Abstract
Stress is commonly regarded as an important trigger for relapse and a significant factor that promotes increased motivation to drink in some individuals. However, the relationship between stress and alcohol is complex, likely changing in form during the transition from early moderated alcohol use to more heavy uncontrolled alcohol intake. A growing body of evidence indicates that prolonged excessive alcohol consumption serves as a potent stressor, producing persistent dysregulation of brain reward and stress systems beyond normal homeostatic limits. This progressive dysfunctional (allostatic) state is characterized by changes in neuroendocrine and brain stress pathways that underlie expression of withdrawal symptoms that reflect a negative affective state (dysphoria, anxiety), as well as increased motivation to self-administer alcohol. This review highlights literature supportive of this theoretical framework for alcohol addiction. In particular, evidence for stress-related neural, physiological, and behavioral changes associated with chronic alcohol exposure and withdrawal experience is presented. Additionally, this review focuses on the effects of chronic alcohol-induced changes in several pro-stress neuropeptides (corticotropin-releasing factor, dynorphin) and anti-stress neuropeptide systems (nocicepton, neuropeptide Y, oxytocin) in contributing to the stress, negative emotional, and motivational consequences of chronic alcohol exposure. Studies involving use of animal models have significantly increased our understanding of the dynamic stress-related physiological mechanisms and psychological underpinnings of alcohol addiction. This, in turn, is crucial for developing new and more effective therapeutics for treating excessive, harmful drinking, particularly stress-enhanced alcohol consumption.
Keywords: Chronic alcohol exposure, alcohol withdrawal, stress, alcohol drinking, neuropeptides
1. Introduction
It is generally acknowledged that stress is an important factor in alcohol abuse and alcohol use disorders. However, the influence of stress on alcohol drinking is complex and not fully understood. On the one hand, alcohol has anti-anxiety properties, serving as an effective anxiety-reducing (anxiolytic) agent. Hence, motivation for drinking may be driven, at least in part, by its ability to alleviate stress, including stress associated with periods of abstinence following bouts of heavy drinking (Cappell and Greeley, 1987; Sayette, 1999). This has been the cornerstone of the tension-reduction hypothesis of alcoholism (Conger, 1956). From a behavioral perspective, this defines how alcohol can serve as a negative reinforcer (i.e., alcohol consumption results in the removal (alleviation) of an aversive or unpleasant (anxiety) state).
At the same time, it is firmly established that alcohol, itself, is a stressor. Acute alcohol exposure activates the hypothalamic-pituitary-adrenocortical (HPA) axis, a major component of the neuroendocrine stress response (Smith and Vale, 2006). This effect has been shown to be mediated through direct stimulation of neurons in the paraventricular nucleus (PVN) of the hypothalamus, leading to the release of corticotropin-releasing factor (CRF) (and vasopressin) that induces secretion of adrenocorticotrophic hormone (ACTH) from the pituitary, which subsequently acts at the adrenal gland to release glucocorticoids into circulation (Lee et al., 2004; Rivier, 2014). It has been suggested that stress may increase motivation to imbibe through synergistic effects on reward circuitry in brain (e.g., mesolimbic dopamine transmission). That is, the activating effects of stress and alcohol on dopamine neurotransmission and on the HPA axis (elevated glucocorticoids) may combine to enhance the rewarding effects of alcohol, thereby facilitating greater propensity to drink (Stephens and Wand, 2012; Uhart and Wand, 2009).
Thus, the interaction between stress and alcohol is very complex. Alcohol can alleviate stress while at the same time provoke a stress response. The dynamic interplay between numerous biological and environmental variables along with experiential factors plays a critical role in defining subjective aspects of stress (i.e., perception and appraisal of a stressful event) as well as how response to stress impacts decisions about alcohol drinking and the manner in which alcohol consumption alters stress responsiveness. Recently, greater attention has focused on examining how a history of chronic alcohol exposure and withdrawal influences the capacity of stress to modulate alcohol consumption. Indeed, stress contributes to dynamic changes underlying transition to alcohol addiction and influences drinking at all stages of the addiction process.
Prolonged excessive alcohol consumption constitutes a potent stressor to the organism, setting in motion a host of neuroadaptive changes within brain reward and stress systems (Becker, 2012; Hansson et al., 2008; Koob, 2013; Koob and Le Moal, 2008; Vengeliene et al., 2008). Stress associated with chronic alcohol exposure and withdrawal experience continually challenges the organism through progressive dysregulation of brain reward and stress systems beyond normal homeostatic limits (Koob, 2003). These neuroadaptive changes are postulated to impact neural and physiological systems integral to the motivational effects of alcohol and, consequently, contribute to escalation of drinking and maintenance of sustained excessive alcohol consumption associated with dependence (Becker, 2012, 2013; Heilig et al., 2010; Koob, 2013). In this vein, alcohol dependence may be viewed as a persistent dysfunctional (allostatic) state, with the organism rendered ill-equipped to exert appropriate behavioral control over alcohol consumption, as well as appropriately respond to other (additional) stressful events that may provoke return to excessive drinking.
This article reviews literature indicating the complex reciprocal relationship between stress and alcohol, with particular emphasis on animal models demonstrating how stress associated with chronic alcohol exposure and withdrawal experience serves as a continual physiological, psychological, and behavioral challenge to the organism. Neuroadaptive (and maladaptive) mechanisms underlying a negative emotional state, altered stress responsiveness, and increased motivation to seek and consume alcohol are key components of the addiction process. The article highlights studies showing that prolonged exposure to alcohol produces perturbations in neuroendocrine and brain stress systems that interface with and influence motivational and reward circuitry in the brain, ultimately rendering subjects more vulnerable to relapse and driving excessive levels of alcohol consumption.
2. Stress Associated with Chronic Alcohol Exposure and Withdrawal
As previously noted, alcohol activates the HPA axis, with the magnitude and response profile influenced by a host of variables (Lu and Richardson, 2014; Rivier, 2000; Wand, 2000). These include a number of alcohol-related factors (e.g., history of use, level and pattern of drinking, timing of accessibility of alcohol in relation to stress experience) as well as stress-related factors (e.g., type, chronicity, intermittency, predictability, controllability) that intersect with a number of biological variables (e.g., genetics, age, sex). As reported in clinical studies, experimental studies have documented profound disturbances in HPA axis function following chronic alcohol exposure and withdrawal. For example, studies in rodents have shown that chronic alcohol consumption results in a general elevation in blood corticosteroid levels, with a typical flattening of normal circadian fluctuations (Kakihana and Moore, 1976; Keith and Crabbe, 1992; Rasmussen et al., 2000; Tabakoff et al., 1978). At the same time, there is a dampened HPA response to subsequent CRF or stress challenge (Lee et al., 2000; Rivier et al., 1990). Additionally, the ability of alcohol to activate the HPA axis is blunted following chronic exposure to the drug (Richardson et al., 2008a), an effect thought to contribute to perpetuation of heavy drinking (Lu and Richardson, 2014).
