Excimer lasers are pulsed gas lasers that use a mixture of a rare gas and halogen as the active medium to generate pulses of short-wavelength, high-energy ultraviolet light. [1] Irradiction to atherosclerotic coronary artery segments allows the culprit lesions to transpirate without heat damaging the normal tissues. We described an effective case of excimer laser coronary atherectomy (ELCA) for a device-uncrossable chronic total occlusion (CTO) lesion below.
A 77-year-old male was found to have a large ischemic defect and redistribution in the inferior wall that was revealed by myocardial perfusion scintigraphy before cerebrovascular surgery. The patient was suspected to have ischemic heart disease, and was transferred to our institution. A coronary angiography (CAG) revealed non-occlusive disease of his left coronary system along with a CTO of the right coronary artery (RCA) (Fig. 1A); he was diagnosed with silent myocardial ischemia (SMI), and percutaneous coronary intervention (PCI) was performed. We performed PCI for the CTO lesion after cerebrovascular surgery because we diagnosed stable ischemic heart disease. A 6 French approach was adopted (JR4.0 guide for the RCA). An antegrade approach was selected because of a lack of appropriate collateral branches for a retrograde approach. A GAIA first guidewire (Asahi Intecc Co. Ltd., Nagoya, Japan), with the support of an Asahi Corsair microcatheter (also called channel dilator, Asahi Intecc Co. Ltd., Nagoya, Japan), was crossed into the distal site of the occluded lesion (Fig. 1B). However, a Corsair microcatheter, Sapphire II balloon 1.0 × 5.0 mm (Orbusneich Medical, Tokyo, Japan) and Tornus 2.6Fr (penetration catheter, Asahi Intecc Co. Ltd., Nagoya, Japan) were not able to pass the lesion. Thereafter, although we tried mother and child technique [2] and buddy wire technique, both techniques failed. Eventually, perforation in the distal RCA lesion by the guidewires was confirmed (Fig. 1C). However, no findings indicated cardiac tamponade and the hemodynamics were not disrupted; all procedures were terminated because of potent concerns of cardiac tamponade.
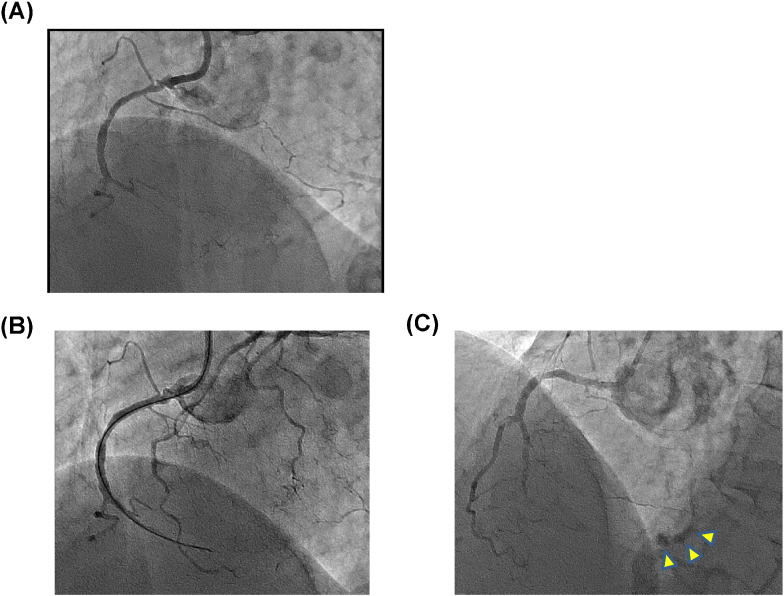
Fig. 1.
(A) Chronic total occlusion (CTO) of the mid-right coronary artery (RCA) revealed by coronary angiography. (B) Guidewire advanced to distal RCA. (C) Extravasation of contrast media (arrowhead).
After 17 months, a second PCI attempt was performed. Single right femoral access was obtained, and a 7Fr AL1.0 SH guiding catheter with side holes (Cordis Corporation, Warren, NJ, USA) was used. Although a GAIA first guidewire, with the support of an Asahi Corsair microcatheter, was crossed into the distal site of the occluded lesion similar to a previous procedure, CAG revealed that the GAIA first guidewire was suspected of crossing a pseudo-lumen. After withdrawing the Corsair microcatheter, Navifocus WR intravascular ultrasound (IVUS) system (Terumo Corp., Japan) [3] was performed through the GAIA first guidewire in that it was suspected to be in the pseudo-lumen. Referencing the IVUS images, a GAIA second guidewire (Asahi Intecc Co. Ltd., Nagoya, Japan) crossed the CTO lesion through a different pathway by an IVUS-oriented method. A Navifocus WR IVUS system over the GAIA first guidewire revealed that the GAIA second guidewire was crossing the true-lumen in the CTO lesion (Fig. 2A). Attempts to cross balloon catheters or penetration catheters for dilatations or microcatheter failed similarly to previous procedures. An excimer laser (Spectranetics, Colorado Springs, Co., USA) [1] was advanced over the GAIA second guidewire; photoablation was performed. We performed the excimer laser technique with a Point 9 catheter (Spectranetics, Colorado Springs, Co., USA) at a maximum energy of 60 fluence and a frequency of 40 Hz. During each activation of the laser, contrast agent was injected into the vessel to amplify the disruptive effect of the laser. This successfully photoablated the lesion which allows the Corsair microcatheter to advance into the distal RCA (Fig. 2B). The GAIA second guidewire was diverted to Neo's SION Blue guidewire (Asahi Intecc Co. Ltd., Nagoya, Japan) using a Corsair microcatheter. Afterwards, sequential balloon expansion occurred, and the lesion was then successfully stented (Fig. 2C).
