Abstract
Aggregation of denatured or unfolded proteins establishes a large energy barrier to spontaneous recovery of protein form and function following traumatic injury, tissue cryopreservation, and biopharmaceutical storage. Some tissues utilize small heat shock proteins (sHSP) to prevent irreversible aggregation, which allows more complex processes to refold or remove the unfolded proteins. We postulated that large, amphiphilic, and biocompatible block copolymers could mimic sHSP function. Reduced and denatured hen egg white lysozyme (HEWL) was used as a model aggregating protein. The poloxamine T1107 prevented aggregation of HEWL at 37 °C by three complimentary measures. Structural analysis of denatured HEWL revealed a partially-folded conformation with preserved or promoted beta-sheet structures only in the presence of T1107. The physical association of T1107 with denatured HEWL, and the ability to prevent aggregation, was linked to the critical micelle temperature of the polymer. The results suggest that T1107, or a similar amphiphilic block copolymer, could find use as a synthetic chaperone to augment the innate molecular repair mechanisms of natural cells.
Keywords: poloxamine, T1107, poloxamer, P188, lysozyme, chaperone
Graphical Abstract
II. Introduction
One important mechanism by which cells protect themselves from biophysical damage following stress is through the upregulation of chaperones1. Small heat shock proteins (sHSPs) recognize and associate with denatured proteins, preventing the formation of unrecoverable aggregates2. A synthetic, biocompatible molecule that behaves as an sHSP may have a variety of uses, including a therapeutic for trauma, a stabilizer in biologic drug formulations, a preservative for organs during transplantation, or as a prophylactic treatment before surgery.
Certain classes of amphiphilic block copolymers act as weak surfactants and are compatible with intravenous injection. Poloxamer 188 (P188) is a 8.4 kDa block copolymer with a 20 wt.% hydrophobic poly (propylene oxide) (PPO) core and hydrophilic poly (ethylene oxide) (PEO) arms (Figure 1a). The surfactant has a long history of safe usage, and evidence suggests that the polymer catalyzes cell membrane resealing by inducing the reorganization and reassembly of lipids3. Because similar forces drive protein aggregation, we hypothesized that P188 also behaved as a synthetic chaperone. Previous investigations have shown that P188 interfered with heat-induced aggregation of lysozyme4,5, and subsequent work showed that the Poloxamine 1107 (T1107, Figure 1a) was even more effective6. The 15 kDa T1107 has PEO-block-PPO arms branching from a ethylene-1,2-diamine core and is 30 wt.% hydrophobic. Our previous work studied block copolymer-protein interactions at temperatures well above thermal burn thresholds7, and it was unclear whether enzyme recovery in the presence of P188 or T1107 was due to the polymers preventing aggregation of denatured proteins, holding proteins in intermediate conformations during stress, or actively assisting refolding. The motivation for this work is to establish the molecular mechanisms by which T1107 prevents aggregation in more physiologic conditions.
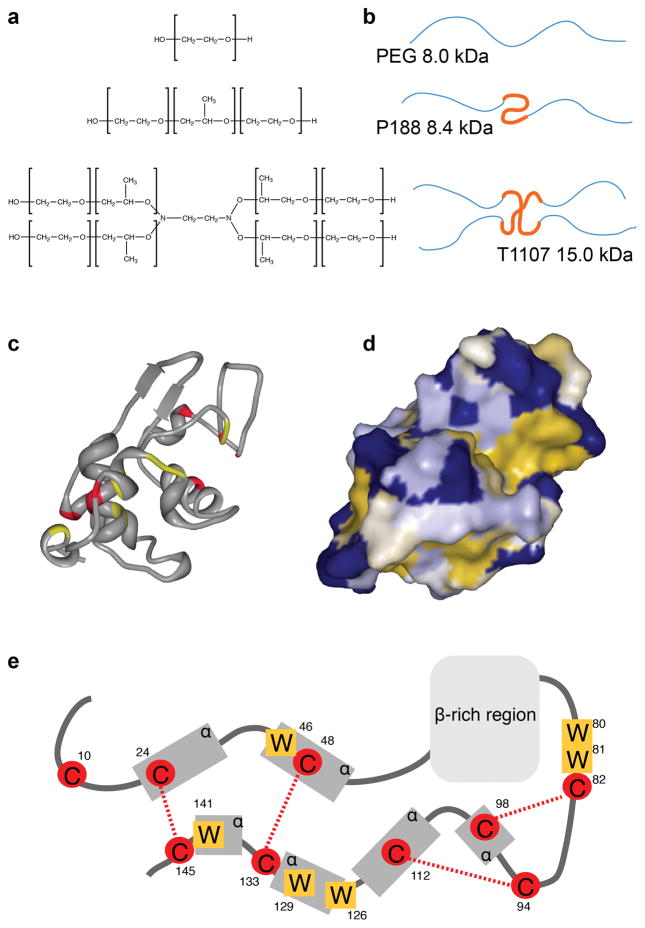
Figure 1.
(a) Chemical structures of PEG, P188, and T1107, and (b) illustrations indicating the relative extent of hydrophilic PEG (thin, blue) and hydrophobic PPG (thick, orange) polymer chains. (c) Ribbon drawing of HEWL with cysteine (red) and tryptophan (yellow) residues highlighted, generated from PDB: 1PDX using RSCB Protein Workshop. (d) Protein surface indicating hydrophilic (blue) and hydrophobic surfaces (yellow). (e) Illustration of HEWL protein indicating residue locations of tryptophan (W) and cysteine (C) residues, disulfide bonds (red lines), alpha helices and beta sheet-rich regions based on UniProt P00698.
