Abstract
Management of obstructive coronary artery disease in patients with aortic stenosis and severe left ventricular dysfunction is challenging. Mechanical circulatory support at the time of percutaneous coronary interventions may be necessary in these extreme-risk patients. We present a case in which the TandemHeart was used to support a patient with severe aortic stenosis, severe protected left main and circumflex disease, and severe cardiomyopathy and review the literature on this subject.
Keywords: aortic stenosis, coronary artery disease, left ventricular dysfunction, mechanical circulatory support
1. INTRODUCTION
Since the introduction of transcatheter aortic valve replacement (TAVR), the number of patients with severe aortic stenosis (AS) undergoing transcatheter or surgical aortic valve replacement has increased substantially.1,2 Significant coronary artery disease (CAD) is present in 40% to 75% of patients with severe AS undergoing TAVR.1,3 Currently, there are no guidelines with regards to percutaneous coronary intervention (PCI) in these patients.3–5 Between 25% and 32% of patients with severe AS undergoing TAVR have reduced left ventricular (LV) ejection fraction (LVEF; <50%), of whom one third have severe LV dysfunction (LVEF <30%).1,3,5,6 Patients with severe AS with LVEF <30% have worse post-PCI outcomes.3 Mechanical circulatory support (MCS) devices have been used for hemodynamic rescue, to facilitate valvuloplasty, and to support high-risk TAVR in patients with severe AS.7–9 Whether utilizing an LV support device improves PCI outcomes in this group of patients is not known. In this review, we describe a patient with severe AS and severe LV dysfunction who was referred for a protected left main (LM) coronary intervention. We discuss the challenges associated with the management of these patients with special emphasis on the utility of MCS and review the literature on this subject.
2. PATIENT PROFILE
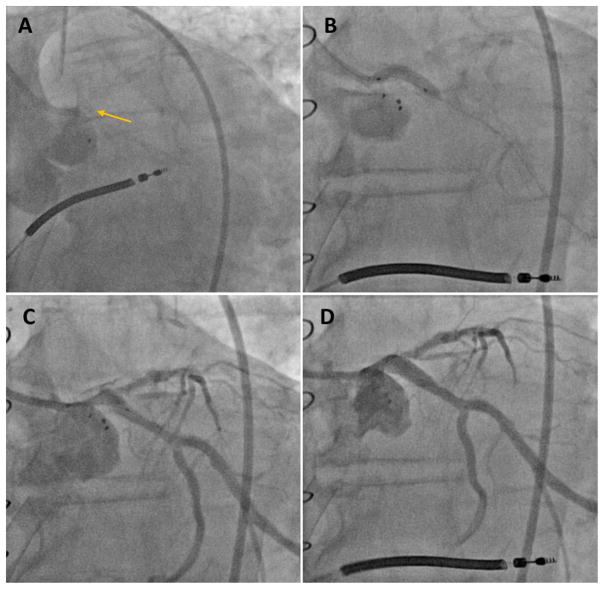
The Institutional Review Board approved the current study and informed consent has been waived. A 72-year-old male was referred due to worsening dyspnea of 6-month duration. He had CAD with a history of coronary artery bypass grafting, ischemic cardiomyopathy, implantable cardioverter defibrillator, sleep apnea, chronic renal insufficiency (glomerular [glow-MAIR-you-lure] filtration rate 48 mL/min), severe carotid artery disease, morbid obesity, and type-II diabetes mellitus. He has been on appropriate guideline-recommended heart failure medications but was only able to tolerate low doses due to borderline blood pressure (Lisinopril 5 mg daily, Spironolactone 25 mg daily). His electrocardiogram showed regular sinus rhythm and right bundle branch block. His transthoracic echocardiogram revealed severe LV systolic dysfunction (LVEF = 29%), an aortic valve area of 0.76 cm2, and a mean transaortic gradient of 18 mmHg with moderate mitral and tricuspid regurgitation and an estimated right ventricular systolic pressure of 57 mmHg. With doubtamine infusion (20 mcg/kg/min), the valve area increased to 0.9 cm2 and mean gradient increased to 34 mmHg. Stroke volume increased by 49%, suggesting an excellent contractile reserve. His coronary angiogram showed severe LM coronary stenosis extending to the left circumflex, mild disease of the left anterior descending (LAD) coronary artery and 60% stenosis in the right coronary artery. It also showed a patent left internal mammary to LAD/diagonal but an occluded saphenous vein graft to the first obtuse marginal coronary artery (Figure 1, Video S1). Computed tomography angiography of the abdomen and pelvis revealed mild tortuosity of the iliac arteries, scattered aortic and iliofemoral calcification, and a minimum luminal diameter >6 mm in the iliac and common femoral arteries (CFA).