Periods of abstinence (i.e., withdrawal) are characterized by elevated glucocorticoid levels that reflect increased HPA axis activity. This, along with increased activity of the sympathetic division of the autonomic nervous system, mediate an array of physiological symptoms of acute alcohol withdrawal (e.g., tachycardia, elevated blood pressure, diaphoresis, body temperature dysregulation) (Becker, 2000; Heilig et al., 2010). While heightened HPA axis activation associated with withdrawal usually resolves within a few days (Kakihana, 1979; Tabakoff et al., 1978), blunted HPA axis responsiveness appears to persist for a protracted period of time (Rasmussen et al., 2000; Zorrilla et al., 2001). In some cases, this may be accompanied by reduced basal levels of circulating corticosteroids (Rasmussen et al., 2000; Richardson et al., 2008a; Zorrilla et al., 2001). These perturbations in HPA axis function align with findings reported in abstinent human alcoholics (Adinoff et al., 1990; Adinoff et al., 1991; Costa et al., 1996; Lovallo et al., 2000; Willenbring et al., 1984). Dysregulation of HPA axis function that extends into protracted phases of withdrawal is thought to contribute to dysphoria and negative affect associated with alcohol dependence (Heilig et al., 2010; Koob and Kreek, 2007; Koob, 2013). Further, persistent changes in HPA neuroendocrine function resulting from chronic alcohol exposure and withdrawal may activate brain stress systems outside the HPA axis (Koob, 2013; Vendruscolo et al., 2012).
Indeed, it is well established that chronic alcohol alters CRF activity independent from the HPA axis. CRF is a 41 amino acid neuropeptide that is widely distributed throughout mammalian brain. As noted above, CRF-containing neurons are found in high concentrations in the PVN of the hypothalamus where they play a primary role in regulating HPA axis activity, which is critical for orchestrating behavioral and physiological responses to stress. CRF-containing neurons are also found outside the neuroendocrine (HPA) axis. The extra-hypothalamic distribution of CRF includes an extensive network of interconnected neural structures (e.g., central amygdala (CeA), bed nucleus of the stria terminalis (BNST), prefrontal cortex) that are intimately associated with brain reward and stress pathways. The actions of CRF (and related peptides urocortin I, II, and III) are modulated by CRF-binding protein and mediated through interaction with two excitatory G-protein-coupled receptor subtypes (CRF1 and CRF2) (Bale and Vale, 2004). CRF1 and CRF2 receptors are distributed in overlapping yet distinct patterns within these brain reward and stress circuits. This anatomical distribution of CRF and its associated binding sites is congruent with the important role of both hypothalamic and extra-hypothalamic CRF in processing and regulating central, autonomic, and emotional/behavioral responses to stress as well as rewarding stimuli/events including alcohol and other drugs of abuse (Bruijnzeel and Gold, 2005; Ryabinin et al., 2002).
A large body of evidence has emerged indicating that CRF plays a critical role in alcohol (and other drug) addiction (Heilig and Koob, 2007; Koob and Zorrilla, 2010; Lowery and Thiele, 2010; Zorrilla et al., 2014). Aside from producing long-lasting dysregulation of HPA function, chronic alcohol exposure produces time-dependent changes in extracellular levels of extra-hypothalamic CRF during withdrawal (Merlo Pich et al., 1995; Olive et al., 2002; Zorrilla et al., 2001). Changes in CRF activity resulting from chronic alcohol exposure (increased CRF release along with an up-regulation in CRF1 receptors) within the extended amygdala network is thought be key to the emergence of withdrawal symptoms reflective of a negative emotional state associated with alcohol dependence. For example, increased behavioral measures of anxiety associated with alcohol withdrawal is reduced by systemic (Breese et al., 2005; Sommer et al., 2008) and central (Baldwin et al., 1991; Rassnick et al., 1993; Valdez et al., 2003) administration of CRF receptor antagonists. This effect appears to be mediated by CRF1 receptors (Overstreet et al., 2004), although a role for CRF2 receptors cannot be ruled out (Valdez et al., 2004). Together, these findings indicate that chronic alcohol exposure and withdrawal experience can be viewed as a potent stressor that disrupts the functional integrity of the HPA axis while at the same time recruiting and sensitizing extra-hypothalamic CRF systems. The resultant allostatic state is characterized by progressive dysregulation of neuroendocrine and brain stress systems along with perturbations in brain reward pathways that contribute to dysphoric and negative affect associated with alcohol dependence. Implications of these changes regarding motivation for alcohol self-administration as well as relapse vulnerability are discussed below.
3. Link Between Chronic Alcohol, Stress Response, and Motivation to Drink
The circumstances and manner in which stress influences alcohol drinking behavior has been extensively studied in humans and animal models (Brady and Sonne, 1999; Pohorecky, 1990, 1991; Sillaber and Henniger, 2004; Sinha, 2001, 2008). Over several decades, a wide array of animal models and experimental procedures have been employed in addressing this important issue. Unfortunately, this large body of literature has yielded equivocal results, most likely due to differences in a number of variables including genetic and other biological factors, environmental conditions, and a host of experimental procedural differences (Becker et al., 2011; Noori et al., 2014; Spanagel et al., 2014).
Recently, greater attention has focused on how stress associated with chronic alcohol exposure and withdrawal experience influences motivation to self-administer alcohol. Studies in mice and rats have demonstrated dependence-related excessive levels of alcohol consumption under a number of conditions (Becker, 2013; Becker and Ron, 2014; Vendruscolo and Roberts, 2014). These models not only demonstrate escalation of drinking, but perturbations in neuroendocrine and brain stress systems induced by chronic alcohol exposure and withdrawal have been linked to enhanced behavioral responsiveness to stress. For example, rats exhibit increased stress responsiveness following withdrawal from chronic alcohol exposure, as measured by several experimental procedures that provoke behavioral measures of stress/anxiety, such as reduced social interaction in a novel environment, reduced exploration in threatening circumstances (e.g., open, brightly illuminated spaces), and greater electroshock-induced suppression of ongoing behavior (Breese et al., 2005; Gehlert et al., 2007; Sommer et al., 2008). In a series of studies involving a mouse model of alcohol dependence and relapse drinking, repeated brief exposure to forced swim stress prior to alcohol drinking sessions significantly increased drinking in alcohol dependent mice, but did not alter intake in nondependent mice (Lopez et al., 2016). Interesting, this stress procedure did not further increase drinking in two other drinking models that typically engender high levels of alcohol intake – the drinking-in-the-dark (DID) model and the intermittent access (‘every-other-day’) model (Anderson et al., 2016a). These results suggest that stress may interact with chronic alcohol exposure and withdrawal in a unique manner to facilitate and further augment escalated drinking in dependent subjects (Anderson et al., 2016b). Further, behavioral sensitization to stress may be critical in rendering subjects more vulnerable to relapse. Indeed, experimental evidence suggests that stress can provoke relapse-like behavior more easily in subjects with a history of dependence (Liu and Weiss, 2002; Sommer et al., 2008).
