Abstract
Gas chromatography-tandem mass spectrometry (GC-MS/MS) was used to detect fungal secondary metabolites. Detection of verrucarol, the hydrolysis product of Stachybotrys chartarum macrocyclic trichothecene (MCT), was confounded by matrix effects associated with heterogeneous indoor environmental samples. In this study, we examined the role of dust matrix effects associated with GC-MS/ MS to better quantify verrucarol in dust as a measure of total MCT. The efficiency of the internal standard (ISTD, 1,12-dodecanediol), and application of a matrix-matched standard correction method in measuring MCT in floor dust of water-damaged buildings was additionally examined. Compared to verrucarol, ISTD had substantially higher matrix effects in the dust extracts. The results of the ISTD evaluation showed that without ISTD adjustment, there was a 280% ion enhancement in the dust extracts compared to neat solvent. The recovery of verrucarol was 94% when the matrix-matched standard curve without the ISTD was used. Using traditional calibration curves with ISTD adjustment, none of the 21 dust samples collected from water damaged buildings were detectable. In contrast, when the matrix-matched calibration curves without ISTD adjustment were used, 38% of samples were detectable. The study results suggest that floor dust of water-damaged buildings may contain MCT. However, the measured levels of MCT in dust using the GC-MS/MS method could be significantly under- or overestimated, depending on the matrix effects, the inappropriate ISTD, or combination of the two. Our study further shows that the routine application of matrix-matched calibration may prove useful in obtaining accurate measurements of MCT in dust derived from damp indoor environments, while no isotopically labeled verrucarol is available.
Keywords: Dust, macrocyclic trichothecenes, matrix effect, mycotoxins, verrucarol, water-damaged buildings
Introduction
Mycotoxins are harmful low-molecular-weight secondary metabolites produced by fungi and have been detected in air, settled dust, and mold contaminated building materials.[1–4] Many fungal metabolites are potentially toxic to humans and animals. Some studies suggested associations between indoor mycotoxin-producing fungi and a variety of adverse health effects that are experienced by building occupants and range from neurologic[5, 6] to respiratory problems.[7–11] To date, there have been few epidemiologic studies that have evaluated exposure to fungal secondary metabolites in the indoor environment due to the limitations of existing measurement platforms.[12,13] Numerous indoor fungi are capable of producing secondary metabolites;[14] however, Stachybotrys chartarum, is most well known for the production of potent macrocyclic trichothecene, such as satratoxins F, G, and H, roridin E, and verrucarin J.[5, 16]
Methods available to measure Stachybotrys chartarum mycotoxins are limited and are generally categorized into either biological assays or chemical analysis. Gas chromatography (GC) or liquid chromatography (LC) tandem mass spectrometry (MS/MS) are chemical analysis techniques that enable the detection and quantification of specific mycotoxins and are based on the mass and ion charges. Verrucarol and trichodermol are the hydrolysis products of macrocyclic trichothecene (MCT) and trichodermin produced by Stachybotrys chartarum.[3,17]
GC-MS/MS approaches offer high sensitivity and specificity; however, the detection of a specific analyte extracted from an environmental sample may be influenced by matrix effects. Co-extracted matrix components may interfere with active sites in the GC inlet liner and column and produce differential analyte signals between the matrix-containing sample extract and matrix-free standard extract.[18–20] Matrix effects associated with heterogeneous components in environmental samples will significantly influence the detection and quantification of an analyte.[21] Sample preparation techniques, including solid phase extraction (SPE) or liquid-liquid extraction (LLE), have been shown to potentially reduce matrix effects; however, these preparative steps may increase sample loss.[22] An internal standard (ISTD) is commonly used to adjust for matrix effects or sample loss during preparative steps; however, if the selected ISTDs do not contain similar ionization properties or chemical structures to the target analyte, they may not always react the same way as the analyte of interest, especially with the presence of matrix.[23]
In this study, we evaluated effects of dust matrix in the analysis of verrucarol using GC-MS/MS to more accurately measure MCT in floor dust of water-damaged buildings. The efficiency of the ISTD (1,12-dodecanediol) used in the reported analytical method[3,17,24] was additionally evaluated.
Methods
Chemicals and reagent
LC-MS grade methanol, dichloromethane (DCM), sodium hydroxide, toluene, and acetonitrile was purchased from Sigma-Aldrich (St. Louis, MO) and N-heptafluorobutyrylimidazole (HFBI, derivatization reagent) from Regis Technology (Morton Glove, IL). Standard verrucarol and ISTD (1,12-dodecanediol) were also purchased from Sigma-Aldrich (St. Louis, MO). Strata™-X polymeric reversed phase solid phase extraction (SPE) cartridges were purchased from Phenomenex (Torrance, CA).
