Abstract
Rationale: The lack of consistent associations between clinical outcomes and microbiological responses to therapy for some infectious diseases has raised questions about the adequacy of microbiological endpoints for tuberculosis treatment trials.
Objectives: To evaluate the association between symptoms and microbiological response to tuberculosis treatment.
Methods: We performed a retrospective analysis of four clinical trials in which participants had culture-positive tuberculosis, standardized symptom assessment, and follow-up mycobacterial cultures. Two trials (studies 22 and 23) followed participants to identify recurrent tuberculosis; participants in studies 27 and 28 were only followed to treatment completion.
Measurements and Main Results: This analysis included 1,978 participants; 39 (2.0%) had culture-confirmed treatment failure, and 75 (3.9%) had culture-confirmed recurrence. Productive cough was associated with indices of increased mycobacterial burden at diagnosis (acid-fast smear grade, severity of radiographic abnormalities). Fever and sweats improved rapidly with treatment, whereas productive cough decreased more slowly and was present in 20% of visits after treatment completion. During treatment, study participants with productive cough more often had concurrent culture positivity compared with those without productive cough (studies 22 and 23: adjusted odds ratio, 1.80; 95% confidence interval [CI], 1.33–2.44). Finally, symptoms during the latter part of treatment and follow-up were associated with culture-confirmed treatment failure and recurrence in studies 22 and 23 (for cough: adjusted hazard ratio, 2.07; 95% CI, 1.23–3.49; for fever: adjusted hazard ratio, 5.05; 95% CI, 2.76–9.19).
Conclusions: There are consistent relationships between symptoms and microbiological indices of tuberculosis, including measures of mycobacterial burden at baseline, culture positivity during treatment, and time to culture-confirmed treatment failure and recurrence.
Keywords: tuberculosis, drug treatment, treatment failure, recurrence, cough
The field of tuberculosis (TB) treatment has emphasized bacteriological outcomes of therapy for several decades (1). The traditional endpoints for clinical trials of TB treatment have been treatment failure (sputum culture-positivity at or near the end of treatment) and recurrent disease (culture positivity after the completion of treatment). However, a recent review of the literature by the Food and Drug Administration revealed a lack of recent attention to the association between these microbiologically defined outcomes of TB treatment and the symptoms of TB (2). The lack of consistent associations between clinical and microbiologically defined responses to therapy in other infectious diseases (e.g., otitis media, bacterial pneumonia [3, 4]) raises questions about the adequacy of microbiological endpoints for TB treatment trials.
Previous studies have established that quality of life is decreased in patients with TB and improves with treatment (5–8). Residual symptoms and pulmonary impairment have been reported among patients with microbiologically cured pulmonary TB (9, 10), presumably reflecting damage to the lungs that occurred before control of the infection or because of concomitant lung disease. Results from these studies are limited because they did not include an assessment of participants in clinical trials and did not compare symptoms with microbiological response to treatment. We used results from four clinical trials performed by the Tuberculosis Trials Consortium (TBTC) to evaluate the association between symptoms and concurrent microbiological status before, during, and after treatment.
Materials and Methods
The TBTC has used a standard set of questions to assess the presence of common symptoms of TB: fever; sweats (soaking clothes or sheets); cough; and, if present, whether the cough is productive. Symptoms and body weight were assessed at enrollment, every 2 to 4 weeks during treatment, every 3 to 6 months during follow-up to detect recurrent TB, and at the time of suspected treatment failure or recurrence. The severity of these symptoms was not uniformly assessed in these studies. Detailed information on the demographic and clinical characteristics of the patients included and for definitions used throughout the analysis is provided in the online supplement.
The four clinical trials used for this analysis were limited to patients with culture-positive TB who were treated using directly observed therapy (Table 1) (11–15). Two trials (studies 22 and 23, conducted in the United States and Canada) were chosen because they included follow-up after completion of therapy to detect recurrent TB. Furthermore, study 23 evaluated TB treatment for patients with HIV coinfection. Studies 27 and 28 (conducted in the United States, Uganda, South Africa, Brazil, and Spain) were included because they included frequent study visits during the first 2 months of therapy and provided additional cases of treatment failure. However, patients were not followed beyond the end of treatment; hence, these trials cannot be used for analyses of the relationship between symptoms and recurrent TB.
Table 1.
