Abstract
Aims
The purpose of this study was to evaluate the effect of the corticosteroid therapy for both treatment of juvenile systemic lupus erythematosus and disease activity on two masticatory muscles and condyle of the temporomandibular joint.
Methods
A total of 21 controls and 48 juvenile systemic lupus erythematosus patients were investigated. Volumes of the temporal and masseter muscles and condyle of the subjects were assessed by using a 3D reconstruction in magnetic resonance imaging. The ITK-SNAP, a medical imaging software, was used for 3D reconstruction. A dental examination with registration of occlusion was performed in subjects. Data were statistically analyzed by means of the Dahlberg’s test associated with paired t-test, Fisher’s exact test and Chi-square.
Results
There was a positive correlation between temporalis muscle and age (p = 0.032), masseter volume (p = 0.029) and condyle volume (p = 0.013). The mean volume measurements of temporal and masseter muscles and condyle were not statistically associated with juvenile systemic lupus erythematosus regarding disease activity and corticosteroid therapy (p > 0.05). There were no significant differences between malocclusion and volume of muscles and condyle.
Conclusion
This study suggested that volume of the target structures has no correlation with cumulative corticosteroid dose, disease activity, and malocclusion.
Keywords: Corticosteroids, MRI, Temporomandibular joint, Masseter muscle, Temporal muscle
1. Introduction
Juvenile systemic lupus erythematosus (JSLE) is a chronic multisystem inflammatory disease of unknown etiology which can affect many organs, including joints, skin, heart, lungs, kidneys, and nervous system. Approximately 15–20% of all cases of JSLE involve children and adolescents,1, 2 with these individuals usually having a more serious course of the disease than adults as the damage increases over time.1
In JSLE, the skeletal muscle involvement is observed in 43%–92% of the patients as they present with weakness, myalgia and atrophy.3, 4, 5Myopathies, with reduced muscle strength and atrophy, may be part of the disease condition or associated with the continuous use of corticosteroid therapy.6, 7 Other complications from the effect of corticosteroid on the musculoskeletal system are avascular necrosis, osteoporosis and growth failure.8
Muscular function plays an important role during growth9. Some authors have found an association between functional capacity of the masticatory muscles and craniofacial morphology,9, 10including a possible etiological factor in the development of malocclusion.
Temporomandibular joint (TMJ) involvement can be affected in patients with systemic lupus erythematosus11 as they have changes in the condyles, including flattening, erosions, osteophytes, and sclerosis.12, 13These can be linked to both disease activity and chronic use of corticosteroids,14 leading to loss of joint cartilage and bone destruction, may causing growth disturbances.
To the best of our knowledge, there are no studies in the literature evaluating facial muscles and imaging of TMJ changes in JSLE patients. The purpose of this study was to evaluate associations of the volumes of masseter and temporal muscles and TMJ condyle with steroid therapy and disease activity in patients with JSLE.
2. Materials and methods
2.1. Study participants
Subjects for this study were a subset of a previously research of the central nervous system manifestations in JSLE patients (the original sample was comprised of 57 subjects). For the current study, a subset of 48 JSLE individuals were studied based on their regular follow-up visits to the pediatric rheumatology outpatient clinic at the university hospital of UNICAMP from the original sample. Clinical and laboratory manifestations were according to an already established protocol.15, 16 Inclusion criteria were: patients with age of onset of disease ≤18; and previous MRI scan. During the initial clinical visit, an examiner explained the study. The control group comprised 21 healthy subjects who had no rheumatic disease history. The study was approved by the Institutional Review Board of the UNICAMP, and informed consent was obtained from each participant and/or legal guardian, prior to their inclusion in the study.
Exclusion criteria included: impaired visualization of the temporal and masseter muscles and condyle; impaired visualization of details; low sharpness images resulting from patients’ movements during scanning; signs of previous trauma; presence of metal prosthetics and accessories, any history of TMJ therapy and orthodontic treatment.
Laboratory findings were recorded at time of subjects were selected to the study.