FIGURE 1.
Baseline coronary and graft angiography. (A) Severe left main stenosis extending into the left circumflex (arrow), (B) right coronary artery stenosis (arrow), (C) patent left internal mammary bypass graft to left anterior descending and diagonal arteries, (D) occluded saphenous vein graft to the obtuse marginal
After a multidisciplinary team evaluation, he was deemed not to be a suitable candidate for concomitant aortic replacement and redo bypass grafting (Society of Thoracic Surgeons score 8.6%). It was therefore decided to proceed with PCI in anticipation of a subsequent TAVR. LV support with TandemHeart (Cardiac Assist, Pittsburgh, PA) was planned. A 6-French sheath was inserted in the left-CFA, two Proglide suture-mediated closure devices (Abbott Vascular, Redwood City, CA) were placed, and the sheath was upsized to a 12-French Cook sheath (Cook, Bloomington, IN). Then, a 7-French 45-cm brite-tip sheath (Cordis, Hialeah, FL) was inserted in the right-CFA, to provide access for the PCI. The right femoral vein was accessed and two Proglide devices were deployed. Transseptal puncture was performed with a Mullins sheath and a Brockenbrough needle (Medtronic, Minneapolis, MN) under biplane-fluoroscopy guidance. Over an Inoue wire (Toray, Tokyo, Japan), the septum was dilated with sequential TandemHeart dilators, and the 21-French LA cannula was placed in the mid LA. The left CFA sheath was exchanged with the 17-French arterial cannula over an Amplatz 0.035” super-stiff wire (Boston Scientific, Marlborough, MA). The system was connected to the TandemHeart centrifugal pump, and the pump was turned on, providing cardiac output support of 4–4.5 L/min.
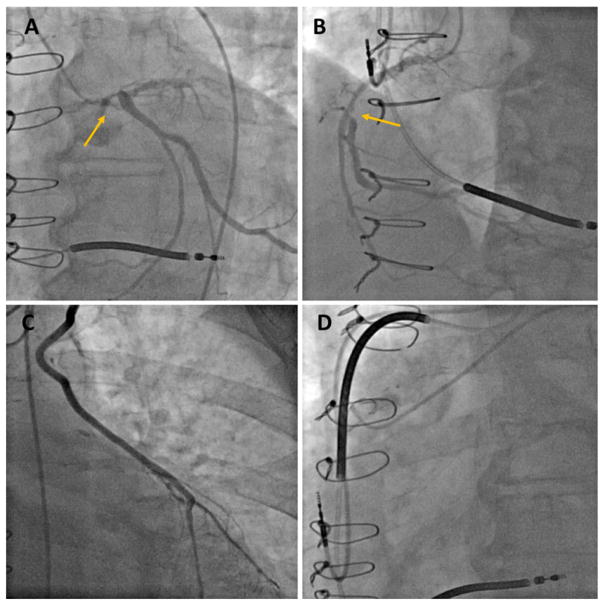
Immediately after engaging the LM with a 7-French JL-4 guiding catheter (Boston Scientific), the LM became completely occluded (Figure 2A, Video S2). The patient remained stable but had very-low pulse pressures suggesting complete dependence on Tandem-Heart support (Figure 3). The guide was exchanged with a 7-French XB3.5 guide (Boston Scientific) and LM was crossed with a 0.014” Whisper wire (Abbott Vascular). Balloon dilatation of the ostial LM was performed with a 2.0 × 12-mm Emerge non-compliant balloon (Boston Scientific) restoring Thrombolysis in Myocardial Infarction-III flow. A Pilot-50 wire (Abbott Vascular) was then used to cross the LM into the Circumflex. The LM-Circumflex lesion was then aggressively dilated with 3.5 × 15-mm and 4 × 20-mm Emerge non-compliant balloons (Figure 2B and C). A 4 × 20-mm Promus drug-eluting stent (Boston Scientific) was deployed and post-dilated with a 4.5 × 15-mm Emerge noncompliant balloon (Figure 2D, Video S3). Hemostasis was achieved with the previously placed per close suture-mediated closure devices. The patient was discharged home the following day on dual antiplatelet therapy (Aspirin 81 mg and Plavix 75 mg daily). The plan was to perform TAVR in 4–6 weeks. However, the patient had a dramatic improvement in his symptoms and opted to continue medical management only. A transthoracic echocardiogram done 3 months later showed a slightly improved LVEF 35%, and a small residual atrial septal defect. The defect was not closed because it was tolerated well and because many of these defects close spontaneously within a few months.10
FIGURE 2.
