Abstract
This article summarizes current data and approaches to assess sodium intake in individuals and populations. A review of the literature on sodium excretion and intake estimation supports the continued use of 24-h urine collections for assessing population and individual sodium intake. Since 2000, 29 studies used urine biomarkers to estimate population sodium intake, primarily among adults. More than half used 24-h urine; the rest used a spot/casual, overnight, or 12-h specimen. Associations between individual sodium intake and health outcomes were investigated in 13 prospective cohort studies published since 2000. Only three included an indicator of long-term individual sodium intake, i.e., multiple 24-h urine specimens collected several days apart. Although not insurmountable, logistic challenges of 24-h urine collection remain a barrier for research on the relationship of sodium intake and chronic disease. Newer approaches, including modeling based on shorter collections, offer promise for estimating population sodium intake in some groups.
Keywords: 24 h, spot, overnight, balance, metabolism, sweat
INTRODUCTION
Excess sodium intake increases the risk for high blood pressure, and high blood pressure, or hypertension, is a leading risk factor for cardiovascular disease (2, 52, 55, 132). Globally, a projected 1 in 10 deaths from cardiovascular causes (1.65 million in 2010) are attributed to excess sodium intake (110). In contrast to randomized controlled trials indicating a positive dose-response relationship between sodium intake and blood pressure, some recent prospective cohort studies suggest that lower and higher sodium intakes are associated with an increased risk of cardiovascular disease and death (51, 113). The Institute of Medicine concluded in its 2013 report, Sodium Intake in Populations: Assessment of Evidence, that the “results of studies linking dietary sodium intake with direct health outcomes were highly variable in methodological quality, particularly in assessing sodium intake” (67, p. 4). Further, an American Heart Association science advisory concludes that these paradoxical findings may in part be explained by the measures used to assess sodium intake, in addition to a number of other biases (24). At the population level, ongoing activities in several countries to reduce sodium in foods require accurate monitoring of intakes of sodium and related nutrients, such as potassium (1, 21, 66, 146).
Twenty-four-hour urine collections are the recommended method of monitoring population sodium intake (149, 66). Assuming no urine voids are missed, about 90% of the sodium consumed (from all sources) is excreted in urine and estimated intake from 24-h urine collection is not subject to recall bias (65). In contrast, dietary methods (e.g., 24-h dietary recalls, food frequency questionnaires, dietary records) can be biased by errors in recall and recording as well as errors in food and nutrient composition tables (140). Dietary methods also do not usually capture the amount of sodium from salt added at the table or the amount of sodium consumed from nondietary sources (95), e.g., water softeners, sodium-containing supplements or antacids, and medications. Although these sources generally contribute a small proportion of population sodium intake, they can contribute substantial amounts among individuals exposed (49). Due to the high participant burden of 24-h urine collection, other urine specimens, such as spot/casual, overnight, and timed 12-h collections, are also used. The methods employed to assess sodium and related nutrient intake through urine biomarkers have not been comprehensively reviewed.
The types of urine biomarkers (e.g., 24 h-urine sodium excretion) used in surveys and studies to assess sodium intake are often noted in systematic reviews of population sodium intake and the associations between sodium intake and health outcomes (2, 14, 24, 67, 120). Study-specific data on the accuracy of the urine biomarkers used (e.g., methods used to assess the completeness of 24-h urine collection) are more limited. The present review of the published literature summarizes and evaluates current approaches to assess sodium intake through urine biomarkers in individuals and populations. This review includes sections on (a) data sources and review methods, (b) factors that affect sodium excretion and estimation of sodium intake from urine biomarkers, (c) approaches used to assess population and individual sodium intake through urine biomarkers in population-based surveys and prospective cohort studies of sodium intake and health, and (d) critical questions and directions for future research.
DATA SOURCES AND REVIEW METHODS
We identified factors affecting sodium balance and excretion through the Institute of Medicine’s 2005 Dietary Reference Intakes report (65). To identify new factors or information related to urinary sodium excretion, we conducted additional database searches using words such as “sodium,” “salt,” “balance,” “homeostasis,” “urine,” and “sweat.”
We identified studies using urine biomarkers to assess sodium intake from recent systematic reviews of population sodium intakes (14) and the associations between sodium intake and health outcomes (2). Search strategies were developed to identify more recent studies not included in these reviews (see Supplemental Appendix; follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). Search strategies included all age groups, no language restrictions, and the EMBASE, Global Health, CINHAL, Cochrane Library, and Lilacs databases. Additional studies were identified through the review of reference lists of studies or systematic reviews identified in the database search and through contact with experts. Two independent reviewers screened titles and abstracts of all references. Abstracts and articles not published in English were translated using an online language translation program or through native speakers. We did not include data from conference abstracts. When multiple publications were available for an included study, we used one publication as the primary publication. The inclusion/exclusion criteria for studies using urine biomarkers to assess (a) population sodium intakes or (b) the association between sodium intake and health outcomes are described below.
Population Sodium Intakes
We included population-based studies on all ages and both sexes from the general noninstitutionalized population or specific subgroups (e.g., males) that estimated group sodium intake (mean or the proportion above or below a specific threshold) using data on urinary sodium excretion. We included cross-sectional surveys and baseline data from cohort or intervention studies representative of the population at any level (national, regional, local). We excluded studies conducted only on a specific subset of the population (e.g., people with hypertension only) and studies in which participants were not randomly selected from a geographic area (e.g., convenience samples, workers, schoolchildren).
Sodium Intake and Health Outcomes
We included randomized controlled trials, other intervention trials, and prospective observational studies on all ages and both sexes with data on urinary excretion of sodium used in the analysis of sodium and health outcomes and data on one or more of the following cardiovascular health indicators: blood pressure, cardiovascular disease events or mortality, and other indicators. We included original study reports or meta-analyses. We excluded studies that restricted analyses to people with a specific acute illness.
FACTORS AFFECTING URINARY SODIUM EXCRETION AND SODIUM INTAKE ESTIMATION
Physiologic Factors
Factors affecting sodium absorption, metabolism, and excretion can alter the amount of sodium consumed that is excreted through urine (65). Normally, almost all of sodium consumed is absorbed through the intestines, and when sweating is not excessive, about half of sodium consumed on a particular day is excreted in urine the next 18–31 hours (12, 41, 137). When intake is held constant for three or more days, the majority of sodium consumed is thought to be excreted through urine (about 90%), regardless of the amount of water consumed (65). Recent balance studies of three-week duration or more confirm that, regardless of intake, 97%–99% of sodium consumed is absorbed, with a small amount (0.1–0.2 g/d) excreted in the feces (65, 80, 116).
Within the body, sodium is the main cation of the extracellular fluid, with the majority sodium found in the extracellular fluid (plasma and interstitial fluid) and the remainder found within cells. Medical physiology and nutrition texts indicate that the concentration of sodium in the plasma (140 mmol/L) and the interstitial fluid (145.3 mmol/L) are similar and constant and more than 10 times that of the concentration within cells, such as in muscles (13 mmol/L) (7, 53). Normal kidneys play a key role in maintaining plasma sodium concentration through excretion and reabsorption of water and sodium based on neural and hormonal signals (53). When sodium intake is low, angiotensin II and aldosterone increase, sodium and water are reabsorbed, and less sodium and water are excreted (53). When sodium intake is high, these hormones decrease, and more sodium and water are excreted in sweat and urine (65). In controlled conditions when substantial sweating does not occur, the amount of sodium excreted in sweat is small, about 0.1–0.3 g (4–12.7 mmol) per day (65, 116, 122). The human body requires almost two days to excrete the amount of water and sodium in an acute isotonic saline infusion, e.g., 30 ml/kg body weight infused over 25 minutes (31). When the amount of sodium intake is decreased or increased and then held constant, it takes about three days for the amount of sodium excreted in urine to equal intake (53) (Table 1).
Table 1.
Recent sodium balance studiesa
| First author (reference) | N | Sex | Age (y) | Race, country | T (°C) | RH (%) | Physical exercise (min/d) | D (d) | Na intake [g/d (mmol/d)] | 24-h urinary Na excretion [g (mmol)] | Excretion/ intake (%)b | Na retentionc [g/d (mmol/d)] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rakova (122) | 4 | M | 33–40 | ND, Russia | 18–25 | 30–85 | 30–60 | 29d | 4.81 (209)e | 4.27 (186) | 89 | 0.5 (23.4) |
| Rakova (122) | 4 | M | 33–40 | ND, Russia | 18–25 | 30–85 | 30–60 | 29d | 3.61 (157) | 3.30 (144) | 91 | 0.3 (13.4) |
| Rakova (122) | 4 | M | 33–40 | ND, Russia | 18–25 | 30–85 | 30–60 | 29d | 2.40 (107) | 2.56 (111) | 107 | −0.1 (−4.4) |
| Rakova (122) | 6 | M | 27–38 | ND, Russia | 18–25 | 30–85 | 30–60 | 29f | 4.43 (193) | 4.19 (182) | 95 | 0.2 (10.6) |
| Rakova (122) | 6 | M | 27–38 | ND, Russia | 18–25 | 30–85 | 30–60 | 29f | 3.27 (142) | 2.92 (127) | 89 | 0.4 (15.5) |
| Rakova (122) | 6 | M | 27–38 | ND, Russia | 18–25 | 30–85 | 30–60 | 29f | 2.17 (96) | 1.89 (82) | 87 | 0.3 (12.3) |
| Rakova (122) | 6 | M | 27–38 | ND, Russia | 18–25 | 30–85 | 30–60 | 29f | 4.35 (189) | 4.02 (175) | 92 | 0.3 (13.9) |
| Heer (58) | 9 | M | 26g | ND, German | 24 | 55 | 0h | 6i | 0.7 mmol NaCl/kg/dj | 1.47(64) | ND | −0.4 (−17) |
| Heer (58) | 9 | M | 26 | ND, German | 24 | 55 | 0 | 6i | 2.8 mmol NaCl/kg/d | 3.82 (166) | ND | 0.7 (32) |
| Heer (58) | 9 | M | 26 | ND, German | 24 | 55 | 0 | 10i | 7.7 mmol NaCl/kg/d | 11.98 (521) | ND | 0.6 (24) |
| Heer (58) | 9 | M | 26 | ND, German | 24 | 55 | 0 | 6i | 0.7 mmol NaCl/kg/d | 1.66 (72) | ND | −0.6 (−26) |
| Kodama (80) | 109 | B | 18–28 | ND, Japan | 22–29 | 40–65 | Varied, up to 120 | 5–12 | 2.21–6.87 (51–158) | NDk | ND | ND |
| Palacios (116) | 19 | F | 11–15 | Black, US | ND | ND | ND | 21 | 1.31 (43) | 0.8 (35) | 61 | 0.4 (17) |
| Palacios (116) | 19 | F | 11–15 | Black, US | ND | ND | ND | 21 | 3.95 (174) | 2.5 (109) | 63 | 1.0 (43) |
| Palacios (116) | 12 | F | 11–15 | White, US | ND | ND | ND | 21 | 1.31 (43) | 0.9 (39) | 96 | 0.2 (9) |
| Palacios (116) | 10l | F | 11–15 | White, US | ND | ND | ND | 21 | 3.95 (174) | 3.3 (143) | 84 | 0.3 (13) |
| Heer (57) | 8 | M | 25g | ND, German | 24 | 60 | 0h | 7 | 0.46 (20)m | ND | ND | NDn |
| Heer (57) | 8 | M | 25 | ND, German | 24 | 60 | 0 | 7 | 1.84 (80) | ND | ND | ND |
| Heer (57) | 8 | M | 25 | ND, German | 24 | 60 | 0 | 7 | 3.68 (160) | ND | ND | ND |
| Heer (57) | 8 | M | 25 | ND, German | 24 | 60 | 0 | 7 | 5.06 (220) | ND | ND | ND |
Abbreviations: B, both male and female; D, duration of balance study; F, female; M, male; N, number of participants; Na, sodium; ND, no data or not determined; RH, relative humidity; T, temperature.
(Average sodium urinary excretion divided by average sodium intake) × 100.
In Rakova et al. (122), Na retention is defined as daily Na intake - daily 24-h urine Na excretion, over 29 days. Estimates do not account for sweat or fecal loss of sodium. In the second study, sweat loss was estimated as 12.7 mmol/d. In Palacios et al. (115), Na retention was based on Na intake - (24-h urine Na excretion + Na in feces + Na in sweat) over 20 days (with sweat collected over the last two weeks of each balance period).
Participants consumed diets with sequentially lower daily average amounts of salt (12 g, 9 g, 6 g) over a period of 105 days, with salt maintained at the given level for at least 29 days. Other nutrients were held constant. Microgravity was not simulated (122).
Information based on average daily Na content of individualized meal plans (122).
Participants consumed diets with sequentially lower daily average amounts of salt (12 g, 9 g, 6 g) and then the average amount of salt was increased 12 g over a period of 205 days, with salt maintained at the given level for at least 29 days (122).
The same participants were sequentially fed diets with the amount of sodium based on body weight for the number of days specified (58).
Authors did not report the average sodium intake for each group but indicated the average body weight of participants was 71.5 kg (116).
In this analysis of 11 previously published balance studies, numeric information on urinary sodium excretion and retention is not available; this information is presented in figures only (80).
Participants were randomly assigned to controlled diets in a crossover design. Two participants did not complete this study arm (57).
In this study, 32 participants were randomly assigned to one of four diets. Here we converted meq NaCl/d to mmol Na/d by multiplying NaCl times 0.4 (57).
Na retention data are provided in figures. Retention levels on the first day of each study period are provided in the text as well as storage from the first to last day of the study period: Participants who consumed 400 mmol NaCl/d stored 338 mmol Na, and those who consumed 550 mmol NaCl/d stored 202 mmol Na from the first to the last day of the study period.
Additional metabolic factors
Sodium metabolism may be even more complex and might include a third fluid compartment (98, 141) and an additional extrarenal regulatory mechanism contributing to sodium and water homeostasis and blood pressure control. Titze (141), in a review of recent studies from his group, suggests substantial amounts of excess sodium are found “bound to glycosaminoglycans in skin and in muscle” (p. 102). Marvar et al. (98) indicate that these studies suggest sodium is present under the skin on proteoglycans in an osmotically inactive state, causing lymph vessel growth. In two small highly controlled balance studies of 105 days or more in young adult men, changes in total body sodium content of ± 200–400 mmol had a monthly or longer duration without parallel changes in total body water content (122). These studies require replication in other groups and, if possible, with larger sample sizes, but results could explain the observed sodium retention with excess sodium intake in recent reports of other controlled balance studies (57, 58, 80, 116) (Table 1). In contrast to this theory, authors of two of these studies (80, 116) suggest sodium could be retained in the water in bones rather than a reservoir in skin.
Demographic Characteristics
On average, urinary sodium excretion varies by sex, age, and race-ethnicity, but these variations may be related to numerous lifestyle (e.g., sodium intake) and environmental factors or differences in the presence of chronic disease conditions and related medication use. As with sodium intake, urinary sodium excretion tends to be higher in males than females, among adults than children, and lower among the elderly than young or middle-aged adults (5, 32, 41, 68). Recent studies suggest 24-h urinary volume is lower and urine osmolality and sodium concentration (mmol/L) higher while the rate of sodium excretion per 24 hours does not differ significantly among black versus white adults (9, 22, 145). When sodium intake and environmental conditions were held constant for several weeks in a balance study among adolescent girls (116), 24-h urine volume did not differ significantly by race with intake of 1.3 g/d or 4.0 g/d, nor did 24-h sodium excretion differ on a diet of 1.3 g/d. When 4.0 g/d was consumed, 24-h sodium excretion was significantly lower for black girls (2.5 g) compared with white girls (3.3 g) (116) (Table 1).
Lifestyle and Environmental Factors
Dietary intake of sodium and potassium, physical activity, and climate can affect the amount of sodium excreted in the urine.