4. Mechanisms Underlying Chronic Alcohol, Stress, and Drinking Relationship
4.1 Corticotropin-Releasing Factor (CRF)
Numerous studies involving rodent models have shown that such changes in brain CRF activity have important ramifications regarding alcohol self-administration behavior. For example, CRF infusion into the ventricles was shown to reduce voluntary alcohol intake in rats (Bell et al., 1998; Thorsell et al., 2005b). Likewise, transgenic CRF over-expressing mice exhibited reduced voluntary alcohol intake compared to non-transgenic controls (Palmer et al., 2004), while CRF deficient mice showed the opposite effect – increased alcohol drinking (Olive et al., 2003). Differences in brain CRF content have been observed in alcohol-naïve rats and mice known to differ in voluntary alcohol intake (Ehlers et al., 1992; George et al., 1990), although a recent report indicated no difference in brain regional expression of CRF between C57BL/6J (high alcohol drinking) and DBA/2J (low alcohol drinking) mice (Hayes et al., 2005). Additional studies in humans, monkeys, and rats suggest an association between genetic variants (single nucleotide polymorphisms) of the CRF and CRF1 receptor genes and alcohol drinking (Barr et al., 2009; Barr et al., 2008; Blomeyer et al., 2008; Hansson et al., 2006; Schmid et al., 2010). Collectively, these findings indicate that genetic variations in the Crf and the CrfR1 genes interact with stressful life events to influence age of drinking onset, progression of heavy drinking in adulthood, and general vulnerbaility to alcohol dependence.
Given its pivotal role in mediating neuroendocrine and brain (outside the HPA axis) responses to stress, it is not surprising that a large number of studies have examined the effect of chronic alcohol on CRF activity in relation to alcohol drinking. The non-selective peptide CRF antagonist (D-Phe-CRF12-41) reduced excessive drinking in dependent animals when administered into the brain ventricles (Finn et al., 2007; Valdez et al., 2002) or into the CeA (Funk et al., 2006). Systemic administration of CRF1 receptor-selective antagonists reduced up-regulated drinking in dependent mice (Chu et al., 2007) and rats (Funk et al., 2007; Gehlert et al., 2007; Gilpin et al., 2008b; Richardson et al., 2008b; Roberto et al., 2010; Sabino et al., 2006; Sommer et al., 2008).
Studies using operant conditioning procedures also have demonstrated an important role for CRF in mediating the ability of stress to trigger relapse-like behavior. For example, CRF antagonists have been shown to block stress-induced reinstatement of alcohol seeking behavior (Gehlert et al., 2007; Le et al., 2000; Liu and Weiss, 2002; Marinelli et al., 2007). This effect appears to be mediated by extra-hypothalamic CRF activity, since adrenalectomy (with or without corticosterone supplementation) did not affect reinstatement of alcohol responding induced by foot-shock stress (Le et al., 2000). In fact, direct infusion of a CRF antagonist into the median raphe nucleus blocked stress-induced alcohol seeking behavior (Funk et al., 2003; Le et al., 2002; Le et al., 2013). CRF1 receptor antagonists injected into the ventral tegmental area reduced alcohol intake in high-drinking models, including stress-enhanced drinking (Hwa et al., 2016; Rinker et al., 2016). Overall, while a role for CRF2 receptors cannot be ruled out (Funk and Koob, 2007; Valdez et al., 2004), the large preponderance of evidence suggests that CRF1 receptors play an important role in regulating alcohol consumption, especially excessive levels of drinking associated with dependence. Taken together, there is a large body of evidence indicating that chronic alcohol-induced changes in CRF function within brain/neuroendocrine systems may directly, and/or through mediating withdrawal-related anxiety and stress/dysphoria, promote excessive levels of drinking as well as influence relapse vulnerability (Heilig and Koob, 2007; Koob and Zorrilla, 2010; Lowery and Thiele, 2010; Zorrilla et al., 2014).
4.2 Dynorphin
Because both stress and chronic alcohol engage the DYN/KOR system, the role of this neuropeptide system in alcohol dependence-related stress/dysphoria and elevated drinking has gained increasing attention (Kissler et al., 2014; Wee and Koob, 2010). Dynorphins (DYN) are peptides derived from the precursor prodynorphin (Pdyn) that preferentially bind to kappa opioid receptors (KOR), producing physiological and behavioral effects via inhibitory G-protein (Gi) coupling and other signaling cascades (Bruchas and Chavkin, 2010; Bruchas et al., 2010; Crowley and Kash, 2015; Wee and Koob, 2010). KOR activation has been shown to produce aversive/dysphoric effects as indicated by measures of conditioned avoidance, anxiety-like, and depression-like behavior (Knoll and Carlezon, 2010; Van't Veer and Carlezon, 2013). Stress exposure activates the DYN/KOR system, eliciting dysphoria-like responses, increasing anxietylike behaviors (Land et al., 2008) and resulting in elevated DYN immunoreactivity in brain regions that are integral to reward and stress circuitries involved in alcohol/drug addiction (Shirayama et al., 2004). Further, pharmacological manipulation of KOR activity alters behavioral responses to stress and motivational effects of alcohol. For example, KOR agonists have been shown to produce a state of stress/dysphoria, mimicking effects of stress on alcohol drinking and conditioned reward (Anderson et al., 2016b; Sperling et al., 2010). Conversely, KOR antagonists attenuate stress-induced potentiation of conditioned alcohol reward (Sperling et al., 2010), yohimbine-induced reinstatement of alcohol seeking behavior (Funk et al., 2014), withdrawal-related negative affect and anxiety (Berger et al., 2013; Gillett et al., 2013; Rose et al., 2015; Schank et al., 2012; Valdez and Harshberger, 2012), and enhanced alcohol self-administration after exposure to a cue associated with a KOR agonist (Berger et al., 2013).
Structures within the extended amygdala circuitry that are intimately involved in mediating negative emotional and motivational states associated with stress and chronic alcohol exposure/withdrawal (e.g., CeA, BNST) are rich in DYN and KORs (Mansour et al., 1994; Marchant et al., 2007; Poulin et al., 2009). There is evidence that stress and chronic alcohol exposure increase DYN/KOR function in the CeA and BNST (Chung et al., 2014; Kissler et al., 2014). A number of studies have shown that this upregulation in DYN/KOR signaling contributes to escalated alcohol intake associated with dependence. For example, systemic administration of the KOR antagonist nor-binaltorphimine reduced escalated drinking in dependent rats while not altering more modest intake in nondependent rats (Kissler et al., 2014; Walker and Koob, 2008; Walker et al., 2011). Similar results were reported in mice (Rose et al., 2015). Pharmacological blockade of KORs by direct injection of a KOR antagonist into the CeA (Kissler et al., 2014) or into the nucleus accumbens (Nealey et al., 2011) also was shown to attenuate elevated alcohol consumption in dependent rats. Additionally, systemic administration of the novel short-acting KOR antagonist, LY2444296, abolished the ability of stress (forced swim) to enhance drinking in dependent mice (Anderson et al., 2016b).
There is also evidence for interaction between the DYN/KOR system and CRF within extended amygdala circuitry that have implications for stress and chronic alcohol consequences. For example, these neuropeptides have been shown to be involved in chronic alcohol-induced alterations in synaptic plasticity in the CeA (Kang-Park et al., 2013, 2015; Roberto et al., 2010). Specifically, KORs were shown to modulate GABAergic transmission in a CRF1 receptor-dependent manner (Kang-Park et al., 2015). Interestingly, CRF infusion into the CeA was reported to increase DYN release in a CRF2 receptor-dependent manner (Lam and Gianoulakis, 2011). The aversive/anxiety-like behavioral effects of central CRF administration are attenuated by pretreatment with a KOR antagonist (Bruchas and Chavkin, 2010; Bruchas et al., 2010; Land et al., 2008). Conversely, a CRF1 receptor antagonist blocked KOR agonist-induced reinstatement of alcohol seeking (Funk et al., 2014). Thus, while the precise nature of DYN/KOR-CRF interactions is not completely understood, behavioral and physiological evidence suggests that an interaction between these two stress-related neuropeptide systems within extended amygdala circuitry plays a significant role in mediating stress and motivational effects of chronic alcohol exposure.
