Abstract
Background
Odontogenic Cysts & tumors originate through some aberration from the normal pattern of odontogenesis. Ameloblastoma is one of the most frequent intraosseous odontogenic tumors. However it is no longer appropriate to use the diagnosis of ameloblastoma without specifying the type. Varied-clinical entities of ameloblastoma differ in their biologic behaviour. Odontogenic cysts like dentigerous and radicular cysts are less aggressive in nature than odontogenic tumors. Recently, podoplanin commonly used as a lymphatic endothelial marker in cancers has recently been found to play a possible role in odontogenic tumorigenesis also. Therefore the purpose of this study was to immunohistochemically analyse the expression of podoplanin in ameloblastomas, KCOTs, dentigerous cysts, radicular cysts & dental follicles.
Methods
Paraffin-embedded tissue specimens of 15 Ameloblastomas (7 follicular, 6 unicystic, 2 desmoplastic),10KCOTs, 5 dentigerous cysts, 5 radicular cysts & 5 dental follicles were immunohistochemically examined using antibody against podoplanin.
Results
All ameloblastomas displayed podoplanin expression in ameloblast-like cells of the epithelial islands while the stellate-reticulum like cells exhibited no or weak immunostaining. Expression of podoplanin in KCOTs was strongly positive in the cells of the basal and suprabasal layers & odontogenic epithelial nests. Positive immunoreaction for podoplanin was observed in the inflammatory radicular cysts and inflamed dentigerous cyst only and negative or weak expression in the lining epithelium of uninflamed dentigerous cysts and dental follicles.
Conclusion
Our results suggest that podoplanin can be used as a potential proliferative marker to observe the aggressive behaviour of ameloblastomas and KCOTs.
Keywords: Ameloblastoma, Dentigerous cyst, Keratocystic odontogenic tumor (KCOT), Radicular cyst, Podoplanin
1. Introduction
A multitude of odontogenic tumors and cysts occurring in the oral and maxillofacial region originate through some aberration from the normal pattern of odontogenesis which reflect their complex multiformity.1 It is no longer appropriate to use the diagnosis of ameloblastoma which is the second most common odontogenic neoplasm without specifying the type in any scientific study.Varied-clinical entities of ameloblastoma differ in their biologic behaviour. The solid / multicystic ameloblastoma is a slowly growing, locally invasive, epithelial odontogenic tumour of the jaws with a high rate of recurrence. The unicystic ameloblastoma on the other hand presents as a cyst with low recurrence rate & less aggressive behaviour. Recently desmoplastic variant of ameloblastoma is also regarded as a separate clinical entity based on its distinct clinicopathologic behaviour.2 The odontogenic keratocyst (OKC) is known to have a more aggressive biologic behavior than other cysts of jaw. In the 2005 WHO classification, odontogenic keratocyst has been redesignated under the name of keratocystic odontogenic tumor (KCOT). Hence in this study this lesion is referred to as KCOT. These benign odontogenic tumors have the property of invasion, resulting in high recurrence rate. The extent of invasion can be analysed by the expression and production of various genes and proteins by lesional cells. However, current clinical parameters lack the potential to predict the neoplastic behavior in KCOTs. Till date, no suitable immunohistochemical marker is available to assess the aggressiveness of KCOT.
Podoplanin which is frequently used as a lymphatic endothelial marker in OSCCs has recently been found to play a possible role in odontogenic tumorigenesis also. Podoplanin is a 38 kDa type-1 transmembrane sialomucin -like glycoprotein which consists of 162 amino acids. In the recent literature, podoplanin expression is also observed in odontogenic tissues like in secretory ameloblasts, developing & mature odontoblasts, Tomes’ fibres & pulp cells.3
The podoplanin expression in tumorous odontogenic cells is a recent topic of interest. Both mitotic activity and podoplanin expression within the ameloblastoma are coincident (i.e., restricted to the peripheral epithelial cells of the tumor cords and strands). This pattern of distribution of podoplanin immunostaining, according to the cellular subtype in ameloblastomas, may be helpful to the classification of odontogenic tumors.4
Various clinical and laboratory studies reviewed in the past provided evidence of the distinctively less aggressive nature of the dentigerous & radicular cysts than odontogenic keratocysts. The expression of podoplanin in these odontogenic cysts can therefore be compared with that of KCOTs. Moreover, the question of whether inflammation in dentigerous cyst might influence its behaviour & may bring about a change in the immunohistochemical expression of podoplanin in the developmental cyst can also be evaluated.