Sodium intake
In the United States and other developed populations, the amount of sodium consumed is highly correlated with calories consumed in populations (66). In these populations, the major source of sodium intake is processed and restaurant foods rather than sodium inherent in foods or salt added at the table or during home cooking (5, 95). In less developed countries, the major source of sodium intake is salt added during home cooking (5). With higher food and caloric intake, such as in men versus women, usually more sodium is consumed and excreted (5, 40). Day-to-day variation in sodium excretion within individuals is generally greater (up to three times) than variation in sodium excretion between persons (9, 27, 32–34, 43, 96, 119).
Potassium
Independent of sodium intake, potassium intake also may increase urinary sodium excretion and can blunt the impact of sodium intake on blood pressure (65). Kanbay et al. (73, p. 1), in a 2013 review of the effects of potassium intake in mediating the effects of dietary sodium on cardiovascular disease, indicate potassium can decrease blood pressure through regulating “vascular sensitivity to catecholamines, promotion of natriuresis, limiting plasmin renin activity, and improving endothelial function.” When high sodium intake was held constant (307.7 mmol or 18 g) in a 2013 randomized controlled trial in China, potassium supplementation (60 mmol or 2.4 g potassium) resulted in a slight increase in average 24-h sodium excretion (88). Average 24-h sodium excretion increased 12 mmol (0.28 g) among 102 participants with prehypertension or hypertension, 7 mmol (0.16 g) in 172 of their siblings, and 8 mmol (0.18 g) in 47 offspring (88). The slight increase in urinary sodium excretion was accompanied by significant reductions in blood pressure (e.g., by 7 mm Hg in systolic blood pressure among participants with prehypertension or hypertension) (88). In a smaller study among 21 healthy Swedish participants consuming either 150 or 200 mmol (3.45 to 4.60 g) of sodium daily, potassium supplements of 50 mmol twice a day (3.9 g) increased mean 24 h sodium excretion by 8 mmol, but the difference was not statistically significant (100). In both randomized controlled trials, the amount of potassium was consumed from supplements rather than foods, and the amount was less than the adequate intake of 4.7 g recommended by the Institute of Medicine (65) to blunt the impact of sodium intake on blood pressure.
Physical activity and climate
Physical activity and climate (temperature, humidity) are important factors related to losses of sodium through sweat, potentially decreasing the amount of sodium intake excreted in urine. The previous Institute of Medicine review (65) and recent studies (Table 2) indicate substantial variation exists in the sodium lost through sweat. On average, sodium sweat losses can vary from 30 mmol to 140 mmol over 1–2 hours of intense exercise, with more lost in conditions of higher heat and humidity (Table 2). In recent studies, average sweat sodium concentrations varied from 17 to 20 mmol/L among 92 male 18-year-old soccer players after three weeks of training (131) to 91 mmol/L among 7 male 47-year-old badminton players (54) (Table 2). Individual variation in sweat sodium concentration was even greater in these studies (6–126 mmol/L) (Table 2).
Table 2.
Sweat sodium concentrations and losses with physical activitya
| First author (reference) | N | Sex | Mean age (y) | Country | Temp (°C) | Relative humidity (%) | Physical activity | Sweat Na concentration | Mean sweat Na loss [g (mmol)] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levela | Type | Duration (min) | Mean [g/L (mmol/L)] | Range [g/L (mmol/L)] | ||||||||
| Gibson (50) | 34 | F | 16 | Canada | 10 | 63 | Elite | Soccer | 90 | 1.1 (48) | 0.6–1.6 (27–68) | 0.7 (30) |
| Kurdak (82) | 22 | M | 20 | Turkey | 34 | 64 | ND | Soccer | 90 | 0.9–1.1 (43–46) | 0.6–1.4 (25–59) | 3.2 (137–140) |
| Horswill (62) | 14 | M | 24 | United States | 29–32 (WGBT) | ND | ND | Football | 132 | 0.9–1.2 (38–53) | ND | ND |
| Kilding (78) | 13 | F | 23 | New Zealand | 6–14 | 71–74 | International | Soccer | 90 | 1.0–1.1 (44–46) | ND | 0.7–0.8 (32–35) |
| Maughan (101) | 9 | M | 19 | Canada | 36 (27 water) | ND | Trained elite | Swimming | 105 | 1.0 (44) | ND | ND |
| Maughan (101) | 8 | F | 21 | Canada | 36 (27 water) | ND | Trained elite | Swimming | 105 | 0.9 (39) | ND | ND |
| Palmer (117) | 44 | M | 18 | Canada | 14 | 66 | Elite | Ice hockey | 60 | 1.2 (54) | 0.6–2.0 (27–88) | 2.3 (98) |
| Shirreffs (131) | 92 | M | 18 | Tunisia | 25–28 | ND | After 3-w trainingb | Soccer | 60–70 | 0.4–0.5 (17–20) | 0.1–1.2 (6–50) | 0.7 (28–29) |
| Hashimoto (54) | 7 | M | 47 | Japan | ND | ND | ND | Badminton | 180 | 2.1 (91) | 1.2–2.9 (52–126) | 2.8 (122) |
| Hashimoto (54) | 5 | F | 50 | Japan | ND | ND | ND | Badminton | 180 | 0.9 (41) | 0.4–1.6 (18–68) | ND |
| Maughan (102) | 20 | M | 21 | England | 6–8 | 50–60 | Premier league | Soccer | 95 | 1.4 (62) | 0.6–2.1 (25–90) | 2.4 (104) |
| Shirreffs (130) | 26 | M | 26 | Seven countriesc | 32 (22 WGBT) | 20 | International professional | Soccer | 90 | 0.7 (30) | 0.4–1.5 (16–66) | 1.5 (67) |
Abbreviations: F, female; M, male; N, number of participants; ND, no data or not determined; WBGT, wet bulb globe temperature, an index used for the assessment of heat stress.
Level of athletes as reported by the investigators. In Shirreffs & Maughan (131), the level is not reported, but measures of sweat were started after three weeks of training among athletes living together in a residential facility.
Athletes from seven countries; countries are not listed (130).
In the studies reviewed above, information on the amount of dietary sodium consumed was unavailable. As indicated by earlier studies, the amount of sodium lost through sweat is directly related to intake (4, 63, 65). When men were exposed to the same level of heat (40°C) on different levels of sodium intake for the last five days of an eight-day intervention diet, men who consumed more sodium excreted more sodium in their sweat (4). Similarly, in a balance study among adolescent girls, when sodium was increased from 1.3 to 4.0 grams per day, the amount of sodium excreted in sweat after two weeks of adaptation and acclimatization increased significantly (from ~ 90 mg to 120 mg/day), 7% and 3% of total dietary sodium intake, respectively (116).
Although substantial amounts of sodium can be lost through sweat, the amount may be reduced over a relatively short period of time with acclimatization to heat and exercise. Buono and colleagues (15) exposed eight healthy male volunteers (average age 26 years) to progressively increasing heat and sometimes differing humidity (36°C and 40% humidity, 40°C and 40% humidity, 42°C and 60% humidity) during 3- to 30-minute exercise bouts of walking on a treadmill in an environmental chamber for 10 days. The average sweat sodium concentration decreased 13 mmol/L over 10 days holding constant the amount of heat and humidity, confirming previous studies that suggested the body acclimates to heat by reducing the amount of sodium lost in sweat over a period of 5–10 days (63, 65).
Chronic Disease Conditions
It is well known that cardiac and kidney conditions (e.g., congestive heart failure, end-stage renal disease) result in fluid and sodium retention, decreasing urinary sodium excretion. Diuretics used to treat these conditions increase water and sodium excretion, with loop diuretics (e.g., furosemide) causing the greatest increases in water and sodium excretion (53). Some chronic conditions may not affect overall sodium excretion but may affect the circadian pattern of excretion. Urinary sodium and water excretion are normally lower at night, during sleep, and higher during the day, with a maximum about midday (9, 32, 91) (Table 3). This circadian pattern is not an issue when measuring 24-h sodium excretion but can affect the measurement when urine is collected for less than 24 hours.
Table 3.
Recent studies on circadian variations in 24-h urinary sodium excretiona
| First author (reference) | Participant characteristics | Urinary sodium excretion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M% | Age (y) | Race- ethnicity | Health characteristics | Time periods | Measure (unit) | Ratio | Pattern | |
| Bankir (9) | 141 | 64 | 18–40 | 29% B, 71% W | Healthy: BMI 18–39, Ccr ≥ 65 ml per min per 1.73 m2, difference in Cr excretion ≤50%, normotensive (not taking anti-HTN med) except 5 (3.5%) | 06:00–22:00, 22:00–06:00 | 24 h (134–159 mmol) to nighttime (ND) | 2.2–4.0 | Lower at night |
| Mill (108) | 109 | 46 | 30–74 | Brazilian | Healthy, 37.6% HTN: nonpregnant, without self-reported heart or kidney disease | 0:700–21:00, 21:00–07:00 | Day (122 mmol) to night (104 mmol) | 1.2 | Similar day and night |
| Bankir (8) | 325 | 45 | 46b | 100% B, East African descent | Family history of HTN: participants from 73 families with ≥1 siblings with HTN, urinary Cr excretion ≤0.4 mmol/kg, usual diet | Day and night | Day (ND) to night (ND) (micromoles per minute) | 0.9 | Similar day and night |
| Fukuda (44) | 65 | 51 | 16–80 | 100% A, Japanese | CKD: GFR <60 w/o diabetic nephropathy or nephrotic syndrome. Not taking anti-HTN medication or diuretics, classified by Ccr into tertilesc | 06:00–21:00, 21:00–06:00 | Day (78 mmol) to night (76 mmol) | 1.0d | Similar day and nighte |
| Agarwal (3) | 22 | 82 | 18–80 | 59% B, 41% W | CKD: GFR <60 or proteinuria of ≥1 g/d, nondialysis, ACE inhibitors and ARB constant dose ≥3 months before enrollment | Day and night | Day (6.7 mmol/h) to night (9.3 mmol/h) | 0.7 | Higher at night |
| Fukuda (45) | 27 | 44 | 20–80 | 100% A, Japanese | CKD: GFR <60/kidney damage for 3+ months, including patients with nephropathy or nephritis but w/o diabetic nephropathy or taking anti-HTN medication or diuretics, classified by Ccr into tertilesf | 06:00–21:00, 21:00–06:00 | Day (4.2 mmol/h) to night (4.1 mmol/h) | 1.0 | Similar day and nightg |
| Fukuda (46) | 26 | 57 | 17–72 | 100% A, Japanese | Glomerulopathy: diagnosed with renal biopsy. Classified by Ccr into tertilesh | 06:00–21:00, 21:00–06:00 | Day (6.7 mmol/h) to night (3.6 mmol/h) | 1.4i | Lower at nightj |
Abbreviations: A, Asian; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; B, black race; BMI, body mass index in kg/m2; Ccr, creatinine clearance in mL/min; GFR, glomerular filtration rate in ml/min/m2; HTN, hypertension; M, male; N, number of participants; ND, not reported; W, white race.
Average age; range not reported (8).
Tertile 1, Ccr 91–164 mL/min; tertile 2, Ccr 50–90 mL/min; tertile 3, Ccr 5–41 mL/min (44).
Average night:day ratio in urine sodium excretion rate (mmol/h) was reported as 1.05 (44).
Average night:day ratios in urinary sodium excretion rates (mmol/h) were 0.59 for tertile 1 of creatinine clearance (highest), 0.86 for tertile 2, and 1.69 for tertile 3 (lowest) (44).
Group A, Ccr > 90 mL/min; Group B, Ccr 30–89 mL/min; Group C, Ccr < 29 mL/min (45).
Nocturnal reduction in urinary sodium excretion rates (mmol/h) significantly different (P < 0.001) by level of creatinine clearance. Nocturnal reduction is highest in the group with the highest creatinine clearance and lowest in the group with the lowest creatinine clearance (45).
Lower Ccr 16–62 mL/min; medium Ccr 75–92 mL/min; higher Ccr 106–15 1 mL/min (46).
In the paper, the night:day ratio for the overall group is 0.75, which is different from that calculated from the average day:night ratio (46).
Nocturnal reduction in urinary sodium excretion rates (mmol/h) significantly different (P <0.012) by level of creatinine clearance. Nocturnal reduction is highest in the group with the highest creatinine clearance (night:day ratio 0.44) and was flat (night:day ratio 0.99) in the group with the lowest creatinine clearance (46).
Hypertension
The diurnal pattern in sodium excretion among healthy individuals can be reversed or altered with hypertension. The usual lower nighttime/higher daytime urinary sodium excretion pattern was flattened among 107 hypertensive men and women aged 41–80 years who were not on diuretics or other antihypertensive medication (34, 134). In more recent reports (8, 108), similar nighttime and daytime rates of urinary sodium excretion were noted in a group of adults among whom 37.6% had hypertension and in a separate group of adults with a family history of hypertension (Table 3).
Chronic kidney disease
In recent studies among patients with chronic kidney disease (3, 44, 45), the usual circadian pattern of nocturnal dipping of urinary sodium excretion was either flattened or reversed (Table 3). Further, the flattening or reversal of the nocturnal dipping in sodium excretion was more pronounced among patients with lower creatinine clearance (44–46). Fukuda et al. (47) recently studied the effects of angiotensin receptor blockers on the nocturnal dipping pattern of blood pressure and the change in dipping pattern on the diurnal patterns of urinary sodium excretion in the 41 adults aged 17–75 years with chronic kidney disease without diabetic nephropathy or nephrotic syndrome. The restoration of the nocturnal dipping pattern in blood pressure with angiotensin receptor blocker administration was correlated with an increase in daytime urinary sodium excretion but no change in the pattern of nighttime sodium excretion (47).
Collection and Laboratory Analysis Methods
Urine collection
For 24-h urine collection, under- and overcollection can bias results. If the start and stop times of the 24-h urine collection are accurate, collecting more urine beyond the 24-h time period can be adjusted in analysis. Missing a urine void or spilling urine voids can result in undercollection. In the global, population-based INTERSALT and INTERMAP studies (39, 126, 135), emphasis was placed on ensuring complete collection through detailed data procedures, such as complete and detailed written and verbal instructions, starting and stopping collection in person, and asking participants to recollect a sample if they reported missing a void or the urine volume was low, defined as total urine volume <250 ml in 24 hours.
Post collection, additional endogenous and exogenous factors are used to identify and exclude potentially incomplete urine collection, the most common of which are urinary creatinine excretion and para-amino benzoic acid (PABA) recovery. Urinary creatinine excretion (endogenous) is used to assess completeness of urine collection because creatinine excretion is considered to be less variable in urine than sodium. When observed creatinine excretion is less than expected based on a person’s age, body size, or sometimes sex, the urine is judged to be potentially incomplete (11). Creatinine excretion can vary substantially from day to day and in relation to age, muscle mass, and dietary factors such as meat consumption, suggesting it can be a poor marker of completeness of collection (11, 38, 79, 111).
PABA is completely absorbed and 93% is excreted in urine within five hours of administration (10). PABA recovery requires the participant to consume three 80-mg doses of (exogenous) PABA, one with each meal, and then the amount of PABA recovered in urine is measured (10). Generally, a 24-h urine collection with 85%–110% of PABA recovered is considered complete (10, 138). Timing and subject age can affect urine excretion (69, 79, 85).
Transport and storage
Preanalytic variables such as transport and storage are not usually an issue in the estimation of sodium from urine. Sodium is stable indefinitely when frozen and for at least 45 days when stored at room temperature (150); however, the latter is not recommended because it will lead to bacterial growth in the urine. If the specimen cannot be immediately frozen it should be stored refrigerated for a limited time period. It has been shown that multiple (up to six) freeze/thawing cycles (four hours at room temperature per cycle) did not affect urine sodium concentrations (118). Also, long-term (20–25 years) frozen storage of urine samples at −70°C did not appear to cause any appreciable specimen desiccation (118).