Lysozyme was employed as a model protein because aggregation could be induced in phosphate-buffered saline (PBS), at physiologically-relevant temperatures, by treatment with the reducing agent dithiothreitol (DTT). Hen egg white lysozyme (HEWL) is a 14.3 kDa enzyme with four disulfide bonds, six distinct hydrophobic domains8, and six tryptophan residues (Figure 1e). Intermediate folding states of lysozyme are prone to aggregation driven by hydrophobic interactions9–11. The aggregates that result from DTT reduction are amorphous, branching structures consisting of globules roughly 100 nm in diameter and rich in cross-beta structures12.
The motivation for this work was to determine the mechanisms by which T1107 behaves as a chaperone in physiologic conditions. The aggregation of HEWL in the presence of DTT was prevented in the presence of equimolar concentrations of T1107, and was shown by three complimentary assays. Dynamic light scattering (DLS) was used to demonstrate physical association of the polymer with the denatured protein, as well as to characterize the micellizaiton behavior of T1107. Fluorescent spectroscopy revealed a loss of tertiary structure and rearrangement of tryptophan residues in all samples exposed to DTT. Circular dichroism (CD) spectroscopy showed that T1107 preserved or promoted beta structures in this intermediate folding state, while control samples lost all detectible secondary structure. Denaturation in the absence of T1107 resulted in aggregates large enough to turn solutions milky white and that stained positive for Thioflavin T (ThT), indicating the formation of intermolecular cross-beta structures. The results showed that T1107 prevented aggregation by physically associating with and stabilizing an intermediate folding state of HEWL when above, but not below, the critical micelle temperature.
III. Experimental Methods
Aggregation assays
The following proteins were purchased from Sigma (St. Louis, MO) and used without further purification: HEWL (L6876), insulin from bovine pancreas (I6634), and ribonuclease A from bovine pancreas (R6513). Research grade P188 and T1107 were purchased from Maroon Biotech (Chicago, IL). At least two separate batches of P188 and T1107 were used in each experiment. Polyethylene glycol (PEG, 8000 MW), used as a negative control, along with DTT and sodium dodecyl sulfate (SDS) were purchased from Sigma.
Aggregation assays consisted of 0.5 mg/ml protein, 0.5 mg/ml polymer, and 5 mM DTT in PBS, pH 7.4, unless otherwise stated. When 35 μM lysozyme is used, protein concentration was verified by absorbance at 280 nm with an extinction coefficient of 36,000 M−1cm−1. All materials were dissolved immediately before use. Unless otherwise stated, samples were incubated in 15 ml sealed, polycarbonate tubes in a 37°C water bath for 3 h. Samples were briefly vortexed to mix and resuspend precipitate before all measurements, which were made at room temperature.
Large aggregates scatter light, making solutions appear turbid. Turbidity was quantified by optical absorbance at 400 nm. The wavelength was chosen because it offered the greatest sensitivity to turbidity without being absorbed by cuvette material. Samples were placed in 1 cm path length acrylic cuvettes within a Beckman DU800 spectrophotometer.
Thioflavin T (ThT) is a dye that fluoresces upon binding to cross-beta structures in aggregated proteins. Although these intramolecular bonds are a hallmark of amyloid fibrils, many aggregates bind and induce ThT fluorescence to varying extents. The assay consisted of mixing 100 μl of 0.04 mg/ml ThT (Sigma), dissolved in PBS and filtered, with 50 μl denatured protein sample within black-walled 96 well plates (Corning Inc., Corning, NY). Plates were incubated for 30 min in a 37°C incubator before reading fluorescence at 400 nm excitation / 482 nm emission using a Biotek Synergy Neo-2 plate reader (Winooski, VT).
High-speed centrifugation separates heavier aggregates from smaller, soluble proteins. Samples were centrifuged at 12,000 g for 15 min. The amount of protein in the supernatant was quantified using a bicinchoninic acid (BCA) assay kit. The BCA-RAC assay (Thermo Fisher, Waltham, MA) was compatible with DTT and used according to manufacturer instructions. Briefly, 9 μl sample was mixed with 4 μl compatibility reagent in a round-bottom 96 well plate and incubated 15 min. Working reagent was added to each well at a volume of 260 μl before incubating another 30 min at 37°C. A Biotek plate reader was used to quantify the absorbance of each well at 562 nm. Standard curves constructed from native proteins were used to calculate the results in terms of mg/ml, with 0.5 mg/ml corresponding to 100% protein solubility.
Fluorescent spectroscopy
Tryptophan exposure was quantified by intrinsic fluorescent spectroscopy. HEWL solutions containing 0.5 mg/ml protein, 0 or 5 mM DTT, and 0.525 mg/ml PEG or T1107 were mixed and maintained at 37°C in a heating block. In thirty minute intervals, 3 ml samples were transferred to a 1 cm quartz cuvette for measurement. Samples were excited with 295 nm light (2 nm bandwidth) and spectra were collected from 300 to 450 nm (2 nm bandwidth) in a FluoroLog-3 spectrofluorometer (Horiba Scientific, Edison, NJ) with 1200 g/mm grating blazed at 330 nm. An LFI-3751 control unit (Wavelength Electronics, Bozeman, MT) was used to maintain temperature at 37°C.