2.2. MRI acquisition
All patients and controls underwent the same protocol for 3D sagittal acquisition on a 3T scanner (Philips Medical Systems, Best, The Netherlands) as follows: T1-weighted images (voxel size = 1 × 1 × 1 mm3), flip angle = 35°, TR = 22, TE = 9, matrix = 256 × 220, and FOV = 230 × 250 cm.
2.3. MRI volumetric analysis
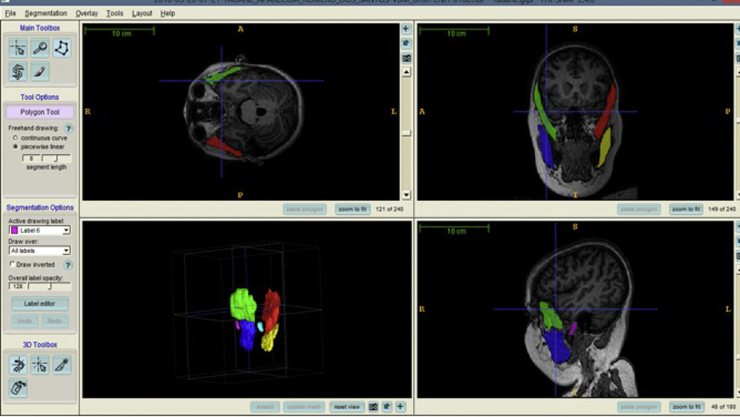
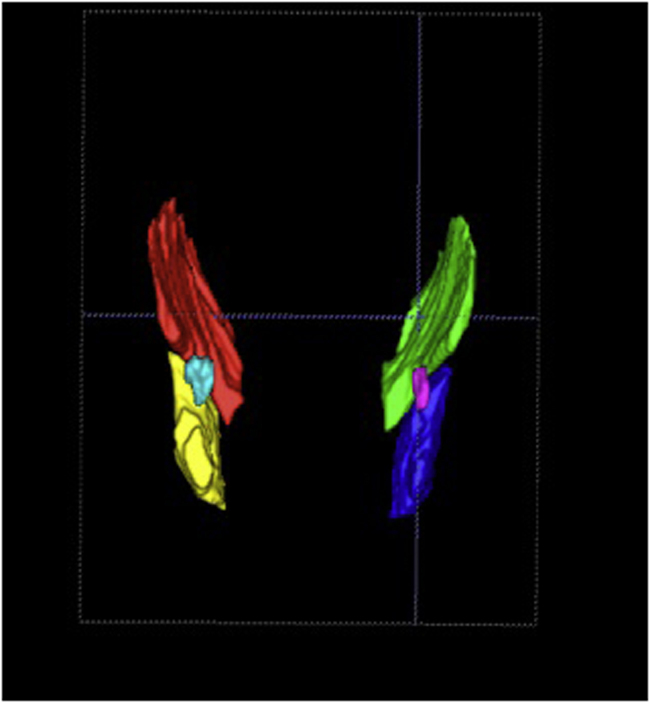
We used the MRIcro software (www.mricro.com) to convert the original DICOM format into ANALYZE format.17After format conversion, the ITK-SNAP software18 was used to analyze the images and to segment the temporal and masseter muscle and TMJ condyle. Temporal and masseter muscles were chosen because they are easily seen in brain MRI scans.19 Segmentation is the process by which image points (voxels) are accordingly assigned to a specific anatomic structure.17 The selected regions were manually traced on the MRI slices by a trained orthodontist (5-years’ experience in MRI of TMJ), blinded to the clinical diagnosis and who used a computer mouse to draw a line around the muscles and condyles borders.20The images were randomized in order to prevent the examiner to be aware of the condition (patient or control) from the scan she was segmenting. Axial, coronal, and sagittal planes were used to ensure anatomical accuracy (Fig. 1). The target structure was manually marked in all selected slices until the structure was fully delimited, following careful realignment of the region of interest, assigning different colors to each anatomic structure. Subsequent to the segmentation, the software generated 3D Images.17 Segmentation consisted only of the condyle and a portion of the ramus, superior limit of the condylar head and sigmoid notch, which were determined for boundary delimitation.17 For the following structures, separate volume measurements were made: right and left masseter muscles, right and left temporal muscles and right and left condyles (Fig. 2). Volume measurements were automatically calculated by multiplying the number of voxels in each region by the voxel volume.18Therefore, the 3D model and the volume in mm3 were acquired with the software.