Laboratory analyses
The standard AOAC method for sodium is ion-selective electrode (ISE) assays. These ISE assays have low imprecision, with between-assay coefficients of variation of usually less than 3% (118). Assay performance can be verified by participating in proficiency testing programs, such as the College of American Pathologists General Urine Chemistry and Urine Chemistry Calibration Verification/Linearity Surveys. Standard Reference Materials are also available from the National Institute of Standards and Technology for calibration verification; e.g., SRM 2201 Sodium Chloride (ISE). As long as standard protocols are used for transport and storage, and the AOAC method is used for analysis with attention to quality control, these variables are not an issue for estimation of sodium intake from urinary excretion (118, 150).
CURRENT APROACHES TO ASSESS SODIUM INTAKE THROUGH URINE BIOMARKERS
Population Sodium Intake
Study characteristics
Our objective was to evaluate urine biomarkers used in recent population-based studies to estimate sodium intake. Of the population-based studies we reviewed, 29 had data collected since 2000 on urine biomarkers to assess sodium intake (Table 4). We grouped the studies by national and subnational level and then from most recent to oldest in relation to last year of data collection (Table 4). Twelve studies were conducted at the national level and 17 at the subnational level (province, region, county, city). Of the national-level studies, all but two (42, 86) were conducted in Europe or North America. All but one (64) included both sexes. Across studies, the age groups ranged from preschool and school-aged children to the elderly, with most data collected among young and middle-aged adults. All but six were conducted among adults only. In three of the studies including children, data were collected from adolescents and combined with data on adults (20, 109, 129); in the three remaining, population level (national or subnational), age group, and type of specimen collection varied. At the national level, in England, in 2008–2009 and 2011–2012, 24-h urine specimens were collected among the population aged 4 years and older and analyzed separately for children aged 4–6, 7–10, and 11–18 years (112). At the subnational level in Iran, spot urine specimens were collected and analyzed in two studies, one among children aged 3–10 years (75), the other among children aged 7–12 years (60).
Table 4.
Urine biomarkers used to assess population sodium intake in national and subnational studies ordered by the last year of data collection since 2000a
| First author (reference) | Country, region, or cityb | Age (y) | M (%) | Data, year | Urine biomarker used to assess sodium intake | |||
|---|---|---|---|---|---|---|---|---|
| Analysis other than Na | Type | Indicators/ equations used to estimate Na intake | Type of population statistics reported for Na and related nutrients | |||||
| National | ||||||||
| The Scottish Government (129) | Scotland | 16+ | 45 | 2012–2013 | K, Cr | Spot random | Na (mmol/L) Na/Cr ratio |
Mean (SE) Median, percentiles |
| National Diet and Nutrition Survey (112) | England | 4+c | 46 | 2008–2009, 2011–2012d | PABA | 24 h | Salt intake (g) = urine Na (mmol/24 h)/17.1 | Mean (SD, SE) Median 2.5th, 97.5th percentiles % Distribution at various thresholds (e.g., >6 g/d) |
| Donfrancesco (30) | Italy | 35–79 | 51 | 2008–2012 | K, I, Cr | 24 h | Na Na/K |
Mean (SD, 95% CI) Frequency distribution |
| Chappuis (20) | Switzerland | 15+ | 49 | 2010–2011 | Cr | 24 h | Salt (1 mmol Na = 0.0584 g NaCl) Volume adjusted for time |
Mean (SD) Distribution % <5 g salt/24 h Linear regression with BP |
| Pfeiffer (118) | United States | 20–59 | 48–53 | 1988–1994e 2003–2006, 2010 | Cl, K, Cr | Spot, random | Estimated Na intake (mg/24 h) based on spot urine Na excretion used with INTERSALT equationsf | Mean (SE) Distribution Temporal trends in mean estimated Na intake adjusted for age, sex, race-ethnicity |
| Lee (86) | Korea | 20+ | 46 | 2009–2010 | Cr, SG, SGU = (SG-1) × 100 | Spot, fasting | Spot Na (mmol/L) Spot Na/Cr Spot Na/spot SGU |
Mean (SD) Quartiles of Na/Cr and Na/SGU Association with BP |
| Scottish Center for Social Research (128) | Scotland | 19–64 | 46 | 2009 | PABA | 24 h | Salt intake (g) = urine Na (mmol/24 h)/17.1 | Mean (SD, 95% CI), median 2.5th and 97.5th percentiles % Distribution at various thresholds (e.g., >6 g/d) |
| Ortega (115) | Spain | 18–60 | 47 | 2009 | K, Cr | 24 h | Na Na/K Na/Cr |
Mean (SD) Median (IQR) Quintiles % >85 mmol Na/d (5 g salt) OR for >200 mmol Na/d |
| Millett (109) | England | 16+ | ND | 2003–2007 | ND | Spot, random | Salt intake (g) = urine Na (mmol/spot)/17.1, 1 g salt = 17.1 mmol Na | Mean (95% CI) Temporal trends |
| Erdem (42) | Turkey | 18+ | 46 | 2007 | K, Ca, U, Cr | 24 h | Na Na/K |
Mean (SD) Correlation with BP |
| Ribic (124) | Slovenia | 25–65 | 43 | 2007 | Cr | 24 h | Salt intake (g/d) = urine Na (g/24 h) × 2.54 Na corrected to 24 h |
Mean (SD) Correlation, salt intake with BMI |
| Subnational | ||||||||
| Kelishadi (75) | Iran, Isfahan | 3–10 | 49 | 2011–2012 | K, Cr | Spot, first morning fasting | Spot (mEq/L) Spot Na/Cr Spot Na/K |
Mean (SD) Median (IQR) Linear regression with BP Correlation |
| Land (84) | Australia, Lithgow | 20+ | 47 | 2011 | Cr | 24 h | Na (g/24 h) Salt (Na × 2.542) |
Mean (SD) |
| Angell (6) | United States, New York City | 18+ | 42 | 2010 | Cr | 24 h | Normalized to 24-h collection time Na Na/K |
Mean (SE) Median 10th and 90th percentiles Percent below threshold |
| Zhang (151) | China, Shandong Province | 18–69 | 52 | 2011 | Cr | 24 h | Na (mmol/24 h) Salt intake (g/24 h) |
Mean (SD, 95% CI) |
| Rhee (123) | Korea, Goyang | 20–65 | 42 | ND | K | 24 h | Na | Mean (SE) |
| Hendriksen (59) | Netherlands, Doetinchem | 19–70 | 43, 45 | 2006, 2010 | K, I | 24 h | Adjusted for% of intake excreted in urine (95%) Salt intake (adjusted Na intake × 2.54) |
Mean (SD) Temporal trends |
| Kudo (81) | Japan, Takahata | 40+ | 45 | 2009 | Cr, Alb, β2–micro globulin | Spot, early morning | Estimated 24-h Na excretion (mEq/L) using the Kawasaki equation with spot Na concentrationg | Mean (SD) As a predictor of urine β2–microglobulin |
| Khosravi (76) | Iran, Isfahan | 19+ | 43 | 2007 | K, Cr | 24 h | Na/Cr | Mean (SD) Temporal trends |
| Cipullo (23) | Brazil, São José do Rio Preto | 18–93 | 49 | 2004–2005 | Cr | 12 h | Na (mEq/L)/12 h | Categories of 12-h Na excretion |
| Honarpisheh (60) | Iran, Kashan | 7–12 | 52 | 2004–2005 | Ca, Cr | Spot, second morning, nonfasting | Na (mmol/l)/Cr (mmol/l) Ca (mmol/l)/Na (mmol/l) |
Mean (SD) Percentiles |
| Hulthen (64) | Sweden, Gothenburg | 19 | 100 | 2005 | K, PABA | 24 h | Na (mmol) Na/K (mmol) |
Mean (SD) Distribution Quartiles |
| Meyerfreund (107) | Brazil, Aracuz Indian Reserve | 29–34 | 48 | 2003–2004 | K | 12 h, 19:00–07:00 | Estimated 24-h Na excretion based on assumption of 45% of sodium excreted in this time periodh | Mean (SD) Correlation with BP Linear regression with BP as outcome |
| Maseko (99) | South Africa, Johannesburg | 21–72 | 37 | 2003 | K, Cr | 24 h | Na (mmol/L) Na (mmol) |
Mean (SD) 24-h urine Na > 65 mmol |
| Laatikainen (83) | Finland | 25–64 | 47 | 1979, 1982, 1987, 2002 | K, Cr | 24 h | Na Na/K Salt |
Mean (SD) Temporal trends |
| Cappuccio (17) | Ghana, Ashanti | 40–75 | 48 | 2001–2002 | K, Cr | 24 h, 2 consecutive, timed | Average of 2 consecutive urine collections, normalized to 24-h collection time Na (mmol/24 h) Na/K, Na/Cr |
Mean (SD) Mean (SD) pre- and postintervention |
| Hedayati (56) | United States, Dallas County | 30–65 | 44 | 2000–2002 | K, Cr, Alb | Spot, first morning, fasting | Na/K | Mean (SD) Median (IQR) Continuous variable Regression with BP as the outcome |
| Rodrigues (125) | Brazil, Vitória | 25–64 | 46 | 1999–2001 | K, Cr | 12 h, timed, 21:00 to 07:00 | Na (12 h) Na/K |
Mean (SD) |
| Ejike (35) | Nigeria, Anyigba | 19–40 | 50 | ND | K | Overnight | Na (mEq/L) Na/K |
Mean (SD) Tertiles Correlation with BP |
Abbreviations: Alb, albumin; BMI, body mass index; BP, blood pressure; CI, confidence interval; Cl, chloride; Cr, creatinine; FM, first morning; I, iodine; IQR, interquartile range; K, potassium; M, male; Na, sodium; NFS, not further specified; PABA, para-amino benzoic acid recovery = % of PABA intake recovered in the urine; SD, standard deviation; SE, standard error; SG, specific gravity.
Region or city name listed when applicable and available for surveys conducted at the subnational level.
Results are reported separately for children aged 4–6, 7–10, and 11–18 years and adults aged 65+ years (112).
Results are reported for all four years combined (112).
Spot urine specimens came from participants in the National Health and Nutrition Examination Survey conducted during each time period. In 1988–1994, specimens came from a convenience sample of participants; in 2003–2006 and 2010, participants were randomly selected (118).
INTERSALT estimation equations, Na intake for males (mg/d) = 23 × {25.46 + [0.46 × spot Na (mmol/L)] − [2.75 × spot Cr (mmol/L)] 2 [0.13 × spot K (mmol/L)] + [4.10 × BMI (kg/m2)] + [0.26 × age (y)]} and for females (mg/d) = 23 × {5.07 + [0.34 × spot Na (mmol/L)] 2 [2.16 × spot Cr (mmol/L)] 2 [0.09 × spot K (mmol/L)] + [2.39 × BMI (kg/m2)] + [2.35 × age (y)] − [0.03 × age2 (y)]} (117).
Kawasaki equation, 24-h urinary Na excretion (mEq/L) = (16.3 × XNa0.5), where XNa = [spot Na (mEq/L)/spot Cr (mg/dl) × 10] × [Pr24hCr (mg/day)] (81).
Based on previous study in an urban population in which two 12-h urine specimens were collected (107).
Urinary biomarkers and types and data collection procedures
Twenty-four-hour urine specimens were collected in 17 studies (8 national, 9 subnational), spot or casual specimens in 8 studies (4 national, 4 subnational), and overnight or 12-h specimens in 4 subnational studies (Table 4). In the three national-level studies, spot urine specimens were collected at variable times throughout the day (109, 118, 129), and in one, a fasting specimen was collected (86); in four subnational studies, spot specimens were collected in the morning, and in two, the timing was not specified. In two studies, a 12-h timed overnight specimen was collected (107, 125); in one, an overnight specimen, “after awakening in the morning and during awakenings at night” (35); and in one, the 12-h duration was specified, but whether it was collected during the day or overnight is unclear (23). In one study, 24-h urine was collected on two consecutive days (17).
Along with sodium, 21 studies included data on urinary creatinine, and 17 included data on potassium excretion. PABA, but not creatinine, was measured in three studies; two national-level studies in the United Kingdom (112, 128) and one subnational study (64).
Instructions for 24-h urine collection varied, but in general, participants were provided instructions and a kit and started and stopped collection on their own (data not shown). In 12 of the studies including 24-h urine collection, participants were instructed to completely empty the bladder upon waking in the morning, discard this urine (i.e., discard the first morning void), and record this as the start time. They were then instructed to collect all urine in the next 24 hours ending the following morning with the first void upon waking. They were instructed to record this as the stop time. The start and stop times could then be used to adjust the amount of sodium collected to 24 hours. In two studies, it appeared that participants started and stopped collection under supervision in a clinic (20, 123). In the report of one study, investigators stated the 24-h urine sample was obtained “based on the INTERSALT protocol,” with urine was collected from 7 AM through 7 AM the next day (76, 77). In two reports, we could not find information about the procedures for 24-h urine collection (17, 30). Information on the types and amounts of “incentives” or “tokens of appreciation” for 24-h urine collection was not reported in the publication of results, with two exceptions. In New York City, participants received 100 US dollars for a 24-h urine sample (6). In the National Diet and Nutrition Survey in England, participants received 15 UK pounds in “high street vouchers” (112).
Indicators of sodium intake
Indicators used to assess sodium intake ranged from spot urine sodium concentrations (e.g., mmol/L) to 24-h sodium excretion (mmol/24 h). Two of the studies estimated 24-h sodium excretion from spot specimens using prediction equations (81, 118). In the remainder of the studies with spot urine collections, sodium or salt (NaCl) intake was based on spot urine sodium concentrations (e.g., mmol/L), mmol per spot urine collection, or sodium as a ratio to potassium or creatinine (Table 4). One estimated 24-h sodium excretion on the assumption that 45% of the sodium was excreted in the timed 12-h specimen (107). Of the studies that collected 24-h urine, only one (59) reported using a factor (95%) to adjust the estimate of intake for the percentage of sodium lost through sweat or stool.
In all but one study, investigators reported the mean urinary sodium concentration or mean amount of sodium excreted per 24 hours (Table 4). In 15, investigators reported the proportion of the population with sodium excretion above or below specific thresholds, in categories (e.g., tertiles), or as a percentile or frequency distribution. In nine, investigators examined individual sodium excretion as a correlate or determinant of blood pressure (n = 6) or other variables.
Participation and completion of urine collection
Among the studies collecting 24-h urine specimens to estimate sodium intake, participation rates varied widely, as did definitions used for participation and completion (Table 5). Among the 13 studies reporting participation rates, 9% to 81% of individuals verbally agreed to collect or physically returned a 24-h urine specimen. In seven, >50% participated. In two, >70% participated (Table 5).
Table 5.