Circular dichroism spectroscopy
Circular dichroism (CD) spectroscopy was used to compare secondary structures in denatured samples. HEWL was treated for 3 h at 37°C in the presence of 0 or 5 mM DTT and 0.575 mg/ml PEG or T1107. All samples were diluted 1:50 in double distilled water in a 1 cm quartz cuvette within a J-715 spectropolarimeter (Jasco, Easton, MD) set to 37°C with at least 5 min equilibration time. Spectra were collected at 50 nm/min with a 5 nm bandwidth and 0.5 nm pitch. Data below 200 nm were not obtainable due to high sample absorbance, and the remaining data smoothed with a five-point moving filter prior to analysis.
Dynamic light scattering
Dynamic light scattering (DLS) measurements, used to quantify particle size distributions, were collected using a Dynapro Nanostar system (Wyatt, Santa Barbara, CA) with a 10 μl cuvette. Block copolymer micelle formation was observed in solutions of 0.5 mg/ml polymer heated at a rate of 1.3 °C/min. Solutions containing DTT-treated HEWL and T1107 were passed through a 0.22 μm syringe filter after 3 h at 37°C and allowed to equilibrate for 5 min in the cuvette before measuring. At least five acquisitions of 30 s each were used for each independent sample of polymer-only solutions, while 20 acquisitions of 5 s each were used for HEWL-polymer solutions. Results are reported as the mean of three independent samples, with particles comprising <1% of total mass excluded from analysis.
Data presentation and statistics
All bar and line charts were created using Excel (Microsoft, Redmond, WA) and display the mean measurements of three independent samples with error bars representing standard error. The ** symbol denotes p < .01, * p < .10, and # and % were employed to denote statistically-significant groupings in multiple comparison tests at p < .05. Histograms were created in Excel from data exported from Dynamics software (Wyatt), with traces that display three independent samples. Statistical analysis was completed using R software (R Foundation for Statistical Computing, Vienna, Austria) and consisted of two-tailed Student’s t-tests or analysis of variance with Tukey post-hoc means comparisons.
IV. Results
Quantitative measures of aggregation
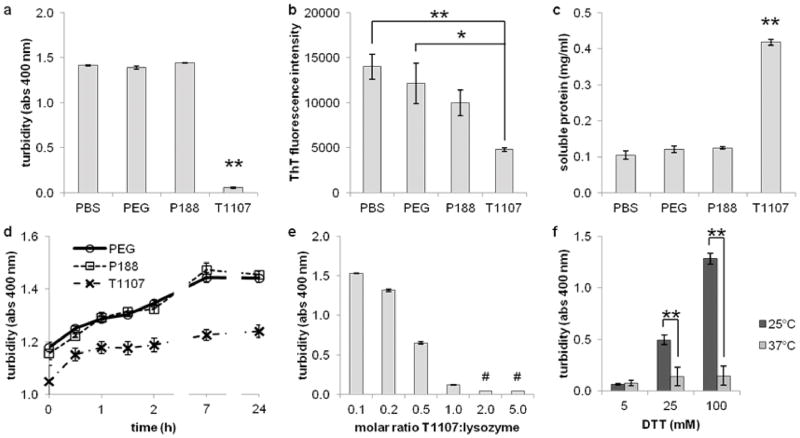
T1107 impaired the aggregation of DTT-reduced HEWL by three measures. A 3h incubation of 0.5 mg/ml HEWL in 5 mM DTT with no treatment (PBS), 0.5 mg/ml PEG, or 0.5 mg/ml P188 each produced a cloudy solution with some precipitate. Treatment in the presence of 0.5 mg/ml T1107 resulted in optically clear solutions. Turbidity was quantified by optical absorbance at 400 nm (Figure 2a). The turbidity of T1107 samples was statistically equivalent to samples treated with an equal mass of SDS, a potent, ionic detergent (data not shown, p = .99). The relative fluorescence of ThT, a dye that binds to cross-beta structures in aggregates, was also reduced from untreated (p < .01) and PEG (p = .01) samples (Figure 2b).
Figure 2.
Aggregation of DTT-reduced HEWL was inhibited in the presence of T1107. (a) Turbidity quantified by optical absorbance at 400 nm. Samples consisting of 0.5 mg/ml HEWL and 5 mM DTT resulted in milky-white solutions in the presence of 0.5 mg/ml PEG, P188, and no treatment (PBS), while samples treated with T1107 remained clear (p < .01). (b) ThT fluorescence decreased with block copolymer treatment (p < .01). (c) The amount of soluble protein recovered from supernatant after high speed centrifugation. T1107-treated samples contained 84% of native controls, while less than 25% of protein remained soluble in all other conditions (p < .01). (d) Addition of T1107 to DTT-denatured lysozyme did not reverse aggregation as measured by turbidity, but did halt further aggregate formation. Turbidity increased after 24 h in samples receiving PEG or P188 (p < .01) but not in samples receiving T1107 (p = .17). (e) Turbidity of HEWL in the presence of DTT and decreased with increasing amounts of T1107 (p < .05 for all pairwise comparisons except 2:1 and 5:1, indicated by #). (f) Aggregation was only prevented at 37°C. Increasing concentrations of DTT at room temperature resulted in increased turbidity, indicating that the anti-aggregation properties of T1107 were temperature-dependent (p < .01).