Sample preparation
The published[3,17] sample preparation method optimized for settled dust and building materials from water-damaged dwellings was modified for our study. Briefly, approximately 30 mg of floor dust was incubated with 1 mL methanol in a borosilicate glass tube closed with a PTFE (polytetrafluoroethylene) screw cap overnight in the dark and at room temperature. The next day, the dust was sonicated in methanol for 1 hr, and centrifuged at 3200 rpm for 5 min. The supernatant was transferred into a new test tube and evaporated until dry under a gentle nitrogen gas stream at room temperature and the ISTD (1,12-dodecanediol, 500 pg) was then added to the dried extract. The extract was hydrolyzed with 200 μL of 0.2 M methanolic NaOH overnight at room temperature (macrocyclic trichothecene is hydrolyzed into verrucarol). On the third day, the solution was evaporated until dry under nitrogen gas and then 1 mL deionized (DI) water was added to each test tube. The test tube was vortexed and the extract was loaded onto a polymeric reversed phase cartridge (Strata-X SPE cartridges) that was pre-conditioned with 2 mL DCM, 2 mL methanol, and then 2 mL DI water. After sample loading, the cartridge was washed with 2 mL DI water, aspirated for 20 min (about 1 ml/min), and then eluted with 2 mL DCM. The eluate was evaporated under nitrogen gas and the dried extract was derivatized with 15 μL HFBI and 200 μL toluene:acetonitrile (4:1, v:v) at 70°C for 1 hr. The final extract containing derivatized verrucarol (Bis-heptafluorobutyrylverrucarol) that we quantified was washed with 1 mL DI water and centrifuged at 3200 rpm, and the upper organic layer was transferred into a GC vial and stored at 4°C overnight before GC-MS/ MS analyses.
GC-MS/MS conditions
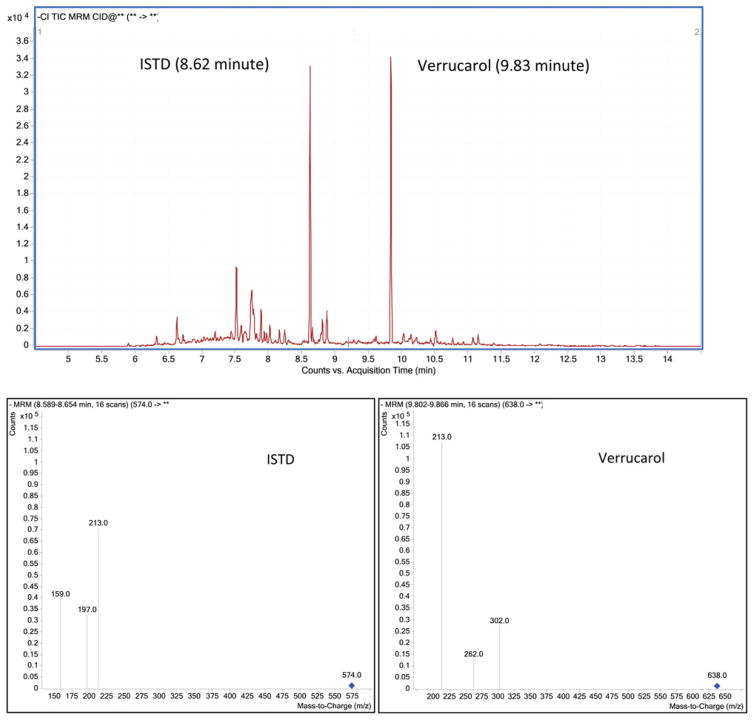
We used a Gas Chromatograph-tandem Mass Spectrometer (GC-MS/MS, 7890A GC system with G7000A Triple Quad MS system, Agilent, Santa Clara, CA) equipped with a fused-silica capillary column (HP-5 ms, 30 m × 0.25 mm i.d.) in the study. Samples (injection volume = 1 μl) were analyzed in negative chemical ionization mode using methane as ionization gas at the energy of 150 eV and the ion source temperature of 150°Cin splitless mode. The oven temperature was programmed as 70°C initial temperature ramped at 20°C/min to 280°C. The precursor ion for verrucarol derivative (retention time: 9.8 min) was m/z 638 that yielded a target product ion of m/z 302 for quantification and two ions of m/z 262 and 213 for qualification. For the ISTD derivative (retention time: 8.6 min), the precursor ion (m/z 574) produced a target ion of m/z 197 for quantification and two ions of m/z 213 and 159 for qualification. All sample extracts and standards were injected into GC in duplicate.
