Abstract
Objective:
To compare the sequential therapy (ST) with the hybrid therapy (HT) for the eradication of Helicobacter pylori.
Materials and Methods:
Patients with peptic ulcer disease and gastritis found to be H. pylori positive were randomized to HT group who received omeprazole (20 mg bid) and amoxicillin (1 g bid) for 7 days followed by omeprazole (20 mg bid), amoxicillin (1 g bid), clarithromycin (500 mg bid), and metronidazole (400 mg tid) for the next 7 days and ST group who received omeprazole and amoxicillin for 5 days followed by omeprazole, clarithromycin, and metronidazole for the next 5 days. Eradication rate, compliance, and complications were compared.
Results:
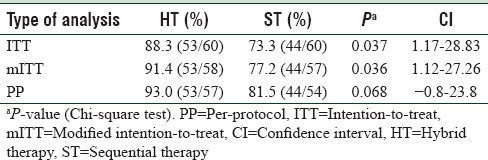
A total of 120 patients were included, sixty in each group. H. pylori eradication rate was significantly higher in HT group on intention-to-treat analysis (88.3% [confidence interval (CI) 78.3%–94.8%] vs. 73.3% [CI 61.1%–83.3%]; P = 0.037). Per-protocol analysis showed higher eradication rate with HT (93% [CI 83.9%–93.7%] vs. 81.5% [CI 69.5%–90.2%]; P = 0.068); however, the difference was insignificant. Compliance and side effects were similar. A complete course of HT costs $10.77, while ST costs only $6.347.
Conclusions:
HT achieves significantly higher H. pylori eradication rate than ST with comparable patient compliance and side effects but at an higher price. However, it can be used in places where ST is ineffective.
Keywords: Antibiotic resistance, gastritis, hybrid therapy, peptic ulcer disease, sequential therapy
INTRODUCTION
Helicobacter pylori is possibly the most common human infection estimated to have infected more than 50% of the world's population.[1] The association of H. pylori with gastric and duodenal ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma is well known. The mean prevalence of H. pylori in peptic ulcer disease patients is found to be as high as 80%–90%, especially in developing countries like India. Several studies have shown that the eradication of H. pylori decreases the recurrence of ulcer in patients with peptic ulcer disease.[2,3]
Standard triple therapy (STT)[4] which includes proton pump inhibitor (PPI), clarithromycin, and amoxicillin or metronidazole is the commonly used regimen for H. pylori eradication.[5] However, lower eradication rate with the STT has become a matter of concern. Graham and Fischbach analyzed the published results of STT and found that only 18% of them could achieve the eradication of more than 85%.[6] With the emergence of antibiotic-resistant strains, there is a need for newer regimens to achieve higher eradication rates.[7]
Sequential therapy (ST)[8] and hybrid therapy (HT) are used as an alternative to STT and have shown promising results in previous studies. A study done earlier in the present institute showed higher eradication of H. pylori by the use of ST than that of STT, but the difference was not statistically significant.[9] At present, STT is the standard of care for the treatment of H. pylori in the present hospital, and the eradication rate was found to be 81.25%. A study conducted in Iran showed that HT is more effective in H. pylori eradication (89.5% vs. 76.7%) when compared with ST, in patients suffering from peptic ulcer disease.[5] Hsu et al. in their recent study showed H. pylori eradication rate of 99.1% with HT.[10]
The factors that affect the outcome of ST resulting in lower eradication rates than expected could be varied dose, duration, etc., which were chosen empirically for this regimen.[11] The possible explanation for better eradication of HT may be due to longer duration of treatment, sequential administration of drugs, and amoxicillin causing lysis of bacterial cell wall which helps in the diffusion of macrolides into the cell, thereby enhancing the efficacy of clarithromycin in the second phase of treatment.[12] Some studies have demonstrated that the eradication rates of ST might improve by increasing the duration and by modifying the drug schedule.[13,14] However, there is only limited data available to substantiate the superiority of HT in comparison to other regimens in achieving target eradication rate for H. pylori. Hence, this study was conducted to compare the potency of HT with that of ST in H. pylori eradication in patients suffering from peptic ulcer disease or gastritis and to access the side effects and the patient compliance with the HT and compare it with that of the ST.