4.3 Other Stress-Related Neuropeptides
Aside from CRF and DYN, several other neuropeptide systems in brain are involved in stress response as well as motivational effects of alcohol (Ciccocioppo et al., 2009; Martin-Fardon et al., 2010; Roberto et al., 2012). In some cases, these neuropeptide systems serve to dampen stress effects associated with chronic alcohol exposure, thereby modulating alcohol consumption in the context of dependence. Here we review three anti-stress neuropeptide systems (nociceptin, neuropeptide Y, and oxytocin) that are influenced by chronic alcohol exposure and contribute to withdrawal-related behavioral measures of dysphoria as well as motivation to self-administer alcohol. Of note, recent studies have pointed to several other neuropeptide systems that contribute to emotional and behavioral sequela that reflect the intersection of stress and chronic alcohol exposure/withdrawal, including orexin/hypocretin, neurokinins, and neuropeptide S (Schank et al., 2012).
4.3.1 Nociceptin
Nociceptin/orphanin FQ is a 17-amino acid peptide classified as being a member of the opioid family, but binds with high affinity to the nociception receptor (NOP; also referred to as the opioid receptor-like 1 - ORL1) rather than mu, delta, or kappa opioid receptors (Meunier et al., 1995; Reinscheid et al., 1995). Dense expression of the peptide and its receptor within cortical and limbic regions suggest its role in emotional and motivational behaviors, particularly those related to stress, chronic alcohol exposure/withdrawal, and drinking. Increased expression of nociception and NOP mRNA in the CeA of rats selectively bred for high alcohol preference is suggested to relate to the high-anxiety and stress responsiveness in these animals (Ciccocioppo et al., 2007; Economidou et al., 2008). Pharmacological studies have demonstrated a role for the nociceptin/NOP system in regulation of alcohol self-administration, as well as withdrawal-related anxiety and drinking. For example, infusion of nociceptin into brain ventricles reduced alcohol conditioned reward as well as relapse-like behavior provoked by either stress or alcohol-related cues (Ciccocioppo et al., 2004; Martin-Fardon et al., 2000). Likewise, systemic administration of brain-penetrant nociception analogues reduced alcohol self-administration (Aziz et al., 2016; Ciccocioppo et al., 2014; Kuzmin et al., 2007). Direct injection of nociceptin into the CeA also was reported to reduce alcohol consumption (Economidou et al., 2008). This effect may be mediated by nociception interacting with alcohol-induced modulation of GABA and glutamate transmission in the CeA (Kallupi et al., 2014a; Kallupi et al., 2014b). Additionally, chronic alcohol exposure appears to enhance sensitivity to NOP activation, as nociceptin (or its analogues) were shown to be more effective as anxiolytics and reducing elevated drinking associated with dependence (Aujla et al., 2013; Aziz et al., 2016; de Guglielmo et al., 2015; Economidou et al., 2011; Martin-Fardon et al., 2010).
Contrary to these findings, recent reports suggest that blocking, rather than activating, NOP receptors may be effective in reducing alcohol self-administration. Genetic deletion of the nociception receptor in rats resulted in lower alcohol consumption compared to wildtype controls, while saccharin intake was unaltered (Kallupi et al., 2017). Further, systemic administration of NOP antagonists reduced alcohol self-administration in wildtype rats but was without effect in NOP-deficient rats (Kallupi et al., 2017). These results align with another report showing that oral administration of a NOP antagonist reduced alcohol consumption, motivation to work for alcohol, and stress (yohimbine)-induced reinstatement of alcohol-seeking behavior in rats (Rorick-Kehn et al., 2016). These effects produced by the nociceptin receptor antagonist were attributed to the drug blocking alcohol-induced dopamine release in the nucleus accumbens. Several explanations have been postulated to address these apparent contradictory results (NOP agonists and antagonists reduce alcohol consumption), including receptor translocation, ligand-biased receptor signaling, and brain regional differences in receptor variants (Rorick-Kehn et al., 2016). Future studies will need to resolve this issue as well as address the therapeutic potential of this target for treating alcohol use disorders and stress-related drinking in particular.
4.3.2 Neuropeptide Y
Neuropeptide Y (NPY) is known to mediate anti-stress effects, typically opposing behavioral actions of CRF (Sajdyk et al., 2006). NPY, a 36-amino acid peptide, produces these effects primarily through actions at Y1 and Y2 receptors in brain. NPY exerts anxiolytic effects in a number of behavioral tasks, an effect thought to be mediated by interaction with Y1 receptors in the amygdala (Heilig et al., 1993; Thorsell, 2008). There is evidence indicating a relationship between NPY activity (primarily in the CeA) and alcohol consumption. For example, rats selectively bred for high alcohol preference exhibit low NPY mRNA and peptide levels in the CeA, an effect that was reversed when the rats were given the opportunity to consume alcohol (Pandey et al., 2005). Viral mediated overexpression of NPY in the amygdala was shown to reduce alcohol drinking in rats identified as being highly anxious (Primeaux et al., 2006) or following periods of forced abstinence (Thorsell et al., 2007). Also, infusion of NPY in brain ventricles (icv.) reduced stress-induced relapse-like behavior in rats (Cippitelli et al., 2010).
Pharmacological studies also support a role for NPY in the regulation of alcohol consumption, although there is some contradictory evidence regarding the role of Y1 and Y2 receptor subtypes. For example, systemic (ip.) and central (icv.) administration of a Y1R antagonist reduced alcohol intake in C57BL/6J mice (Sparta et al., 2004). In contrast, another study showed that central injection (icv.) of a Y1R agonist reduced binge-like alcohol intake in mice in a dose-related manner (Sparrow et al., 2012). Further, a Y1R antagonist (icv.) increased alcohol consumption (Sparrow et al., 2012), supporting an earlier report indicating that Y1R knockout mice exhibited increased alcohol intake (Thiele et al., 2002). A Y2 antagonist given directly into brain (icv.) reduced ethanol intake in rats (Thorsell et al., 2002), and sensitivity to this effect was greater is dependent rats (Rimondini et al., 2005). A similar finding was reported in mice (Sparrow et al., 2012), corroborating results from a study showing reduced ethanol intake in Y2R knockout mice (Thiele et al., 2002). However, in another study systemic (sc.) or central (icv.) administration of a brain-penetrant Y2 antagonist did not alter operant responding for alcohol or stress (foot-shock)-induced reinstatement of alcohol responding in rats, although the drug was effective reducing withdrawal-related anxiety (Cippitelli et al., 2011).