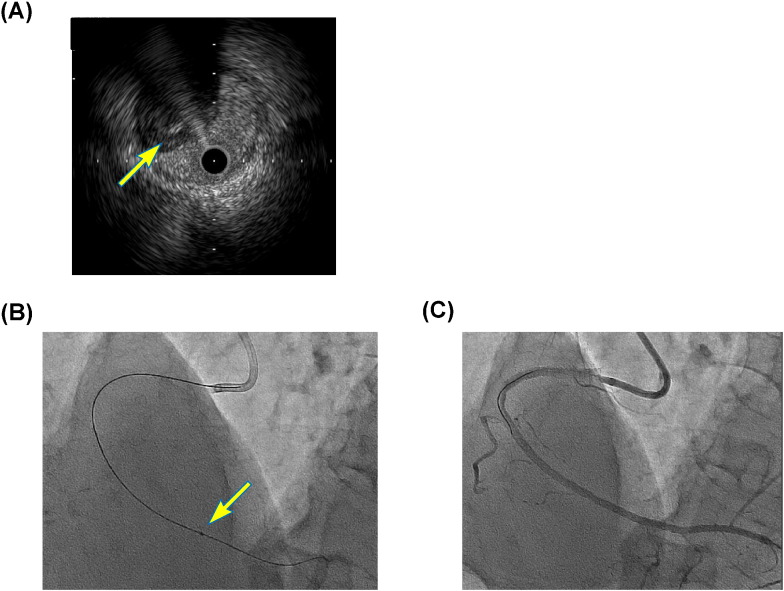
Fig. 2.
(A) Presence of guidewire in true-lumen revealed by intravascular ultrasound images (arrow). (B) Irradiction to CTO segments by excimer laser. (C) Post-stent deployment, with excellent results.
In this session, although a guidewire crossed into a false-lumen, this cross allowed us to cross another guidewire into the true-lumen by an IVUS-guided technique. After we confirmed that the guidewire was present in true-lumen, we performed the ELCA. A previous report [4] by Brilakis et al. showed that the use of Rotablator® was an alternative; however, it was impossible to use a Rotawire® because it was not realistic in the present case.
The laser catheter was used to have the ability to cross the heavily calcified CTO lesion that the balloon was not able to cross. However, the advent of a smaller (0.9 mm tip diameter) excimer laser coronary catheter (Point 9, Spectranetics Corporation, USA) allowed us to cross the complex lesions, as described in the case above.
The Point 9 xenon chloride excimer laser catheter is a pulsed ultraviolet laser catheter. It differs from longer-wavelength continuous-wave lasers in that it causes minimal thermal injury to tissue and is capable of ablating calcified plaque. Early generation 1.6-mm catheters were made of twelve 100-μm fibers. The 0.9 mm consisted of two rows of 50-μm fibers arranged concentrically around the PTFE (polyfluoroethylene) walled guide wire tubing [5].
The major findings of this report are as follows: 1) balloon expansions and stent deployments were successfully achieved by performing ELCA for a CTO lesion where a balloon or penetration catheter would not pass or when the procedure was limited to an antegrade approach, and 2) a PCI guidewire was able to cross the true-lumen by utilizing a parallel wire technique and IVUS-guided technique. After we confirmed that the PCI guidewire was present in the true-lumen, ELCA was performed.
Funding sources.
None.
Potential conflict of interest.
None.
References
- 1.Isner J.M., Donaldson R.F., Deckelbaum L.I., Clarke R.H., Laliberte S.M., Ucci A.A. The excimer laser: gross, light microscopic and ultrastructural analysis of potential advantages for use in laser therapy of cardiovascular disease. J. Am. Coll. Cardiol. 1985;6:1102–1109. doi: 10.1016/s0735-1097(85)80316-8. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi S., Saito S., Tanaka S., Miyashita Y., Shiono T., Arai F. New method to increase a backup support of a 6 French guiding coronary catheter. Catheter. Cardiovasc. Interv. 2004;63:452–456. doi: 10.1002/ccd.20223. [DOI] [PubMed] [Google Scholar]
- 3.Okamura A., Iwakura K., Date M., Nagai H., Sumiyoshi A., Fujii K. Navifocus WR is the promising intravascular ultrasound for navigating the guidewire into true lumen during the coronary intervention for chronic total occlusion. Cardiovasc. Interv. Ther. 2014;29:181–186. doi: 10.1007/s12928-013-0212-x. [DOI] [PubMed] [Google Scholar]
- 4.Brilakis E.S., Grantham J.A., Rinfret S., Wyman R.M., Burke M.N., Karmpaliotis D. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 2012;5:367–379. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Ohlow M.A., Lotze U., Lauer B. Excimer laser coronary atherectomy in septal collaterals during retrograde recanalization of a chronic total occlusion. Heart Int. 2011;6 doi: 10.4081/hi.2011.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]