MATERIALS AND METHODS
The study was conducted in the department of general surgery at a tertiary care hospital in South India from October 2013 to June 2015. The study was approved by the ethics committee of the institute and has been performed in accordance with the ethical standards laid down in an appropriate version of the Declaration of Helsinki (as revised in Brazil 2013). The study has been registered in the Clinical Trial Registry (URL – http://ctri.nic.in/Clinicaltrials/login.php) with the registration no-CTRI/2016/04/006881.
All consecutive patients attending the department of surgery with peptic ulcer disease or gastritis who were positive for H. pylori infection were included in the study. The details of the patients and the findings were recorded. Assessment of eradication of H. pylori was carried out at the end of 6 weeks following the completion of therapy. Completion of therapy included the duration of administration of ST or HT followed by the course of PPI for ulcer healing.
Patients who had received any form of H. pylori eradication therapy in the past, those with associated findings in endoscopy such as malignancy, portal hypertensive gastropathy, etc., and with gastric ulcers confirmed to be malignant by endoscopic biopsy were excluded from the study.
The study was designed as a prospective, open-labeled, parallel arm, randomized controlled trial (RCT). Block randomization was carried out using a computer program with randomly selected block sizes of four and six. Allocation concealment was ensured by serially numbered opaque-sealed envelope (SNOSE) technique.
All patients who fulfilled the inclusion criteria underwent upper gastrointestinal endoscopy (UGIE), after obtaining written informed consent. The procedure (UGIE) was well tolerated by the study population. No procedure-related complications and withdrawal of consent due to inconvenience caused by the study procedure has been encountered during the study period. Two percent lignocaine viscous or spray was used as a local anesthetic, allowing a contact time of 5 min. Gastric ulcer or duodenal ulcer on endoscopy was defined as a breach in the continuity of mucosa of the size of 5 mm or more with an apparent depth. Gastritis was diagnosed on endoscopy as localized redness or beefy red mucosa in the stomach without the presence of any gastric or duodenal ulcer disease. For the diagnosis of H. pylori infection, urease test, histology by Giemsa stain, and stool antigen test (SAT) for H. pylori were used. Although urea breath test is a noninvasive and safe method for the diagnosis of H. pylori, the kit is not available in the present institute, hence, it was not used in the study. Two biopsy samples from pyloric antrum and corpus each were taken for urease test and histology by Giemsa stain. A positive H. pylori state was defined if one, two, or all the three tests were positive for H. pylori. A negative H. pylori test was defined if all the three tests were negative. The study participants and the principal investigator were aware about the treatment given. However, the consultants who performed endoscopy and the consultants who evaluated the biopsy samples were unaware of the treatment given.
Urease test[15,16] was done using a solution earlier standardized in the present institute,[1] and positive urease test for H. pylori was defined as the change of the color of the solution from yellow to pink.[17]
SAT is done by an enzyme immunoassay or immunochromatographic assay. It detects H. pylori antigen in stool sample with the use of monoclonal or polyclonal anti-H. pylori antibody. Monoclonal SAT is superior to polyclonal in the initial diagnosis and eradication of H. pylori after treatment.[15] The sensitivity and specificity of SAT in predicting H. pylori are 59.4% and 72.6%, respectively.[18] Although the sensitivity is low, it can be used in low-resource settings, relatively younger patients (below 45 years) with no signs of complications. Each patient was asked to collect freshly passed stool sample in a sterile, wide-mouthed, screw-capped, leak proof, vial that was already labeled with the patient's name, hospital number, and date of collection, and SAT was performed as per standard instructions.[15,18]
Patients with H. pylori positivity were randomized into two groups using the SNOSE technique. One group received HT while the other group received ST. The HT comprised omeprazole 20 mg bid, amoxicillin 1 g bid for 7 days followed by omeprazole 20 mg bid, metronidazole 400 mg tid, amoxicillin 1 g bid, and clarithromycin 500 mg bid for the next 7 days. The ST comprised omeprazole 20 mg bid, amoxicillin 1 g bid for 5 days followed by omeprazole 20 mg bid, clarithromycin 500 mg bid, and metronidazole 400 mg tid for the next 5 days. In all peptic ulcer disease patients, PPI was continued for 4 weeks for ulcer healing.