Podoplanin has been also found to modulate the actin cytoskeleton, thereby suggesting an important role in tumor invasion and metastasis.5 It may be involved in the process of local expansion of developmental, inflammatory and neoplastic odontogenic lesions. The pattern of staining for podoplanin in KCOT could be related to its neoplastic nature, and may suggest a role of this protein in tumor invasiveness.
Therefore, this study aims to analyse the expression of podoplanin in the ameloblastomas and keratocystic odontogenic tumors (KCOTs), further to elucidate and reinforce the importance of this molecule in the growth of these tumors & to compare their pattern of expression with dentigerous & radicular cysts.
1.1. Materials & methods
The paraffin embedded study specimens which included fifteen cases of Ameloblastomas (seven follicular, six unicystic & two desmoplastic), ten cases of Keratocystic Odontogenic Tumors, ten cases of odontogenic cysts (five dentigerous cysts & five radicular cysts) & five sections of dental follicles (taken as control) were retrieved from the archives of Department of Oral & Maxillofacial Pathology, Kanti Devi Dental College, Mathura, U.P.
2. Immunohistochemistry
Deparaffinized sections were immersed in 0.01 M citrate buffer, pH 6.0, and heated in a microwave oven for 5 min at high voltage and then for 15 min at low voltage for antigen retrieval. The tissue was then immersed in peroxide block for 20 min at room temperature to block endogenous peroxidase activity. After PBS washing, tissue was covered with power block reagent for 20 minutes. Ready to use mouse monoclonal anti-human D2–40 (anti-podoplanin) antibody(Dako Flex)was applied to the sections for 1 h at room temperature and the slides were then covered with Post primary Block for 25 minutes.The sections were then covered with secondary antibody conjugated with peroxidise.This was followed by application of freshly prepared substrate chromogen (DAB) solution for 2 minutes and then counterstained with Mayer’s hematoxylin.
3. Immunostaining evaluation
Scoring was based on:
Intensity of the podoplanin expression in the epithelial odontogenic Cells (A): 0 = absent, 1 = weak, 2 = moderate,3 = strong, and 4 = very strong.
Percentage of podoplanin positive odontogenic cells (B): 0 = 0% positive cells,1 = <25% positive cells,2 = 25–50% positive cells,3 = 50–75% positive cells,4 = >75% positive cells.
Final score (A + B): 0 = absent,1–4 = weak, and 5–8 = strong.
Final scores ranged from 0 to 8: (0 = absent, 1–4 = weak,5–8 = strong).
For statistical analysis,the independent t- test was used to compare the mean scores among the study groups. The level of significance was set at 5% for all tests.