Aggregation properties were quantified by two additional measures. Aggregates treated with ThT, a dye that fluoresces upon binding to cross-beta structures in aggregated proteins, was highest in untreated samples and lowest in T1107-treated samples (Figure 2b). Finally, aggregates were separated by high-speed centrifugation, and protein in the supernatant was quantified using a DTT-compatible BCA assay (Figure 2c). Significantly more protein remained soluble after treatment with T1107 – 84% of the total protein content – compared to other treatments (p < .01). Although we observed that T1107 interfered with other BCA assay kits, it had no effect on blank wells in this procedure (data not shown, p = .52).
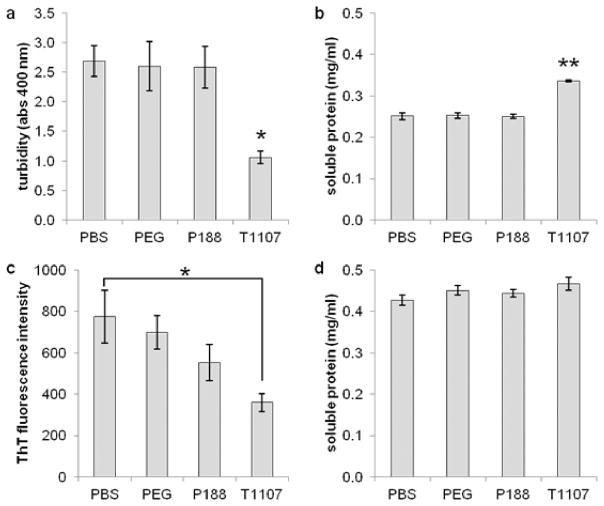
Two other model proteins were used to verify the anti-aggregation properties of T1107. Insulin is a 5.7 kDa protein with three disulfide bonds. At 0.5 mg/ml, insulin solutions became highly turbid upon DTT denaturation (Figure 3a). Treatment of T1107 significantly reduced turbidity (p = .02) and increased soluble protein recovery (Figure 3b, p < .01). Untreated insulin samples contained so much precipitate that accurate measures of ThT binding were not possible with this procedure. In contrast, the 13.7 kDa ribonuclease did not result in turbid solutions in any case. Fluorescence from ThT was lower in T1107-treated samples compared to untreated controls (Figure 3c, p = .05), but differences in soluble protein were not statistically significant (p = .63, Figure 3d).
Figure 3.
T1107 mitigates the aggregation of two other disulfide-containing proteins in the presence of 5 mM DTT. (a) Turbidity of 0.5 mg/ml insulin solutions was reduced but not eliminated (p = .02), and (b) recovery of soluble protein was increased (p < .01) in the presence of T1107. (c) A decrease in ThT fluorescence of DTT-treated ribonuclease was observed compared to untreated controls (p = .05), while (d) an increase in soluble protein was observed but did not reach statistical significance (p = .63).
Treatment with T1107 inhibited DTT-induced aggregation, but did not reverse it. HEWL was denatured for 1 h before adding PEG, P188, or T1107 to a final concentration of 0.5 mg/ml treatment, 0.5 mg/ml protein, and 5 mM DTT. Turbidity measurements were taken immediately after mixing and at several time points thereafter (Figure 2d). Turbidity at all points after 30 min treatment with T1107 were not significantly increasing (p = .17). Control PEG- and P188-treated samples continued to increase in turbidity (p < .01) and were statistically higher than T1107 at each point (p < .01).
The effects of T1107 on aggregation were dose-dependent (p < .01). HEWL was dissolved at 35 μM and denatured in the presence of varying quantities T1107 (Figure 2e). Significant differences (p < .05) were detected between all pairs except 2:1 vs. 5:1 molar ratios.
The ability of T1107 to mitigate aggregation depended on temperature. The aggregation assay was run with 5, 25, and 100 mM DTT and at room temperature or 37°C (Figure 2f). Turbidity increased with DTT concentration at room temperature (p < .01) but not at 37°C (p = .77). At 5 mM DTT, turbidity was minimal in both samples (p = .99). However, the turbidity of room temperature samples with DTT at 25 mM (p = .01) and 100 mM (p <.01) was significantly higher than those at 37°C.
The observed effects were not due to crowding, as comparisons with an equivalent mass concentration of purely-hydrophilic PEG did not reduce aggregation compared to untreated controls. Further, the results were likely not due purely to the detergent properties of T1107, as an equivalent mass concentration of P188 did not significantly reduce aggregation in any test. The linear P188 has a similar hydrophobe:hydrophile ratio to the four-arm T1107, and with roughly half the molecular weight should have fewer steric barriers to associating with exposed hydrophobic surfaces on denatured proteins. Taken together, the results suggest that T1107 interacted with DTT-denatured HEWL in a way that prevented aggregate growth and limited cross-beta bond formation.