Preparation of spiked samples and calibration curves
We analyzed 21 dust samples and nine spiked samples in 5 separate experiments. Each of the experiments included two sets of standard curves—one prepared in neat solvent and the other in dust extract (as detailed below). Nine dust samples were randomly selected from the 21 samples for spike sample analysis. To examine percent recovery of spiked verrucarol in those samples, known amounts of verrucarol were spiked into dust before extraction. Each spike experiment included six samples: a spiked dust and an un-spiked dust for each of the three different samples. The spiking experiment was repeated at three spiking levels (10, 25, or 50 ng loadings) with a total of nine dust samples. The spiked and un-spiked samples were extracted using the same sample preparation procedure as described above, and the extract was analyzed with the GC-MS/MS. The percent recovery was calculated based on: 100×[(measured amount in spiked dust–measured amount in un-spiked dust)/(spiked level of verrucarol)].
To evaluate and adjust for matrix effects using the calibration technique, two sets of standard curves were prepared for each of the five experiments: one in neat solvent with no dust matrix (traditional calibration); and the other in dust extract (matrix-matched calibration). We prepared the traditional standard solutions for calibration by adding 500 pg of ISTD and a series of four verrucarol standard solutions into empty glass tubes (0.1, 1, 10, and 100 ng/tube), and then dried under nitrogen gas. The dried extract was then derivatized by adding 200 μL toluene:acetonitrile (4:1, v:v) and 15 μL HFBI at 70°C for 1 hr, washed with DI water, and centrifuged as described above. We prepared the matrix-matched standard solutions by adding the series of four verrucarol standards into four sets of glass tubes containing dried dust extract with ISTD prepared from the same dust using the sample preparation procedure above. After drying under nitrogen gas, the samples were then derivatized, washed with DI water, and centrifuged. For both neat and matrix-matched standards, the upper organic portion of the liquid was transferred into an autosampler vial and stored at refrigerator for overnight before injection. The dust selected for the matrix-matched calibration contained non-detectable amount of ergosterol (by GC-MS)[25] and culturable Stachybotrys chartarum and Myrothecium species (by culture method)[25,26] and was homogenized for 2 hr before it was made into aliquots for the experiments.
Dust sample analysis
We analyzed floor dust samples collected from two water-damaged buildings in the northeastern area of the United States in 2006 and 2007, respectively. A total of 21 samples (eight from building H and 13 from building B) were selected based on the availability of dust and the high level of contamination with total culturable fungi. A detailed method for quantifying total culturable fungi has been described elsewhere.[26] To collect floor dust, a 2 m2 area of floor was vacuumed around occupant’s workstation for 5 min. For each dust sample, we used a polyethylene filter sock (Midwest Filtration Company, Fairfield, OH, USA) with a pre-cleaned crevice tool on a L’il Hummer™ backpack vacuum sampler (100 ft3/min, 1.5 horse power; Pro- Team Inc., Boise, ID, USA).[25,26] We homogenized and partitioned the samples after hair, fluff, and other larger objects had been removed. The collected and processed dust samples had been stored in a −80°C freezer. Prior to the sample preparation for the GC-MS/MS analysis, the bulk dust samples were homogenized for 2 hr and aliquots of the selected samples were prepared in a new sterile test tube.
Statistical analyses
We examined response factors that were computed by dividing peak area (response) by concentration of verrucarol (ng/μl) or ISTD (ng/μl). Matrix effect was calculated by (peak area for standard spiked into dust extract)/(peak area for standard spiked into neat solvent).[27] Percent coefficient of variation (%CV) was calculated by dividing standard deviation by average value and then multiplying 100. We used geometric mean (GM) and geometric standard deviation (GSD) if there are outliers in measurements; otherwise, we used arithmetic mean and standard deviation as a point estimate. Analysis of Variance (ANOVA) was used to compare slope of verrucarol standard curve prepared in neat solvent to that in dust extract. The limit of detection (LOD) for each of the five experiments was estimated from an average concentration of blank samples (n = 3–5 per experiment) plus three times their standard deviation. Median LOD was 0.016 ng/sample (range: 0.012–0.053) when the matrix-matched calibration curves were applied without ISTD adjustment.