4. Results
The immunohistochemical results for Ameloblastomas, KCOTs, Dentigerous cysts, Radicular cysts & Dental Follicles are summarised in Table 1. The immunohistochemical results for odotogenic tumors, odontogenic cysts & dental follicles are given in Table 2. The strong expression of podoplanin in follicular ameloblastomas was predominantly observed in the peripheral columnar cells of tumoral islands, while the stellate-reticulum like cells exhibited virtually no or weak immunostaining. The acanthomatous areas did not express podoplanin (Fig. 1, Fig. 2). The expression of podoplanin was quite variable in the unicystic ameloblastomas. Its positivity was restricted to the basal and supra-basal layers of the cystic lining in some tumors and spread throughout the extension of the tumoral epithelium in others (Fig. 3). Out of two desmoplastic specimens, one showed moderate immunostaining score for podoplanin whereas the other one showed strong immunostaining score (Fig. 4). Expression of podoplanin in KCOTs was strongly positive in the cell membrane and cytoplasm of most of the cells in the basal and suprabasal layers & areas of odontogenic epithelial nests (Fig. 5). In 4 out of 5 cases of dentigerous cysts, the immunoreaction for podoplanin was negative or weak in some areas of lining epithelium (Fig. 6). Interestingly, positive immunoreactivity for podoplanin in basal cells in only one case of inflamed dentigerous cyst was found (Fig. 7). Radicular cysts presented with moderate to strong podoplanin expression within the basal epithelial layers only (Fig. 8). The immunoreaction for podoplanin in the reduced enamel epithelium of dental follicles was also found to be weak (Fig. 9).
Table 1.
Showing the t-values & p-values of the intensity of the Podoplanin immunostaining(A), Percentage of Podoplanin positive odontogenic cells(B) & the Final immunostaining score(A + B) comparing between 15 cases of Ameloblastomas,10 cases of KCOTs,5 cases of Dentigerous cysts, 5 cases of Radicular cysts & 5 cases of Dental Follicles.
| Group | Immunostaining Intensity (A) |
% of Podoplanin Positive Cells (B) |
Total (A + B) |
|||
|---|---|---|---|---|---|---|
| t-value | p- value | t-value | p-value | t-value | p-value | |
| Ameloblastomas vs KCOTs | 0.335 | >0.05(ns) | 1.515 | >0.05(ns) | 0.473 | >0.05(ns) |
| Ameloblastomas vs Dentigerous cysts | 3.623 | <0.05 | 3.964 | <0.05 | 4.157 | <0.05 |
| Ameloblastomas vs Radicular cysts | 1.767 | >0.05(ns) | 1.699 | >0.05(ns) | 1.938 | >0.05(ns) |
| Ameloblastomas vs Dental Follicles | 3.797 | <0.05 | 5.196 | <0.05 | 4.838 | <0.05 |
| KCOTs vs Dentigerous Cysts |
4.869 | <0.05 | 3.726 | <0.05 | 5.256 | <0.05 |
| KCOTs vs Radicular Cysts | 2.757 | <0.05 | 0.745 | >0.05(ns) | 2.360 | <0.05 |
| KCOTs vs Dental Follicles | 5.513 | <0.05 | 5.507 | <0.05 | 6.687 | <0.05 |
| Dentigerous Cysts vs Radicular Cysts | 2.631 | <0.05 | 2.581 | <0.05 | 2.840 | <0.05 |
| Dentigerous Cysts vs Dental Follicles | 0.000 | >0.05(ns) | 1.414 | >0.05(ns) | 0.631 | >0.05(ns) |
| Radicular Cysts vs Dental Follicles |
3.953 | <0.05 | 4.242 | <0.05 | 4.348 | <0.05 |
ns: not significant, p -value set at 5%.
Table 2.
Comparison of immunostaining between Odontogenic tumors, Odontogenic cysts and dental follicles.
| Group | Immunostaining Intensity (A) |
% of Podoplanin Positive Cells (B) |
Total (A + B) |
|||
|---|---|---|---|---|---|---|
| t-value | p- value | t-value | p-value | t-value | p-value | |
| Odontogenic tumors vs Odontogenic cysts |
4.185 | <0.05 | 3.405 | <0.05 | 4.356 | <0.05 |
| Odontogenic tumors vs Dental follicles |
4.392 | <0.05 | 5.243 | <0.05 | 5.460 | <0.05 |
| Odontogenic cysts vs Dental follicles |
1.331 | >0.05(ns) | 2.566 | <0.05 | 1.965 | >0.05(ns) |
Fig. 1.