DLS of block copolymers
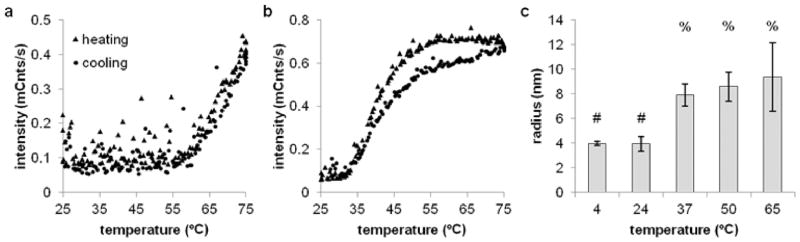
The temperature-dependent behavior of T1107 was linked to temperature-dependent micelle formation using DLS. Measurements were made at 0.5 mg/ml in PBS while increasing the temperature. The critical micelle temperature of P188 was observed at 60°C and was reversible (Figure 4a) Measurements of particle radius were highly variable, indicating a heterogeneous mixture of micelle size. In contrast, the critical micelle temperature of T1107 was 32°C (Figure 4b), and the resulting micelles were comparatively smaller and homogenous in size (Figure 4c). Assuming spherical particles, the density of T1107 at room temperature and below is approximately 0.09 g/cm3 – less than a typical protein and consistent with these largely hydrophilic molecules being monomeric in solution. Assuming the same density, the micelles would contain eight molecules.
Figure 4.
Temperature-dependent micelle formation measured by DLS. (a) P188 forms irregularly shaped micelles above 60°C at 0.5 mg/ml in PBS. Micelle formation was reversible upon cooling. In contrast, (b) T1107 had a critical micelle temperature of 32°C, and (c) the resulting micelles were uniform in size. Symbols denote statistical groupings (p < .05).
Fluorescent spectroscopy of denatured HEWL
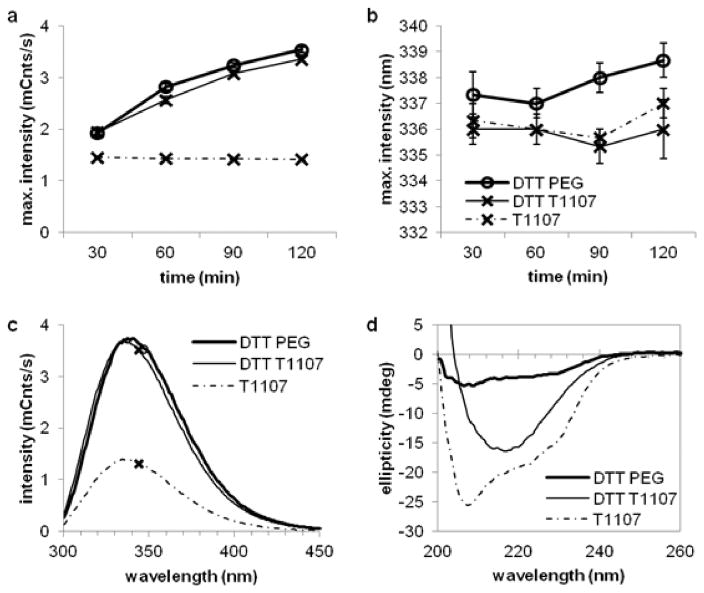
Tryptophan fluorescence emission increased at a steady rate for the first two hours of DTT denaturation in the presence of both T1107 and PEG (Figure 5a), reflecting a progressive change in tertiary conformation of the protein population. Samples denatured in the presence of PEG had slightly higher intensity (p < .01) and red shift in the emission maximum (p < .01) at each point compared with T1107. No differences in maximum intensity were observed between PEG and T1107 treatments of either denatured (p = .34) or native (p = .99) HEWL by 3h (Figure 5c), though the slight red shift remained (p < .01). Despite reaching statistical significance, the rates T1107 only slightly alter the kinetics of tertiary conformational changes.
Figure 5.
Denaturation with DTT resulted in increased tryptophan fluorescence and a loss of alpha-helical structure in all samples, while T1107 preserved or promoted beta-sheet structures in HEWL. (a) Fluorescent intensity and (b) emission maxima over time. (c) Representative fluorescent spectra after 3 h DTT treatment, indicating little difference between PEG- and T1107-treated samples. (d) Representative CD spectra showed that T1107 treatment allowed for beta-sheet structures in denatured HEWL, while PEG did not prevent the loss of either beta-sheet or alpha-helical structures. Plots show HEWL incubated with DTT and PEG (round markers, thick lines), DTT and T1107 (X markers, thin lines), and T1107 alone (X markers, dashed lines). Native HEWL in the presence of PEG was similar to T1107 and omitted for clarity.
CD spectroscopy of denatured HEWL
HEWL denatured in the presence of PEG lost nearly all evidence of secondary structure by three hours (Figure 5d). T1107 appeared to preserve or enhance the beta structures in the protein, but not alpha structures. A negative peak at 218 nm, characteristic of beta structures13, was a mean of −18.5 mdeg from native controls in the presence of T1107, −15.5 mdeg from denatured samples with T1107 (p = .05 compared to native), and −3.4 mdeg from denatured samples with PEG (p < .01 compared to native). In contrast, the negative peak at 208 nm characteristic of alpha structures13 decreased in both denatured samples, from a mean of −23.7 mdeg in native samples with T1107, to −10.1 mdeg and −4.25 mdeg for denatured samples with T1107 (p < .01) and PEG (p < .01), respectively. No differences were observed between native samples in the presence of T1107 compared to PEG (p > .88 at both wavelengths). Note that no tryptophan residues were present in the beta domain (Figure 1e), and that intermolecular bonds formed in these regions may be the sites of ThT binding observed in aggregates (Figure 2b).