Studies have shown that chronic alcohol exposure alters NPY function and this may contribute to increased stress/anxiety associated with dependence as well as increased propensity to drink. Early studies indicated that NPY administration reduced elevated drinking in dependent animals (Thorsell et al., 2005a, c). More recent work has focused on NPY actions in the CeA in the context of dependence (Gilpin et al., 2015). For example, increased anxiety during withdrawal from chronic alcohol exposure was associated with decreased NPY expression in CeA (Roy and Pandey, 2002). Further, direct injection of NPY into the CeA reduced excessive alcohol consumption in dependent rats (Gilpin et al., 2008a), an effect possibly related to modulation of GABA transmission in the CeA (Gilpin et al., 2011). Recent work also points to interactions between NPY and CRF within the BNST as a site important for mediating stress influences on alcohol drinking (Pleil et al., 2015).
4.3.3 Oxytocin
A growing body of literature suggests that oxytocin plays a significsant role in alcohol (and othe drug) addiction, as well as neuropsychiatric disorders that involve deficits in social behaviors (Baskerville and Douglas, 2010; Lee and Weerts, 2016). Oxytocin, a nonapeptide, is an endogenous neurohormone synthesized in the paraventricular and supraoptic nuclei of the hypothalamus and released by the posterior pituitary into peripheral circulation. In addition, oxytocin is released by neurons in the hypothalamus that project to numerous extra-hypothalamic regions in the brain (e.g., cortical, limbic, basal ganglia structures) where it mediates an array of behavioral effects via interaction with G(q)-coupled oxytocin receptors (Lee et al., 2016). Aside from its known hormonal role in parturition and maternal behaviors, oxytocin also regulates a number of behaviors that involve social interactions (e.g., pair-bonding, social reward processing, aggression) and nonsocial behaviors, including anxiety and stress responses (Baskerville and Douglas, 2010; Bowen et al., 2011; Neumann and Landgraf, 2012).
Preclinical evidence indicates that oxytocin influences a number of behavioral and physiological effects of alcohol, including tolerance, withdrawal, and motivational effects (Lee and Weerts, 2016). For example, systemic administration of oxytocin reduces alcohol preference and intake in a variety of drinking models in rats (Bowen et al., 2011; MacFadyen et al., 2016; McGregor and Bowen, 2012) and mice (King et al., 2017; Peters et al., 2013). Direct injection of oxytocin into brain ventricles reduced alcohol consumption and alcohol-induced dopamine efflux in the nucleus accumbens in rats (Peters et al., 2016). Few studies have examined the role of oxytocin receptors in mediating the neuropeptide's effects on motivational actions of alcohol. Recent studies involving viral-mediated overexpression of oxytocin receptors in the nucleus accumbens core have implicated a role for these receptors in alcohol drinking and conditioned reward (Bahi, 2015; Bahi et al., 2016). However, it is noteworthy that oxytocin was shown to block alcohol-induced ataxic and sedative/hypnotic effects via an apparent direct interaction with delta-subunit containing GABA-A receptors (Bowen et al., 2015). Thus, there remains some abiguity as to the role of oxytocin receptors in mediating the neuropeptide's effects on physiological effects of alcohol and, in particular, alcohol-related reward and self-administration behavior.
Oxytocin is known to exert stress-bufferring effects, and this may be of relevance to its role in influencing stress-alcohol interactions. For example, oxytocin decreases stress-induced HPA axis activation and behavioral (anxiety) responses (Neumann et al., 2000; Windle et al., 1997). Systemic oxytocin treatment was also shown to temper stress-related increases in alcohol consumption (Peters et al., 2013). Finally, in a recent clinical study, Pedersen and colleagues demonstrated that intranasal oxytocin treatment attenuated alcohol withdrawal symptoms in treatment-seeking human subjects compared to placebo (Pedersen et al., 2013). Taken together, there is emerging evidence that oxytocin may hold promise as a therapeutic for treating alcohol use disorders and, in particular for mitigating stress effects on alcohol drinking and relapse.
4.4 Glucocorticoids
Alcohol-induced activation of the HPA axis results in elevated circulating glucocorticoids and it has been suggested that this may contribute to amplified motivation to drink through an interaction with brain reward circuitry (Piazza and Le Moal, 1997; Uhart and Wand, 2009). Central and systemic administration of corticosterone has been shown to increase alcohol consumption, whereas adrenalectomy or administration of a corticosteroid synthesis inhibitor (i.e., metyrapone) decreased alcohol intake in rodents (Fahlke et al., 1995; Fahlke et al., 1996). Likewise, a glucocorticoid receptor antagonist (i.e., mifepristone) reduced alcohol self-administration behavior in rats (Koenig and Olive, 2004). Furthermore, mifepristone administered systemically or into the central nucleus (but not the basolateral nucleus) of the amygdala attenuated stress-induced reinstatement of alcohol seeking behavior (Simms et al., 2012).
Stress associated with chronic alcohol exposure and withdrawal results in a dysregulated HPA axis. Resultant elevated glucocorticoids dampen HPA activity through negative feedback, but there is evidence that glucocorticoids induce CRF activity in extra-hypothalamic sites such as the amygdala (Sawchenko, 1987). Thus, chronic alcohol exposure may ultimately dampen HPA axis while accentuating extra-hypothalamic CRF activity. This, in turn, may contribute to chronic alcohol-induced negative affect and increased motivation to drink (Koob, 2013; Lu and Richardson, 2014).
There is also evidence that chronic alcohol exposure alters corticosteroid levels and glucocorticoid receptors in brain. Recent studies have shown that glucocorticoid receptor expression (mRNA levels) and phosphorylation were elevated in the central (but not basolateral) amygdala in dependent compared to nondependent rats (Vendruscolo et al., 2012; Vendruscolo et al., 2015). Further, systemic and direct (central amygdala) injection of mifepristone was shown to reduce alcohol self-administration in dependent but not nondependent rats (Vendruscolo et al., 2015). Mifepristone treatment was also reported to reduce alcohol craving and consumption in a double-blind clinical laboratory-based study of alcohol dependent human subjects (Vendruscolo et al., 2015). Finally, studies in mice and rats have shown that withdrawal following prolonged alcohol consumption produced elevated corticosterone levels in certain brain regions (i.e., the prefrontal cortex and hippocampus) that persisted long after plasma corticosterone levels returned to baseline levels (Little et al., 2008). Elevations in brain glucocorticoid concentrations following chronic alcohol exposure and withdrawal not only may have significant implications for motivation to drink, but also may contribute to the cognitive deficits and neurotoxic damage that is commonly associated with alcohol dependence (Rose et al., 2010).
4.5 Autonomic Nervous System
An important component of stress associated chronic alcohol exposure and withdrawal is activation of the autonomic nervous system. In particular, increased noradrenergic output from locus coeruleus activation plays an important role in mediating both somatic and affective aspects of alcohol withdrawal (Heilig et al., 2010). Beyond withdrawal symptoms, changes in central noradrenergic activity also has been implicated in stress-related psychiatric illnesses as well as alcohol use disorders. For example, the noradrenergic alpha-1 receptor antagonist prazosin has been shown to be efficacious in treating various PTSD-related symptoms (Germain et al., 2012; Raskind et al., 2007; Raskind et al., 2013). Another noradrenergic alpha-1 receptor antagonist with a more favorable pharmacokinetic profile, doxazosin, was shown to reduce symptoms of PTSD as well (De Jong et al., 2010).