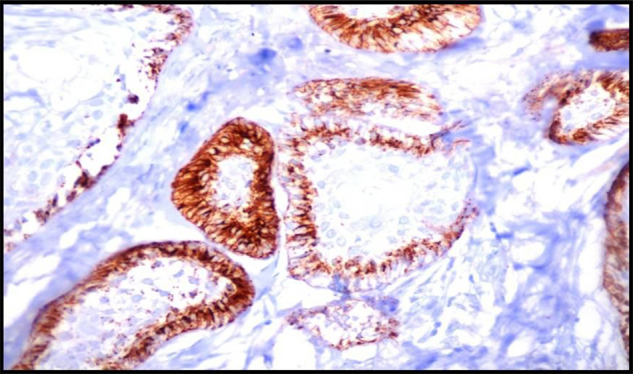
Immunohistochemically Stained Section Of Follicular Ameloblastoma Showing Very Strong Podoplanin Expression In The Peripheral Cells Of Follicular Islands,10X.
Fig. 2.
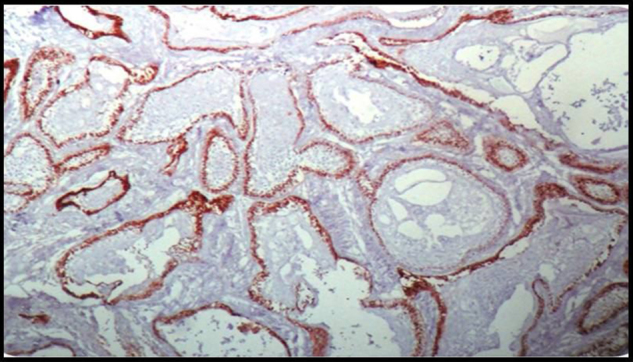
Immunohistochemically Stained Section Of Follicular Ameloblastoma Showing Negative Podoplanin Expression In The Acanthomatous Areas Of The Follicular Islands,10X.
Fig. 3.
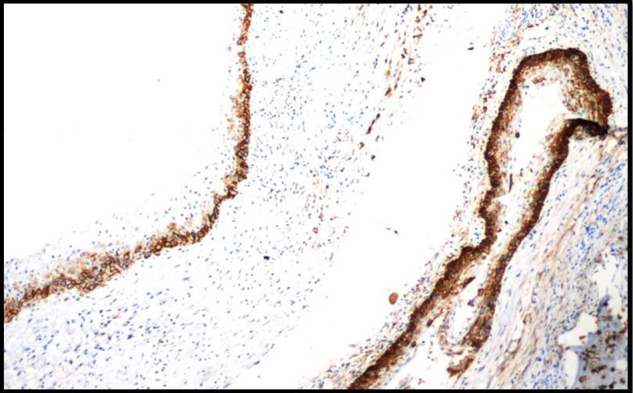
Immunohistochemically Stained Section Of Unicystic Ameloblastoma Showing Moderate To Strong Podoplanin Expression In The Basal & Suprabasal Layers Of The Epithelium,10X.
Fig. 4.
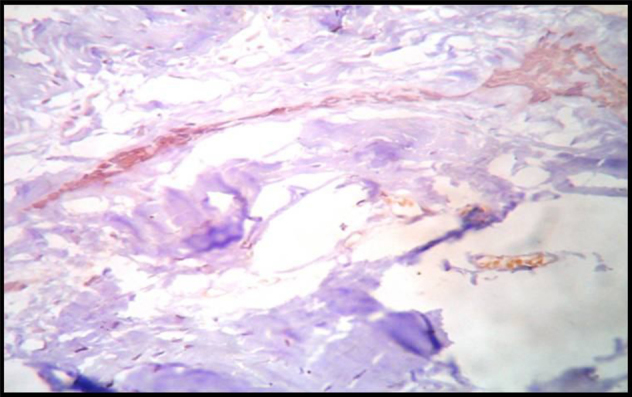
Immunohistochemically Stained Section Of Desmoplastic Ameloblastoma Showing Moderate Expression Of Podoplanin In The Tumor Islands,10X.