Participation and completion of 24-h urine collection in recent population-based surveys of sodium intakea
| First author (reference) | Selected | Agreed to participate | Completion of 24-hour urine collection | ||||
|---|---|---|---|---|---|---|---|
| Nb | Nc | % | Criteria for completion | Nd | %e | %e1 | |
| National Diet and Nutrition Survey (112) | 6,224 | 3,844 | 61.8 | 85%–119% PABAe recovery by colorimetry, 70%–104% PABA recovery HPLC If chose not to take PABA or took less than 3 doses, report no missed doses and collection time between 23–25 h |
1,971 | 31.7 | 51.3 |
| Land (84) | 2,018f | 327g | 16.2 | 24-h urine volume ≥500 ml 24-h urine Cr ≥4 mmol for women and 6 mmol for men 24-h urine Cr within ± 3 SD from the mean |
306 | 15.2 | 93.6 |
| Angell (6) | 5,830 | 2,333 | 40.0 | 24-h urine volume ≥500 ml 24-h urine Cr ≥6.05 mmol for men or 3.78 mmol for women Self-report of a complete collection (no missing voids) |
1,656 | 28.4 | 71.0 |
| Zhang (151) | 2,184 | 2,061h | 94.4 | 24-h urine volume 24-h urine Cr relative to body weight was ≥3.81 mmol for men and 4.57 mmol for women (greater than or equal to 2 SD below the population mean) |
1,938 | 88.7 | 94.0 |
| Donfrancesco (30) | ND | 2,400 | ND | 24-h urine volume ≥500 ml 24-h urine Cr relative to body weight was within <2 SD from population mean |
2,212 | ND | 92.2 |
| Rhee (123) | 875 | 496 | 56.7 | Self-reported urine loss ≤100 ml Self-reported urine loss zero or one time Cr index ≥0.7. Cr index is 24-h urine creatinine (mg/dl) / [21 × body weight (kg)]. |
368 | 42.1 | 74.2 |
| Hendriksen (59) | 1,686i | 437 | 25.9 | Urine Cr >5.0 mmol/24 h Urine Cr >6.0 mmol/24 h Urine Cr 5–6 mmol/24 h and urine volume >1 liters ≤One missing void ≤One over collection (outside of 24 h) |
342 | 20.2 | 81.7 |
| Khosravi (77) | ND | 842h | ND | Incorrect collection of 24-h urine samples based on creatinine values, NFS Indicated INTERSALT protocol used for urine collection |
806 | ND | 95.7 |
| Scottish Center for Social Research (128) | 1,691 | 937h | 50.2 | PABA (% recovery of consumed PABA) 85%–110%, colorimetric assay; or If PABA >110% colorimetric, PABA by HPLC 78%–110%; or If PABA 70%–85%, colorimetric and PABA by HPLC 75%–77% HPLC with correction |
702 | 37.6 | 74.9 |
| Ortega (115) | 1,835 | 492 | 26.8 | Estimated FFM compared with FFM from bioelectrical impedance (criteria NFS). Estimated FFM (kg) = 0.02908 × 24-h urine Cr (mg/d) + 7.38 | 418 | 22.8 | 85.0 |
| Chappuis (20) | 14,928 | 1,448h | 9.7 | 24-h urinary volume ≥300 ml No report of missed doses (collected all 24-h urine) Urinary Cr excretion by weight (kg) >5th percentile by sex (i.e., 0.121 mmol/kg/24 h in men and 0.082/kg/24 h in women) |
1,351 | 9.1 | 93.3 |
| Ribic (124) | 600 | 158 | 26.3 | Urinary Cr index ≥120 men or ≥124 for women. Urinary Cr index (mmol/kg/d) = 24-h urine Cr (mmol/L) × 24-h urine volume (L)/body weight (kg) × 1,000 | 143 | 23.8 | 90.5 |
| Erdem (42) | ND | 1,775h | ND | Urinary Cr relative to body weight (10.7–26.0 g/kg for women and 12.1–28.9 g/kg for men) No reported HTN (n = 267 with reported HTN) Not taking anti-HTN medication (n = 192 reported taking anti-HTN medication)j |
816 | 45.9 | ND |
| Hulthen (64) | 106k | 86 | 81.1 | PABA recovery ≥85% | 79 | 74.5 | 91.9 |
| Cappuccio (17) | 1,896 | 1,013h | 53.4 | Baseline, ND | 1,013 | 53.4 | 100 |
| Maseko (99) | ND | 438 | ND | Urine volume ≥300 ml/24 h 24-h urine Cr 3.5–35 mmol for males and 3.5–30 mmol for females |
310l | ND | 70.8 |
| Laatikainenm (83) | 2,240 | 1,564n | 69.8 | Urinary Cr >6.0 mmol/d Urinary Cr 5.0–6.0 mmol/d and urine volume ≥1,000 ml |
909o | 40.6 | 58.1 |
Abbreviations: Cr, creatinine; FFM, fat-free mass; HPLC, high-performance liquid chromatography; HTN, hypertension; INTERSALT, an international study on electrolytes and blood pressure; in this protocol, participants who did not return a complete sample were asked if they would repeat a 24-h urine collection; ND, not determined or no data; NFS, not further specified; PABA, para-aminobenzoic acid, SD, standard deviation.
Number of individuals asked to collect a 24-h urine specimen.
Number of individuals who agreed to collect a 24-h urine specimen.
Number of individuals who returned a complete 24-h urine specimen.
Percent of individuals selected or asked to collect a sample who returned a complete sample.
Percent of individuals who agreed to participate (agreed to collect a 24-h urine specimen) who returned a complete sample.
Original sample was 2,152; 2,019 excludes 5 who had died or 117 moved away before contact and 11 aged <20 years (84).
A total of 329 agreed; 327 excludes 2 participants aged <20 years (did not meet age criteria) (84).
Agreed to participate and collected a 24-h urine specimen; the proportion who agreed to participate and did not provide a specimen is unclear or not available (17, 20, 42, 77, 128, 151).
Data shown for 2010 only; data for 2006 similar (59).
Unclear if these reported HTN and taking anti-HTN medications are mutually exclusive (42).
Number not reported in text; it is based on the reported random 10% selection of participants from a sample of 1,068 participants randomly selected and participating in a population-based study (107).
Number not reported in text; it is based on a sample of 438 enrolled participants, of whom 128 of the 438 did not “meet prespecified quality criteria for 24-h urine collection” (99).
Data reported here for 2002 only (83).
Specifies the number selected who participated in the baseline survey; unclear if all were asked to participate in the 24-h urine collection (83).
A total of 919 participants completed urine collection, but 10 did not meet completion criteria in relation to urine volume and creatinine (83).
For all the studies except two, more than 70% of those who agreed to participate completed their 24-h urine collection (Table 5). In two, completion rates were 51% (112) and 58% (83), one of which included children (112). Although participation rates did not vary significantly by age in that study, completion rates were 40% among children aged 4–18 years and 60% among adults 19+ years (112). Indicators to assess completion of 24-h urine collection included self-report of missing urine collection, urine volume, collection time, 24-h urine creatinine criteria, and PABA recovery rates. In 16, either creatinine or PABA was used. No two definitions for completion of 24-h urine collection were exactly the same. In relation to potential overcollection, in one study (59), participants were excluded if they reported collecting specimens outside the 24 hours. In two (20, 123), collection start and stop were supervised.
In the studies included in this review, participation in spot urine collection, for studies reporting this information, generally ranged from 73% to 100%, with lower rates in national-level surveys in Scotland (67%) (129) and England (9%–41.3%) (109) corresponding with participation in the particular survey component in which urine collection was offered (data not shown).
Strengths and Limitations: Population Sodium Intake
Strengths
The majority of recent national- and subnational-level studies using urine biomarkers to assess population sodium intake collected 24-h urine. Twenty-four-hour specimens are recommended for assessing population-wide mean sodium intake (66, 149) and were chosen as the primary metric of sodium intake in the most recent systematic analyses of global sodium intake (110, 120) because of the “known larger measurement errors” in self-reported dietary methods (120, p. 2).
We identified new reports (not included in previous systematic reviews) of national and subnational population-based studies (6, 30, 59, 64, 76, 84, 112, 123, 151) using 24-h urine specimens. All but two, which were subnational (76, 151), were conducted in high-income countries. This suggests the cost of collecting and processing 24-h urine specimens may be a barrier to estimating sodium intake. Furthermore, only one study included separate estimates for children.
Limitations
Other than cost, potential limitations in the use of 24-h urine collection to assess sodium intake in population-based studies include bias due to the high participant burden of collection, underestimation due to missed or lost urine, and under- or overcollection due to incorrect timing. Assessment of population sodium intake using spot urine specimens is potentially limited by variation in the rate of sodium excreted during the day versus the night. In addition, the within-individual day-to-day variability in urinary sodium excretion is an issue for any type of urine collection. We discuss each of these issues in more detail below.
Participation bias
In most studies using 24-h urine collection, low participation is noted as a limitation. Our review suggests that the proportion of people selected in recent population-based studies who agreed to collect and/or return a 24-h urine specimen was generally lower than 70%, and among those who participated, the proportion with potentially complete urine collection was generally higher than 70%. Even when the majority (>50%) of those selected participated in a study, further exclusions of potentially incomplete urine specimens resulted in a smaller and more select sample, with often less than 50% of those selected included in the final analysis. Some argue that using a convenience sample may be less costly and as useful as a population-based sample where diets are high in sodium (84). However, using a convenience sample could result in other biases (e.g., healthy worker) and may not be appropriate for monitoring temporal trends because selection may be difficult to replicate over time.
Few studies included data to determine whether the final participants represent the population of interest or systematically differ from those excluded. A recent study in New York City (6) suggested those completing 24-h urine collection were less likely than the target population (differences of 5 percentage points or more) to be aged 18–24 years (6% versus 13%) or Asian (5% versus 10%) and more likely to be aged 65 and older (21% versus 16%), female (58% versus 53%), have higher income (33% versus 28%), or have hypertension (36% versus 30%). However, participants did not differ, by 5 percentage points or more, from New York City residents in the distribution of country of birth, body mass index, diet quality, smoking status, diabetes, or chronic kidney disease (6). A study conducted in the Netherlands indicated participants were more likely than the general Dutch population to be highly educated and nonsmokers (59). How differences in the distributions of these characteristics might affect estimates depends on their associations with sodium intake within the specific population. A useful approach used by studies was to apply sample weights to account for differences in demographic characteristics due to sampling and nonresponse (6, 84, 112, 118, 123, 128, 129, 151).
Completion of 24-h urine collection
As noted from the studies included in this review, no single standard exists for excluding potential incomplete urine collection. Further, few studies include criteria related to overcollection. Accurately timed urine collection, urine volume, and reports of “missing more than a few drops” were used to determine complete collection in INTERSALT and INTERMAP, but not creatinine or PABA recovery criteria. Creatinine criteria were considered but rejected because of poor sensitivity/specificity, and PABA criteria for logistic reasons and because of limited information on use in different countries. In contrast, in recent population-based studies creatinine criteria were commonly used, whereas urine volume and reported missing urine collection were used less frequently. In most studies, it is unclear how the application of completion criteria affects population estimates of sodium excretion, though exclusions of seemingly incomplete collections will likely result in higher population estimates of sodium intake. In Switzerland (20), excluding potentially incomplete urine excretion did not substantially increase the estimate of average sodium intake, 9.4 g/d versus 9.1 g/d. However, only about 7% of participants were excluded for incomplete urine collection. Whereas completion also may be an issue for 12-h or overnight urine collection, it is not an issue for spot or casual urine samples, which measure urine sodium concentration or ratios (sodium-creatinine or sodium-potassium) unless the amount of sodium in the void is of interest.
Intraindividual day-to-day variability in sodium excretion
All but one of the population-based studies in this review used a single day to assess sodium intake. Elliott & Brown (38, p. 9) suggest that, “by including sufficient numbers of people, mean [group] sodium excretion can be estimated from single 24-h urine collections, with little error around the mean.” As long as 24-h urine is collected across seasons and on different days of the week to balance variability in day-to-day sodium intake, mean group sodium intake is unlikely to be biased (38).
As noted previously, considerable day-to-day variability exists in 24-h sodium excretion even under controlled environmental conditions and on a constant sodium intake consumed over long periods of time (122). This variation can be substantially increased by day-to-day fluctuations in diet by people eating their usual fare, given the large amounts of sodium present in many manufactured and restaurant foods. Fluctuations in urinary sodium excretion may also reflect sweat excretion due to increased temperature and vigorous or sustained moderate physical activity, especially in the absence of acclimatization. These variations, if random, can increase measurement error and subsequently the variability in sodium intake within a group.
About half of the studies in this review, in addition to mean intake, also estimated the group prevalence of excess or lower levels of intake and population percentiles using a single day or the mean of two consecutive days (one report). The increased variability in urinary sodium excretion due to measurement error from a single collection, or the mean of a small number of collections, results in inaccurate percentiles and overestimates the proportion of individuals in the tails of the distribution, e.g., the proportion of individuals consuming <5 g of salt per day (18, 29, 147). Similar to dietary intake, when either a single day or a mean of a few days is used to estimate the population distribution of sodium intake, the spread of the estimated distribution of sodium intake will be wider than the actual distribution (18, 147). Adjusting estimates for within-individual variation requires a second measurement in a substantial number of individuals, with enough time between measurements to appropriately account for changes in individual intake (147). Few investigators acknowledged this limitation in the discussion of their results.
Diurnal variability in sodium excretion
As discussed previously, sodium excretion and concentration (mmol/L) vary across the day, which adds further intraindividual variability to the assessment of average population sodium intake if a collection period of less than 24 hours is used. As long as spot urine samples are collected across the day (morning, afternoon, evening, nighttime), the use of spot urine would result in random, rather than systematic, error. Individual spot and 12-h urine sodium concentration (mmol/l) selected at a specific time of day may differ from 24-h sodium concentration, leading to under- or overestimation of sodium intake and an inconsistent pattern among individuals. Some investigators (35, 75, 86, 125, 128) use the ratio of spot or 12-h urine sodium concentrations relative to creatinine or potassium concentrations (Table 4). In general, these ratios are used to correct for variable urine dilution (97), assuming a similar diurnal dilution pattern for sodium and the additional analyte (e.g., creatinine or potassium), which may not be the case. If the characteristics of the population remain consistent over time, prediction equations using spot urine sodium concentrations might be used to account for the differences in these characteristics between individuals in estimated mean sodium intake.
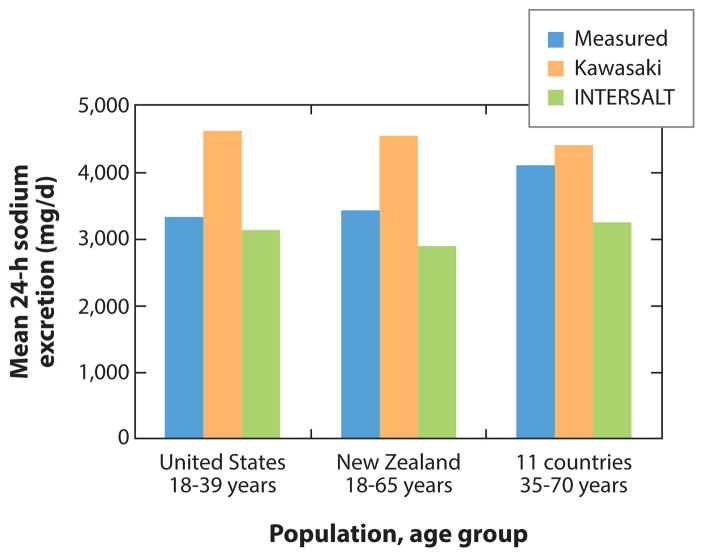
In two (81, 118) of the eight population-based studies using spot urine sodium concentrations, investigators used prediction equations (13, 74) adjusting for creatinine excretion along with other factors to estimate mean 24-h sodium excretion. The validity of the two prediction equations was recently evaluated. Results of three studies suggest that average population sodium intake is relatively unbiased when estimated among adults in the age range of 18 to 65 years using spot urine sodium concentrations with INTERSALT prediction equations (13, 25, 103). Further, the observed bias in mean estimated population sodium intake is consistent from low to high levels of sodium excretion (13). In contrast, a recent study (105) reported that the INTERSALT equation (developed for Western populations) is significantly biased among adults aged 35–70 years from different populations including groups in South America, South Africa, East Asia, and India. Moreover, the average bias in estimated sodium intake was smallest using the Kawasaki equation in comparison with two other studies (25, 103) (Figure 1). Brown and colleagues (13) recommend evaluating study-specific calibration equations against 24-h urine collection in small homogenous subgroups at baseline to ensure the validity of using spot or other partial urine specimens to monitor average population sodium intake.
Figure 1.