Drugs targeting the noradrenergic system also has been reported to reduce alcohol consumption in a number of preclinical studies. Prazosin reduced alcohol drinking in rats selectively bred for high alcohol preference (Froehlich et al., 2013a; Rasmussen et al., 2009). The drug also was effective in reducing intake in relapse models involving repeated alcohol deprivation periods (Froehlich et al., 2015) and stress-induced reinstatement of alcohol seeking behavior (Le et al., 2011). Prazosin treatment prior to stress (restraint) exposure during repeated deprivation periods prevented increased anxiety-like behaviors during a subsequent deprivation period (Rasmussen et al., 2017). Additionally, several studies found prazosin in combination with naltrexone to be more effective in reducing drinking than either drug given alone (Froehlich et al., 2013b; Rasmussen et al., 2015; Verplaetse and Czachowski, 2015). Studies also have shown prazosin reduces alcohol self-administration in dependent rats (Walker et al., 2008), and a similar effect was obtained with the beta-adrenergic antagonist propranolol (Gilpin and Koob, 2010). Given this preclinical and clinical evidence, there is active interest in the potential for drugs targeting central adrenergic receptors in the treatment of alcohol use disorders and the high prevalence of its comorbidity with stress-related illnesses such as PTSD (Kenna et al., 2016; Simpson et al., 2015).
5. Summary
The relationship between stress and alcohol is complex, with a wide array of factors (genetic, biological, and environmental) playing a role in contributing to the outcome of stress-alcohol interactions. While stress may interact with alcohol during the initial stages of drinking to enhance its rewarding effects, persistent excessive alcohol consumption serves as a potent stressor itself, continually challenging and ultimately compromising the physiological integrity of the subject. Prolonged alcohol exposure leads to fundamental changes in brain reward and neuroendocrine/stress systems beyond normal homeostatic limits (i.e., a state of allostasis), which, in turn, impacts physiological and brain motivational systems that are integral to control and regulation of ethanol consumption. As reviewed in this article, substantial evidence has accrued demonstrating that chronic alcohol exposure produces profound dysregulation in the neuroendocrine (HPA axis) stress system while at the same time recruiting and sensitizing extra-hypothalamic stress circuitry in the brain. Use of animal (primarily rodent) models has been critical to advancing our understanding about how chronic alcohol-induced changes in neuroendocrine and brain stress systems, particularly those intertwined with reward circuitry, underlie expression of withdrawal symptoms reflective of a negative affective state (i.e., dysphoria, anxiety), along with increased motivation to self-administer alcohol (Figure 1). As highlighted in this review, elevated glucocorticoids, along with activation of several pro-stress neuropeptides (CRF, DYN) and anti-stress neuropeptide systems (nociceptin, NPY, oxytocin) have been shown to play a significant role in these dynamic aspects of the addiction process. Heightened autonomic (sympathomimetic) effects also contribute to the stress, negative emotional, and motivational consequences of chronic alcohol exposure. Future studies aimed at elucidating mechanisms of engagement and timing of these (and other) stress-related systems will be critical in providing a more comprehensive understanding of the dynamic physiological and psychological underpinnings of alcohol addiction. Use of animal models also will be key to identifying new targets and evaluating potential therapeutics for treating problem drinking, particularly stress-related excessive alcohol consumption. This research also has important implications for developing more effective treatments for those individuals presenting with comorbidity of alcohol use disorder and a stress-related illness, such as PTSD.
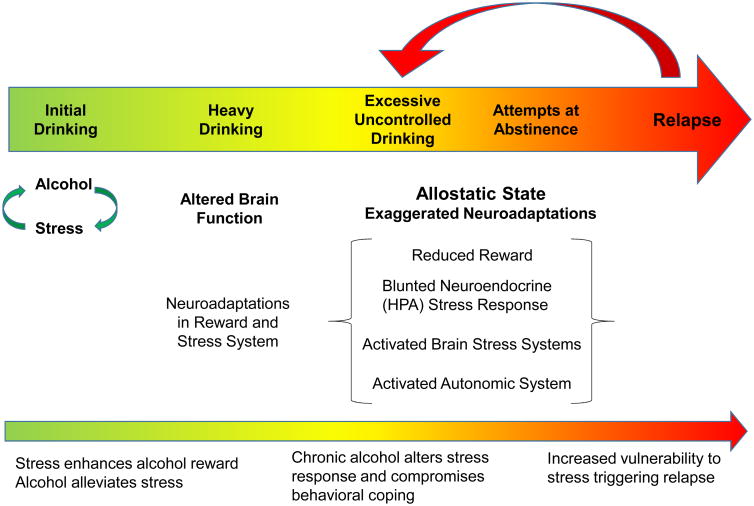
Figure 1.
Stress Influences on the Progression of Alcohol Addiction.
When drinking is first initiated and regulated, alcohol and stress interact in a complex manner. Stress may promote alcohol consumption via glucocorticoid interactions with brain reward systems and, for some individuals, the anxiolytic (stress-alleviating) effect alcohol may influence motivation to drink. Continued heavy drinking results in fundamental changes in brain function. Under assault from continued alcohol exposure, the brain engages a host of adaptations in brain reward and stress systems that contribute to the perpetuation of excessive levels of drinking. Stress associated with heavy bouts of drinking and repeated failed attempts at abstinence fuel progressive dysregulation of these brain systems beyond normal homeostatic limits, setting the stage for a persistent dysfunctional (allostatic) state. This is characterized by exaggerated neuroadaptations that manifest as reduced reward processing, a blunted neuroendocrine stress response, engagement of extra-hypothalamic stress (e.g., CRF, dynorphin) and anti-stress (e.g., nociceptin, NPY, oxytocin) systems, and heightened autonomic nervous system (sympathomimetic) function. Collectively, these changes contribute to a persistent negative emotional (affective) state along with compromised executive function that renders individuals ill-equipped to exert appropriate behavioral control over alcohol consumption, as well as appropriately respond to stressful events that may provoke return to excessive drinking.
Highlights.
Stress alters drinking and progression of addiction in a complex manner
Prolonged excessive alcohol consumption is a potent stressor
Chronic alcohol produces persistent dysregulation of brain reward and stress systems
Chronic alcohol engages stress and anti-stress neuropeptide systems
chronic alcohol adaptations underlie stress, negative affect, and motivational behaviors
Acknowledgments
This work was supported by the National Institutes of Health [National Institute on Alcohol Abuse and Alcoholism grants: P50 AA010761, U01 AA014095, and U01 AA020929], Department of Defense/U.S. Army Institute for Molecular Neuroscience [grant: 803-94], and Department of Veterans Affairs [grant: BX000813].