Fig. 1.
Opening screen of the ITK/SNAP software showing multiple planar views (sagittal, coronal and axial) with segmentation and 3D surface model displaying volume rendering.
Fig. 2.
3D view of the muscles and condyle; right and left condyles are shown in pink and light blue, respectively; left and right masseter and temporal muscles in yellow, red, green and blue, respectively.
The same examiner (SLG) performed all the delimitation procedures. Calibration was performed twice aiming to analyze the intra-examiner agreement. After an interval of 21 days, the sample was re-evaluated in the same manner for assessment of the reproducibility of the method.
2.4. Cumulative corticosteroid dose
The mean cumulative corticosteroid (CC) dose was calculated from medical charts for every patient. All patients were using oral corticosteroids and at time blood withdrawal no patients were taking any other medication.
2.5. Evaluation of disease activity and cumulative damage
Disease activity was measured by using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).21 The SLEDAI scores range between 0 and 105, with scores of >3 being considered active disease.22, 23 Active nephritis was diagnosed on the basis of renal items of the SLEDAI (proteinuria exceeding 0.5 g/L, abnormal urinary sediment, low complement levels). Cumulative SLE-related damage in all patients was determined using the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index (SDI)23 at time of blood withdrawal.
2.6. Clinical dental examination
The patient group participated in a standardized clinical dental examination24, 25, 26 performed by a calibrated dentist. Patients were evaluated after MRI scan (12 months). The examinations were executed using mouth mirrors and disposable wooden spatulas under natural light and with the subjects sitting on an ordinary upright chair; linear measurements were taken using a Vernier Caliper. Each patient was evaluated for Class I, Class II, and Class III malocclusion (according to: the Class II or Class III molar sagittal relationship (permanent dentition) or canine relationship (deciduous and mixed dentition)), and presence of other conditions such as posterior or anterior crossbite, overjet (>3 mm), overbite (<1 mm or >4mm), anterior and posterior crossbite, midline deviation (>2 mm), and crowding (or spacing larger than 3 mm in the permanent dentition) were observed as well.
We were unable to include the clinical examination for the control group. The subjects declined the invitation to come to our medical center, and a short telephone interview was conducted to find information about previous orthodontic treatment.
2.7. Data analysis
Comparisons of the volumetric data were performed by using averages. Intra-examiner agreement for volume measurements was assessed by using the Dahlberg’s test associated with paired “t” test. Distribution of frequencies and continuous variables were evaluated by using Fisher’s exact test and Chi-square, with paired t-test comparing volumes in the left and right sides. Pearson’s correlation coefficients were calculated to evaluate the association of the variables (i.e. age, SLEDAI and CC) with median volumes of muscles and condyle. To assess the relationship among patient's malocclusion with median volumes of muscles and condyle was studied with Pearson’s correlation coefficients. We fitted a linear regression model for volumes of the structures and effect of the variables (i.e. age, SLEDAI and CC). For all analyses, p < 0.05 was considered statistically significant. Data were entered into a database and the SPSS software (Statistical Package for Social Sciences, v. 13.0, SPSS Inc, Chicago, IL, USA) was used.
3. Results
3.1. Demographics
We included 48 consecutive childhood-onset SLE patients (45 females, 3 males, median age of 17.4 years, and age range of 9–30 years old). The control group consisted of 21 healthy volunteers (14 females, 7 males, median age of 20 years, and age range of 10 to 28 years old). Disease duration was 4.71 years (SD ± 4.57; range 026).
3.2. Clinical, laboratory, and treatment features
All patients had disease onset before the age of 16. At the time of study entry, 40 patients had active disease (SLEDAI ⩾ 3) with median SLEDAI scores of 8 (range 4–18), whereas 8 inactive patients had a median SLEDAI score of 0 (range 0–2).
Cumulative SLE-related damage in all patients was determined using the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index (SDI) measured at the time of blood withdrawal. The SDI scores ranged from 0 to 47.