Mean 24-h sodium excretion based on 24-h urine collection (measured), and spot urine sodium concentration used to estimate 24-h sodium excretion based on prediction equations (74), for 339 adults (50% black race-ethnicity) aged 18–39 years living in the United States (25), 98 adults aged 18–65 years living in New Zealand (103), and 448 adults aged 35–70 years from 11 diverse countries in South America, Africa, India, East Asia, and the Middle East (105).
Sodium intake and blood pressure in cross-sectional studies
Six recent population-based cross-sectional studies examined the correlations or associations of estimates of individual sodium intake with blood pressure (Table 4), as did a recent large multicountry study (106). Because 24-h urinary sodium excretion varies from day to day within individuals, investigators estimate that anywhere from three to more than ten 24-h urine collections, and relatively more 12-h collections, may be needed to accurately estimate individual sodium intake even when individual intake is grouped broadly (89–94, 96). Given the substantial within-individual random variability in day-to-day sodium excretion, it is expected that associations with blood pressure would be attenuated when a sodium excretion is assessed on a single day or urinary measure. Despite this attenuation, in four studies, urinary sodium excretion was positively correlated with blood pressure (20, 56, 86, 115). In Angell and colleagues’ study (6) among 1,656 New York City adults, 24-h sodium excretion was positively associated with systolic (0.82 mm Hg/1,000 mg sodium/d) and diastolic (0.36 mm Hg/1,000 mg/d) blood pressure, but the association with diastolic blood pressure was not statistically significant. In Kelishadi and colleagues’ study (75) among 241 Iranian children aged 3–10 years, neither first morning spot urine sodium/creatinine nor potassium/creatinine excretion was significantly associated with blood pressure. Mente and colleagues (106) evaluated the relationship between sodium intake as measured in study participants in 18 countries by estimated 24-h sodium intake and blood pressure measured at the same point in time. Estimated 24-h sodium intake was based on spot urine sodium and creatinine concentrations. Results of this study suggested the association between sodium intake and blood pressure was steeper among participants with estimated sodium intake >5 g per day (2.6 mm Hg systolic blood pressure per gram sodium) compared with persons with estimated sodium intake <3 g per day (0.74 mm Hg per gram sodium) (106). These findings are paradoxical to those of Dietary Approaches to Stop Hypertension (DASH)-Sodium randomized controlled trial, which indicated that the linear association between sodium intake and blood pressure was stronger among individuals with sodium intake <2.4 g per day compared with intake 2.4 g/d and greater (127). Angell and colleagues (6) discussed intraindividual variability as a limitation. Mente and colleagues (106) attempted to adjust for within-individual variability in a separate sensitivity analysis. The use of a single 24-h or spot urine specimen to assess individual intake is discussed further in the next section.
Individual Sodium Intake and Health Outcomes: Prospective Cohort Studies
Study characteristics
For the purpose of this review we identified 13 recent prospective cohort studies (published since 2000) from articles using urine biomarkers to assess sodium intake in relation to health outcomes. We ordered the studies in our table by cohort type (six general population and seven pre-existing disease) and within type by publication date (Table 6). The number and type of participants ranged from 232 patients with heart failure (133) to 101,945 participants from general population samples (113). All identified studies were conducted among adults with age ranges between 18 and 97 years. Inclusion and exclusion criteria varied widely among the studies; however, the Trials of Hypertension Prevention follow-up study (26) is unique in the careful screening and exclusion of participants with pre-existing cardiovascular disease or family history of cardiovascular disease, as well as other diseases that have the potential to affect both diet and mortality (e.g., cancer or gastrointestinal disease). The duration and frequency of follow-up from baseline also varied widely among the studies, e.g., in the general population cohorts, from an average 3.7 years (113) to 10–15 years (26).
Table 6.
Study characteristics, urine biomarker methods, and qualitative results of recent prospective cohort studies of sodium intake and health outcomes, by type of cohorta
| Study population and follow-up | Urine biomarker used to assess Na intake | Health outcome | Association with Na intake | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author (reference) | N | Age (y) | Follow-upb (y) | Type | Duration of Na assessmentc (d) | Number of specimens per person | Adjustment/equation used to estimate individual sodium intake | Na intake levels assessed (g/d) | ||
| General population | ||||||||||
| Cook (26) | 2,275 | 30–54 | 10–15 | 24 h | 547–1,460 | 1–7e | Mean of 1–7 measured 24-h urine Na, Na/K, Na/Cr excretion | q 1.0 <2.3 2.3–<3.6 3.6–<4.8 (ref) ≥4.8 |
CVD CVD |
P* N |
| Joosten (72) | 7,543 | 28–75 | 10–11 | 24 h | 2 (48 h) | 2 | Mean of 2 measured 24-h urine Na excretions | q 1.0, sex-specific quartiles, lowest (ref) | CHD | N (total) P (HTN) P (NT- proBNP level >median) |
| O’Donnell (113) | 101,945 | 35–70 | 3.7, 1.7–2.7f | Spot, fasting, morning | 1 (30–90 in 448)g | 1 (up to 2 in 448)g | Estimated 24-h urine Na and K excretion, Kawasaki prediction equationsh |
<3.0 3.0–3.9 4–5.9 (ref) 6–6.9 ≥7.0 |
CVD CVD + death Total death Stroke |
J J J J |
| Stolarz-Skrzypek (136) | 3,681 | 41i | 7.9 | 24 h | 1 | 1 | No adjustment | Tertiles | Stroke CHD CVD CVD death Total death |
N N N I N |
| Geleijnse (48) | 7,983 | 55+ | 5.0 | Overnight, timed | 1 | 1 | Standardized to 24 hours using recorded collection time, Na, and Na/K | 1 SD Na (1.6 g), 1 unit Na/K ratio | MI Stroke CVD death Total death |
N N N N |
| Tuomilehto (142) | 2,436 | 25–64 | 8–13 | 24 h | 1 | 1 | Adjusted for regression dilution bias using data from other studies | q 2.3 g | Stroke CHD CVD death Total death |
N P P P |
| Pre-existing disease | ||||||||||
| Cardiovascular disease or diabetes mellitus | ||||||||||
| O’Donnell (114) | 28,880 | 67i | 4.7 | Spot, morning, fasting | 1 (up to 730 on 2,625)j | 1 (up to 2 on 2,625)j | Estimated 24-h urine Na and K excretion using Kawasaki prediction equationsh |
<2.0 2.0–3.9 4–5.9, ref 6–8 >8 |
CVD/CHF Stroke MI CHF CVD death Total death |
J N(L), P(H) N(L), P(H) J J N(L), P(H) |
| Diabetes mellitus | ||||||||||
| Thomas (139) | 2,807 | 39i | 10 | 24 h | 1 | 1 | No adjustment |
<2.3 2.3–4.3 (ref) >4.3 |
Total death ESRD |
J I |
| Ekinci (36) | 638 | 64i | 9.9 | 24 h | Up to 365k | 1–5k | Mean of 1–5 measured 24-h urine Na excretions | q 2.3 | CVD death Total death |
I I |
| Chronic heart failure | ||||||||||
| Lennie (87) | 302 | 23–97 | 1 | 24 h, timed | 1 | 1 | No adjustment |
>3 g ≤3 g |
Cardiac event | I (I/II) P (III/IV) |
| Son (133) | 232 | 40–87 | 1 | 24 h, timed | 1 | 1 | No adjustment |
<3.0 ≥3.0 |
Cardiac event | P |
| Chronic kidney disease | ||||||||||
| McQuarrie (104) | 448 | 51 | 1 | 24 h | 385 | Up to 2 on 154 | Na/Cr ratio |
<16.0 ≥16.0 Stratified by albumin excretion |
Total death RRT RRT + death |
P* N P* |
| Vegter (144) | 500 | 18–70 | 4.25 | 24 h | 796l | 5.4l | Mean of multiple measured 24-h urinary Na/Cr ratio, mEq/g |
<2.9m 2.9–5.75 >5.75 |
ESRD | P |
Abbreviations: CHF, chronic heart failure; CHD, coronary heart disease; Cr, creatinine; ref, referent (comparison) group; CVD, cardiovascular disease; ESRD, end-stage renal disease; H, high sodium; HTN, hypertension; I/II or III/IV correspond to New York Heart Association class of heart failure, III/IV is advanced; K, potassium; L, low sodium; MI, myocardial infarction; N, number of participants; Na, sodium; NT-ProBNP, N-terminal pro-B-type natriuretic peptide; RRT, renal replacement therapy.
Follow-up time from baseline Na assessment to outcome assessment in years.
Duration in days from first to last time Na is assessed, i.e., the duration of Na exposure period.
Pattern of association with sodium intake: P, a direct positive association; N, null or not statistically significant; J, J-shaped association increased risk at low and high sodium intake; I, inverse association;
some groups or analyses the association is not significant, parentheses indicate subgroups related to the association.
Median of five measurements per person over a period of 18 to 48 months (26).
Post hoc sensitivity analysis excluding people with CA at baseline; with CVD, CA at baseline or follow-up; DM, who were prescribed CVD medications or who were smokers; with CVD events in years 1 and 2 (113).
In a separate analysis, investigators reported within-person day-to-day variability was adjusted based on a <1% sample of 448 participants with a second spot urine collected at an unspecified time after the first measurement (113).
Kawasaki prediction equation for sodium is as follows: 24-h urinary Na = 23 × (16.3 × XNa0.5), where XNa = [spot Na (mmol/L)/spot Cr (mg/dl) × 10] × [Pr24hCr (mg/day)] (113, 114).
In a subsample of 9% of study participants, a second spot urine was measured two years after the first and used to adjust sodium intake for within-person day-to-day variability in secondary sensitivity analysis (114).
One to five (median two) 24-hour urine collections were used to assess average sodium excretion for each individual. The timing of measurements were not specified but occurred anytime during the year 2001 (36).
Twenty-four-hour urine was collected at baseline, and then at 3 months, 6 months, and every 6 months thereafter for a period of 26.2 months. The minimum and maximum number of measures was not specified, but the average number of collections per person was 5.4 (144).
Based on <100 mEq/g, 100–200 mEq/g, and >200 mEq/g, which according to the authors is about 125 and 250 mEq Na/d (144).
Urine biomarkers used to assess sodium intake
Urine biomarkers used in recent prospective cohort studies to assess the association between individual sodium intake and health outcomes varied in type and number of days collected (Table 6). The spectrum ranged from the long-term average daily amount of sodium excreted from multiple measured 24-h urine specimens (1–7, median 5) collected over a period of up to four years (26) to sodium and creatinine concentrations excreted in a single spot fasting morning specimen collected on one day (113, 114) (Table 6). Five investigators used a single 24-h urine specimen to estimate sodium intake (Table 6). Geleijnse et al. (48) used a single overnight specimen and standardized the amount of sodium excreted in 24 hours based on the collection time. In the three remaining studies (36, 72, 144), the mean of two or more 24-h urine collections was used to estimate sodium intake. Joosten and colleagues (72), however, collected 24-h urine over 48 hours, representing short-term intake. Individual sodium intake was classified into broad categories or examined as a continuous variable.
Completion of 12-h and 24-h urine collection
Only three of the studies in which 12-h (48) or 24-h urine specimens (87, 133) were collected included details in the study reports on instructions for collecting urine and recording start and stop times. Potential under- or overcollection of urine specimens was addressed through exclusion criteria in five studies (48, 87, 133, 136, 142) and in separate post hoc sensitivity analyses in two studies (26, 72). In the five studies that excluded participants for incomplete collection a priori, the proportion excluded ranged from 5% to 10%. Criteria for completion included accurately recorded collection times and urine volume (48), self-report (133, 142), urine collection logs or urinary sodium ≥40 mmol/24 h (87), actual-to-expected urine volume (expected volume based on serum and urine creatinine) (72), and creatinine criteria (26, 136). In one report (144), urinary sodium excretion was normalized to creatinine excretion to correct for errors in collection of 24-h urine. In the remaining four reports, we did not find specific criteria used to assess or account for under- or overcollection of urine specimens, and in two of the studies (36, 104), participants were identified because they had collected 24-h urine as part of their clinical care.
Urine processing, storage, and laboratory analysis
In one multisite study, a variety of instruments and methods were used to analyze urinary sodium, with varying degrees of precision at each laboratory and with one laboratory’s CV >3% (106, 113). Analyses were conducted and common factors applied in an attempt to account for potential error.
Associations with health outcomes
Overall, the observed associations of sodium intake with cardiovascular events were mixed, with some studies suggesting direct positive associations (e.g., linear dose response), others showing J-shaped relationships, some suggesting no relationship or an inverse relationship between sodium intake and health outcomes, and some showing different results for the overall cohort versus subgroups or with different categories of sodium intake (Table 6). The types of and definitions of outcomes varied, with some examining incidence of events (26, 48, 72, 142).
Strengths and Limitations: Individual Sodium Intake and Health Outcomes
The mixed results of recent prospective cohort studies cause confusion. Well-designed prospective cohort studies can be helpful in guiding policy when examined along with other evidence. However, as Cobb and colleagues (24) note in a recent American Heart Association science advisory, significant design and methods flaws can bias results of cohort studies on sodium intake and cardiovascular disease outcomes, including but not limited to:
systematic and random error in sodium intake assessment,
reverse causality,
residual confounding caused by imbalance among groups or inadequate adjustment,
follow up of <80% of participants, and
inadequate statistical power due to small sample size.
Five of the studies included in this review were not previously evaluated by Cobb and colleagues (24). Four were published since their evaluation (26, 72, 104, 113). Two focused on end-stage renal disease and not cardiovascular disease or death (104, 144). Below we discuss systematic and random error in sodium intake assessment, reverse causality, and residual confounding as they relate to the urine biomarkers used to assess sodium intake in these studies.
Systematic error in sodium intake assessment
According to Cobb and colleagues (24, p. 1174), studies with a lower risk of systematic error are those assessing sodium intake with “24 h urine collections not collected as part of routine clinical practice that report quality assurance or exclude incomplete collections” and excluding shorter urine collections (e.g., 12 h or spot). Of the three studies in our report that were also previously reviewed by Cobb and colleagues (24), investigators in one general population study (142) and in two studies conducted in cohorts with pre-existing congestive heart failure (87, 133) collected 24-h urine as part of the study (not as part of routine clinical practice) and reported either quality assurance measures or excluded incomplete collections. One additional study (136) also excluded potentially incomplete 24-h urine specimens. Of the new studies reviewed, separate post hoc analyses excluding or adjusting for potentially incomplete 24-h collections did not affect results (26, 72, 144); however, the quality assurance criteria used to ensure complete urine collection are not clear in these studies.
Some might argue that spot or overnight urine specimens are a valid indicator of individual 24-h urine sodium excretion, but evidence suggests otherwise. In a study conducted by O’Donnell and colleagues (113), results from the investigator’s validation substudy suggest that individual estimated 24-h urine sodium excretion predicted with the Kawasaki equation using a spot urine is systematically biased (105) compared with a single 24-h urine specimen: Bias in estimated individual 24-h sodium excretion is not random but is subject to overestimation at lower levels and underestimation at higher levels when compared with measured 24-h sodium excretion. Bland-Altman plots of individual bias in estimated 24-h sodium excretion using a spot urine specimen and the Kawasaki prediction equation suggest estimated individual sodium intake can under- or overestimate measured sodium intake by ±3,000 mg/d. At higher sodium intakes, underestimation by as much as −7,000 mg/d occurred in some individuals (105, 113). Thus, some study participants who have high measured sodium intake were misclassified as having low estimated sodium intake based on the spot urine.
Relying on a single spot urine collection or one 24-h urine collection to estimate sodium intake does not adequately represent a person’s long-term sodium intake, nor does it represent what would happen if the individual’s dietary sodium intake were reduced. When a single 24-h urine or spot urine specimen is used to determine long-term usual individual sodium intake, estimated sodium intake may be low or high when actual sodium intake is not.