Fig. 5.
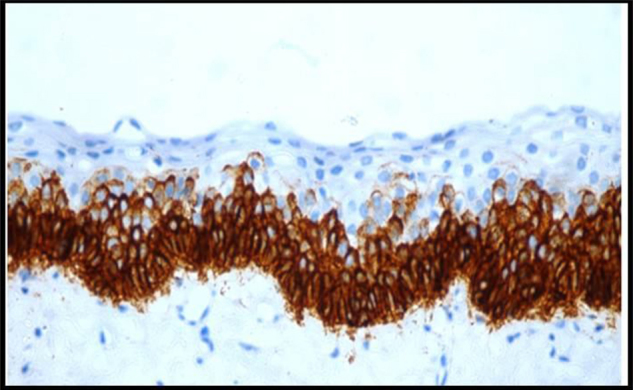
Immunohistochemically Stained Section Of Keratocystic Odontogenic Tumor Showing Very Strong Expression Of Podoplanin In Basal And Suprabasal Layers Of The Epithelium,10X.
Fig. 6.
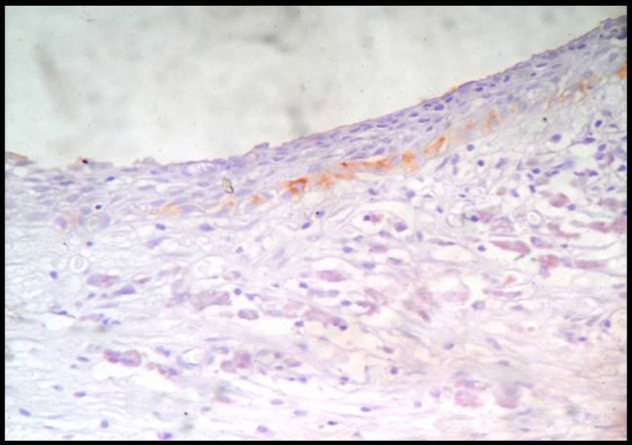
Immunohistochemically Stained Section Of Dentigerous Cyst Showing Weak Or Negative Podoplanin Expression In The Epithelial Lining,40X.
Fig. 7.
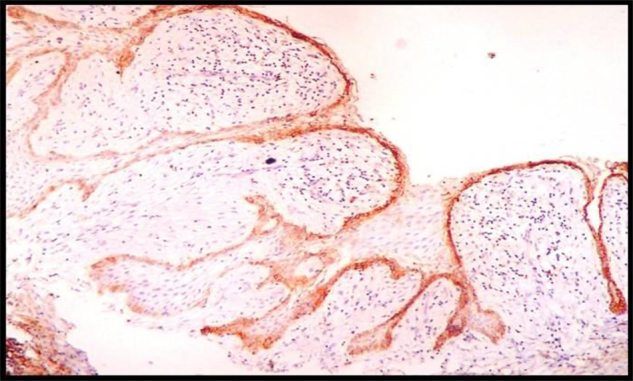
Immunohistochemically Stained Section Of Inflamed Dentigerous Cyst Showing Strong Podoplanin Expression In The Epithelium.
Fig. 8.
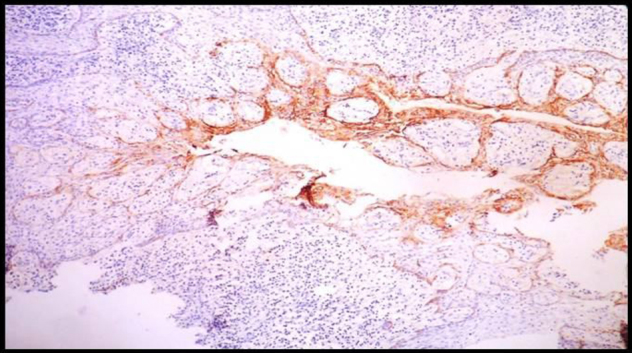
Immunohistochemically Stained Section Of Radicular Cyst Showing Moderate To Strong Expression At The Areas Of Intense Inflammation,10X.