Results
Dust matrix effect on verrucarol
Chromatographic separation of derivatized verrucarol and an ISTD in GC is presented in the total ion chromatogram (Figure 1). In order to evaluate the matrix effect, we examined peak areas for four levels of standard verrucarol spiked into neat solvent and dust extract. When we used peak area relative to the ISTD (peak area for verrucarol standard divided by that for the ISTD) to construct a standard curve for each experiment, the slope of the standard curve prepared in the dust extract was significantly (p < 0.001) lower than that in neat solvent (average ratio of slope in dust extract to that in neat solvent= 0.04, SD=0.005), indicating an ion suppression in dust matrix. However, when peak area of verrucarol standard was not adjusted for the ISTD, slope of the standard curve prepared in the dust extract was 2.8-fold (range: 2.4–3.1, SD = 0.3) significantly (p < 0.001) higher than that in neat solvent, indicating an ion enhancement in dust matrix by 280%. These two results appeared to be contradictory. Therefore, we further evaluated effectiveness of the ISTD used in the experiments.
Figure 1.
Total ion chromatogram (upper panel) and MSMS spectra (lower panels) for derivatized 1,12-dodecanediol (internal standard, ISTD) and verrucarol.
Dust matrix effect on internal standard
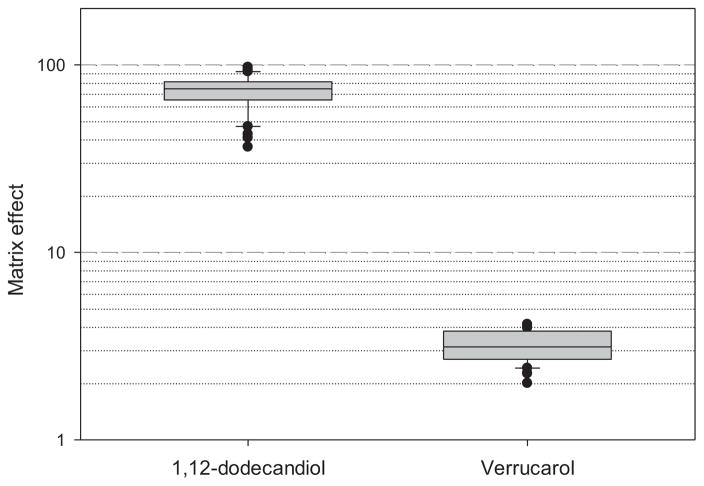
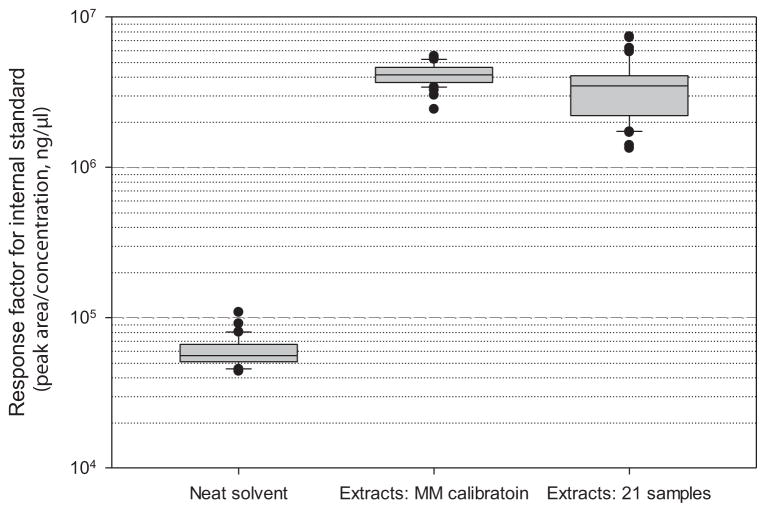
We examined the matrix effect of dust extract on responses to the ISTD as well as verrucarol standard. For each of the verrucarol and internal standards, we calculated a ratio of peak area for the standard measured in dust extract to that in neat solvent as a measure of matrix effect (Figure 2). We found that the matrix effect (ion enhancement) of dust extract on 1,12-dodecanediol was much larger (mean ratio = 71.8, SD = 14.8) than that on verrucarol (mean ratio = 3.2, SD = 0.6). Dissimilar matrix effect on verrucarol and the internal standard implies that adjustment of response for verrucarol with 1,12-dodecanediol as an internal standard would yield results which incorrectly show an ion suppression (Figure 2). Thus, the dust matrix effect on response for verrucarol is not an ion suppression but an ion enhancement. We also compared response factors for ISTD in neat solvent of 20 standard samples, extracts of 20 aliquots of one dust sample selected for the matrix-matched calibration, and extracts of 21 dust samples from all five experiments (Figure 3). The results show that the average response factor for ISTD was 58-fold (in extracts of 21 dust samples) or 70-fold (in extracts of 20 aliquots of one dust sample) higher than that in neat solvent (p-values < 0.001). In contrast to ISTD, the average response factor for verrucarol in dust extracts using the matrix-match calibration was only about three-fold higher than that in neat solvent as presented in the previous section and Figure 2.