Measurement error and regression dilution bias
As discussed previously, multiple days are needed to accurately assess individual sodium intake using 24-h urine sodium excretion. Because of the short half-life of dietary sodium, a single day of sodium excretion is not an indicator of long-term sodium intake. The use of spot urine adds additional error due to diurnal variability in sodium excretion. Within-individual day-to-day variability (random measurement error) can falsely result in no association when using an indicator of sodium intake based on one day or part of one day. Worse, Elliott (37) illustrates that when a curvilinear relationship exists, estimated optimal sodium intake in relation to minimal cardiovascular risk may be artificially shifted to the right (higher intake) if this bias remains uncorrected in a quadratic relationship. Thus, without correction, it may seem that lower sodium intake is associated with increased risk or an inverse association may even appear to exist.
The best method for minimizing random error in sodium intake assessment is through collecting multiple measurements on each individual with several days between the measurements, such as in three studies in this review (26, 36, 144). In addition, despite the multiple 24-h urine specimens, in one study among patients with diabetes (36) these specimens were collected as part of clinical care and investigators did not exclude specimens with incomplete collection, resulting in potential systematic bias. In the studies conducted by Vegter et al. (144) and Cook et al. (26), 24-h urine specimens were collected at regular intervals as part of a clinical trial, decreasing the potential for systematic bias and random error in sodium assessment.
If multiple days of collection are not possible, methods to assess and adjust for intraindividual variability in measurement might be applied. Investigators attempted to adjust for regression dilution bias (due to within-person day-to-day variability in 24-h sodium excretion) in three of the prospective cohort studies included in this review, two of which used a spot urine sodium specimen (113, 114) and one of which used a single 24-h urine specimen (142). Tuomilehto (142) used external estimates of within-individual variance because only a few participants had more than one 24-h urine sample. Correcting the linear slopes of the adjusted hazards ratios for regression dilution resulted in a fourfold increase in the hazards ratios associated with a 100 mmol change in 24-h sodium excretion. O’Donnell et al. (113, 114) used information from a validation substudy of 448 (0.4% of 101,945) participants with a repeat measurement at 30–90 days after baseline to correct for day-to-day variability in estimated 24-h sodium excretion. Participants in this substudy (105) were from a subset of 11 of the 17 countries in the overall study and were older (average age 57 years versus 51 years for the larger cohort study). In analyses of the full cohort study, data investigators (106, 113) report the same methods were applied as those used in a meta-analysis of studies on cholesterol and vascular mortality, which included repeat measures in 40,313 (4%) participants (121). The magnitude of the regression dilution shrinkage factor and how it was applied are unclear (106, 113). When adjusted for regression dilution, there was a slight increase in the risk of total and cardiovascular disease mortality with low estimated sodium intake and no change in the relationship with high estimated sodium intake (113). Similar methods to adjust for regression dilution bias were applied to O’Donnell and colleagues’ previous study (114). As indicated by Willett (147) and others (28), these methods require careful application and assume that the observed association is related to random rather than systematic measurement error; as discussed previously, the error in estimation of 24-h sodium excretion using spot urine appears to be systematic rather than random.
Reverse causality
If investigators do not exclude participants with high blood pressure, heart disease, stroke, chronic kidney disease, diabetes, or a family history of heart disease and stroke, a possibility exists that these participants are lowering their sodium intake because of these conditions, i.e., reverse causality. In this case, it is not clear which came first—the low sodium intake or the risk of heart disease and stroke leading people to reduce their sodium intake for health reasons. The majority of the studies examined in this review were conducted among participants with pre-existing chronic disease conditions or with little to no information about family history of hypertension or cardiovascular disease.
Adults in cohorts with pre-existing cardiovascular disease, diabetes mellitus, or kidney disease have a higher risk of cardiovascular or total mortality, whereas the general population cohorts (with the exception of the Trials of Hypertension Prevention follow-up study) included substantial percentages of participants with hypertension (26%–42%), cardiovascular disease (up to 17%), and diabetes (up to 10%). As indicated by Cobb and colleagues (24), “conducting analyses by excluding known sick individuals or events at the beginning of follow up may not fully account for reverse causality” (p. 1176). They considered reverse causality to be reduced only when such analyses were conducted and “proportional hazards assumptions were not violated” (p.1176). Joosten and colleagues (72) conducted sensitivity analyses after excluding participants with cardiovascular disease in the first 2 years of the 10.5-year follow-up period. Results were similar, but it was not clear if proportional hazards assumptions were violated. Reverse causality is a particular concern when the duration of follow-up is relatively short. In the general population studies reviewed, follow-up was shortest in the Prospective Urban Rural Epidemiology study (113). In fact, investigators analyzed the data using logistic regression, which is ordinarily used in retrospective or cross-sectional studies rather than proportional hazards. Subanalysis excluding participants with chronic conditions in the years since baseline resulted in a subset of participants with an even shorter follow-up, i.e., 1.7–2.7 years (113).
Potential confounding
Cohort studies assessing sodium intake and health outcomes can be confounded (leading to bias) since, unlike randomized controlled trials, participants are not randomly assigned to different levels of sodium intake for a defined time period. Thus factors other than sodium intake (physiologic, demographic, lifestyle, and environmental factors, or chronic disease conditions) that are associated with the health outcome of interest may occur more frequently among participants with low or high sodium intake. Inadequate control for these factors can lead to erroneous results (potential confounding).
Cobb and colleagues (24) indicate that studies using urine biomarkers to assess sodium intake should control for weight, body mass index, or creatinine excretion to help control for systematic error related to inaccurate collection such as lower estimated sodium intake falsely appearing to be associated with higher risk of heart disease, stroke, and mortality. In addition, they suggest that studies should include the following potential confounding factors in their models of sodium intake and cardiovascular disease: body mass index or weight, cholesterol, diabetes mellitus, age, sex, race, socioeconomic status (e.g., income or education), smoking, cholesterol, and treatment status in observational analyses conducted as follow-up to clinical trials (24). Further, they indicate that the distributions of age, sex, and race should be balanced across levels of sodium intake. If the distributions are not balanced, the association between sodium intake and health outcomes should not differ when stratified by these characteristics. From our review of factors affecting urinary biomarkers of sodium intake, we suggest additional adjustment for physical activity (e.g., through work, transportation, leisure time), and if studies are conducted across time and space (e.g., multisite studies), some adjustment may be needed for location or seasonality as an indicator of climate. Furthermore, adjusting for family history of cardiovascular disease or hypertension and conducting a separate analysis excluding participants with chronic kidney disease may be important. Participants with these risk factors could be reducing sodium intake and have a higher risk of cardiovascular disease.
Of the eight studies in our review that were also included in Cobb and colleagues’ review (24), only one was classified as having a moderate rather than a high or unknown level of potential bias (114), according to Cobb et al.’s criteria. Among the five new studies, only two (72, 113) could be classified as having a moderate level of potential bias related to confounding by these criteria. Of the three studies with a moderate, rather than high, level of potential bias (72, 113, 114), two also controlled for some level of physical activity in either the main analysis or subanalysis (113, 114). The newest study conducted by O’Donnell and colleagues (113) included population samples from 628 communities in 17 countries. Although the investigators used an analytic method to account for clustering, whether they accounted for clustering at the household, community, or country level is unclear. In addition, neither study (113, 114) excluded participants with chronic kidney disease or a family history of cardiovascular disease or adjusted for these risk factors in analysis. The remaining 10 studies in this review were classified as having a high or unknown level of potential confounding.
In an attempt to address variability in urine dilution or incomplete collection, some investigators use the ratio of sodium to creatinine, essentially cancelling the dilution factor and assuming the ratio of these analytes is constant across the day (104, 144). The basis for the Kawasaki prediction of 24-h sodium excretion is the spot sodium:creatinine ratio and estimated 24 h creatinine excretion using age, weight, height, and sex (74, 113, 114). However, Van Dam & Hunter (143, p. 164) in Willett’s textbook (147) state, “Confounding of the association between the analyte-to-creatinine ratio and health outcomes by determinants of creatinine concentration is a concern.” Van Dam & Hunter (143) suggest instead adding creatinine as a variable to models assessing sodium intake and health outcomes or using the residuals from a regression of urinary creatinine on sodium. It is possible that the J-shaped associations with cardiovascular disease or mortality observed in some cohort studies when using an estimation equation (74) for 24-h sodium excretion that relies on spot and estimated creatinine excretion are related not to sodium intake but rather to factors associated with creatinine excretion (16).
CRITICAL QUESTIONS AND FUTURE DIRECTIONS
This review of current studies raises several critical questions about using urine biomarkers to assess sodium intake at the population and individual levels. On the population level, a 24-h urine collection is recommended for assessing population mean sodium intake. As indicated by Van Dam & Hunter, 24-h urine collection is “more likely to be representative of intake than a random urine sample” (143, p. 154). However, the degree to which incomplete participation and urine collection bias the estimates of mean population sodium intake remains unclear. In population surveys, the assessment of participation bias associated with 24-h urine collection and its effects on the overall estimates of mean sodium intake could increase the accuracy of estimates and our understanding of the impact of this potential bias (148). In the reports reviewed, few investigators included information on the incentive used or instructions provided to potential participants, both of which can affect participation and completeness of urine specimens. Explicit instructions and potentially starting and ending collection in person, as in the INTERSALT and INTERMAP studies (39, 135), could help ensure complete collection. Information on the types and costs of methods used to increase participation and completion is needed to increase the use of 24-h urine collection. Also, the best methods to assess whether 24-h urine collection is complete merit further investigation, including whether requiring PABA supplementation affects participation and the effects of incomplete urine collection on estimates of sodium intake. In the OPEN study, for example, excluding potentially incomplete 24-h urine specimens based on PABA recovery did not affect estimates of or variation in population potassium or protein intakes (138). The high variability in creatinine excretion limits its use for assessing completeness of urine collection (11, 38) at the individual level and may limit its use at the population level. Consensus on the use of creatinine-based criteria for completeness of urine collection would facilitate cross-study comparisons.
Further, several studies and national surveys included estimates of the proportion of the population with sodium intake above or below specific thresholds. None of these studies assessed or adjusted for within-person day-to-day variability in sodium intake and excretion. This could bias estimates of the proportion of the population at risk for excess sodium intake. In the 2014 National Health and Nutrition Examination Survey, one-half of participants who collected an initial 24-h urine specimen were randomly selected to collect a second specimen 3 to 10 days later. The second specimen will be used to assess the intraindividual variability and to better estimate the population distribution using measurement error models, as is done now for sodium intake estimated using 24-h dietary recalls (18, 19, 29).
Spot or other partial (e.g., 12-h) urine specimens seem like an attractive alternative to 24-h urine collection because of the decreased burden on the participant and lack of issues related to complete collection. Using partial urine specimens (i.e., spot, overnight, or 12 h) to compare mean population sodium intake across groups requires the assumption that the diurnal pattern of sodium concentration within individuals is either consistent or varies randomly across individuals. However, as discussed previously, evidence suggests that the diurnal pattern of sodium excretion is neither consistent nor random across individuals. The nocturnal dipping in sodium excretion and concentration may be blunted or reversed in certain population subgroups (e.g., people with hypertension or chronic kidney disease). If these subgroups represent a large portion of the population or if the distribution of these subgroups changes in the population over time, then average spot sodium concentration may change regardless of sodium intake. Still, within generally healthy young adults aged 18–39 years without chronic diseases, some evidence suggests spot urine might be used along with other variables, such as age, sex, and creatinine, to estimate average group sodium intake with an equation developed using INTERSALT data (13, 25, 103). Among adults aged 35–40 years and older (70, 105), studies suggest differential bias in estimates of sodium intake from spot urine specimens across population subgroups. Little evidence is available among children (71). In addition, we do not know how estimates of sodium intake from 24-h urine and partial urine specimens compare in the same individuals with changes in sodium intake over time, e.g., as part of long-term randomized controlled sodium-reduction trials. This method is recommended for evaluating the usefulness of biomarkers (61, 147). Resolution of these knowledge gaps is important before partial urine specimens are widely used to assess and compare sodium intake across populations and time.
On the individual level, multiple days of 24-h urine collections across time are recommended to assess individual intake measured as a continuous variable or in broad categories such as tertiles. As with population estimates of sodium intake, the same issues exist with individual estimates of sodium intake in relation to collection and completion of 24-h urine collection. A single 24-h urine collection is a measure of sodium intake over the last 1–3 days. As indicated by Van Dam & Hunter (143), “The power of a single measurement to predict long-term average concentration is low if the within-person variation is large” (pp. 154–55). As indicated previously in this review, the ratio of within-person to between-person variation in urine sodium excretion is large. It may be possible to correct attenuation (regression dilution bias) due to the use of a single 24-h urine collection or other specimen by assessing and adjusting for intraindividual variation (24, 89). However, the accurate application of this correction assumes accurate assessment of intraindividual variation within the population of interest and that the bias in the assessment is random across the distribution of sodium intake (28, 147).
The use of spot or 12-h urine specimens is not currently recommended to assess individual sodium intake in relation to health outcomes (24, 147). Further, the use of spot creatinine excretion and related variables in the equations used to estimate individual 24-h sodium excretion from spot urine could confound the associations with health outcomes. Although spot urine specimens are easier to collect and have the advantage of increasing sample size and subsequently statistical power in general population cohort studies, questions remain regarding systematic and random error in individual assessment as well as potential confounding through the use of creatinine in prediction equations. Additional work is needed to better understand these issues and their potential to distort or confound relationships of sodium to health outcomes.
Alternative approaches to 24-h urine collection, such as modeling based on shorter urine collections, may offer promise for estimating population mean sodium intake in some groups. However, questions remain about the utility of these approaches (e.g., spot urine specimens) for estimating population sodium intake among different age groups and the use of alternative approaches for estimating individual sodium intake. Furthermore, it is unclear whether the prevalence of high or low population sodium intake can be accurately estimated. On the basis of this review, we conclude that 24-h urine collections remain the recommended approach for assessing population and individual sodium intake.
Supplementary Material
Acknowledgments
The authors thank Katherine John, Joanna Taliano, and Lauren Clark for their help with the literature search and references, and Christine Pfeiffer, PhD for the helpful review and comments on the section on data collection and laboratory analysis. This work was supported by the USDHHS. Dr. Elliott acknowledges support from the NIHR Biomedical Research Center at Imperial College Healthcare NHS Trust and Imperial College, the NIHR Health Protection Research Unit on Health Impact of Environmental Hazards, and the MRC-PHE Center for Environment and Health. He is an NIHR Senior Investigator.