Fig. 9.
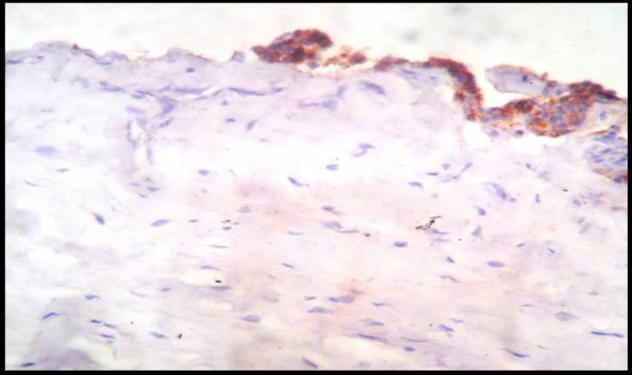
Immunohistochemically Stained Section Of Dental Follicle Showing Weak To Moderate Podoplanin Expression In The Reduced Epithelium,10X.
5. Discussion
Odontogenic cysts and tumors constitute an unusually diverse group of lesions. Many studies have been done in an attempt to explore the exact molecular mechanism of invasion in ameloblastomas and KCOTs but nothing well has been elucidated till date. In the recent past podoplanin – a small mucin-like protein has evolved as a potential molecular marker in the phenomenon of cell invasion. Therefore it is said to have potential implications for diagnosis and prognosis.
Podoplanin is a 38 kDa type-1 transmembrane sialomucin -like glycoprotein which consists of 162 amino acids. In human neoplasms, podoplanin expression has been reported in squamous cell carcinoma (SCC) of the oral cavity and various other malignancies. In addition, podoplanin is reportedly associated with tumor-induced platelet aggregation and tumor metastasis. As all these studies have showed increased expression of podoplanin in various malignant tumors, its potential role in tumor progression has been suggested. Although the protein has been considered as a specific marker for lymphatic endothelial cells, its expression is also found in odontogenic epithelial and mesenchymal tissues. Recently, podoplanin has been found to play a probable role in odontogenic tumorigenesis also.3
We therefore conducted this study to analyse the expression of podoplanin in the ameloblastomas & KCOTs further to elucidate and reinforce the importance of this molecule in the growth of these tumors & to compare their pattern of expression with dentigerous cysts, radicular cysts & dental follicles. Through this study, we also tried to ascertain whether podoplanin could be used as a novel marker to assess the neoplastic behaviour in KCOTs.
We observed from our study that the intensity of podoplanin expression, percentage of podoplanin positive odontogenic epithelial cells & the final immunostaining score were found to be significantly higher in follicular ameloblastomas than in unicystic ameloblastomas (Table 3).
Table 3.
Comparison of immunostaining between Follicular Ameloblastomas and unicystic ameloblastomas.
| Group | Immunostaining Intensity (A) |
% of Podoplanin Positive Cells (B) |
Total (A + B) |
|||
|---|---|---|---|---|---|---|
| t-value | p- value | t-value | p-value | t-value | p-value | |
| Follicular Ameloblastomas vs unicystic ameloblastomas |
3.507 | <0.05 | 3.889 | <0.05 | 4.268 | <0.05 |
We found that out of two desmoplastic specimens, one showed weak immunostaining score for podoplanin whereas the other one showed strong immunostaining score. Since this study had only two specimens of desmoplastic ameloblastoma, the data could not be used for statistical analysis.
Our findings were in accordance with Gonzalez-Alva et al.,6 who also observed similar expression of podoplanin. Similar findings were observed by Gonzalez-Alva et al.,7 in odontomas. It can therefore be inferred that the podoplanin is expressed during intense proliferative activity in odontogenic cells and when these cells reach maturity or a stable state, there is a reduction or absence of podoplanin expression.4 Our observation of negative podoplanin expression in acanthomatous ameloblastomas could be possibly due to squamous metaplasia and terminal differentiation of odontogenic cells. Our results reinforce this hypothesis, also proposed by Gonzalez-Alva et al.,6 that this pattern of distribution of podoplanin immunostaining, according to the cellular subtype in ameloblastomas, may be helpful to the classification of odontogenic tumors.