Left coronary angiography before and after percutaneous coronary intervention. (A) Left main occlusion after guide engagement (arrow), (B) balloon dilatation of the left main/circumflex, (C) post-balloon dilatation angiogram, (D) post-stenting angiogram
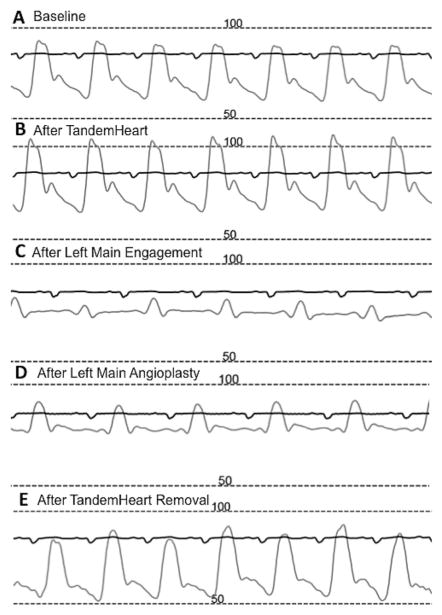
FIGURE 3.
Peri-procedural arterial pressure monitoring. (A) Low blood pressure at the beginning of the procedure, (B) improvement in blood pressure after TandemHeart insertion, (C) near complete obliteration of the pulse pressure after left main occlusion, (D) partial recovery of the blood and pulse pressures after balloon angioplasty, (E) blood pressure returns to baseline post-percutaneous coronary intervention and after TandemHeart removal
3. DISCUSSION
This case illustrates the challenges of managing CAD in patients with severe AS and severe LV dysfunction. Several important considerations should be integrated in the multidisciplinary evaluations of these patients including: the need for MCS at the time of the PCI, the types of MCS devices to be used, the timing of the PCI in relation to TAVR, and the potential role for concomitant balloon valvuloplasty.
Large registry data have confirmed the safety of PCI (including LM disease) in the setting of severe AS, but does not address patients with severe LV dysfunction.3–5,11,12 In a study by Goel et al, patients with severe symptomatic AS who had LVEF <30% had a much higher post-PCI mortality than patients with preserved LVEF (hazard ratio 2.83, confidence interval 1.79–4.49, p < 0.001).3 As illustrated in this case, our patient would probably not have survived without mechanical circulatory support (Figure 3).