3.3. Clinical occlusion
The percentages and types of malocclusion in patients group can be seen in Table 1. The most common malocclusion features were decreased overbite (26.7%), increased overjet (13.3%) and crossbite (13.3%). Regarding the overall prevalence of malocclusion class, 70% of the subjects had Class I malocclusion. The frequencies of Class II and Class III, malocclusions were 16.7% and 13.3%, respectively.
Table 1.
Frequency of different types of malocclusion in patients group.
| Malocclusion | % | |
|---|---|---|
| Overjet | increased | 13,3 |
| decreased | 16,7 | |
| Overbite | increased | 16,7 |
| decreased | 26,7 | |
| Malocclusion-Class | Class I | 70 |
| Class II | 16,7 | |
| Class III | 13,3 | |
| Dental Midline | shift | 6,7 |
| Crossbite | Unilateral | 3,3 |
| Bilateral | 13,3 | |
| Crowding | Superior | 10 |
| Inferior | 6,7 | |
| Superior e Inferior | 3,3 | |
| Diastema | Superior | 6,7 |
| Inferior | 6,7 | |
| Superior and Inferior | 6,7 | |
3.4. Volumetric features
As no significant differences between the right and left sides were observed in the volume values of the structures (t-test, P-value >0.05), the analyses were conducted by using the average of both sides for each volume measurement as dependent variable.
The mean volume measurements of temporal and masseter muscles and of the condyle are listed in Table 2. The patient group had structures with lower mean volume. With regard to the masseter muscle, the volume was significantly lower than in the control group (P = 0.020), and the difference in the volume of the condyle was not significant. As can be seen, temporal muscle and condyle values in the JSLE group were not statistically different compared to those in the control group (P = 0,139 and P = 0.096, respectively).
Table 2.
Mean volumetric measurements in patients and controls.
| Group | N | Mean (mm3) | S.D. | P | |
|---|---|---|---|---|---|
| Muscle Temporal | Patients | 48 | 29883,396 | 7598,9665 | 0,139 |
| Controls | 21 | 32904,310 | 7951,6208 | ||
| Muscle Masseter | Patients | 48 | 21922,542 | 5575,8893 | 0.02 |
| Controls | 21 | 26792,810 | 6342,0383 | ||
| Condyle | Patients | 48 | 1077,563 | 352,7240 | 0,096 |
| Controls | 21 | 1238,381 | 388,9481 | ||
Significant difference between groups at P < .005.
Intra-examiner agreement was evaluated by using the Dahlberg’s test. High intra-examiner indexes were found (Table 3) and there were no significant differences between patients and controls regarding age (P = 0.89) and gender (P = 1).
Table 3.
Intra-examiner evaluation at two different times (T1 e T2) by application of the Dahlberg’s test associated to the paired T test.
| Volume (mm3) measurement | S1 |
S2 |
T test | Dalhlberg | ||
|---|---|---|---|---|---|---|
| mean | SD | mean | SD | |||
| Left temporal muscle | 35589,40 | 7783,35 | 35792,27 | 8348,31 | 0,554 | 897,015 |
| Right temporal muscle | 35852,27 | 8152,53 | 35453,13 | 7895,00 | 0,169 | 781,661 |
| Left masseter muscle | 27246,00 | 6255,53 | 26811,27 | 5898,47 | 0,382 | 1310,925 |
| Right masseter muscle | 27738,87 | 6544,05 | 26774,87 | 6474,00 | 0,046 | 2,194 |
| Left condyle | 1023,67 | 273,79 | 989,40 | 271,71 | 0,253 | 79,783 |
| Right codyle | 1093,33 | 371,54 | 1057,33 | 372,17 | 0,186 | 72,989 |
S1: first time; S2: second time; SD: standard deviation.
Significance level set at p < 0.05.
This study was performed to correlate the volumetric statistical analysis of the condyle and of the masseter and temporalis muscles with age, SLEDAI index, and total corticosteroid doses (i.e. total CC). Significant correlations were found between temporalis muscle volume and age (P = 0.032) as well as between masseter muscle volume (P = 0.029) and condyle volume (P = 0.013). Pearson’s correlation and P values for all correlation analyses are presented in Table 4. There was no correlation between SLEDAI and total CC in any of the structures (P > 0.05).
