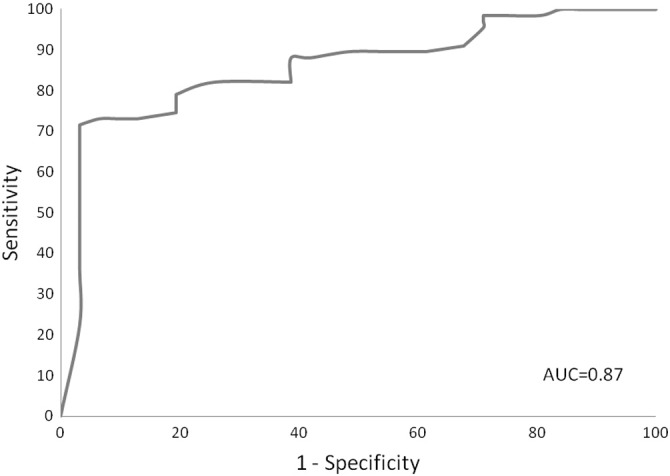
ROC curve (ROC — receiver–operator characteristic).
7.1. Comparison of males vs. females
Based on the chi-square test, diastolic dysfunction was found to be significantly more prevalent (p = 0.0007) in females (82.1%) than in males (50.0%) (Table 3). Via the t-test, mean ejection fraction was found to be significantly (p < 0.0001) higher for females than for males (Table 3). These findings are in accordance with the observation that women are more likely to develop heart failure with preserved ejection fraction (diastolic dysfunction) than men (9). It is felt that this may be because women experience more concentric left ventricular remodeling and less ventricular dilatation in response to systemic arterial hypertension. Also, both ventricular and arterial stiffness increase with age more in women than in men. The systolic and diastolic function and functional reserve become more compromised in postmenopausal women than in men of similar age (9).
Table 2.
Distribution of intervals of ejection fraction and diastolic dysfunction.
| Ejection fraction on stress RNMPI | N with EF in this range | N with diastolic dysfunction on echocardiography | % |
|---|---|---|---|
| 50–54 | 8 | 0 | 0 |
| 55–59 | 15 | 7 | 46.7 |
| 60–64 | 21 | 11 | 52.4 |
| 65–69 | 8 | 4 | 50.0 |
| 70–74 | 19 | 19 | 100.0 |
| 75–79 | 11 | 11 | 100.0 |
| 80–84 | 11 | 10 | 90.9 |
| 85–89 | 0 | 0 | 0 |
| 90–94 | 5 | 5 | 100.0 |
Table 3.
Distribution of mean ejection fraction and incidence of diastolic dysfunction among males and females.
| Males | Females | p-Value | |
|---|---|---|---|
| Total subjects | 42 | 56 | |
| Diastolic dysfunction | |||
| Yes | 21 (50.0%) | 46 (82.1%) | 0.0007 |
| No | 21 (50.0%) | 10 (17.9%) | |
| Ejection fraction | |||
| Mean | 62.0 | 72.9 | < 0.0001 |
7.2. Relationship between mean ejection fraction and diastolic dysfunction
A two-way analysis of variance (ANOVA) model was created to detect differential patterns of ejection fraction across diastolic dysfunction and gender. The interaction term of diastolic dysfunction by gender is statistically significant (p = 0.0106). As shown in Fig. 3, the relationship between diastolic dysfunction and ejection fraction is more pronounced for females than for males.
Fig. 3.
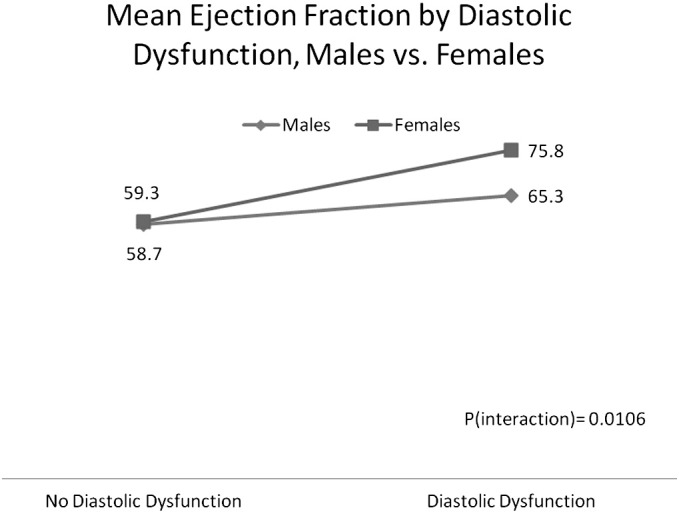
The relationship between diastolic dysfunction and mean ejection fraction is more pronounced for females compared to males (females with diastolic dysfunction have a greater mean ejection fraction).