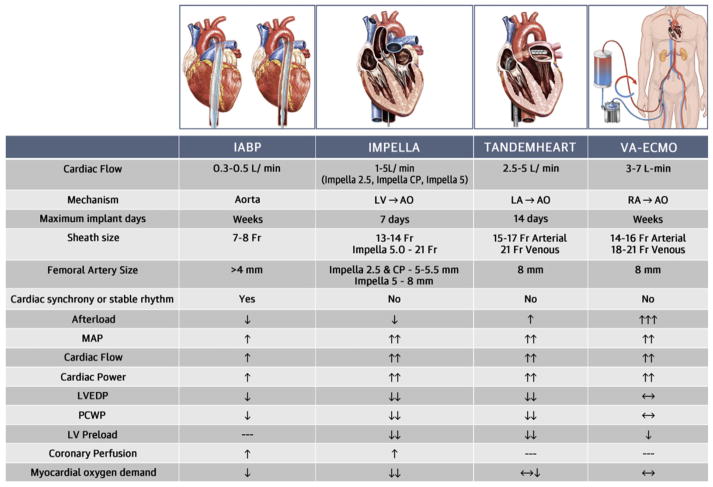
An algorithmic approach to CSD in high-risk patients undergoing complex PCI has been outlined in an expert opinion document from the interventional Scientific Council of the American College of Cardiology.13 In this algorithm, patients who have severe AS or significantly reduced LVED (<30%) should be considered for possible MCS prior to their PCI. There are generally four MCS systems commonly used in clinical practice: Intra-aortic balloon pump (IABP), the Impella device (Abiomed, Danvers, Massachusetts), TandemHeart, and extracorporeal membrane oxygenation (ECMO).13 IABPs are widely available, low profile, and easy to insert, and are therefore considered the first-line MCS for many PCIs. Although IABPs decrease after load and increase coronary flow, they provide only minimal cardiac output support (0.3–0.5 L/min) and they require a stable or synchronous cardiac rhythm. Continuous-flow devices such as the Impella and TandemHeart offer a greater level of LV support for high-risk cases. The Impella device is also relatively easy to insert, does not increase after load, and improves coronary flow. The TandemHeart provides higher cardiac output support compared with the IABP and Impella, but it is not widely available and its implantation is more intricate due to the need for transseptal puncture, and a larger bore access. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides complete heart and lung support, but can lead to significant increase in ventricular after load and often requires additional measures for LV unloading.14 Potential complications of all MCS devices are access site bleeding, embolization, limb ischemia, infection, stroke, and hemolysis.15 IABPs carry a very small risk of aortic dissection and device entrapment.15 Device entanglement and/or fracture, and interactions with the mitral valve are uncommon but well described complications of the Impella device.16–20 TandemHeart insertion carries the risk of adverse events related to transseptal catheterization (tamponade, air embolism, iatrogenic atrial septal defect, etc.).21 Lung over-ventilation, relative lung ischemia, brain hypoperfusion, and intraventricular thrombus formation are rare complications of MCS specific to VA-ECMO.15
The utility of various MCS in patients with severe AS undergoing planned or rescue PCI, balloon aortic valvuloplasty (BAV), or TAVR has been demonstrated.9,22–27 However, no comparative effectiveness, safety, or cost data between these MCS is available in the literature. IABPs have been used on a limited basis in patients with severe AS due to the limited cardiac output support they provide.22 The Impella device has been successfully used to support high-risk PCI and/or BAV in patients with severe AS (Video S4). Impella insertion across very stenotic aortic valves is feasible, but occasionally requires predilatation with a 7–10-mm balloon.9,24 While VA-ECMO has been mostly used to salvage AS patients due to clinical decompensation or following TAVR, the use of the TandemHeart has been mostly limited to elective high risk PCI ± BAV due to the complexity and the required expertise for this procedure. A comparison between these MCS systems is illustrated in (Figure 4). In this case, the IABP was felt to provide insufficient support for the PCI. ECMO was deemed to be less preferable due to the increase in afterload. We elected to use the TandemHeart over Impella because it provides higher cardiac output.
FIGURE 4.
Comparison of mechanical circulatory support systems. AO, aorta; IABP, intra-aortic balloon pump; LA, left atrium; LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; MAP, mean arterial pressure; PCWP, pulmonary capillary wedge pressure; RA, right atrium; VA-ECMO, venoarterial extracorporeal membrane oxygenation. Reprinted with Permission, Atkinson et al, JACC Cardiovasc Interv. 2016 9;9(9):871–83
BAV has been suggested in patients with severe AS at the time of high-risk PCI.11 However, BAV carries substantial risk due to the use of large-bore femoral access, the risk of developing significant aortic regurgitation, the risk of embolization and the potential compromise of the ostial LM stent during the BAV28,29 (Video S5). The risk of rapid ventricular pacing in these high-risk patients can be mitigated by the use of newer valvuloplasty balloons such as the Intervalve V8 hourglass shaped balloon (Maquet Medical, Wayne, NJ) and the True balloon (Bard, Covington, GA).30 We elected to perform the PCI without a concomitant BAV in this case because of the associated risks.