Table 4.
Pearson’s correlation among temporal and masseter muscles and condyle volumes with age, cumulative Sledai and CC.
| Temporal Muscle |
Masseter Muscle |
Condyle |
||||
|---|---|---|---|---|---|---|
| p | Pearson’s correlation | p | Pearson's correlation | p | Pearson’s correlation | |
| age | 0,023* | 0,312 | 0,029* | 0,301 | 0,013* | 0,338 |
| cumulative Sledai | 0,800 | -0,044 | 0,849 | -0,033 | 0,624 | 0,086 |
| CC | 0,091 | 0,261 | 0,128 | 0,236 | 0,068 | 0,281 |
CC: cumulative corticosteroid dose.
Statistical significance by adopting p < 0.05.
In Table 5, volume of target structures did not significantly correlate with malocclusion Class and malocclusion features (Pearson correlation coefficients, P > 0.05).
Table 5.
Pearson’s correlation between volumetric target structures and malocclusion.
| Temporal Muscle |
Masseter Muscle |
Condyle |
||||
|---|---|---|---|---|---|---|
| p | Pearson’s correlation | p | Pearson’s correlation | p | Pearson’s correlation | |
| Overjet | 0,827 | 0,057 | 0,949 | −0,017 | 0,454 | 0,195 |
| Overbite | 0,705 | 0,099 | 0,806 | 0,064 | 0,794 | 0,069 |
| Malocclusion-Class | 0,827 | 0,057 | 0,949 | −0,017 | 0,454 | 0,195 |
| Midline shift | 0,176 | 0,344 | 0,233 | 0,306 | 0,874 | -0,041 |
| Crossbite | 0,175 | −0,345 | 0,233 | −0,306 | 0,684 | 0,106 |
| Crowding | 0,798 | 0,067 | 0,883 | 0,039 | 0,91 | 0,03 |
| Diastema | 0,259 | −0,29 | 0,419 | −0,21 | 0,595 | −0,139 |
Statistical significance by adopting p < 0.05.
Linear regression revealed an association between age and volume of the structures (Table 6).
Table 6.
Linear Regression Analyses of temporal and masseter muscles and condyle volumes (mm3).
| age | N | temporal muscle |
masseter muscle |
condyle |
|||
|---|---|---|---|---|---|---|---|
| R2 | Sig. | R2 | Sig. | R2 | Sig. | ||
| patients | 48 | 0,097 | 0,032 | 0,08 | 0,0459 | 0,114 | 0,020 |
| controls | 21 | 0,219 | 0,019 | 0,248 | 0,22 | 0,002 | 0,867 |
Significance level set at p < 0.05.
4. Discussion
Although JSLE is a chronic disease, early reductions in the disease activity have improved the survival rate over the last two decades.1 Corticosteroid therapy is widely used in the treatment of JSLE due to its anti-inflammatory and immunosuppressive capacities. Therefore, it is expected a decrease in bone-mineral density8 as well as myopathies (e.g. muscular atrophy)5, 8 in patients with JSLE.
Corticosteroid-induced muscle atrophy is initiated by catabolic conditions and the actions of the medication during muscle deterioration are complex, reflecting a regulation of several mechanisms at molecular level inducing both synthesis and degradation of muscle proteins.27 The long-term use of corticosteroids can cause a breakdown of the cartilage matrix in the joint as this affects protein synthesis, inhibiting osteoblast proliferation/differentiation and increasing the apoptosis rates of mature osteoblasts and osteocytes.28, 29
Varying degrees of muscle atrophy and changes in joints have been observed in rheumatic diseases, and it was demonstrated that many JSLE patients developed them following steroid therapy.30
Our analysis validated the viability of the quantitative measurements of MRI masticatory muscles and condyle in a sample with JSLE patients. Despite the small sample size, as far as we are concerned this is the first comprehensive study on data of these structures so far. The present study examined the possible factors influencing the volume of some masticatory muscles and condyle.