Wicki et al.,5 proposed that podoplanin is often expressed in a one-cell layer at the invasive edge of the tumours in about 80% of human squamous cell carcinomas. We found that in our study also, the odontogenic epithelial tumoral cells which were in intimate contact with the stroma stained strongly positive for podoplanin. This restricted immunoexpression of podoplanin at the front of human squamous cell carcinomas prompted the question whether factors of the surrounding tissue could influence podoplanin expression. Scholl et al.,8 & Wicki et al.,5 suggested that podoplanin expression can be induced by epidermal growth factor, basic fibroblast growth factor (FGF2) and transforming growth factor α in MCF7 breast cancer cells thereby strongly implying the possible role of stroma around the tumor cells in the invasive behaviour of ameloblastomas. Active remodelling of the actin cytoskeleton is responsible for the invasion of cells into surrounding tissues. Martin-Villar E et al.,9 suggested that podoplanin modulates the actin cytoskeleton that helps in tumor invasion. Yu et al.,10 stated that ezrin which is one of the ERM proteins mediates filopodia formation & induces metastasis. Indeed, Scholl et al.,8 found that podoplanin physically associates with ezrin. Thus, their results suggested the role of podoplanin in cell migration or invasiveness.
We also found that expression of podoplanin was significantly higher in KCOTs like ameloblastomas (t-0.473; p > 0.05) than in dentigerous cysts (t-5.256; p < 0.05). Our findings are consistent with the findings of Okamoto et al.,3 who also observed similar immunohistochemical reactivity for podoplanin in KCOTs. They have also reported that podoplanin is expressed in limited myoepithelial elements of pleomorphic adenomas which has a tendency to invade surrounding tissues.10 Agaram NP et al.,11 observed that a significant number of OKCs showed clonal loss of heterozygosity of tumor suppressor genes like p16, p53, PTCH. Hence they also supported the hypothesis that OKCs are neoplastic rather than developmental in origin. Our observation of strong expression of podoplanin in basal & suprabasal layers in KCOTs suggests the proliferative activity of these cells, increasing their potential for intrinsic growth and making them locally invasive & aggressive. We therefore believe that podoplanin probably plays a role along with other proteins and growth factors, in increasing the proliferative activity of the lining epithelium in KCOT thereby making it to behave more like a neoplasm rather than like a cystic lesion. Hence, we suggest that podoplanin can be used as a potential proliferative marker to indicate the neoplastic behaviour of KCOTs. Our study further supports the change in nomenclature of Odontogenic Keratocyst to Keratocytic Odontogenic Tumor by WHO & claims that it should be regarded as benign cystic neoplasm rather than cyst.
The immunoreaction for podoplanin was negative or weak in some areas of lining epithelium of dentigerous cysts. We observed positive immunoreactivity for it in only one case of inflamed dentigerous cyst. This immunoreaction was also seen by Yuji Miyazaki et al.,12 in the inflamed gingival tissues. Podoplanin expression in epithelial lining of radicular cysts was statistically significant when compared with KCOTs, dentigerous cysts, and dental follicles. The inflammatory mediators released by the inflammatory component which is present in the connective tissue capsule of the radicular cysts might have influenced the expression of podoplanin in the basal epithelial layers of radicular cysts. However, the reason why podoplanin expression is enhanced in the presence of inflammation remains unknown. It may be however hypothesised that the various stromal growth factors like EGFR, FGF2, TGFα which are known to induce podoplanin expression in the epithelial cells are also released in large amounts during chronic inflammatory conditions.