Footnotes
CONFLICT OF INTEREST
The authors acknowledge no conflict of interest in the submission.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Holmes DR, Jr, Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 2.Reinohl J, Kaier K, Reinecke H, et al. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med. 2015;373:2438–2447. doi: 10.1056/NEJMoa1500893. [DOI] [PubMed] [Google Scholar]
- 3.Goel SS, Agarwal S, Tuzcu EM, et al. Percutaneous coronary intervention in patients with severe aortic stenosis: implications for transcatheter aortic valve replacement. Circulation. 2012;125:1005–1013. doi: 10.1161/CIRCULATIONAHA.111.039180. [DOI] [PubMed] [Google Scholar]
- 4.Penkalla A, Pasic M, Drews T, et al. Transcatheter aortic valve implantation combined with elective coronary artery stenting: a simultaneous approach dagger. Eur J Cardiothorac Surg. 2015;47:1083–1089. doi: 10.1093/ejcts/ezu339. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarty T, Sharma R, Abramowitz Y, et al. Outcomes in patients with transcatheter aortic valve replacement and left main stenting: the TAVR-LM registry. J Am Coll Cardiol. 2016;67:951–960. doi: 10.1016/j.jacc.2015.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron SJ, Arnold SV, Herrmann HC, et al. Impact of ejection fraction and aortic valve gradient on outcomes of transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67:2349–2358. doi: 10.1016/j.jacc.2016.03.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh V, Patel SV, Savani C, et al. Mechanical circulatory support devices and transcatheter aortic valve implantation (from the National Inpatient Sample) Am J Cardiol. 2015;116:1574–1580. doi: 10.1016/j.amjcard.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Singh V, Yarkoni A, O’Neill WW. Emergent use of Impella CP during transcatheter aortic valve replacement: transaortic access. Catheter Cardiovasc Interv. 2015;86:160–163. doi: 10.1002/ccd.25784. [DOI] [PubMed] [Google Scholar]
- 9.Martinez CA, Singh V, Londono JC, et al. Percutaneous retrograde left ventricular assist support for interventions in patients with aortic stenosis and left ventricular dysfunction. Catheter Cardiovasc Interv. 2012;80:1201–1209. doi: 10.1002/ccd.24303. [DOI] [PubMed] [Google Scholar]
- 10.Alkhouli M, Sarraf M, Zack CJ, et al. Iatrogenic atrial septal defect following transseptal cardiac interventions. Int J Cardiol. 2016;209:142–148. doi: 10.1016/j.ijcard.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Paradis JM, Fried J, Nazif T, et al. Aortic stenosis and coronary artery disease: what do we know? What don’t we know? A comprehensive review of the literature with proposed treatment algorithms. Eur Heart J. 2014;35:2069–2082. doi: 10.1093/eurheartj/ehu247. [DOI] [PubMed] [Google Scholar]
- 12.Virk SA, Tian DH, Liou K, et al. Systematic review of percutaneous coronary intervention and transcatheter aortic valve implantation for concomitant aortic stenosis and coronary artery disease. Int J Cardiol. 2015;187:453–455. doi: 10.1016/j.ijcard.2015.03.391. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson TM, Ohman EM, O’Neill WW, et al. Interventional Scientific Council of the American College of C. A practical approach to mechanical circulatory support in patients undergoing percutaneous coronary intervention: an interventional perspective. JACC Cardiovasc Interv. 2016;9:871–883. doi: 10.1016/j.jcin.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Alkhouli M, Narins CR, Lehoux J, et al. Percutaneous decompression of the left ventricle in cardiogenic shock patients on venoarterial extracorporeal membrane oxygenation. J Card Surg. 2016;31:177–182. doi: 10.1111/jocs.12696. [DOI] [PubMed] [Google Scholar]