The segmentation of masticatory muscles and condyle have already been carried out in previous successful works.19, 20, 31, 32 Our analysis of the manual muscles and condyle contours did not showed that the volume difference between patients and controls. Yasuda et al.20 demonstrated the reduction of temporal muscle volume due to atrophy caused by craniotomy. Ny et al.19 calculated the correlation between the volumes of the individual masticatory muscles as well as the correlation between the total volumes of the masticatory muscles, on both sides of the face.
The results did not demonstrate that children with JSLE present a reduced muscle volume compared to healthy subjects. Except for the masseter muscle volume, there was not statistically significant difference in the volumes of the structures.
The prevalence of different types of malocclusions may show great variability. In the present study, the assessment of malocclusion shows no malocclusion in 70% cases. This is similar to results of others studies in a normal population.33, 34
It has also been advised that prolonged systemic corticosteroid treatment cause an alteration in growth hormone release increase the severity of the type of malocclusion and induce significant muscle atrophy.35, 36 The data from this work do not seem to support in the JSLE group studied. Our results did not reveal any factors contribute to malocclusion development and the frequency of occlusal features in the present study are consistent with demonstrated by others studies in normal population.37
Linear regression analyses showed no evidence that SLEDAI and CC might have an effect on the target volume of the structures. In the present study, age affected the muscle volume. This finding may reflect the natural trend of growth. The changes in muscle during growth are dominated by the increasing levels of muscle mass and strength.38, 39
Unfortunately, we do not have data on the MRI, SLEDAI and CC of these patients during their early childhood, which could be used to understand the progressive decline in muscles and condyle.
Jonsson et al.11 concluded that the TMJ is not an uncommon site for SLE-induced deformities. Previous literature has suggested that SLE patients may have TMJ pathologic findings.12, 13 In our study, we did not find any condyle pathology.
Masseter muscle volume was smaller in patients group. The volume of the temporal muscle was not different between the two groups. A thinner masseter muscle is associated with a lower local bone density.40 Long-term systemic corticosteroid therapy is associated with bone loss.41 We suggest that the reason for this is the corticosteroid therapy not with muscle, but with bone density.
Potential limitations of the study include the low participation rate, the absence of concurrent clinical examination in a matched control group, and its retrospective nature in MRI scans and bias.
In conclusion, both disease activity and influence of corticosteroid therapy did not correlate with the volume values of masticatory muscles and condyle in our study. A direct association with malocclusion involvement caused by JSLE was not demonstrated.
A larger number of subjects and a heterogeneous patient group with disease activity ranging from mild to severe would be required to support a valid conclusion. Further analyses should be performed with a longitudinal study that follows patients from the diagnostic phase of the disease (childhood) to adulthood.
Conflict of interest
The authors have none to declare.
Acknowledgements
This work was supported by the São Paulo Research Foundation −FAPESP (grant number 12/16953-4).
References
- 1.Brunner H.I., Gladman D.D., Ibanez D., Urowitz M.D., Silverman E.D. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58:556–562. doi: 10.1002/art.23204. [DOI] [PubMed] [Google Scholar]
- 2.Morgan T.A., Watson L., McCann L.J., Beresford M.W. Children and adolescents with SLE: not just little adults. Lupus. 2013;22:1309–1319. doi: 10.1177/0961203313502863. [DOI] [PubMed] [Google Scholar]