Key features of the Tuberculosis Trials Consortium clinical trials included in this analysis
| Trial | Study Population | Intervention Evaluated | Time of Enrollment | Timing of Study Visits at which Symptoms Were Assessed | Primary Endpoint | Duration of Follow-up |
|---|---|---|---|---|---|---|
| Study 22 (11, 12) | Pulmonary TB, susceptible to INH and RIF | Once-weekly rifapentine plus INH vs. twice-weekly rifampin plus INH in the continuation phase | 2 mo (end of the intensive phase of therapy) | Time of TB diagnosis (retrospectively); monthly during Months 2–6 of treatment, then at 3, 6, 9, 12, 18, and 24 mo after completing treatment | Culture-positive treatment failure or recurrent TB | 24 mo after completing treatment |
| Study 23 (15) | Pulmonary or extrapulmonary TB, susceptible to RIF | Twice-weekly rifabutin plus INH in the continuation phase | At any time during the first 2 mo of treatment | Monthly during months 2–6 of treatment, then at 3, 6, 9, 12, 18, and 24 mo after completing treatment | Culture-positive treatment failure or recurrent TB | 24 mo after completing treatment |
| Study 27 (13) | Smear-positive pulmonary TB, susceptible to RIF | Moxifloxacin vs. ethambutol (both in combination with INH, RIF, and PZA) | Within 7 d of starting TB treatment | Weeks 0, 2, 4, 6, and 8 and then monthly during the remainder of treatment | 2-mo sputum culture status | Completion of TB therapy |
| Study 28 (14) | Smear-positive pulmonary TB, susceptible to INH and RIF | Moxifloxacin vs. INH (both in combination with RIF, PZA, and EMB) | Within 7 d of starting TB treatment | Weeks 0, 2, 4, 6, and 8 and then monthly during the remainder of treatment | 2-mo sputum culture status | Completion of TB therapy |
Definition of abbreviations: EMB = ethambutol; HIV = human immunodeficiency virus; INH, isoniazid; PZA = pyrazinamide; RIF = rifampin; TB = tuberculosis.
Microbiological Techniques and Outcome Definitions
All four trials used both liquid and solid culture media results at each time point to determine microbiological outcomes. If either media type was positive for growth of Mycobacterium tuberculosis, the sputum culture was considered positive for that time point. “Treatment failure” was defined as a positive culture at or beyond 4 months of therapy but before its completion. “Recurrent tuberculosis” was defined as a positive culture after the completion of therapy and during 18 months of follow-up (Table 1). Paired baseline and failure/recurrence isolates underwent DNA fingerprinting. Though uncommon in these studies, three cases of apparent reinfection were included in this analysis (16).
Data Analysis
We used several methods to evaluate the association between symptoms and concurrent microbiological status before, during, and after treatment, including contingency tables, graphical summaries, regression with correlated data, and time to event.
We used contingency tables with Pearson’s chi-squared test to evaluate the association between symptoms at baseline and the following measures of the mycobacterial burden at baseline at or very near the initiation of treatment: sputum smear grade (high bacillary load was defined as ≥ 1 acid-fast bacilli/field at 1,000×) and the severity of chest radiographic abnormalities.
We used graphical methods to depict the change in frequency of symptoms and culture positivity over the first 24 weeks after starting therapy. We evaluated the association between concurrent symptoms and positive sputum cultures during therapy using logistic regression analysis with generalized estimating equations to compensate for statistical dependence among multiple observations on individuals (17) (additional details are provided in the online supplement).
We used Cox proportional-hazard regression models to quantify the association of time to treatment failure or recurrence with concurrent symptoms. We assessed potential confounding by study and HIV status. Because the frequency of study visits differed, we performed all of these analyses separately for studies 22 and 23 and then for studies 27 and 28. Statistical analyses were performed using SAS (version 9.2; SAS Institute, Inc., Cary, NC) and R (version 2.10.1; The R Foundation for Statistical Computing) (18).
Results
TBTC studies 22, 23, 27, and 28 collectively enrolled 2,025 patients (Table 2). Of these, 27 patients had baseline cultures that were either negative or grew drug-resistant M. tuberculosis; these patients were discontinued from study therapy and not included in this analysis. The median age of the remaining cohort of 1,998 patients was 38.5 years (interquartile range 29, 49 yr), 73% were male, and 18% had HIV coinfection. Of the 1,998 patients in the analysis, 39 had culture-confirmed treatment failure, and 75 had culture-confirmed recurrence.
Table 2.