- 15.Myat A, Patel N, Tehrani S, et al. Percutaneous circulatory assist devices for high-risk coronary intervention. JACC Cardiovasc Interv. 2015;8:229–244. doi: 10.1016/j.jcin.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Saleh QA, Foster M, Abi Rafeh N. First experience with successful percutaneous retrieval of retained-fractured impella device. JACC Cardiovasc Interv. 2017;10:e15–e16. doi: 10.1016/j.jcin.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Bhamidipati CM, Mathur M, Hira RS, et al. Impella 5.0 fracture and transcatheter retrieval. JACC Cardiovasc Interv. 2016;9:2568–2570. doi: 10.1016/j.jcin.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Eftekhari A, Eiskjaer H, Terkelsen CJ, et al. Perforation of the anterior mitral leaflet after impella LP 5.0 therapy in cardiogenic shock. Am J Cardiol. 2016;117:1539–1541. doi: 10.1016/j.amjcard.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Toggweiler S, Jamshidi P, Erne P. Functional mitral stenosis: a rare complication of the Impella assist device. Eur J Echocardiogr. 2008;9:412–413. doi: 10.1093/ejechocard/jen029. [DOI] [PubMed] [Google Scholar]
- 20.Elhussein TA, Hutchison SJ. Acute mitral regurgitation: unforeseen new complication of the Impella LP 5.0 ventricular assist device and review of literature. Heart Lung Circ. 2014;23:e100–e104. doi: 10.1016/j.hlc.2013.10.098. [DOI] [PubMed] [Google Scholar]
- 21.Alkhouli M, Rihal CS, Holmes DR., Jr Transseptal techniques for emerging structural heart interventions. JACC Cardiovasc Interv. 2016;9:2465–2480. doi: 10.1016/j.jcin.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Sakata Y. Transvenous Inoue balloon aortic valvuloplasty with intra-aortic balloon pump in treating cardiogenic shock due to critical calcific aortic stenosis. Cardiovasc Interv Ther. 2017 doi: 10.1007/s12928-016-0453-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Tomasello SD, Boukhris M, Ganyukov V, et al. Outcome of extracorporeal membrane oxygenation support for complex high-risk elective percutaneous coronary interventions: a single-center experience. Heart Lung. 2015;44:309–313. doi: 10.1016/j.hrtlng.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Spiro J, Venugopal V, Raja Y, et al. Feasibility and efficacy of the 2.5 L and 3.8 L impella percutaneous left ventricular support device during high-risk, percutaneous coronary intervention in patients with severe aortic stenosis. Catheter Cardiovasc Interv. 2015;85:981–989. doi: 10.1002/ccd.25355. [DOI] [PubMed] [Google Scholar]
- 25.Makdisi G, Makdisi PB, Wang IW. Use of extracorporeal membranous oxygenator in transcatheter aortic valve replacement. Ann Transl Med. 2016;4:306. doi: 10.21037/atm.2016.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu P, Fearon WF, Raleigh LA, et al. Salvage extracorporeal membrane oxygenation prior to “Bridge” transcatheter aortic valve replacement. J Card Surg. 2016;31:403–405. doi: 10.1111/jocs.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav P, Singh V, Eng M, et al. TCT-136 outcomes of hemodynamic support with impella in very high-risk patients undergoing balloon aortic aortic valvuloplasty: results from the global cVAD registry. J Am Coll Cardiol. 2016;68:B55. doi: 10.1016/j.ijcard.2017.03.071. [DOI] [PubMed] [Google Scholar]
- 28.Witzke C, Don CW, Cubeddu RJ, et al. Impact of rapid ventricular pacing during percutaneous balloon aortic valvuloplasty in patients with critical aortic stenosis: should we be using it? Catheter Cardiovasc Interv. 2010;75:444–452. doi: 10.1002/ccd.22289. [DOI] [PubMed] [Google Scholar]
- 29.Saia F, Marrozzini C, Ciuca C, et al. Emerging indications, in-hospital and long-term outcome of balloon aortic valvuloplasty in the transcatheter aortic valve implantation era. EuroIntervention. 2013;8:1388–1397. doi: 10.4244/EIJV8I12A212. [DOI] [PubMed] [Google Scholar]
- 30.Latib A, Pedersen W, Maisano F, et al. Initial findings using the V8 hourglass-shaped valvuloplasty balloon for postdilatation in treating paravalvular leaks associated with transcatheter self-expanding aortic valve prosthesis. Catheter Cardiovasc Interv. 2016;87:1306–1313. doi: 10.1002/ccd.26462. [DOI] [PubMed] [Google Scholar]