Figure 2.
Matrix effect calculated by peak area for standard spiked into dust extract divided by that spiked into neat solvent. Data for the box plots are from 4 different concentrations for verrucarol and one fixed concentration for 1,12-dodecanediol (internal standard) in each standard curve of the five experiments. Dust extract was prepared with one sample with no detectable amount of ergosterol, and culturable Stachybotrys and Myrothecium species. Box=25–75 percentile; solid line in the box=median; whiskers=5 and 95 percentiles; and filled circles = outliers.
Figure 3.
Response factors of internal standard (1,12-dodecanediol) measured in neat solvent, dust extracts prepared from one dust for matrix-matched (MM) calibration in each of the five experiments, and extracts of 21 dust samples spiked with known amount of internal standard (0.5 ng/sample). Y-axis is in a log scale. Box = 25–75 percentile; solid line in the box = median; whiskers = 5 and 95 percentiles; and filled circles = outliers.
Percent recovery of spiked dust samples
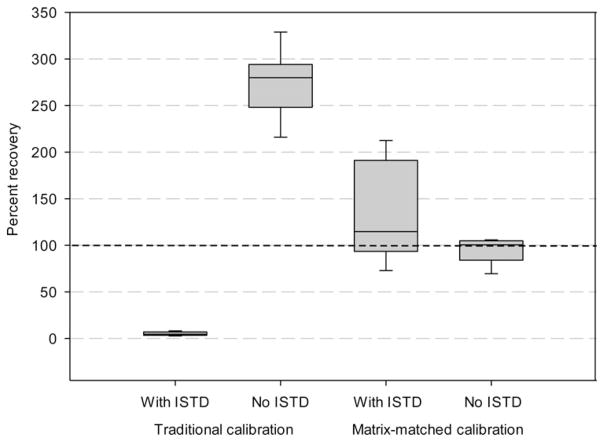
Spike sample analysis shows that percent recovery was extremely low [mean = 5%, percent coefficient of variation (%CV) = 39] when the traditional calibration curve (prepared in neat solvent) adjusted with ISTD was applied to calculate sample concentration (Figure 4). When the matrix-matched calibration curve adjusted for ISTD was applied, the recovery rate was improved (mean = 135%) but still as variable (%CV = 37) as that in neat solvent. However, the matrix-matched calibration curve that was not adjusted with ISTD resulted in the most accurate and reliable recovery rates (mean=94%, %CV=14) whereas the traditional calibration method without ISTD adjustment resulted in a large overestimation (mean = 273%, %CV = 12) in recovery rate. The finding of 135% average recovery rate with large CV for the spiked samples calculated with ISTD-adjusted matrix-matched standard curve may also indicate that 1,12-dodecanediol is an inappropriate ISTD to adjust for variable response for verrucarol due to the matrix-effect as well as an analyte loss during sample preparation in the analysis of dust samples using GC-MS/MS.
Figure 4.
Percent recovery of verrucarol in nine spiked dust samples calculated using calibration curves prepared in neat solvent (traditional calibration) or dust extract (matrix-matched calibration) with or without internal standard (ISTD) adjustment. Box=25–75 percentile; solid line in the box = median; whiskers= 5 and 95 percentiles.
Measurement of MCT and culturable Stachybotrys chartarum in building floor dust
The geometric mean (GM) level of total culturable fungi for the 21 samples analyzed for MCT was 153,000 cfu/g of dust [range: 15,000–5,944,000 cfu/g]. When we used the traditional calibration curve prepared in neat solvent with the ISTD adjustment, the levels of verrucarol in all 21 dust samples were below the LOD. However, when we reanalyzed the same samples using the matrix-matched calibration curves with no ISTD adjustment, we were able to detect verrucarol in 8 of the 21 samples (38%). The average level of verrucarol measured in these 8 dust samples was 2.5 ng/g of dust (range: 0.6–36.7 ng/g of dust). Of the eight samples, Stachybotrys chartarum was cultured (GM = 11,000 cfu/g) in four samples (Table 1). In contrast, we did not detect verrucarol in three of the seven dust samples from which Stachybotrys chartarum was cultured (mean = 18,000 cfu/g). Myrothecium species were not cultured from any of those 21 samples.
Table 1.