Number of patients included in the analysis and the number of microbiologically defined treatment endpoints
| Trial | Number Enrolled | Number Included in this Analysis | HIV Coinfection, n (%) | Treatment Failure, n (%) | Recurrence, n (%) |
|---|---|---|---|---|---|
| Study 22 | 1,075 | 1,075 | 71/1075 (6.6) | 11/1,075 (1.0) | 69/1,075 (6.6) |
| Study 23 | 181 | 181 | 181/181 (100) | 3/181 (1.7) | 6/181 (3.3) |
| Study 27 | 336 | 322 | 71/322 (22.0) | 11/322 (3.3) | not assessed |
| Study 28 | 433 | 420 | 45/420 (10.7) | 14/420 (3.2) | not assessed |
| Total | 2,025 | 1,998 | 368/1,998 (18.4) | 39/1,998 (2.0) | 75/1,256 (6.1) |
Association between Symptoms and Mycobacterial Burden at Baseline
Productive cough was more common among patients with high-grade sputum smear or pulmonary cavitation in studies 22, 27, and 28 (those studies with consistently collected baseline data) (Table 3). For example, in studies 27 and 28, 94% (450/480) of patients whose sputum had high-grade smear positivity had productive cough, compared with 86% (198/230) of patients whose sputum smear grade was less than 1 bacillus per high-power field (P < 0.001). The relationship between fever and sweats and measures of mycobacterial burden was less consistent, with a significant association in study 22 but not in studies 27 and 28.
Table 3.
Associations between symptoms and sputum smear grade and radiographic indices of disease severity at the time of tuberculosis treatment initiation (week 0) among patients in Tuberculosis Trials Consortium Studies 22, 27, and 28
| Study | Index of Severity | Fever |
Sweats |
Productive Cough |
|||
|---|---|---|---|---|---|---|---|
| n (%) | P Value | n (%) | P Value | n (%) | P Value‡ | ||
| 22* (n = 1,075)† | Smear grade | ||||||
| Smear ≥ 1 per high power field | 317/427 (74) | 302/426 (71) | 378/438 (86) | ||||
| Smear < 1 per high power field | 278/570 (49) | <0.001 | 243/565 (43) | <0.001 | 362/577 (63) | <0.001 | |
| Chest radiographic features | |||||||
| Cavitation | 307/463 (66) | 286/460 (62) | 394/475 (83) | ||||
| No cavitation | 275/522 (53) | <0.001 | 250/518 (48) | <0.001 | 341/527 (65) | <0.001 | |
| Bilateral abnormalities | 348/530 (66) | 330/524 (63) | 427/539 (79) | ||||
| Unilateral abnormalities or normal | 272/501 (54) | <0.001 | 239/501 (48) | <0.001 | 343/509 (67) | <0.001 | |
| 27 and 28 (n = 742) | Smear grade | ||||||
| Smear ≥ 1 per high power field | 276/480 (58) | 296/480 (62) | 450/480 (94) | ||||
| Smear < 1 per high power field | 140/230 (61) | 0.39 | 152/230 (66) | 0.25 | 198/230 (86) | <0.001 | |
| Chest radiographic features | |||||||
| Cavitation | 313/545 (57) | 342/545 (63) | 510/545 (94) | ||||
| No cavitation | 120/197 (61) | 0.40 | 124/197 (63) | 0.96 | 168/197 (85) | <0.001 | |
| Bilateral abnormalities | 249/424 (59) | 281/424 (66) | 391/424 (92) | ||||
| Unilateral abnormalities or normal | 184/318 (58) | 0.81 | 185/318 (58) | 0.02 | 287/318 (90) | 0.35 | |
Study 22 only because enrollment in Study 23 occurred after beginning of treatment.
Missing values for symptoms, smear grade, or chest radiographic features may reduce denominator totals.
P value for Pearson’s chi-squared test.