DLS of denatured HEWL and T1107
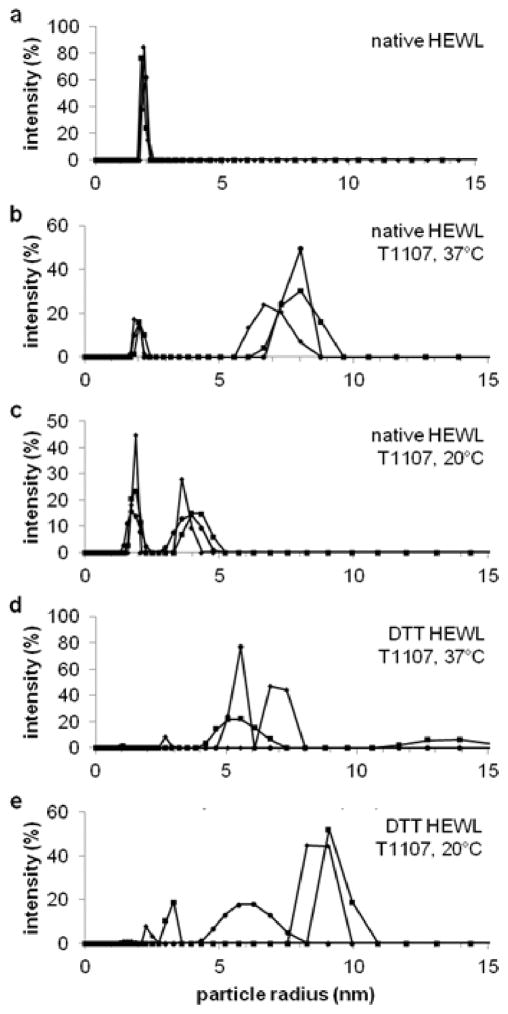
The block copolymer T1107 formed a complex with DTT-denatured HEWL at 37°C, but dissociated at 20°C. Solutions containing 0.5 mg/ml HEWL and an equimolar amount of T1107, with or without DTT, were incubated for 3 h at 37°C and filtered before DLS. Native HEWL alone in solution had a mean hydrodynamic radius of 1.9 nm and mean polydispersity of 3.9% (Figure 6a). Solutions of native HEWL in the presence of T1107 had similarly-sized particles at 20°C (p = .71) and 37°C (p = .08), along with a second population of particles with mean hydrodynamic radii of 3.9 nm (6.3% polydispersity, Figure 6b) and 7.6 nm (7.6% polydispersity, Figure 6c), which were close in size to monomeric and micellized T1107 reported above (Figure 4d). These results indicated there were weak or no physical interaction between T1107 and the native protein. In contrast, solutions containing DTT-denatured HEWL and T1107 had a dominant peak at 5.9 nm with 6.8% polydispersity (Figure 6d). This peak, being larger than native HEWL (p = .02) and monomeric T1107 (p = .04), but smaller than micellized T1107 (p = .07), is indicative of a stable complex between T1107 and the denatured protein. The presence of a single dominant peak with a radius that didn’t match native protein or T1107 indicated the complexed structure was equimolar. The same solutions cooled to 20°C for 1 h, had at least two populations of particle sizes and greater polydispersity (Figure 6e), indicating the dissociation of T1107 from denatured HEWL below the critical micelle temperature.
Figure 6.
Particle size distributions from DLS demonstrated the reversible association of T1107 and denatured HEWL. (a) DLS of native lysozyme measured a hydrodynamic radius of 1.9 nm. (b) Native lysozyme (1.9 nm radius) in the presence of T1107 micelles (8.0 nm radius) indicated that the block copolymer does not interact with the native protein at 37C or (c) at 20C, in the presence of monomeric T1107. (d) T1107 formed a complex with DTT-denatured HEWL with a hydrodynamic radius of 5.5 nm; the size low polydispersity suggested this was a single polymer molecule interacting with a single protein. (e) The association was reversible upon cooling, demonstrating the temperature-dependent behavior of T1107.
V. Discussion
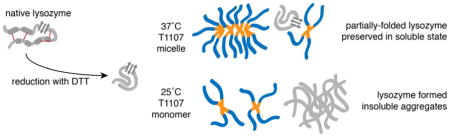
This work established two important molecular mechanisms that underlie T1107 chaperone behavior. First, T1107 stabilized a partially folded form of HEWL. Specifically, the block copolymer preserved or promoted the beta-sheet structures in the center of the sequence after disulfide cleavage induced collapse of alpha-helical structures and tertiary reorganization. By stabilizing this folding intermediate, T1107 prevented the formation of insoluble and light-scattering aggregates. Second, the ability of T1107 to associate with denatured protein and prevent aggregation was tied to its critical micelle temperature. The hydrophobicity of PPO in the core of the molecule increases with temperature. At room temperature and below, T1107 was in monomeric form. Only at 37°C and above was T1107 capable of associating with and stabilizing a partially folded species of HEWL.
Disulfide scissions lead to the rearrangement of the alpha helical structures that comprise significant fractions of the protein from 23–54 and 98–138. Four of six tryptophan residues were in these stretches, and the remaining two were adjacent to a disulfide-bonded cysteine outside of the beta-rich region. The dramatic increase in tryptophan emission may be explained by hydrophobic-driven collapse and internalization of the fluorescing residues, or by other changes in tertiary structure that altered quenching characteristics. The ThT assays suggested that in the absence of a chaperone, the intermediate states formed intermolecular beta structures as they lost intramolecular beta-sheets. Previous work has provided evidence for an intermediate state of lysozyme between native and aggregate-forming states that lacks alpha helical structure, has significantly increased tryptophan fluorescence, and has increased capacity to retain hydrophobic dyes12,14.