- 3.Lim K.L., Abdul-Wahab R., Lowe J., Powell R.J. Muscle biopsy abnormalities in systemic lupus erythematosus: correlation with clinical and laboratory parameters. Ann Rheum Dis. 1994;53:178–182. doi: 10.1136/ard.53.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal S., Sher M.R., Good R.A., Cawkwell G.D. Diversity in presenting manifestations of systemic lupus erythematosus in children. J Pediatr. 1999;135:500–505. doi: 10.1016/s0022-3476(99)70174-5. [DOI] [PubMed] [Google Scholar]
- 5.Abdwani R., Rizvi S.G., El-Nour I. Childhood systemic lupus erythematosus in Sultanate of Oman: demographics and clinical analysis. Lupus. 2008;17:683–686. doi: 10.1177/0961203307087611. [DOI] [PubMed] [Google Scholar]
- 6.Hilario M.O., Yamashita H., Lutti D., Len C., Terreri M.T., Lederman H. Juvenile idiopathic inflammatory myopathies: the value of magnetic resonance imaging in the detection of muscle involvement. Sao Paulo Med J. 2000;118:35–40. doi: 10.1590/S1516-31802000000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padeh S., Passwell J.H. Intraarticular corticosteroid injection in the management of children with chronic arthritis. Arthritis Rheum. 1998;41:1210–1214. doi: 10.1002/1529-0131(199807)41:7<1210::AID-ART10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Stichweh D., Pascual V. Systemic lupus erythematosus in children. An Pediatr (Barc) 2005;63:321–329. doi: 10.1157/13079833. [DOI] [PubMed] [Google Scholar]
- 9.Vargervik K. Morphologic evidence of muscle influence on dental arch width. Am J Orthod. 1979;76:21–28. doi: 10.1016/0002-9416(79)90296-3. [DOI] [PubMed] [Google Scholar]
- 10.Kiliaridis S., Georgiakaki I., Katsaros C. Masseter muscle thickness and maxillary dental arch width. Eur J Orthod. 2003;25:259–263. doi: 10.1093/ejo/25.3.259. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson R., Lindvall A.M., Nyberg G. Temporomandibular joint involvement in systemic lupus erythematosus. Arthritis Rheum. 1983;26:1506–1510. doi: 10.1002/art.1780261213. [DOI] [PubMed] [Google Scholar]
- 12.Liebling M.R., Gold R.H. Erosions of the temporomandibular joint in systemic lupus erythematosus. Arthritis Rheum. 1981;24:948–950. doi: 10.1002/art.1780240714. [DOI] [PubMed] [Google Scholar]
- 13.Gerbracht D., Shapiro L. Temporomandibular joint erosions in systemic lupus erythematosus. Arthritis Rheum. 1982;25:597. doi: 10.1002/art.1780250519. [DOI] [PubMed] [Google Scholar]
- 14.Caramaschi P., Biasi D., Dal Forno I., Adami S. Osteonecrosis in systemic lupus erythematosus: an early, frequent, and not always symptomatic complication. Autoimmune Dis. 2012;2012:725249. doi: 10.1155/2012/725249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan E.M., Cohen A.S., Fries J.F. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 17.Costa A.L., Yasuda C.L., Appenzeller S., Lopes S.L., Cendes F. Comparison of conventional MRI and 3D reconstruction model for evaluation of temporomandibular joint. Surg Radiol Anat. 2008;30:663–667. doi: 10.1007/s00276-008-0400-z. [DOI] [PubMed] [Google Scholar]
- 18.Yushkevich P.A., Piven J., Hazlett H.C. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Ng H.P., Foong K.W., Ong S.H. Quantitative analysis of human masticatory muscles using magnetic resonance imaging. Dentomaxillofacial Radiol. 2009;38:224–231. doi: 10.1259/dmfr/75198413. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda C.L., Costa A.L., Franca M., Jr. Postcraniotomy temporalis muscle atrophy: a clinical, magnetic resonance imaging volumetry and electromyographic investigation. J Orofac Pain. 2010;24:391–397. [PubMed] [Google Scholar]
- 21.Bombardier C., Gladman D.D., Urowitz M.B., Caron D., Chang C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 22.Yee C.S., Farewell V.T., Isenberg D.A. The use of systemic lupus erythematosus disease activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology (Oxf) 2011;50:982–988. doi: 10.1093/rheumatology/keq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladman D.D., Urowitz M.B., Goldsmith C.H. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–813. doi: 10.1002/art.1780400506. [DOI] [PubMed] [Google Scholar]