Change in Symptoms during Treatment
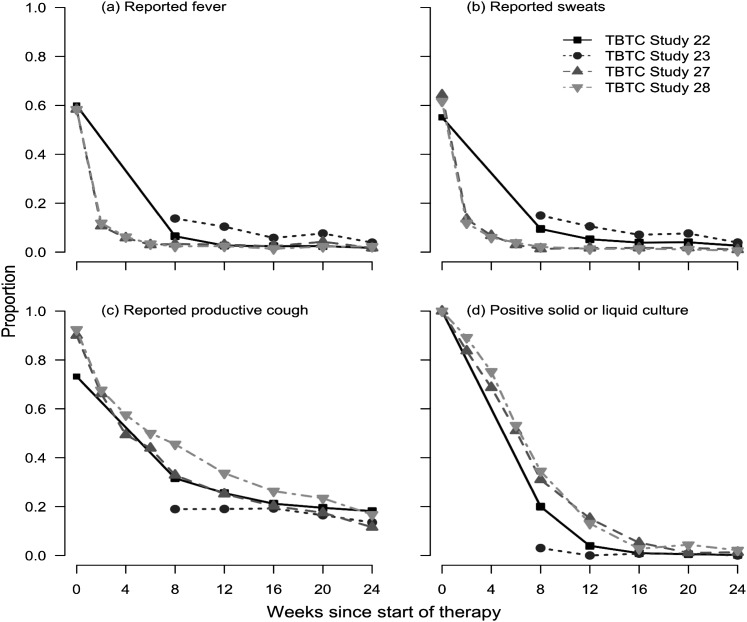
Fever and sweats improved rapidly with treatment (Figures 1A and 1B). By 6 to 8 weeks of treatment, fewer than 10% of patients had either of these symptoms. The prevalence of productive cough decreased steadily with treatment but was present in 35 to 50% of study patients at Week 8 of therapy (Figure 1C). Thereafter, the prevalence of productive cough remained at approximately 20% during or after therapy. For all three symptoms, the curves of the decrease in prevalence of symptoms were similar across studies. Sputum culture positivity declined steadily in the first 12 weeks of therapy, and very few patients had positive cultures at or after 16 weeks (Figure 1D).
Figure 1.
Frequency of fever (A), sweats (B), productive cough (C), and culture positivity (by solid or liquid culture) (D) during Weeks 0 to 24 after initiation of tuberculosis treatment among patients in Tuberculosis Trials Consortium (TBTC) Studies 22, 23, 27, and 28. Study 23 data are not included for Week 0 because enrollment occurred after the beginning of treatment.
Association between Symptoms and Mycobacterial Burden at Week 8 of Treatment
We initially focused on the 8-week time point, given the use of sputum culture status at that time point as a surrogate marker of the sterilizing effect of a therapeutic regimen (19). Productive cough at Week 8 was more common among patients with a positive sputum culture (Table 4). For example, in study 22, productive cough was present in 51% of those with a positive 8-week culture, compared with 29% of those with a negative culture (P < 0.001). Furthermore, productive cough was consistently associated with high sputum smear grade. The association between productive cough and radiographic measures of disease severity was less consistent, being present in study 22 but not in studies 27 and 28. Fever and sweats were uncommon at Week 8 and showed no significant association with measures of mycobacterial burden (data not shown).
Table 4.
Associations between productive cough and culture results, sputum smear grade, and radiographic indices of disease severity at 8 weeks of therapy among patients in Tuberculosis Trials Consortium Studies 22, 23, 27, and 28
| Study | Laboratory and Radiographic Features of Tuberculosis | Percent Reporting Productive Cough, n (%) | P Value* |
|---|---|---|---|
| 22 and 23 (n = 1,246)† | Sputum culture result | ||
| Positive | 103/208 (50) | ||
| Negative | 261/971 (27) | <0.001 | |
| Smear grade | |||
| Smear ≥ 1 per high-power field | 24/35 (69) | ||
| Smear < 1 per high-power field | 339/1,135 (30) | <0.001 | |
| Chest radiographic features | |||
| Cavitation | 161/394 (41) | ||
| No cavitation | 186/766 (24) | <0.001 | |
| Bilateral abnormalities | 205/574 (36) | ||
| Unilateral abnormalities or normal | 159/613 (26) | <0.001 | |
| 27 and 28 (n = 742)† | Sputum culture result | ||
| Positive | 114/207 (55) | ||
| Negative | 144/417 (35) | <0.001 | |
| Smear grade | |||
| Smear ≥ 1 per high-power field | 36/44 (82) | ||
| Smear < 1 per high-power field | 219/571 (38) | <0.001 | |
| Chest radiographic features | |||
| Cavitation | 203/506 (40) | ||
| No cavitation | 69/175 (39) | 0.89 | |
| Bilateral abnormalities | 170/386 (44) | ||
| Unilateral abnormalities or normal | 102/295 (35) | 0.01 |
P value for Pearson’s chi-squared test.
Missing values for symptoms, smear grade, or chest radiographic features may reduce denominator totals.