Abbreviations
- HPA
hypothalamic-pituitary-adrenocortical
- CRF
corticotropin-releasing factor
- DYN
dynorphin
- KOR
kappa opioid receptor
- CeA
central nucleus of the amygdale
- BNST
bed nucleus of the stria terminalis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Archives of General Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. American Journal of Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC. Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology (Berl) 2016a;233:2035–2043. doi: 10.1007/s00213-016-4257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC. Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Front Cell Neurosci. 2016b;10:45. doi: 10.3389/fncel.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–479. doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz AM, Brothers S, Sartor G, Holm L, Heilig M, Wahlestedt C, Thorsell A. The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacology (Berl) 2016;233:3553–3563. doi: 10.1007/s00213-016-4385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A. The oxytocin receptor impairs ethanol reward in mice. Physiol Behav. 2015;139:321–327. doi: 10.1016/j.physbeh.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, Al Maamari E. Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice. Physiol Behav. 2016;164:249–258. doi: 10.1016/j.physbeh.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual Review of Pharmacology and Toxicology. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, Kling MA, Gold PW, Higley D, Heilig M, Suomi SJ, Goldman D. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65:934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H, Lopez MF, Doremus-Fitzwater T. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Research & Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Becker HC, Ron D. Animal models of excessive alcohol consumption: recent advances and future challenges. Alcohol. 2014;48:205–208. doi: 10.1016/j.alcohol.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Reynolds JG, Thiele TE, Gan J, Figlewicz DP, Woods SC. Effects of third intracerebroventricular injections of corticotropin-releasing factor (CRF) on ethanol drinking and food intake. Psychopharmacology. 1998;139:128–135. doi: 10.1007/s002130050697. [DOI] [PubMed] [Google Scholar]
- Berger AL, Williams AM, McGinnis MM, Walker BM. Affective cue-induced escalation of alcohol self-administration and increased 22-kHz ultrasonic vocalizations during alcohol withdrawal: role of kappa-opioid receptors. Neuropsychopharmacology. 2013;38:647–654. doi: 10.1038/npp.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Peters ST, Absalom N, Chebib M, Neumann ID, McGregor IS. Oxytocin prevents ethanol actions at delta subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proc Natl Acad Sci U S A. 2015;112:3104–3109. doi: 10.1073/pnas.1416900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Research & Health. 1999;23:263–271. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Research Reviews. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Cappell H, Greeley J. Alcohol and tension reduction: An update on research and theory. Guilford; New York: 1987. [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Kim HJ, Kim HJ, Choi SH, Cho JH, Cho YH, Kim DH, Shin KH. Desipramine and citalopram attenuate pretest swim-induced increases in prodynorphin immunoreactivity in the dorsal bed nucleus of the stria terminalis and the lateral division of the central nucleus of the amygdala in the forced swimming test. Neuropeptides. 2014;48:273–280. doi: 10.1016/j.npep.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 2007;61:4–12. doi: 10.1016/j.biopsych.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de Guglielmo G, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 2009;43:491–498. doi: 10.1016/j.alcohol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, Roberto M, Weiss F, Teshima K. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology. 2014;39:2601–2610. doi: 10.1038/npp.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Rezvani AH, Robinson JE, Eisenberg L, Levin ED, Bonaventure P, Motley AT, Lovenberg TW, Heilig M, Thorsell A. The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 45:567–576. doi: 10.1016/j.alcohol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–275. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Crowley NA, Kash TL. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2015;62:51–60. doi: 10.1016/j.pnpbp.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol. 2015;20:643–651. doi: 10.1111/adb.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong J, Wauben P, Huijbrechts I, Oolders H, Haffmans J. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30:84–85. doi: 10.1097/JCP.0b013e3181c827ae. [DOI] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ, Nemeroff CB. Corticotropin releasing factor (CRF): studies in alcohol preferring and non-preferring rats. Psychopharmacology. 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ, Engel JA, Hansen S. Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology. 1995;117:216–224. doi: 10.1007/BF02245190. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Hansen S. Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology. 1996;127:133–139. doi: 10.1007/BF02805986. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcoholism, clinical and experimental research. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Fischer S, Wise B, Rasmussen DD. Prazosin Reduces Alcohol Intake in an Animal Model of Alcohol Relapse. Alcohol Clin Exp Res. 2015;39:1538–1546. doi: 10.1111/acer.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2013a;37:1552–1560. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol Clin Exp Res. 2013b;37:1763–1770. doi: 10.1111/acer.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. Journal of Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Le AD. The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav. 2014;4:356–367. doi: 10.1002/brb3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Li Z, Shaham Y, Le AD. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122:1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. Journal of Neuroscience. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Roldan L, Naranjo CA. Corticotropin-releasing factor is altered in brains of animals with high preference for ethanol. Alcoholism: Clinical & Experimental Research. 1990;14:425–429. doi: 10.1111/j.1530-0277.1990.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Germain A, Richardson R, Moul DE, Mammen O, Haas G, Forman SD, Rode N, Begley A, Nofzinger EA. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res. 2012;72:89–96. doi: 10.1016/j.jpsychores.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett K, Harshberger E, Valdez GR. Protracted withdrawal from ethanol and enhanced responsiveness stress: regulation via the dynorphin/kappa opioid receptor system. Alcohol. 2013;47:359–365. doi: 10.1016/j.alcohol.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 2010;212:431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008a;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008b;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proceedings of the National Academy of Sciences. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DM, Knapp DJ, Breese GR, Thiele TE. Comparison of basal neuropeptide Y and corticotropin releasing factor levels between the high ethanol drinking C57BL/6J and low ethanol drinking DBA/2J inbred mouse strains. Alcoholism Clinical & Experimental Research. 2005;29:721–729. doi: 10.1097/01.ALC.0000164375.16838.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology (Berl) 2016;233:681–690. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakihana R. Alcohol intoxication and withdrawal in inbred strains of mice: behavioral and endocrine studies. Behav Neural Biol. 1979;26:97–105. doi: 10.1016/s0163-1047(79)92933-9. [DOI] [PubMed] [Google Scholar]
- Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976;46:301–305. doi: 10.1007/BF00421118. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Oleata CS, Luu G, Teshima K, Ciccocioppo R, Roberto M. MT-7716, a novel selective nonpeptidergic NOP receptor agonist, effectively blocks ethanol-induced increase in GABAergic transmission in the rat central amygdala. Front Integr Neurosci. 2014a;8:18. doi: 10.3389/fnint.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Scuppa G, de Guglielmo G, Calo G, Weiss F, Statnick MA, Rorick-Kehn LM, Ciccocioppo R. Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology. 2017;42:695–706. doi: 10.1038/npp.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M. Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology. 2014b;39:1081–1092. doi: 10.1038/npp.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. kappa-Opioid receptors in the central amygdala regulate ethanol actions at presynaptic GABAergic sites. J Pharmacol Exp Ther. 2013;346:130–137. doi: 10.1124/jpet.112.202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala. J Pharmacol Exp Ther. 2015;355:206–211. doi: 10.1124/jpet.115.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith LD, Crabbe JC. Specific and nonspecific effects of ethanol vapor on plasma corticosterone in mice. Alcohol. 1992;9:529–533. doi: 10.1016/0741-8329(92)90092-o. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, Leggio L. Role of the alpha1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2016;21:904–914. doi: 10.1111/adb.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC. Oxytocin reduces ethanol self-adminsitartion in mice. Alcohol Clin Exp Res. 2017 doi: 10.1111/acer.13359. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry. 2014;75:774–782. doi: 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American journal of psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Current opinion in investigational drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- Lam MP, Gianoulakis C. Effects of corticotropin-releasing hormone receptor antagonists on the ethanol-induced increase of dynorphin A1-8 release in the rat central amygdala. Alcohol. 2011;45:621–630. doi: 10.1016/j.alcohol.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Harding S, Juzytsch W, Fletcher P, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. Journal of Neuroscience. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2013;18:448–451. doi: 10.1111/j.1369-1600.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, Leggio L. Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs. 2016;30:109–123. doi: 10.1007/s40263-016-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Weerts EM. Oxytocin for the treatment of drug and alcohol use disorders. Behav Pharmacol. 2016;27:640–648. doi: 10.1097/FBP.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcoholism: Clinical & Experimental Research. 2000;24:110–122. [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O'Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. Journal of Neuroscience. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol. 2016;51:17–23. doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism: Clinical & Experimental Research. 2000;24:651–658. [PubMed] [Google Scholar]