The expression of podoplanin in odontogenic epithelial cells of dental follicle was similar to that of dentigerous cyst lining which was found to be statistically insignificant (t-value:0.631;p > 0.05). Our results are in accordance with the findings of Tjioe KC et al.,4. According to them, the ameloblasts reduce their size and the number of cellular organelles after the conclusion of their secreting and proliferative activities. Their data therefore supports the concept that podoplanin may be necessary for the proliferative activity of the epithelial odontogenic cells.
6. Conclusion
Our study therefore shows positive expression of podoplanin in odontogenic epithelium of ameloblastomas and KCOTs in comparison with epithelial lining of dentigerous cysts and dental follicles. This suggests that the podoplanin influences the proliferative activity of these cells increasing their potential for intrinsic growth and making them locally invasive and aggressive. Our finding also justifies the nomenclature of keratocystic odontogenic tumor rather than odontogenic keratocyst. Hence we suggest that podoplanin can be used as a potential proliferative marker to observe the aggressive behaviour of ameloblastomas and KCOTs. However further studies can be done to understand the exact molecular mechanisms by which podoplanin plays a role in these aggressive tumors by studying larger sample specimens.
Conflict of interest
The author declare no conflict of interest.
References
- 1.Rajendran R., Shivapathasundharam B., editors. 7th Ed. Elseviers; 2006. Shafer’s textbook of oral pathology. [Google Scholar]
- 2.Reichart Peter A., Philipsen Hens P. Odontogenic tumors and allied lesions. Quintessence. 2004 [Google Scholar]
- 3.Okamoto E., Kikuchi K., Miyazaki Y. Significance of podoplanin expression in keratocystic odontogenic tumor. J Oral Pathol Med. 2010;39:110–111. doi: 10.1111/j.1600-0714.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- 4.Tjioe K.C., Oliveira D.T., Soares C.T., Lauris J.R., Damante J.H. Is podoplanin expression associated with the proliferative activity of ameloblastomas. Oral Dis. 2012;18:673–679. doi: 10.1111/j.1601-0825.2012.01924.x. [DOI] [PubMed] [Google Scholar]
- 5.Wicki A., Christofori G. The potential role of podoplanin in tumour invasion. Br J Cancer. 2007;96:1–5. doi: 10.1038/sj.bjc.6603518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Alva Patricia, Tanaka Akio, Oku Yuka. Enhanced expression of podoplanin in ameloblastomas. J Oral Pathol Med. 2010;39:103–109. doi: 10.1111/j.1600-0714.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez Alva Patricia, Inoue Harumi, Miyazaki Yuji, Tsuchiya Hozumi, Noguchi Yoshihiro. Podoplanin expression in odontomas: clinicopathological study & immunohistochemical analysis of 86 cases. J Oral Sci. 2011;53:67–75. doi: 10.2334/josnusd.53.67. [DOI] [PubMed] [Google Scholar]
- 8.Vered Marilena, Shohat Izhar, Buchner Amos. Epidermal growth factor receptor expression in ameloblastoma. Oral Oncol. 2003;39(2):138–143. doi: 10.1016/s1368-8375(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 9.Martín-Villar Ester, Megías Diego, Castel Susanna, Yurrita Maria Marta, Vilaró Senén. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci. 2006;119:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- 10.Oku Y., Tanaka A., Gonzalez-Alva P., Sakashita H., Kusama K. Podoplanin expression in human pleomorphic adenomas. Oral Oncol. 2008;12:251–253. [Google Scholar]
- 11.Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 3. Immunocytochemistry of cytokeratin and other epithelial cell markers. Oral Oncol. 2002;38(5):407–415. doi: 10.1016/s1368-8375(01)00067-7. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki Yuji, Okamoto Eri, González-Alva Patricia, Hayashi Joichiro, Ishige Toshiyuki, Kikuchi Kentaro. The significance of podoplanin expression in human inflamed gingiva. J Oral Sci. 2009;51:283–287. doi: 10.2334/josnusd.51.283. [DOI] [PubMed] [Google Scholar]