Association between Symptoms and Concurrent Culture Positivity over the Course of Treatment
We used logistic regression analysis with generalized estimating equations to evaluate the association between productive cough and concurrent sputum culture positivity at any point during treatment. In an initial bivariate analysis adjusting for time on treatment, study participants with cough had twice the odds of having concurrent sputum culture positivity (studies 22 and 23: odds ratio [OR], 2.23; 95% confidence interval [CI], 1.70–2.92; studies 27 and 28: OR, 2.27; 95% CI, (1.90–2.72) (Tables 5 and 6). These odds ratios were minimally attenuated (studies 22 and 23: adjusted OR, 1.80; 95% CI, 1.33–2.44 [P < 0.001]; studies 27 and 28: OR, 1.71; 95% CI, 1.41–2.07 [P < 0.001]) in multivariate models adjusting for factors that have previously been associated with treatment outcome (being underweight at diagnosis, weight change in the first 8 weeks of treatment, baseline bacillary load on sputum smear, bilateral and cavitary disease on chest radiography at baseline, HIV status, and enrollment at an African site [studies 27 and 28]).
Table 5.
Partially and fully adjusted models of the association between the presence of symptoms and concurrent culture positivity during tuberculosis therapy in Tuberculosis Trials Consortium studies 22 and 23
| Term | Partially Adjusted Model |
Fully Adjusted Model |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Symptoms | ||||
| Productive cough | 2.23 (1.70–2.92) | <0.001 | 1.80 (1.33–2.44) | <0.001 |
| Fever | 1.24 (0.74–2.07) | 0.42 | 1.42 (0.77–2.63) | 0.26 |
| Sweats | 0.64 (0.38–1.05) | 0.078 | 0.70 (0.40–1.22) | 0.21 |
| Time on therapy* | ||||
| Weeks 8–16 | 0.65 (0.61–0.69) | <0.001 | 0.63 (0.59–0.68) | <0.001 |
| Weeks 16–24 | 0.88 (0.77–1.01) | 0.074 | 0.90 (0.78–1.04) | 0.14 |
| Weight† | ||||
| Underweight at diagnosis | 1.54 (1.10–2.15) | 0.011 | ||
| Gained > 4 kg | 1.14 (0.78–1.67) | 0.50 | ||
| Any weight loss | 0.93 (0.56–1.54) | 0.77 | ||
| Missing weight change | 1.79 (1.01–3.14) | 0.045 | ||
| Severity of disease at baseline | ||||
| Smear ≥ 1 AFB/field at 1,000× | 2.31 (1.64–3.24) | <0.001 | ||
| Cavitary disease | 2.75 (1.91–3.98) | <0.001 | ||
| Bilateral disease | 1.78 (1.25–2.53) | 0.001 | ||
| Other | ||||
| HIV positive | 0.33 (0.16–0.66) | 0.002 | ||
Definition of abbreviations: AFB = acid-fast bacilli; CI = confidence interval; OR = odds ratio.
Time on therapy was modeled as two separate continuous variables, each with different slopes. The odds ratio represents weekly change in odds during the indicated time period since start of therapy.
Weight change over Weeks 0 to 8, with a gain 0–4 kg as referent.
Table 6.
Partially and fully adjusted models of the association between the presence of symptoms and concurrent culture positivity during tuberculosis therapy in Tuberculosis Trials Consortium studies 27 and 28
| Term | Partially Adjusted Model |
Fully Adjusted Model |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Symptoms | ||||
| Productive cough | 2.27 (1.90–2.72) | <0.001 | 1.71 (1.41–2.08) | <0.001 |
| Fever | 1.26 (0.83–1.91) | 0.28 | 1.25 (0.80–1.94) | 0.33 |
| Sweats | 0.95 (0.66–1.38) | 0.81 | 0.96 (0.63–1.46) | 0.84 |
| Time on therapy* | ||||
| Weeks 2–8 | 0.66 (0.63–0.68) | <0.001 | 0.60 (0.57–0.63) | <0.001 |
| Weeks 8–16 | 0.75 (0.71–0.79) | <0.001 | 0.73 (0.70–0.77) | <0.001 |
| Weeks 16–24 | 0.90 (0.82–1.00) | 0.042 | 0.90 (0.82–0.99) | 0.024 |
| Weight† | ||||
| Underweight at diagnosis | 1.22 (0.96–1.57) | 0.11 | ||
| Gained > 4 kg | 0.88 (0.59–1.31) | 0.53 | ||
| Any weight loss | 0.93 (0.58–1.48) | 0.75 | ||
| Missing weight change | 0.81 (0.62–1.07) | 0.14 | ||
| Severity of disease at baseline | ||||
| Smear ≥ 1 AFB/field at 1,000× | 1.93 (1.49–2.51) | <0.001 | ||
| Cavitary disease | 1.85 (1.41–2.43) | <0.001 | ||
| Bilateral disease | 1.59 (1.25–2.03) | <0.001 | ||
| Other | ||||
| African site | 2.73 (2.05–3.64) | <0.001 | ||
| HIV positive | 0.67 (0.47–0.97) | 0.032 | ||
Definition of abbreviations: AFB = acid-fast bacilli; CI = confidence interval; OR = odds ratio.