The levels of culturable total fungi and Stachybotrys chartarum, and macrocyclic trichothecene measured as equivalent to verrucarol.
| Analyte | # of samples (>LOD/total) | Geometric mean | Geometric standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Total culturable fungi (cfu/g) | 21/21 | 153,000 | 4.0 | 15,000 | 5,944,000 |
| Cuturable Stachyborys chartarum (cfu/g) | 7/21 | 11,000 | 9.9 | 200 | 150,000 |
| Macrocyclic trichothecene (ng/g) | 8/21 | 2.5 | 4.3 | 0.6 | 36.7 |
Discussion
Matrix effects of indoor dust samples
We found significant matrix effects (enhancement) associated with the GC-MS/MS method for the analysis of verrucarol (hydrolysis product of macrocyclic trichothecene) in extracts derived from floor dust from water-damaged buildings. These matrix effects produce inaccurate results in the GC-MS/MS measurement of MCTs in dust samples as we found no dust sample above the LOD when the traditional calibration method was used. The method we modified for the study has been reported previously in the literature[3,17] to analyze for verrucarol in settled dust samples or building materials collected from various indoor environments including office buildings in Europe. Matrix components in dust extracts that coelute with the target analyte may suppress or enhance the response (ion intensity) of mass spectrometer to the analyte. This limitation adversely affects the reproducibility and accuracy of the method. The matrix effects are unavoidable unless active sites in the GC inlet and column or matrix components in extracted sample solution are eliminated, which is nearly impossible.[18,28] Therefore, it is essential to evaluate matrix effects of dust or other environmental samples on target mycotoxins in the development of methods using GC-MS/MS.
Several strategies have been suggested to compensate matrix effects. They include improvement in sample preparation and chromatographic separation, modifying the mass spectrometric condition, or applying calibration techniques.[29] Among these suggestions, calibration methods including ISTD adjustment have been widely applied to compensate matrix effects. The ISTD normally adjusts for losses during sample preparation and variations in sample amount injected into the GC. In addition, if the ISTD is adequately chosen for the analysis, variation of the analyte signal between matrix-containing sample and matrix-free external standard extracts (prepared in neat solvent) may be compensated by equivalent disturbance on the ISTD in both extracts. Our study results indicate that the disturbance of the analytical signal on ISTD between dust extract and neat solvent was not equivalent to that on verrucarol in GC-MS/MS analysis. Dust matrix effects on response for ISTD was much greater than those for verrucarol. Recovery rate of verrucarol spiked in dust calculated using the traditional calibration curve prepared in neat solvent adjusted with ISTD was only 5%. This result indicates that 1,12-dodecandiol may not be an appropriate ISTD for our office dust samples that could contain various biological and chemical agents. Our finding is similar to that of Sordillo et al.[23] who showed that recovery of spiked ergosterol in house dust was poor (36–54%) when 7-dehydrocholesterol was used as an ISTD. Use of an isotope-labeled internal standard of verrucarol will most effectively adjust for these matrix effects;[29,30] however, there is currently no isotope-labeled ISTD available for the analysis of MCT mycotoxins using GC-MS/MS, and our attempt of developing isotope-labeled verrucarol was not successful.
There are other suggested calibration methods to compensate for matrix effects if there is no isotope-labeled ISTD available. Of these, a matrix-matched standard technique has been widely used. In our study, we selected dust with no detectable level of ergosterol and culturable Stachybotrys chartarum and Myrothecium species to create matrix-matched standard solutions. Although the specific floor dust selected for the adjustment in our study was only analogy reference to all other floor dust samples with some variation in components of dust extract, matrix-matched calibration curves using the selected dust substantially increased accuracy in measurements of verrucarol as indicated from the spike experiment using nine different dust samples. To exactly match individual dust sample matrix in calibration, we can use a standard addition technique to correct the matrix effect; however, this technique will increase the number of samples (at least two times) to be analyzed, which results in substantial increase of analytical time and cost per sample, and thus makes this method impractical.
Presence of macrocyclic trichothecene in dust samples
Application of the chemical analysis to detect MCT may be especially helpful for epidemiologic or toxicological studies to examine health or biologic effects, respectively, related to exposure to Stachybotrys. MCT-producing Stachybotrys spp. showed higher cytotoxicity than those that do not produce MCT.[31] In our study, we have detected low levels of MCT in several of the analyzed floor dust samples with and without presence of culturable Stachybotrys chartarum that were collected from water-damaged buildings. In contrast, we did not detect MCT in some dust samples with presence of culturable S. chartarum. This may be explained by study findings of a research group[32] that identified MCT in only 33 of 84 S. chartarum isolates and 2 of 2 S. dichroa isolates, indicating that presence of MCT differs by species of Stachybotrys and strains of S. chartarum in dust samples.