The main effect of diastolic dysfunction was significant (p < 0.0001), indicating that when considering both genders combined, mean EF on stress RNMPI was significantly higher among those with evidence of diastolic dysfunction on echocardiography (mean EF = 70.6) as compared to those with no diastolic dysfunction (mean EF = 59.0). But it is more appropriate to describe this relationship with respect to gender because of the significant interaction term (i.e., a differential relationship across genders).
8. Discussion
Our study has shown that patients who present with the chief complaint of dyspnea or shortness of breath on exertion and who have undergone stress radionuclide myocardial perfusion imaging and are found to have normal perfusion and hyperdynamic left ventricle (LVEF ≥ 70%) are more likely to have evidence of diastolic dysfunction on transthoracic echocardiography. This relationship between high ejection fraction and diastolic dysfunction is more pronounced in females. Based on these results, referring physicians should have heightened suspicion of diastolic dysfunction among individuals (particularly women) with hyperdynamic left ventricle noted on stress radionuclide myocardial perfusion imaging.
As the prevalence of heart failure with preserved ejection fraction (and diastolic dysfunction) relative to heart failure with reduced ejection fraction is expected to increase at the rate of 1% annually, there is an increased need for information with regard to evaluation, diagnosis and treatment of this condition.
9. Limitations of the study
As with any other retrospective study, our study has the potential for selection bias especially as most of the patients were selected from clinical referrals for stress myocardial perfusion imaging. Though the echocardiographic parameters of diastolic dysfunction, which we have used in our study, were based on the guidelines laid down by the American Society of Echocardiography we have not graded diastolic dysfunction. This is because we have felt that using a general simple approach for diagnosing diastolic dysfunction on echocardiography, which we have used in our trial, has a high feasibility and reproducibility and is thus more suited for clinical trials. Also ours is a rural health center and so our study population is older with a mean age of 69 years. This may impact the generalizability of our study.
10. Conclusion
A hyperdynamic left ventricle (ejection fraction ≥ 70%) seen on stress radionuclide myocardial perfusion imaging bears strong association with diastolic dysfunction and is therefore a marker of heart failure with preserved ejection fraction in appropriate clinical setting.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Meyer Theo, MD DPhil, Jeffrey Shih M.D., Gerard Aurigemma M.D. Heart failure with preserved ejection fraction (diastolic dysfunction) Ann Intern Med. 2013;158(1) doi: 10.7326/0003-4819-158-1-201301010-01001. [ITC1-1] [DOI] [PubMed] [Google Scholar]
- 2.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. Jul 20 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Lam C.S., Donal E., Kraigher-Krainer E., Vasan R.S. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. Jan 2011;13(1):18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancy C.W., Lopatin M., Stevenson L.W., De Marco T., Fonarow G.C. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Hoekstra T., Lesman-Leegte I., Van Veldhuisen D.J., Sanderman R., Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail. 2011;13(9):1013–1018. doi: 10.1093/eurjhf/hfr072. [DOI] [PubMed] [Google Scholar]
- 6.Zile M.R., Baicu C.F., Gaasch W.H. Diastolic heart failure — abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. May 6 2004;350(19):1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 7.Nagueh S.F., Appleton C.P., Gillebert T.C., Marino P.N., Oh J.K., Smiseth O.A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Brun P., Tribouilloy C., Duval A.M., Iserin L., Meguira A., Pelle G. Left ventricular flow propagation during early filling is related to wall relaxation: a color M-mode Doppler analysis. J Am Coll Cardiol. 1992;20:420–432. doi: 10.1016/0735-1097(92)90112-z. [DOI] [PubMed] [Google Scholar]
- 9.Borlaug B.A. Why are women more likely than men to develop heart failure with preserved ejection fraction? Scantlebury DC1. Curr Opin Cardiol. Nov 2011;26(6):562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]