Time on therapy was modeled as three separate continuous variables, each with different slopes. The odds ratio represents weekly change in odds during the indicated time period since start of therapy.
Weight change over Weeks 0 to 8, with a gain of 0–4 kg as referent.
Association between Symptoms and Culture-Confirmed Treatment Failure or Recurrence
Of the 89 patients who experienced culture-positive treatment failure or recurrence in studies 22 and 23, 85 (96%) had concurrent information on symptoms. Of these 85 participants, 56 (66%) had at least one symptom at the time of culture-confirmed treatment failure or recurrence. An additional 1,156 participants did not experience treatment failure or relapse and had symptom information at Week 16 or later. Fever (hazard ratio [HR], 5.68; 95% Wald CI, 3.15–10.24 [P < 0.001]) and productive cough (HR, 2.58; 95% CI, 1.58–4.22 [P = 0.002]) were associated with culture-confirmed treatment failure or recurrent TB (Table 7). These associations attenuated only slightly when adjusted for other factors previously shown to be associated with treatment failure or recurrence in study 22 (11). In studies 27 and 28, 25 participants experienced treatment failure, and an additional 666 participants with symptom information between Weeks 12 and 24 did not experience treatment failure. Productive cough was associated with culture-positive treatment failure (HR, 4.70; 95% CI, 2.11–10.47 [P < 0.001]). None of the patients with treatment failure reported fever or sweats between Weeks 12 and 24. Because there were few visits at risk for treatment failure, we did not construct a multivariate hazard regression model for studies 27 and 28.
Table 7.
Hazard regression analysis from Cox regression analysis of the association between the presence of symptoms and time to treatment failure or recurrence after week 12 in Studies 22 and 23
| Term | Partially Adjusted Model |
Fully Adjusted Model |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Symptoms | ||||
| Productive cough | 2.58 (1.58–4.22) | <0.001 | 2.07 (1.23–3.49) | 0.006 |
| Fever | 5.68 (3.15–10.24) | <0.001 | 5.04 (2.76–9.19) | <0.001 |
| Sweats | 1.61 (0.88–2.96) | 0.12 | 2.26 (1.22–4.16) | 0.009 |
| Weight* | ||||
| Underweight at diagnosis | 1.64 (1.03–2.59) | 0.036 | ||
| Gained > 4 kg | 1.05 (0.64–1.71) | 0.85 | ||
| Any weight loss | 0.60 (0.28–1.29) | 0.19 | ||
| Missing weight change | 0.62 (0.24–1.60) | 0.33 | ||
| Severity of disease at baseline | ||||
| Smear ≥ 1 AFB/field at 1,000× | 1.22 (0.77–1.93) | 0.39 | ||
| Cavitary disease | 3.34 (1.91–5.84) | <0.001 | ||
| Bilateral disease | 2.11 (1.25–3.56) | 0.005 | ||
| Other | ||||
| HIV positive | 1.12 (0.58–2.17) | 0.73 | ||
Definition of abbreviations: AFB = acid-fast bacilli; CI = confidence interval; OR = odds ratio.
Weight change over Weeks 0 to 8, with a gain 0 to 4 kg as referent.
Discussion
Using data from four prospective clinical trials, we have shown consistent relationships between symptoms and microbiological outcomes of TB treatment. Symptoms were more common among patients with a greater burden of M. tuberculosis at the time of diagnosis. During treatment, the presence of concurrent productive cough was consistently associated with sputum culture positivity, including in models that adjusted for other factors that have been associated with disease severity. Finally, symptoms (productive cough and fever) were associated with the two microbiological outcomes that have been used in clinical trials over the past 40 years: culture-confirmed treatment failure and recurrence. Although there were minor differences in these associations in different clinical trials, the consistency in the response of symptoms to treatment and the consistency of the associations with culture positivity during treatment are impressive.