- 24.Bjoerk A., Krebs A., Solow B. A method for epidemiological registration of malocclusion. Acta Odontol Scand. 1964;22:27–41. doi: 10.3109/00016356408993963. [DOI] [PubMed] [Google Scholar]
- 25.Krooks L., Pirttiniemi P., Kanavakis G., Lahdesmaki R. Prevalence of malocclusion traits and orthodontic treatment in a Finnish adult population. Acta Odontol Scand. 2016;74:362–367. doi: 10.3109/00016357.2016.1151547. [DOI] [PubMed] [Google Scholar]
- 26.al-Emran S., Wisth P.J., Boe O.E. Prevalence of malocclusion and need for orthodontic treatment in Saudi Arabia. Commun Dent Oral Epidemiol. 1990;18:253–255. doi: 10.1111/j.1600-0528.1990.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 27.Clarke B.A., Drujan D., Willis M.S. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Manolagas S.C., Weinstein R.S. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res. 1999;14:1061–1066. doi: 10.1359/jbmr.1999.14.7.1061. [DOI] [PubMed] [Google Scholar]
- 29.El-Hakim I.E., Abdel-Hamid I.S., Bader A. Tempromandibular joint (TMJ) response to intra-articular dexamethasone injection following mechanical arthropathy: a histological study in rats. Int J Oral Maxillofac Surg. 2005;34:305–310. doi: 10.1016/j.ijom.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Ravelli A., Duarte-Salazar C., Buratti S. Assessment of damage in juvenile-onset systemic lupus erythematosus: a multicenter cohort study. Arthritis Rheum. 2003;49:501–507. doi: 10.1002/art.11205. [DOI] [PubMed] [Google Scholar]
- 31.Ng H.P., Ong S.H., Liu J. 3D segmentation and quantification of a masticatory muscle from MR data using patient-specific models and matching distributions. J Digit Imaging. 2009;22:449–462. doi: 10.1007/s10278-008-9132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cevidanes L.H., Hajati A.K., Paniagua B. Quantification of condylar resorption in temporomandibular joint osteoarthritis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:110–117. doi: 10.1016/j.tripleo.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dias P.F., Gleiser R. Orthodontic treatment need in a group of 9-12-year-old Brazilian schoolchildren. Braz Oral Res. 2009;23:182–189. doi: 10.1590/s1806-83242009000200015. [DOI] [PubMed] [Google Scholar]
- 34.Burden D.J., Holmes A. The need for orthodontic treatment in the child population of the United Kingdom. Eur J Orthod. 1994;16:395–399. doi: 10.1093/ejo/16.5.395. [DOI] [PubMed] [Google Scholar]
- 35.Kjellberg H., Fasth A., Kiliaridis S., Wenneberg B., Thilander B. Craniofacial structure in children with juvenile chronic arthritis (JCA) compared with healthy children with ideal or postnormal occlusion. Am J Orthod Dentofacial Orthop. 1995;107:67–78. doi: 10.1016/s0889-5406(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 36.Fischer J.R., Baer R.K. Acute myopathy associated with combined use of corticosteroids and neuromuscular blocking agents. Ann Pharmacother. 1996;30:1437–1445. doi: 10.1177/106002809603001213. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes E.G., Guissa V.R., Saviolli C., Siqueira J.T., Valente M., da Silva C.A. Osteonecrosis of the jaw on imaging exams of patients with juvenile systemic lupus erythematosus. Rev Bras Reumatol. 2010;50:3–15. [PubMed] [Google Scholar]
- 38.Fricke O., Schoenau E. The ‘Functional muscle-bone unit’: probing the relevance of mechanical signals for bone development in children and adolescents. Growth Horm IGF Res. 2007;17:1–9. doi: 10.1016/j.ghir.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Bechtold S., Roth J. Natural history of growth and body composition in juvenile idiopathic arthritis. Horm Res. 2009;72(Suppl. 1):13–19. doi: 10.1159/000229758. [DOI] [PubMed] [Google Scholar]
- 40.Kiliaridis S., Bresin A., Holm J., Strid K.G. Effects of masticatory muscle function on bone mass in the mandible of the growing rat. Acta Anat (Basel) 1996;155:200–205. doi: 10.1159/000147805. [DOI] [PubMed] [Google Scholar]
- 41.Jayasena A., Atapattu N., Lekamwasam S. Treatment of glucocorticoid-induced low bone mineral density in children: a systematic review. Int J Rheum Dis. 2015;18:287–293. doi: 10.1111/1756-185X.12560. [DOI] [PubMed] [Google Scholar]