Footnotes
DISCLAIMER
The findings and conclusions in this review are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Heart, Lung, and Blood Institute (NHLBI) and the National Institutes of Health, or the US Department of Health and Human Services.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–95. doi: 10.1016/j.mayocp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal R. Relationship between circadian blood pressure variation and circadian protein excretion in CKD. Am J Physiol Renal Physiol. 2007;293:F655–59. doi: 10.1152/ajprenal.00188.2007. [DOI] [PubMed] [Google Scholar]
- 4.Allsopp AJ, Sutherland R, Wood P, Wootton SA. The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration. Eur J Appl Physiol. 1998;78:516–21. doi: 10.1007/s004210050454. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc. 2010;110:736–45. doi: 10.1016/j.jada.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angell SY, Yi S, Eisenhower D, Kerker BD, Curtis CJ, et al. Sodium intake in a cross-sectional, representative sample of New York City adults. Am J Public Health. 2014;104:2409–16. doi: 10.2105/AJPH.2013.301542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey JL, Sands JM, Franch HA. Water, electrolytes and acid-base metabolism. In: Ross A, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern Nutrition in Health and Disease. Philadelphia: Lippincott Williams & Wilkins; 2014. pp. 102–32. [Google Scholar]
- 8.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–98. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 9.Bankir L, Perucca J, Weinberger MH. Ethnic differences in urine concentration: possible relationship to blood pressure. Clin J Am Soc Nephrol. 2007;2:304–12. doi: 10.2215/CJN.03401006. [DOI] [PubMed] [Google Scholar]
- 10.Bingham S, Cummings JH. The use of 4-aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clin Sci (Lond) 1983;64:629–35. doi: 10.1042/cs0640629. [DOI] [PubMed] [Google Scholar]
- 11.Bingham SA, Cummings JH. The use of creatinine output as a check on the completeness of 24-hour urine collections. Hum Nutr Clin Nutr. 1985;39:343–53. [PubMed] [Google Scholar]
- 12.Black DA. Salt and hypertension. Br J Nutr. 1952;6:428–32. doi: 10.1079/bjn19520051. [DOI] [PubMed] [Google Scholar]
- 13.Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, et al. INTERSALT Co-Op. Res. Group. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol. 2013;177:1180–92. doi: 10.1093/aje/kwt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. doi: 10.1093/ije/dyp139. [DOI] [PubMed] [Google Scholar]
- 15.Buono MJ, Ball KD, Kolkhorst FW. Sodium ion concentration versus sweat rate relationship in humans. J Appl Physiol. 2007;103:990–94. doi: 10.1152/japplphysiol.00015.2007. [DOI] [PubMed] [Google Scholar]
- 16.Campbell N. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single-morning fasting urine compared to 24-h measures in 11 countries. J Hypertens. 2014;32:1005–15. doi: 10.1097/HJH.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 17.Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643] BMC Public Health. 2006;6:13. doi: 10.1186/1471-2458-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carriquiry AL. Estimation of usual intake distributions of nutrients and foods. J Nutr. 2003;133:601–8S. doi: 10.1093/jn/133.2.601S. [DOI] [PubMed] [Google Scholar]
- 19.Cent. Dis. Control Prev. (CDC) Trends in the prevalence of excess dietary sodium intake—United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2013;62:1021–25. [PMC free article] [PubMed] [Google Scholar]
- 20.Chappuis A, Bochud M, Glatz N, Vuistiner P, Burnier M. Swiss Survey on Salt Intake: Main Results. Lausanne, Switz: Serv. Nephrol. Inst. Univ. Med. Soc. Prev. Cent. Hosp. Univ. Vaudois (CHUV); 2011. [Google Scholar]
- 21.Choi YM, Jung KC, Jo HM, Nam KW, Choe JH, et al. Combined effects of potassium lactate and calcium ascorbate as sodium chloride substitutes on the physicochemical and sensory characteristics of low-sodium frankfurter sausage. Meat Sci. 2014;96:21–25. doi: 10.1016/j.meatsci.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Chun TY, Bankir L, Eckert GJ, Bichet DG, Saha C, et al. Ethnic differences in renal responses to furosemide. Hypertension. 2008;52:241–48. doi: 10.1161/HYPERTENSIONAHA.108.109801. [DOI] [PubMed] [Google Scholar]
- 23.Cipullo JP, Martin JF, Ciorlia LA, Godoy MR, Cacao JC, et al. Hypertension prevalence and risk factors in a Brazilian urban population. Arq Bras Cardiol. 2010;94:519–26. doi: 10.1590/s0066-782x2010005000014. [DOI] [PubMed] [Google Scholar]
- 24.Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–86. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 25.Cogswell ME, Wang CY, Chen TC, Pfeiffer CM, Elliott P, et al. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18–39 y. Am J Clin Nutr. 2013;98:1502–13. doi: 10.3945/ajcn.113.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–89. doi: 10.1161/CIRCULATIONAHA.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook NR, Kumanyika SK, Cutler JA. Effect of change in sodium excretion on change in blood pressure corrected for measurement error. The Trials of Hypertension Prevention, Phase I. Am J Epidemiol. 1998;148:431–44. doi: 10.1093/oxfordjournals.aje.a009668. [DOI] [PubMed] [Google Scholar]
- 28.Davey Smith G, Phillips AN. Intersalt data. Correction for regression dilution bias in Intersalt study was misleading. BMJ. 1997;315:485–87. [PMC free article] [PubMed] [Google Scholar]
- 29.Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, et al. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. 2006;106:1640–50. doi: 10.1016/j.jada.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Donfrancesco C, Ippolito R, Lo Noce C, Palmieri L, Iacone R, et al. Excess dietary sodium and inadequate potassium intake in Italy: results of the MINISAL study. Nutr Metab Cardiovasc Dis. 2013;23:850–56. doi: 10.1016/j.numecd.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Drummer C, Gerzer R, Heer M, Molz B, Bie P, et al. Effects of an acute saline infusion on fluid and electrolyte metabolism in humans. Am J Physiol. 1992;262(5 Part 2):F744–55. doi: 10.1152/ajprenal.1992.262.5.F744. [DOI] [PubMed] [Google Scholar]
- 32.Dyer AR, Martin GJ, Burton WN, Levin M, Stamler J. Blood pressure and diurnal variation in sodium, potassium, and water excretion. J Hum Hypertens. 1998;12:363–71. doi: 10.1038/sj.jhh.1000601. [DOI] [PubMed] [Google Scholar]
- 33.Dyer AR, Shipley M, Elliott P. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT study. I Estimates of reliability The INTERSALT Cooperative Research Group. Am J Epidemiol. 1994;139:927–39. doi: 10.1093/oxfordjournals.aje.a117099. [DOI] [PubMed] [Google Scholar]
- 34.Dyer AR, Stamler R, Grimm R, Stamler J, Berman R, et al. Do hypertensive patients have a different diurnal pattern of electrolyte excretion? Hypertension. 1987;10:417–24. doi: 10.1161/01.hyp.10.4.417. [DOI] [PubMed] [Google Scholar]
- 35.Ejike CE, Ugwu CE. Association between blood pressure and urinary electrolytes in a population of nonurban-dwelling Nigerians. Niger J Clin Pract. 2012;15:258–64. doi: 10.4103/1119-3077.100617. [DOI] [PubMed] [Google Scholar]
- 36.Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34:703–9. doi: 10.2337/dc10-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott P. Regression dilution bias. Lancet. 1990;335:1230–31. [PubMed] [Google Scholar]
- 38.Elliott P, Brown I. Sodium intakes around the world. Backgr. doc. prep. for Forum Tech. Meet. Reduc. Salt Intake Popul; Paris. 5–7th Oct. 2006; Geneva: World Health Org; 2007. http://www.who.int/dietphysicalactivity/Elliot-brown-2007.pdf. [Google Scholar]
- 39.Elliott P, Stamler R. Manual of operations for “INTERSALT,” an international cooperative study on the relation of sodium and potassium to blood pressure. Control Clin Trials. 1988;9:1–117S. [PubMed] [Google Scholar]
- 40.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ. 1996;312:1249–53. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein M, Hollenberg N. Age as a determinant of renal sodium conservation in normal man. J Lab Clin Med. 1976;87:411–17. [PubMed] [Google Scholar]
- 42.Erdem Y, Arici M, Altun B, Turgan C, Sindel S, et al. The relationship between hypertension and salt intake in Turkish population: SALTURK study. Blood Press. 2010;19:313–18. doi: 10.3109/08037051003802541. [DOI] [PubMed] [Google Scholar]
- 43.Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, et al. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol. 2001;153:996–1006. doi: 10.1093/aje/153.10.996. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda M, Mizuno M, Yamanaka T, Motokawa M, Shirasawa Y, et al. Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension. 2008;52:1155–60. doi: 10.1161/HYPERTENSIONAHA.108.115329. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda M, Motokawa M, Miyagi S, Sengo K, Muramatsu W, et al. Polynocturia in chronic kidney disease is related to natiuresis rather than to water diuresis. Nephrol Dial Transplant. 2006;21:2172–77. doi: 10.1093/ndt/gfl165. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda M, Munemura M, Usami T, Nakao N, Takeuchi O, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65:621–25. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda M, Uzu T, Kimura G. Duration until nighttime blood pressure fall indicates excess sodium retention. Chronobiol Int. 2012;29:1412–17. doi: 10.3109/07420528.2012.728663. [DOI] [PubMed] [Google Scholar]
- 48.Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: the Rotterdam Study. Eur J Epidemiol. 2007;22:763–70. doi: 10.1007/s10654-007-9186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George J, Majeed W, Mackenzie IS, Macdonald TM, Wei L. Association between cardiovascular events and sodium-containing effervescent, dispersible, and soluble drugs: nested case-control study. BMJ. 2013;347:f6954. doi: 10.1136/bmj.f6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibson JC, Stuart-Hill LA, Pethick W, Gaul CA. Hydration status and fluid and sodium balance in elite Canadian junior women’s soccer players in a cool environment. Appl Physiol Nutr Metab. 2012;37:931–37. doi: 10.1139/h2012-073. [DOI] [PubMed] [Google Scholar]
- 51.Graudal N, Jurgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low-and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014;27:1129–37. doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]
- 52.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011;11:CD004022. doi: 10.1002/14651858.CD004022.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Hall GA. Guyton and Hall Textbook of Medical Physiology. 12 Philadelphia: Saunders Elsevier; 2011. [Google Scholar]
- 54.Hashimoto Y, Watanabe N, Futamura A. Amounts of sweat and salt loss due to sweating during a three-hour badminton practice in summer. Rinsho Byori. 2007;55:1015–18. [PubMed] [Google Scholar]
- 55.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;4:CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, et al. Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences. Clin J Am Soc Nephrol. 2012;7:315–22. doi: 10.2215/CJN.02060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000;278:F585–95. doi: 10.1152/ajprenal.2000.278.4.F585. [DOI] [PubMed] [Google Scholar]
- 58.Heer M, Frings-Meuthen P, Titze J, Boschmann M, Frisch S, et al. Increasing sodium intake from a previous low or high intake affects water, electrolyte and acid-base balance differently. Br J Nutr. 2009;101:1286–94. doi: 10.1017/S0007114508088041. [DOI] [PubMed] [Google Scholar]
- 59.Hendriksen MA, van Raaij JM, Geleijnse JM, Wilson-van den Hooven C, Ocké MC, van der ADL. Monitoring salt and iodine intakes in Dutch adults between 2006 and 2010 using 24 h urinary sodium and iodine excretions. Public Health Nutr. 2013;17:1431–38. doi: 10.1017/S1368980013001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Honarpisheh A, Hooman N, Taghavi A. Urinary calcium excretion in healthy children living in Kashan/Iran. Iran J Pediatr. 2009;19:154–58. [Google Scholar]
- 61.Hooper L, Ashton K, Harvey LJ, Decsi T, Fairweather-Tait SJ. Assessing potential biomarkers of micronutrient status by using a systematic review methodology: methods. Am J Clin Nutr. 2009;89:1953–59S. doi: 10.3945/ajcn.2009.27230A. [DOI] [PubMed] [Google Scholar]
- 62.Horswill CA, Stofan JR, Lacambra M, Toriscelli TA, Eichner ER, Murray R. Sodium balance during U.S. football training in the heat: cramp-prone versus reference players. Int J Sports Med. 2009;30:789–94. doi: 10.1055/s-0029-1234056. [DOI] [PubMed] [Google Scholar]
- 63.Hubbard RW, Armstrong LE, Evans PK, DeLuca JP. Long-term water and salt deficits—a military perspective. Predicting Decrements in Military Performance Due to Inadequate Nutrition. Proc. workshop; Oct. 22–24, 1984; Washington, DC: Natl. Acad. Press; 1986. [Google Scholar]
- 64.Hulthen L, Aurell M, Klingberg S, Hallenberg E, Lorentzon M, Ohlsson C. Salt intake in young Swedish men. Public Health Nutr. 2010;13:601–5. doi: 10.1017/S1368980009991431. [DOI] [PubMed] [Google Scholar]
- 65.Inst. Med. Dietary Reference Intake for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: Natl. Acad. Press; 2005. [Google Scholar]
- 66.Inst. Med. Strategies to Reduce Sodium Intake in the United States. Washington, DC: Natl. Acad. Press; 2010. [Google Scholar]
- 67.Inst. Med. Sodium Intake in Populations: Assessment of Evidence. Washington, DC: Natl. Acad. Press; 2013. [Google Scholar]
- 68.Intersalt. An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion Intersalt Cooperative Research Group. BMJ. 1998;297:319–28. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jakobsen J, Ovesen L, Fagt S, Pedersen AN. Para-aminobenzoic acid used as a marker for completeness of 24 hour urine: assessment of control limits for a specific HPLC method. Eur J Clin Nutr. 1997;51:514–19. doi: 10.1038/sj.ejcn.1600434. [DOI] [PubMed] [Google Scholar]
- 70.Ji C, Miller MA, Venezia A, Strazzullo P, Cappuccio FP. Comparisons of spot versus 24-h urine samples for estimating population salt intake: validation study in two independent samples of adults in Britain and Italy. Nutr Metab Cardiovasc Dis. 2014;24:140–47. doi: 10.1016/j.numecd.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 71.Ji C, Sykes L, Paul C, Dary O, Legetic B, et al. Sub-Group Res. Surveill. PAHO-WHO Reg. Expert Group Cardiovasc. Dis. Prev. Through Popul.-Wide Diet. Salt Reduct. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica. 2012;32:307–15. doi: 10.1590/s1020-49892012001000010. [DOI] [PubMed] [Google Scholar]
- 72.Joosten MM, Gansevoort RT, Mukamal KJ, Lambers Heerspink HJ, Geleijnse JM, et al. Sodium excretion and risk of developing coronary heart disease. Circulation. 2014;129:1121–28. doi: 10.1161/CIRCULATIONAHA.113.004290. [DOI] [PubMed] [Google Scholar]
- 73.Kanbay M, Bayram Y, Solak Y, Sanders PW. Dietary potassium: a key mediator of the cardiovascular response to dietary sodium chloride. J Am Soc Hypertens. 2013;7:395–400. doi: 10.1016/j.jash.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20:7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 75.Kelishadi R, Gheisari A, Zare N, Farajian S, Shariatinejad K. Salt intake and the association with blood pressure in young Iranian children: first report from the Middle East and North Africa. Int J Prev Med. 2013;4(4):475–83. [PMC free article] [PubMed] [Google Scholar]
- 76.Khosravi A, Kelishadi R, Sarrafzadegan N, Boshtam M, Nouri F, et al. Impact of a community-based lifestyle intervention program on blood pressure and salt intake of normotensive adult population in a developing country. J Res Med Sci. 2012;17:235–41. [PMC free article] [PubMed] [Google Scholar]
- 77.Khosravi A, Toghianifar N, Sarrafzadegan N, Gharipour M, Azadbakht Salt intake, obesity, and pre-hypertension among Iranian adults: a cross-sectional study. Pak J Med Sci. 2012;28:297–302. [Google Scholar]