Our results are similar to data from a clinical trial done in Uganda, Brazil, and the Philippines (20). In that study of 394 HIV-negative patients with culture-positive pulmonary TB, symptoms were associated with measures of mycobacterial burden at baseline, and symptoms improved quickly with therapy. Furthermore, symptoms (fever, cough, and chest pain) were more common at the time of recurrent culture positivity (microbiologic recurrence) than among patients who had negative cultures during follow-up.
The United States Food and Drug Administration is charged with assuring that new medications are both safe and effective. Demonstration of effectiveness requires improvement in how the patient feels, functions, or survives. The relationship between clinical symptoms and microbiological indices of TB had not been emphasized in clinical trials over the past 40 years, and the resultant lack of data has led to questions about the validity of sputum culture as an appropriate surrogate endpoint for licensure of new drugs
Appropriate concerns have been raised about using microbiological culture as the primary outcome of treatment trials for infectious diseases such as otitis media; there is a weak relationship between conversion of cultures to negative and the clinical outcomes of such treatment (4). TB has several factors that make it quite different from bacterial pneumonia and similar acute bacterial infections. First, the pathogens that commonly cause acute bacterial infections (e.g., Streptococcus pneumoniae) can be commensal bacteria; therefore, culture positivity from cultures of nonsterile sites (e.g., sputum) at baseline or during treatment may represent colonization. In contrast, M. tuberculosis is not a commensal bacterium, so its presence in a culture is nearly always associated with disease (if laboratory cross-contamination has been ruled out). Furthermore, we have shown consistent relationships between symptoms of TB and a number of microbiological outcomes of therapy.
Despite the consistent relationship between productive cough and microbiological outcome of TB treatment, cough would be a problematic endpoint for TB treatment. Productive cough was present in approximately 20% of patients at any time point during the latter part of treatment and during follow-up after completion of therapy. It is likely that residual cough after microbiologically successful treatment represents residual pulmonary damage from TB, conditions associated with cough and risk for TB (e.g., tobacco use or silicosis), and/or other pulmonary diseases (e.g., asthma). However, the consistent relationship between productive cough and culture positivity demonstrates that it is appropriate to use microbiologically defined endpoints as the primary endpoints for TB treatment trials (21).
This study had at least four limitations. First, our analyses were limited to symptoms and other clinical features that were consistently measured across all four studies. Because the severity of symptoms was not uniformly recorded, we were not able to analyze the relationship between symptom severity and microbiological findings. Second, the four studies did not uniformly collect data on other potential causes of cough, such as tobacco use or history of specific pulmonary diseases, such as obstructive pulmonary disease or asthma. Third, mycobacterial culture techniques were not standardized other than requiring that specimens be cultured in a certified lab and include use of approved broth and solid culture media. Finally, only two of the four studies included in this analysis followed patients for recurrent TB, limiting the power of this key association.
Conclusions
There are consistent relationships between symptoms and microbiological indices of TB, including measures of mycobacterial burden at baseline, culture positivity during treatment, and culture-confirmed treatment failure and recurrence. These consistent relationships demonstrate that the use of microbiological outcomes is appropriate in clinical trials of new agents for TB treatment.
Acknowledgment
The authors thank the many patients who contributed to the success of these trials, Dr. Kenneth Castro for continued support within the CDC, local TB program staff who assisted in the clinical management of the participants, and Awal Khan for assistance with legacy data.
Footnotes
Supported by the Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia.
Author Contributions: Contributions to the conception and design of the study: all authors; data analysis: C.M. Hales and C.M. Heilig; drafting the article: all authors; final approval: all authors.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3:S231–S279. [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Issues in the design of clinical trials of antimycobacterial drugs for treatment of tuberculosis; public workshop [Internet]. 2009. [updated September 23, 2009; accessed January 25, 2011]. Available from: http://www.fda.gov/Drugs/NewsEvents/ucm168975.htm.