- Lowery EG, Thiele TE. Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets. 2010;9:77–86. doi: 10.2174/187152710790966605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience. 2014;277:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J. Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2016;140:27–32. doi: 10.1016/j.pbb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in neurosciences. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addict Biol. 2014;19:225–232. doi: 10.1111/adb.12125. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology Biochemistry & Behavior. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Koenig HN, Camarini R, Kim JA, Nannini MA, Ou CJ, Hodge CW. A role for corticotropin releasing factor (CRF) in ethanol consumption, sensitivity, and reward as revealed by CRF-deficient mice. Psychopharmacology. 2003;165:181–187. doi: 10.1007/s00213-002-1248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacology Biochemistry & Behavior. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Sharpe AL, Burkhart-Kasch S, McKinnon CS, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology. 2004;176:386–397. doi: 10.1007/s00213-004-1896-5. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Flor PJ, Neumann ID, Reber SO. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addict Biol. 2013;18:66–77. doi: 10.1111/adb.12001. [DOI] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict Biol. 2016 doi: 10.1111/adb.12362. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Research. Brain Research Reviews. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, Olson DP, Lowell BB, Grant KA, Thiele TE, Kash TL. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals-an update. Alcohol and Alcoholism. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1356–1365. doi: 10.1016/j.pnpbp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O'Connell J, Taylor F, Gross C, Rohde K, McFall ME. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J, Millard SP, Rohde K, O'Connell J, Pritzl D, Feiszli K, Petrie EC, Gross C, Mayer CL, Freed MC, Engel C, Peskind ER. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. The American journal of psychiatry. 2013;170:1003–1010. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcoholism: Clinical & Experimental Research. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rasmussen DD, Kincaid CL, Froehlich JC. Prazosin + Naltrexone Decreases Alcohol Drinking More Effectively Than Does Either Drug Alone in P Rats with a Protracted History of Extensive Voluntary Alcohol Drinking, Dependence, and Multiple Withdrawals. Alcohol Clin Exp Res. 2015;39:1832–1841. doi: 10.1111/acer.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Kincaid CL, Froehlich JC. Prazosin prevents increased anxiety behavior that occurs in response to stress during alcohol deprivations. Alcohol Alcoholism. 2017;52:5–11. doi: 10.1093/alcalc/agw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Research. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008a;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008b;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Supression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Lett. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Effects of alcohol on the neuroendocrine system. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research portfolio: NIAAA Research Monograph No 34. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 61–81. [Google Scholar]
- Rivier C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:221–233. doi: 10.1016/j.yfrne.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med. 2012;2:a012195. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Ciccocioppo R, Wong CJ, Witkin JM, Martinez-Grau MA, Stopponi S, Adams BL, Katner JS, Perry KW, Toledo MA, Diaz N, Lafuente C, Jimenez A, Benito A, Pedregal C, Weiss F, Statnick MA. A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin Exp Res. 2016;40:945–954. doi: 10.1111/acer.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Shaw SG, Prendergast MA, Little HJ. The importance of glucocorticoids in alcohol dependence and neurotoxicity. Alcohol Clin Exp Res. 2010;34:2011–2018. doi: 10.1111/j.1530-0277.2010.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Karkhanis A, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR. Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduced dopamine signaling in the nucleus accumbens. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Heinrichs SC, Lee S, Rivier C, Olive MF, Mehmert KK, Camarini R, Kim JA, Koenig HN, Nannini MA, Hodge CW, Roberts AJ, Koob GF. The corticotropin-releasing factor/urocortin system and alcohol. Alcoholism Clinical & Experimental Research. 2002;26:714–722. [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9:21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain Res. 1987;437:253–263. doi: 10.1016/0006-8993(87)91641-6. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Does drinking reduce stress? Alcohol Research & Health. 1999;23:250–255. [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Henniger MS. Stress and alcohol drinking. Annals of medicine. 2004;36:596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, Varon D, Raskind M, Saxon AJ. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39:808–817. doi: 10.1111/acer.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends in neurosciences. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, Rinker JA, Jijon AM, Pena J, Navarro M, Kash TL, Thiele TE. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology. 2012;37:1409–1421. doi: 10.1038/npp.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Fee JR, Hayes DM, Knapp DJ, MacNeil DJ, Thiele TE. Peripheral and central administration of a selective neuropeptide Y Y1 receptor antagonist suppresses ethanol intake by C57BL/6J mice. Alcohol Clin Exp Res. 2004;28:1324–1330. doi: 10.1097/01.ALC.0000139829.67958.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468–483. [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. Journal of Pharmacy & Pharmacology. 1978;30:371–374. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Naveilhan P, Ernfors P. Assessment of ethanol consumption and water drinking by NPY Y(2) receptor knockout mice. Peptides. 2004;25:975–983. doi: 10.1016/j.peptides.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Thorsell A. Central neuropeptide Y in anxiety- and stress-related behavior and in ethanol intake. Annals of the New York Academy of Sciences. 2008;1148:136–140. doi: 10.1196/annals.1410.083. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O'Dell LE, Chen SA, King AR, Lekic D, Koob GF, Sanna PP. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–1337. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Rimondini R, Heilig M. Blockade of central neuropeptide Y (NPY) Y2 receptors reduces ethanol self-administration in rats. Neurosci Lett. 2002;332:1–4. doi: 10.1016/s0304-3940(02)00904-7. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005a;161:133–140. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behavioural Brain Research. 2005b;161:133–140. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in wistar rats with a history of ethanol exposure. Alcohol Clin Exp Res. 2005c;29:584–590. doi: 10.1097/01.alc.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Harshberger E. kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol Biochem Behav. 2012;102:44–47. doi: 10.1016/j.pbb.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased Ethanol Self-Administration and Anxiety-Like Behavior During Acute Ethanol Withdrawal and Protracted Abstinence: Regulation by Corticotropin-Releasing Factor. Alcoholism Clinical & Experimental Research. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcoholism Clinical & Experimental Research. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Van't Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Czachowski CL. Low-dose prazosin alone and in combination with propranolol or naltrexone: effects on ethanol and sucrose seeking and self-administration in the P rat. Psychopharmacology (Berl) 2015;232:2647–2657. doi: 10.1007/s00213-015-3896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G. Hypothalamic-pituitary-adrenal axis: Changes and risk for alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research portfolio: NIAAA Research Monograph No 34. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 397–415. [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbring ML, Morley JE, Niewoehner CB, Heilman RO, Carlson CH, Shafer RB. Adrenocortical hyperactivity in newly admitted alcoholics: prevalence, course and associated variables. Psychoneuroendocrinology. 1984;9:415–422. doi: 10.1016/0306-4530(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]