- 78.Kilding AE, Tunstall H, Wraith E, Good M, Gammon C, Smith C. Sweat rate and sweat electrolyte composition in international female soccer players during game specific training. Int J Sports Med. 2009;30:443–47. doi: 10.1055/s-0028-1105945. [DOI] [PubMed] [Google Scholar]
- 79.Knuiman JT, Hautvast JG, van der Heyden L, Geboers J, Joossens JV, et al. A multi-centre study on completeness of urine collection in 11 European centres. I Some problems with the use of creatinine and 4-aminobenzoic acid as markers of the completeness of collection. Hum Nutr Clin Nutr. 1986;40:229–37. [PubMed] [Google Scholar]
- 80.Kodama N, Morikuni E, Matsuzaki N, Yoshioka YH, Takeyama H, et al. Sodium and potassium balances in Japanese young adults. J Nutr Sci Vitaminol (Tokyo) 2005;51:161–68. doi: 10.3177/jnsv.51.161. [DOI] [PubMed] [Google Scholar]
- 81.Kudo K, Konta T, Mashima Y, Ichikawa K, Takasaki S, et al. The association between renal tubular damage and rapid renal deterioration in the Japanese population: the Takahata study. Clin Exp Nephrol. 2011;15:235–41. doi: 10.1007/s10157-010-0392-y. [DOI] [PubMed] [Google Scholar]
- 82.Kurdak SS, Shirreffs SM, Maughan RJ, Ozgünen KT, Zeren C, et al. Hydration and sweating responses to hot-weather football competition. Scand J Med Sci Sports. 2010;20:133–39. doi: 10.1111/j.1600-0838.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- 83.Laatikainen T, Pietinen P, Valsta L, Sundvall J, Reinivuo H, Tuomilehto J. Sodium in the Finnish diet: 20-year trends in urinary sodium excretion among the adult population. Eur J Clin Nutr. 2006;60:965–70. doi: 10.1038/sj.ejcn.1602406. [DOI] [PubMed] [Google Scholar]
- 84.Land MA, Webster J, Christoforou A, Praveen D, Jeffery P, et al. Salt intake assessed by 24 h urinary sodium excretion in a random and opportunistic sample in Australia. BMJ Open. 2014;4:e003720. doi: 10.1136/bmjopen-2013-003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leclercq C, Maiani G, Polito A, Ferro-Luzzi A. Use of PABA test to check completeness of 24-h urine collections in elderly subjects. Nutrition. 1991;7:350–54. [PubMed] [Google Scholar]
- 86.Lee SG, Lee W, Kwon OH, Kim JH. Association of urinary sodium/creatinine ratio and urinary sodium/specific gravity unit ratio with blood pressure and hypertension: KNHANES 2009–2010. Clin Chim Acta. 2013;424:168–73. doi: 10.1016/j.cca.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 87.Lennie TA, Song EK, Wu J-R, Chung ML, Dunbar SB, et al. Three gram sodium intake is associated with longer even-free survival only in patients with advanced heart failure. J Card Fail. 2011;17:325–30. doi: 10.1016/j.cardfail.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu F, Zheng S, Mu J, Chu C, Wang L, et al. Common variation in with no-lysine kinase 1 (WNK1) and blood pressure responses to dietary sodium or potassium interventions—family-based association study. Circ J. 2013;77(1):169–74. doi: 10.1253/circj.cj-12-0900. [DOI] [PubMed] [Google Scholar]
- 89.Liu K, Cooper R, McKeever J, McKeever P, Byington R, et al. Assessment of the association between habitual salt intake and high blood pressure: methodological problems. Am J Epidemiol. 1979;110:219–26. doi: 10.1093/oxfordjournals.aje.a112806. [DOI] [PubMed] [Google Scholar]
- 90.Liu K, Cooper R, Soltero I, Stamler J. Variability in 24-hour urine sodium excretion in children. Hypertension. 1979;1:631–36. doi: 10.1161/01.hyp.1.6.631. [DOI] [PubMed] [Google Scholar]
- 91.Liu K, Dyer AR, Cooper RS, Stamler R, Stamler J. Can overnight urine replace 24-hour urine collection to assess salt intake? Hypertension. 1979;1:529–36. doi: 10.1161/01.hyp.1.5.529. [DOI] [PubMed] [Google Scholar]
- 92.Liu K, Stamler J. Assessment of sodium intake in epidemiological studies on blood pressure. Ann Clin Res. 1984;16(Suppl 43):49–54. [PubMed] [Google Scholar]
- 93.Liu LS, Zheng DY, Jin L, Liao YL, Liu K, Stamler J. Variability of urinary sodium and potassium excretion in north Chinese men. J Hypertens. 1987;5:331–35. doi: 10.1097/00004872-198706000-00011. [DOI] [PubMed] [Google Scholar]
- 94.Liu LS, Zheng DY, Lai SH, Wang GQ, Zhang YL. Variability in 24-hour urine sodium excretion in Chinese adults. Chin Med J. 1986;99:424–26. [PubMed] [Google Scholar]
- 95.Loria CM, Obarzanek E, Ernst ND. Choose and prepare foods with less salt: dietary advice for all Americans. J Nutr. 2001;131:536–51S. doi: 10.1093/jn/131.2.536S. [DOI] [PubMed] [Google Scholar]
- 96.Luft FC, Fineberg NS, Sloan RS. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension. 1982;4:805–8. doi: 10.1161/01.hyp.4.6.805. [DOI] [PubMed] [Google Scholar]
- 97.Mage DT, Allen RH, Gondy G, Smith W, Barr DB, Needham LL. Estimating pesticide dose from urinary pesticide concentration data by creatinine correction in the Third National Health and Nutrition Examination Survey (NHANES-III) J Expo Anal Environ Epidemiol. 2004;14:457–65. doi: 10.1038/sj.jea.7500343. [DOI] [PubMed] [Google Scholar]
- 98.Marvar PJ, Gordon FJ, Harrison DG. Blood pressure control: Salt gets under your skin. Nat Med. 2009;15:487–88. doi: 10.1038/nm0509-487. [DOI] [PubMed] [Google Scholar]
- 99.Maseko MJ, Majane HO, Milne J, Norton GR, Woodiwiss AJ. Salt intake in an urban, developing South African community. Cardiovasc J S Afr. 2006;17:186–91. [PubMed] [Google Scholar]
- 100.Matthesen SK, Larsen L, Vase H, Lauridsen TG, Pedersen EB. Effect of potassium supplementation on renal tubular function, ambulatory blood pressure and pulse wave velocity in healthy humans. Scand J Clin Lab Invest. 2012;72:78–86. doi: 10.3109/00365513.2011.635216. [DOI] [PubMed] [Google Scholar]
- 101.Maughan RJ, Dargavel LA, Hares R, Shirreffs SM. Water and salt balance of well-trained swimmers in training. Int J Sport Nutr Exerc Metab. 2009;19:598–606. doi: 10.1123/ijsnem.19.6.598. [DOI] [PubMed] [Google Scholar]
- 102.Maughan RJ, Watson P, Evans GH, Broad N, Shirreffs SM. Water balance and salt losses in competitive football. Int J Sport Nutr Exerc Metab. 2007;17:583–94. doi: 10.1123/ijsnem.17.6.583. [DOI] [PubMed] [Google Scholar]
- 103.McLean R, Williams S, Mann J. Monitoring population sodium intake using spot urine samples: validation in a New Zealand population. J Hum Hypertens. 2014;28:657–62. doi: 10.1038/jhh.2014.10. [DOI] [PubMed] [Google Scholar]
- 104.McQuarrie EP, Traynor JP, Taylor AH, Freel M, Fox JG, et al. Association between urinary sodium, creatinine, albumin, and long term survival in chronic kidney disease. Hypertension. 2014;64:111–17. doi: 10.1161/HYPERTENSIONAHA.113.03093. [DOI] [PubMed] [Google Scholar]
- 105.Mente A, O’Donnell MJ, Dagenais G, Wielgosz A, Lear SA, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J Hypertens. 2014;32:1005–15. doi: 10.1097/HJH.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 106.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–11. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 107.Meyerfreund D, Goncalves C, Cunha R, Pereira AC, Krieger JE, Mill JG. Age-dependent increase in blood pressure in two different Native American communities in Brazil. J Hypertens. 2009;27:1753–60. doi: 10.1097/hjh.0b013e32832e0b2b. [DOI] [PubMed] [Google Scholar]
- 108.Mill JG, Silva AB, Baldo MP, Molina MC, Rodrigues SL. Correlation between sodium and potassium excretion in 24- and 12-h urine samples. Braz J Med Biol Res. 2012;45:799–805. doi: 10.1590/S0100-879X2012007500114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Millett C, Laverty AA, Stylianou N, Bibbins-Domingo K, Pape UJ. Impacts of a national strategy to reduce population salt intake in England: serial cross sectional study. PLOS ONE. 2012;7:e29836. doi: 10.1371/journal.pone.0029836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–34. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 111.Murakami K, Sasaki S, Takahashi Y, Uenishi K, Watanabe T, et al. Sensitivity and specificity of published strategies using urinary creatinine to identify incomplete 24-h urine collection. Nutrition. 2008;24:16–22. doi: 10.1016/j.nut.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Natl. Diet Nutr. Surv. Results from Years 1 to 4 (Combined) of the Rolling Programme for 2008 and 2009 to 2011 and 2012. London: Public Health Engl; 2014. https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012. [Google Scholar]
- 113.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 114.O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–38. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 115.Ortega RM, Lopez-Sobaler AM, Ballesteros JM, Perez-Farinos N, Rodriguez-Rodriguez E, et al. Estimation of salt intake by 24h urinary sodium excretion in a representative sample of Spanish adults. Br J Nutr. 2011;105:787–94. doi: 10.1017/S000711451000423X. [DOI] [PubMed] [Google Scholar]
- 116.Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, et al. Sodium retention in black and white female adolescents in response to salt intake. J Clin Endocrinol Metab. 2004;89:1858–63. doi: 10.1210/jc.2003-031446. [DOI] [PubMed] [Google Scholar]
- 117.Palmer MS, Spriet LL. Sweat rate, salt loss, and fluid intake during an intense on-ice practice in elite Canadian male junior hockey players. Appl Physiol Nutr Metab. 2008;33:263–71. doi: 10.1139/H08-011. [DOI] [PubMed] [Google Scholar]
- 118.Pfeiffer CM, Hughes JP, Cogswell ME, Burt VL, Lacher DA, et al. Urine sodium excretion increased slightly among U.S. adults between 1988 and 2010. J Nutr. 2014;144:698–705. doi: 10.3945/jn.113.187914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pietinen PI, Findley TW, Clausen JD, Finnerty FA, Jr, Altschul AM. Studies in community nutrition: estimation of sodium output. Prev Med. 1976;5:400–7. doi: 10.1016/0091-7435(76)90056-6. [DOI] [PubMed] [Google Scholar]
- 120.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prospect. Stud. Collab. Lewington S, Whitlock G, Clarke R, Sherliker P, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 122.Rakova N, Juttner K, Dahlmann A, Schroder A, Linz P, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na+ balance. Cell Metab. 2013;17:125–31. doi: 10.1016/j.cmet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 123.Rhee MY, Shin SJ, Park SH, Kim SW. Sodium intake of a city population in Korea estimated by 24-h urine collection method. Eur J Clin Nutr. 2013;67:875–80. doi: 10.1038/ejcn.2013.87. [DOI] [PubMed] [Google Scholar]
- 124.Ribic CH, Zakotnik JM, Vertnik L, Vegnuti M, Cappuccio FP. Salt intake of the Slovene population assessed by 24 h urinary sodium excretion. Public Health Nutr. 2010;13:1803–9. doi: 10.1017/S136898001000025X. [DOI] [PubMed] [Google Scholar]
- 125.Rodrigues SL, Baldo MP, de Sa Cunha R, Andreao RV, Del Carmen Bisi Molina M, et al. Salt excretion in normotensive individuals with metabolic syndrome: a population-based study. Hypertens Res. 2009;32:906–10. doi: 10.1038/hr.2009.122. [DOI] [PubMed] [Google Scholar]
- 126.Rose G, Stamler J. The INTERSALT study: background, methods and main results. INTERSALT Co-operative Research Group. J Hum Hypertens. 1989;3:283–88. [PubMed] [Google Scholar]
- 127.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 128.Scott. Cent. Soc. Res. A Survey of 24 Hour Urinary Sodium Excretion in a Representative Sample of the Scottish Population as a Measure of Salt Intake. 2011 http://www.food.gov.uk/scotland/researchscot/scotlandresearch/ScotlandProjectList/s14047.
- 129.Scott. Gov. Scottish Health Survey 2013, Vol. 2: Technical Report. 2014 http://www.scotland.gov.uk/Publications/2014/12/6634/20.
- 130.Shirreffs SM, Aragon-Vargas L, Chamorro M, Maughan RJ, Serratosa L, Zachwieja JJ. The sweating response of elite professional soccer players to training in the heat. Int J Sports Med. 2005;26:90–95. doi: 10.1055/s-2004-821112. [DOI] [PubMed] [Google Scholar]
- 131.Shirreffs SM, Maughan RJ. Water and salt balance in young male football players in training during the holy month of Ramadan. J Sports Sci. 2008;26(Suppl 3):S47–54. doi: 10.1080/02640410802428097. [DOI] [PubMed] [Google Scholar]
- 132.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLOS ONE. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Son YJ, Lee Y, Song EK. Adherence to a sodium-restricted diet is associated with lower symptom burden and longer cardiac event-free survival in patients with heart failure. J Clin Nurs. 2011;20:3029–38. doi: 10.1111/j.1365-2702.2011.03755.x. [DOI] [PubMed] [Google Scholar]
- 134.Staessen J, Broughton PM, Fletcher AE, Markowe HL, Marmot MG, et al. The assessment of the relationship between blood pressure and sodium intake using whole-day, daytime and overnight urine collections. J Hypertens. 1991;9(11):1035–40. doi: 10.1097/00004872-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 135.Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, et al. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary) J Hum Hypertens. 2003;17:591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–85. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 137.Strauss MB, Lamdin E, Smith WP, Bleifer DJ. Surfeit and deficit of sodium; a kinetic concept of sodium excretion. AMA Arch Intern Med. 1958;102:527–36. doi: 10.1001/archinte.1958.00260210013003. [DOI] [PubMed] [Google Scholar]
- 138.Subar AF, Midthune D, Tasevska N, Kipnis V, Freedman LS. Checking for completeness of 24-h urine collection using para-amino benzoic acid not necessary in the Observing Protein and Energy Nutrition study. Eur J Clin Nutr. 2013;67:863–67. doi: 10.1038/ejcn.2013.62. [DOI] [PubMed] [Google Scholar]
- 139.Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34:861–66. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thompson FE, Subar A. Dietary assessment methodology. In: Coulston AM, Boushey CJ, Ferruzzi M, editors. Nutrition in the Prevention and Treatment of Disease. 3 Chapter 1. Oxford, UK: Academic/Elsevier; 2013. pp. 5–46. [Google Scholar]
- 141.Titze J. Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens. 2014;23:101–5. doi: 10.1097/01.mnh.0000441151.55320.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–51. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 143.Van Dam RM, Hunter D. Biochemical indicators of dietary intake. 2012:150–212. See Ref. 147. [Google Scholar]
- 144.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–73. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, et al. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J Nutr. 2013;143:1276–82. doi: 10.3945/jn.113.175927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Webster J, Trieu K, Dunford E, Hawkes C. Target salt 2025: a global overview of national programs to encourage the food industry to reduce salt in foods. Nutrients. 2014;6:3274–87. doi: 10.3390/nu6083274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Willett W. Nutritional Epidemiology. 3 New York: Oxford Univ. Press; 2012. [Google Scholar]
- 148.Wolf HK, Kuulasmaa K, Tolonen H, Ruokokoski E for the WHO MONICA Proj. Participation Rates, Quality of Sampling Frames and Sampling Fractions in the MONICA Surveys. Geneva: World Health Organ; 1998. http://www.thl.fi/publications/monica/nonres/nonres.htm#rate. [Google Scholar]
- 149.World Health Organ./Pan Am. Health Organ. Reg. Expert Group Cardiovasc. Dis. Prev. Through Popul.-Wide Diet. Salt Reduct. Protocol for Population Level Sodium Determination in 24-Hour Urine Samples. 2010 http://new.paho.org/hq/dmdocuments/2010/pahosaltprotocol.pdf.
- 150.Young DS. Effects of Preanalytic Variables on Clinical Laboratory Tests. 2 Washington, DC: AACC Press; 1997. [Google Scholar]
- 151.Zhang J, Temme EH, Sasaki S, Kesteloot H. Under- and overreporting of energy intake using urinary cations as biomarkers: relation to body mass index. Am J Epidemiol. 2000;152:453–62. doi: 10.1093/aje/152.5.453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.