- 3.Wunderink RG. Surrogate markers and microbiologic end points. Clin Infect Dis. 2010;51(Suppl 1):S126–S130. doi: 10.1086/653061. [DOI] [PubMed] [Google Scholar]
- 4.Johann-Liang R, Zalkikar J, Powers JH. Correlation between bacteriologic and clinical endpoints in trials of acute otitis media. Pediatr Infect Dis J. 2003;22:936–937, author reply 937. doi: 10.1097/01.inf.0000092637.66762.c6. [DOI] [PubMed] [Google Scholar]
- 5.Dhuria M, Sharma N, Narender Pal S, Ram Chander J, Saha R, Gopal Krishan I. A study of the impact of tuberculosis on the quality of life and the effect after treatment with dots. Asia Pac J Public Health. 2009;21:312–320. doi: 10.1177/1010539509336242. [DOI] [PubMed] [Google Scholar]
- 6.Marra CA, Marra F, Colley L, Moadebi S, Elwood RK, Fitzgerald JM. Health-related quality of life trajectories among adults with tuberculosis: differences between latent and active infection. Chest. 2008;133:396–403. doi: 10.1378/chest.07-1494. [DOI] [PubMed] [Google Scholar]
- 7.Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis: a systematic review. Health Qual Life Outcomes. 2009;7:14. doi: 10.1186/1477-7525-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang B, Wu AW, Hansel NN, Diette GB. Quality of life in tuberculosis: a review of the English language literature. Qual Life Res. 2004;13:1633–1642. doi: 10.1007/s11136-004-0374-1. [DOI] [PubMed] [Google Scholar]
- 9.Miller TL, McNabb SJ, Hilsenrath P, Pasipanodya J, Weis SE. Personal and societal health quality lost to tuberculosis. PLoS ONE. 2009;4:e5080. doi: 10.1371/journal.pone.0005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasipanodya JG, Miller TL, Vecino M, Munguia G, Bae S, Drewyer G, Weis SE. Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest. 2007;132:1591–1598. doi: 10.1378/chest.07-0755. [DOI] [PubMed] [Google Scholar]
- 11.Tuberculosis Trials Consortium. Once-weekly rifapentine and isoniazid versus twice-weekly rifampin and isoniazid in the continuation phase of therapy for drug-susceptible pulmonary tuberculosis: a prospective, randomized clinical trial among HIV-negative persons. Lancet. 2002;360:528–534. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 12.Vernon A, Burman W, Benator D, Khan A, Bozeman L. Relapse with rifamycin monoresistant tuberculosis in HIV-infected patients treated with supervised once-weekly rifapentine and isoniazid. Lancet. 1999;353:1843–1847. doi: 10.1016/s0140-6736(98)11467-8. [DOI] [PubMed] [Google Scholar]
- 13.Burman WJ, Goldberg S, Johnson JL, Muzanye G, Engle M, Mosher AW, Choudhri S, Daley CL, Munsiff SS, Zhao Z, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:331–338. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- 14.Dorman SE, Johnson JL, Goldberg S, Muzanye G, Padayatchi N, Bozeman L, Heilig CM, Bernardo J, Choudhri S, Grosset JH, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis Am J Respir Crit Care Med 2009. 180:273–280. [DOI] [PubMed] [Google Scholar]
- 15.Burman W, Benator D, Vernon A, Khan A, Jones B, Silva C, Lahart C, Weis S, King B, Mangura B, et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med. 2006;173:350–356. doi: 10.1164/rccm.200503-417OC. [DOI] [PubMed] [Google Scholar]
- 16.Jasmer RM, Bozeman L, Schwartzman K, Cave MD, Saukkonen JJ, Metchock B, Khan A, Burman WJ. Recurrent tuberculosis in the united states and canada: Relapse or reinfection? Am J Respir Crit Care Med. 2004;170:1360–1366. doi: 10.1164/rccm.200408-1081OC. [DOI] [PubMed] [Google Scholar]
- 17.Diggle P, Liang K-Y, Zeger SL.Analysis of longitudinal data. New York: Clarendon Press; 1994 [Google Scholar]
- 18.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2009. R: A language and environment for statistical computing. 2.10.1 ed. [Google Scholar]
- 19.Mitchison D. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis. 1993;147:1062–1063. doi: 10.1164/ajrccm/147.4.1062. [DOI] [PubMed] [Google Scholar]
- 20.Bark CM, Dietze R, Okwera A, Quelapio MI, Thiel BA, Johnson JL. Clinical symptoms and microbiological outcomes in tuberculosis treatment trials. Tuberculosis (Edinb) 2011;91:601–604. doi: 10.1016/j.tube.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopewell P, Cynamon M, Starke J, Iseman M, O'Brien R. Evaluation of new anti-infective drugs for the treatment and prevention of tuberculosis. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992;15:S282–S295. doi: 10.1093/clind/15.supplement_1.s282. [DOI] [PubMed] [Google Scholar]