Conclusions
Our study shows that exposure to mycotoxins may occur in water-damaged indoor environments and suggest that fungal toxins may be an important exposure contributing to various health effects in building occupants. Our study also demonstrated that there were substantial matrix effects in an available published method using GC-MS/MS which resulted in substantial underestimation or overestimation in levels of MCT in dust and thus should be compensated to obtain accurate results. Although isotope-labeled ISTD may be the best way to overcome the matrix effects for accurate measurements of such mycotoxins, the isotope-labeled verrucarol is currently unavailable. Meanwhile, improvement of extraction efficiency in sample preparation steps and use of other calibration methods such as matrix-matched standard curves may be useful practices to obtain accurate measurements of MCT in dust with the method using GC-MS/MS.
Acknowledgments
Funding
This study was supported in part by an interagency agreement between NIOSH and NIEHS (AES12007001-1-0-6) as a collaborative National Toxicology Program research activity.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/uoeh.
References
- 1.Polizzi V, Delmulle B, Adams A, et al. Fungi, mycotoxins and microbial volatile organic compounds in mouldy interiors from water-damaged builinds. J Environ Monit. 2009;11:1849–1858. doi: 10.1039/b906856b. [DOI] [PubMed] [Google Scholar]
- 2.Vishwanath V, Sulyok M, Labuda R, Bicker W, Krska R. Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/ tandem mass spectrometry. Anal Bioanal Chem. 2009;395:1355–1372. doi: 10.1007/s00216-009-2995-2. [DOI] [PubMed] [Google Scholar]
- 3.Bloom E, Bal K, Nyman E, Larsson L. Optimizing a GC-MS method for screening of Stachybotrys mycotoxins in indoor environments. J Environ Monit. 2007;9:151–156. doi: 10.1039/b613853e. [DOI] [PubMed] [Google Scholar]
- 4.Bloom E, Nyman E, Must A, Pehrson C, Larsson L. Molds and Mycotoxins in Indoor Environments — A Survey in Water-Damaged Buildings. J Occup Environ Hyg. 2009;6:671–678. doi: 10.1080/15459620903252053. [DOI] [PubMed] [Google Scholar]
- 5.Baldo J, Ahmad L, Ruff R. Neuropsychological performance of patients following mold exposure. App Neuropsych. 2002;9:193–202. doi: 10.1207/S15324826AN0904_1. [DOI] [PubMed] [Google Scholar]
- 6.Rea WJ, Didriksen N, Simon TR, Pan Y, Fenyves EJ, Griffiths B. Effects of toxic exposure to molds and mycotoxins in building-related illness. Arch Environ Health. 2003;58:399–405. doi: 10.1080/00039896.2003.11879140. [DOI] [PubMed] [Google Scholar]
- 7.Stark PC, Burge HA, Ryan LM, Milton DK, Gold DR. fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am J Respir Crit Care Med. 2003;168:232–237. doi: 10.1164/rccm.200207-730OC. [DOI] [PubMed] [Google Scholar]
- 8.Bünger J, Westphal G, Mönnich A, Hinnendahl B, Hallier E, Müller M. Cytotoxicity of occupationally and environmentally relevant mycotoxins. Toxicology. 2004;202:199–211. doi: 10.1016/j.tox.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Hossain MA, Ahmed MS, Ghannoum MA. Attributes of Stachybotrys chartarum and its association with human disease. J Allergy Clin Immunol. 2004;113:200–208. doi: 10.1016/j.jaci.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Jarvis BB, Miller JD. Mycotoxins as harmful indoor air contaminants. Appl Microbiol Biotech. 2005;66:367–372. doi: 10.1007/s00253-004-1753-9. [DOI] [PubMed] [Google Scholar]
- 11.Shariat C, Collard HR. Acute lung injury after exposure to Stachybotrys chartarum. Respir Med Extra. 2007;3:74–75. [Google Scholar]
- 12.United States Government Accountability Office. INDOOR MOLD - Better Coordination of Research on Health Effects and More Consistent Guidance Would Improve Federal Efforts. Sep, 2008. Report to the Chairman, Committee on Health, Education, Labor and Pensions, U.S. Senate. (GAO-08-980) [Google Scholar]
- 13.Douwes J. WHO guidelines for indoor air quality: dampness and mould. World Health Organization; Copenhagen, Denmark: WHO; 2009. Building dampness and its effect on indoor exposure to biological and non-biological pollutants; pp. 23–24. [Google Scholar]
- 14.Miller JD, McMullin DR. Fungal secondary metabolites as harmful indoor air contaminants: 10 years on. Appl Microbiol Biotechnol. 2014;98:9953–9966. doi: 10.1007/s00253-014-6178-5. [DOI] [PubMed] [Google Scholar]