Further work is necessary to quantify the thermodynamics, kinetics, and precise molecular nature of T1107-protein interactions. The micelle results suggested that the interactions are driven by entropic association of hydrophobic PPG core15 with residues in HEWL’s beta-rich region, while the dose-response and DLS experiments suggested this is a 1:1 interaction. Other interactions may be involved. Though widely considered to be protein-inert, polyethylene glycol does interact with hydrophobic surfaces in native lysozyme16 and specific intermediate states of other proteins17,18. Electrostatic forces may also play a role, as the population of T1107 in PBS likely contains molecules with deprotonated and monoprotonated diamine cores at physiological pH19,20, and there are a number of aspartate residues in this region.
The temperature-dependence of T1107-protein association suggested that its activity could be triggered or modulated by external stimulus. Certain protein chaperones have been reported to have stimuli-responsive behavior21–23, and some synthetic chaperones have been designed around the concept24–26. T1107 could be directed to “catch-and-release” denatured proteins by cycling the temperature. Similar block copolymers could be designed with stimuli-responsive hydrophobic cores triggered by light, pH, or ionic concentration27.
Several attempts were made to determine functional recovery of HEWL following DTT-induced aggregation using various methods to remove or de-activate DTT. In most cases, there was no difference in enzyme activity between T1107-treated solutions and controls. This was likely due to the failure to re-form disulfide bonds, or disulfide shuffling between the nine cysteine residues in the protein. Testing the hypothesis that T1107 is capable of holding denatured proteins in refoldable conformation at 37°C will require a more reversible denaturation procedure.
The term chaperone is used to describe a variety of behaviors, including preventing aggregation through various mechanisms, breaking up aggregates after they have formed, and actively renaturing proteins28. The evidence presented here suggests that a single T1107 prevented aggregation by binding and sequestering denatured lysozyme. In this capacity, T1107 appeared to share characteristics with sHSPs. These <50 kDa chaperones maintain denatured proteins until larger, ATP-driven chaperones are responsible for refolding2. Small HSPs also have oligomerization dynamics that are directly linked to their functionality as chaperones29. It’s possible that if dynamics of micelle formation relevant to chaperone abilities are identified, they could be used to screen synthetic chaperone candidates.
The poloxamine T1107 is commonly used in pharmaceutical formulations and contact lens solutions. It is also under investigation as an anti-malarial drug to prevent membrane fusion and release of the parasite30. Further, it has been proven to seal radiation-damaged erythrocyte membranes31 and protects tissue following ischemia-reperfusion injury32. This work suggests the beneficial effects of T1107 are maybe due, in part, to partial preservation of protein secondary structure and prevention of aggregation.
VI. Conclusions
The pentablock amphiphilic copolymer T1107 prevented the aggregation of DTT-reduced HEWL. Structural characterization showed that T1107 supported the presence of beta-sheet structures in a partially folding species of HEWL, and that loss of these secondary structures in the absence of T1107 resulted in the formation of large aggregates. T1107 manifested this attribute of chaperones when used above the critical micelle temperature. The results suggest that T1107 mimics the behavior of sHSPs, and that these or similar biocompatible block copolymers may be used to preserve protein structure in a variety of conditions.
Acknowledgments
Funding Sources
This research was supported by the National Institutes of Health, National Institute of General Medical Sciences through T32 Training Grant GM099697 (RCL), RO1 GM0055694 (TRS) and the National Center for Advancing Translational Sciences through Grant UL1 TR000430 (RCL).
Microplate assay and DLS data were collected in the University of Chicago BioPhysics Core Facility. The authors thank Elena Solomaha and Michael Baxa for technical assistance. The authors also thank Michael Baxa, Joshua Riback, and Adam Zmyslowski for discussions regarding protein denaturation and refolding, and Devkumar Mustafi, Kristen Jakubowski, Colin Mcfaul, and Jaemin Chin for constructive input regarding T1107 as a synthetic chaperones.
ABBREVIATIONS
- BCA
bicinchoninic acid
- CD
circular dichroism
- DLS
dynamic light scattering
- DTT
dithiothreitol
- HEWL
hen egg white lysozyme
- P188
Poloxamer 188
- PBS
phosphate-buffered saline, pH 7.4
- PEG
poly(ethylene glycol)
- PEO
poly(ethylene oxide)
- PPO
poly(propylene oxide)
- sHSP
small heat shock protein
- T1107
Poloxamine 1107
- ThT
thioflavin T
Footnotes
Author Contributions
MJP, TRS, SCM, and RCL conceived the experiments, analyzed the results, and edited the manuscript. MJP conducted the experiments. All authors have given approval to the final version of the manuscript.
References
- 1.Richter K, Haslbeck M, Buchner J Molecular Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J Nature Structural & Molecular Biology. 2005;12(10):842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 3.Poellmann MJ, Lee RC. submitted. [Google Scholar]
- 4.Walsh AM, Mustafi D, Makinen MW, Lee RC. Annals of the New York Academy of Sciences. 2004;1066:321–327. doi: 10.1196/annals.1363.029. [DOI] [PubMed] [Google Scholar]
- 5.Lee RC, Despa F, Guo L, Betala P, Kuo A, Thiyagarajan P. Annals of Biomedical Engineering. 2006;34(7):1190–1200. doi: 10.1007/s10439-006-9139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustafi D, Smith CM, Makinen MW, Lee RC. Biochimica et Biophysica Acta. 2008;1780:7–15. doi: 10.1016/j.bbagen.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Despa F, Orgill DP, Neuwalder J, Lee RC. Burns. 2005;31(5):568–577. doi: 10.1016/j.burns.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Wirmer J, Schlorb C, Klein-Seetharaman J, Hirano R, Ueda T, Imoto T, Schwalbe H. Angew Chem It Ed. 2004;43:5780–5785. doi: 10.1002/anie.200460907. [DOI] [PubMed] [Google Scholar]
- 9.Fischer B, Sumner I, Goodenough P. Archives of Biochemistry and Biophysics. 1993;306(1):183–187. doi: 10.1006/abbi.1993.1498. [DOI] [PubMed] [Google Scholar]
- 10.Raman B, Ramakrishna T, Rao CM. Journal of Biological Chemistry. 1996;271(29):17067–17072. doi: 10.1074/jbc.271.29.17067. [DOI] [PubMed] [Google Scholar]
- 11.De Bernardez Clark E, Hevehan D, Szela S, Maachupalli-Reddy J Biotechnology Progress. 1998;14(1):47–54. doi: 10.1027/bp970123w. [DOI] [PubMed] [Google Scholar]
- 12.Gu Z, Xiaonan Z, Ni S, Su Z, Zhou HM. The International Journal of Biochemistry & Cell Biology. 2004;36:795–805. doi: 10.1016/j.biocel.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Greenfield NJ. Nat Protoc. 2006;1(6):2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain N, Bhattacharya M, Mukhopadhyay S. Journal of Fluorescence. 2011;21:615–625. doi: 10.1007/s10895-010-0749-3. [DOI] [PubMed] [Google Scholar]
- 15.Alexandridis P, Holzwarth JF, Hatton TA. Macromolecules. 1994;27:2414–2425. [Google Scholar]
- 16.Furness EL, Ross A, Davis TP, King GC. Biomaterials. 1998;19(15):1361–1369. doi: 10.1016/s0142-9612(98)00007-6. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JL, Randolph TW. Journal of Biological Chemistry. 1992;267(5):3147–3153. [PubMed] [Google Scholar]
- 18.Ahmad B, Ansari MA, Sen P, Khan RH. Biopolymers. 2006;81(5):350–359. doi: 10.1002/bip.20424. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong JK, Chowdhry BZ, Snowden MJ, Dong J, Leharne SA. International Journal of Parmaceutics. 2001;229(1–2):57–66. doi: 10.1016/S0378-5173(01)00816-X. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Lorenzo C, Gonzalez-Lopez J, Fernandez-Tarrio M, Sandez-Macho I, Concheiro A. European Journal of Pharmaceutics and Biopharmaceutics. 2007;66(2):244–252. doi: 10.1016/j.ejpb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Tapley TL, Franzmann TM, Chakraborty S, Jakob U, Bardwell JCA. PNAS. 2010;107(3):1071–1076. doi: 10.1073/pnas.0911610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichmann D, Xu Y, Cremers CM, Ilbert M, Mittelman R, Fitzgerald MC, Jakob U. Cell. 2012;148(5):947–957. doi: 10.1016/j.cell.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmieri V, Maulucci G, Maiorana A, Papi M, De Spirito M. ChemBioChem. 2013;14(17):2362–2370. doi: 10.1002/cbic.201300447. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto N, Hashimoto T, Felix MM, Umakoshi H, Kuboi R. Biomacromolecules. 2003;4(6):1530–1538. doi: 10.1021/bm015662a. [DOI] [PubMed] [Google Scholar]
- 25.Hirakura T, Nomura Y, Aoyama Y, Akiyoshi K. Biomacromolecules. 2004;5:1804–1809. doi: 10.1021/bm049860o. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Sreekumar PG, Valluripalli V, Shi P, Wang J, Lin YA, Cui H, Kannan R, Hinton DR, MacKay JA. Journal of Controlled Release. 2014;191:4–14. doi: 10.1016/j.jconrel.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen Stuart MA, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Nature Materials. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Zvi AP, Goloubinoff P. Journal of Structural Biology. 2001;135:84–93. doi: 10.1006/jsbi.2001.4352. [DOI] [PubMed] [Google Scholar]
- 29.Stengel F, Baldwin AJ, Painter AJ, Jaya N, Basha E, Kay LE, Vierling E, Robinson CV, Benesch JLP. PNAS. 2010;107(5):2007–2012. doi: 10.1073/pnas.0910126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glushakova S, Humphrey G, Leikina E, Balaban A, Miller J, Zimmerberg J. Current Biology. 2010;20(12):1117–1121. doi: 10.1016/j.cub.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannig J, Yu J, Beckett M, Weichselbaum R, Lee RC. International Journal of Radiation Biology. 1999;75(3):370–385. doi: 10.1080/095530099140555. [DOI] [PubMed] [Google Scholar]
- 32.Palmer JS, Cromie WJ, Lee RC. The Journal of Urology. 1998;159:2136–2139. doi: 10.1016/S0022-5347(01)63295-6. [DOI] [PubMed] [Google Scholar]