- 15.Bata A, Harrach B, Ujszászi K, Kis-Tamás A, Lásztity R. Macrocyclic trichothecene toxins produced by Stachybotrys atra strains isolated in Middle Europe. Appl Environ Microbiol. 1985;49:678–681. doi: 10.1128/aem.49.3.678-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis BB, Lee YW, Comezoglu SN, Yatawara CS. Trichothecenes produced by Stachybotrys atra from Eastern Europe. Appl Environ Microbiol. 1986;51:915–918. doi: 10.1128/aem.51.5.915-918.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloom E, Bal K, Nyman E, Must A, Larsson L. Mass Spectrometry-Based Strategy for Direct Detection and Quantification of Some Mycotoxins Produced by Stachybotrys and Aspergillus spp. in Indoor Environments. Appl Environ Microbiol. 2007;73:4211–4217. doi: 10.1128/AEM.00343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastovska K, Lehotay SJ, Anastassidiades M. Combination of analyte protectants to overcome matrix effects in routine GC analysis of pesticide residues in food matrix. Anal Chem. 2005;77:8129–8137. doi: 10.1021/ac0515576. [DOI] [PubMed] [Google Scholar]
- 19.Erney DR, Gillespie AM, Gilvydis DM, Poole CF. Explanation of the matrix-induced chromatographic response enhancement of organophosphorus pesticides during open tubular column gas chromatography with splitless or hot on-column injection and flame photometric detection. J Chromatogr. 1993;638:57–63. [Google Scholar]
- 20.Erney DR, Pawlowski TM, Poole CF. Matrix-induced peak enhancement of pesticides in gas chromatography: Is there a solution? J High Resol Chromatogr. 1997;20:375–378. [Google Scholar]
- 21.Sánchez-Brunete C, Albero B, Martín G, Tadeo JL. Determination of Pesticide Residues by GC-MS Using Analyte Protectants to Counteract the Matrix Effect. Analyt Sci. 2005;21:1291–1296. doi: 10.2116/analsci.21.1291. [DOI] [PubMed] [Google Scholar]
- 22.Hyötyläínen T. Critical evaluation of sample pretreatment techniques. Anal Bioanal Chem. 2009;394:743–758. doi: 10.1007/s00216-009-2772-2. [DOI] [PubMed] [Google Scholar]
- 23.Sordillo J, Vespa D, Haggerty L, Youngs F, Gold D, Milton D. Development of a new isotopically labeled internal standard for ergosterol measurement by GC/MS. J Environ Monit. 2009;11:1513–1517. doi: 10.1039/b901824g. [DOI] [PubMed] [Google Scholar]
- 24.Anderson B, Nielsen KF, Jarvis BB. Characterization of Stachybotrys from water-damaged buildings based on morphology, growth, and metabolite production. Mycologia. 2002;94:392–403. [PubMed] [Google Scholar]
- 25.Park J-H, Cox-Ganser JM, Kreiss K, White SK, Rao CY. Hydrophilic fungi and ergosterol associated with respiratory illness in water-damaged building. Environ Health Perspect. 2008;116:45–50. doi: 10.1289/ehp.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J-H, Cox-Ganser JM, Rao CY, Kreiss K. Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air. 2006;16:192–203. doi: 10.1111/j.1600-0668.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 27.Matuszewski BK, Constanzer ML, Chavez CM. Engineering strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/ MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 28.Schenck FJ, Lehotay SJ. Does further clean-up reduce the matrix enhancement effect in gas chromatographic analysis of pesticide residues in food? J Chromatogr A. 2000;868:51–61. doi: 10.1016/s0021-9673(99)01137-1. [DOI] [PubMed] [Google Scholar]
- 29.Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev. 2011;30:491–509. doi: 10.1002/mas.20298. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Salistad J, Shah VP, Skelly JP, Swann PG, Weiner R. Workshop/conference report – quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. AAPS J. 2007;9:E30–42. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen KF, Huttunen K, Hyvärinen A, Andersen B, Jarvis BB, Hirvonen M-R. Metabolite profiles of Stachybotrys isolates from water-damaged buildings and their induction of inflammatory mediators and cytotoxicity in macrophages. Mycopathologia. 2001;154:201–205. doi: 10.1023/a:1016383402963. [DOI] [PubMed] [Google Scholar]
- 32.Andersen B, Nielsen KF, Thrane U, Szaro T, Taylor JW, Jarvis BB. Molecular and phenotypic descriptions of Stachybotrys chlorohalonata sp. Nov. and two chemotypes of Stachybotrys chartarum found in water-damaged buildings. Mycologia. 2003;95:1227–1238. doi: 10.1080/15572536.2004.11833031. [DOI] [PubMed] [Google Scholar]