Follow-up endoscopy was done after 6 weeks of completion of complete therapy to confirm ulcer healing and H. pylori eradication. For eradication, two biopsy samples were taken each from the corpus and the antrum of the stomach for the two tests. Eradication was defined as negative urease test or histology for H. pylori. Any one or both tests when positive were considered positive H. pylori status. If peptic ulcer was present at 6-week follow-up or, if the patient was positive for H. pylori, the patient was considered for rescue therapy for H. pylori infection. The rescue therapy consisted of 10-day course of levofloxacin (500 mg bid), amoxicillin (1 g bid), and omeprazole (20 mg bid). If the patient was negative for H. pylori on follow-up endoscopy, appropriate anti-secretory therapy measures were carried out. The medication was given to the patient by the principal investigator after verifying the availability of the drugs for completing the course. At the end of drug therapy, the telephonic conversation was established with the patient to access the compliance. The reason for noncompliance if any also was documented. The most common reason for noncompliance in both the groups was intolerability to drug therapy due to metallic taste. The cost of drug therapy was calculated per course by adding individual cost of the drug included in each regimen which was provided from the hospital.
Statistical analysis
The sample size was calculated using OpenEPI software.[19] Considering the detection of eradication rate more than 15% between the two regimens on two-tail basis with 95% confidence interval (CI) and power of the study >80% and expected dropout rate of 10%, the sample size was calculated to be 64 in each group. P < 0.05 was considered statistically significant.
RESULTS
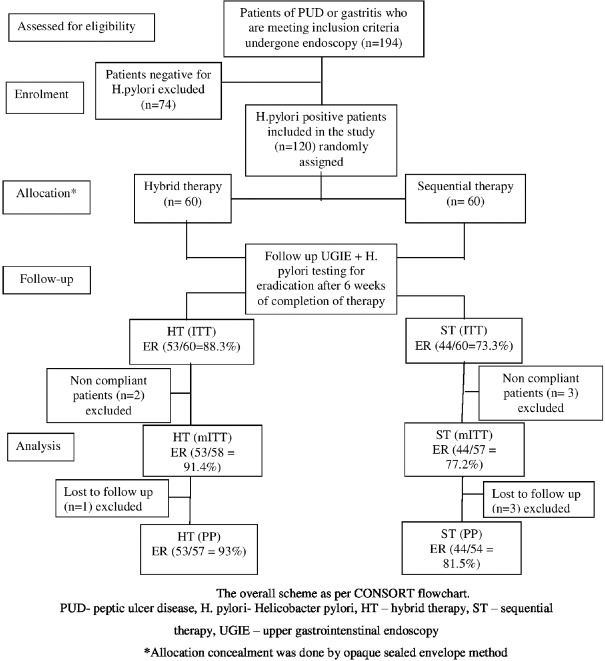
In the present study, out of 194 patients present for initial endoscopy, 120 patients were H. pylori positive (61.85%) [Figure 1].
Figure 1.
CONSORT flowchart
Mean age in HT and ST groups was 39.90 ± 10.715 and 40.80 ± 12.977 years, respectively. There were 29 males (48.3%) and 31 females (51.7%) in the HT arm while the sequential arm comprised 27 males (45%) and 33 females (55%). The gender distribution between the groups was also comparable and the male-to-female ratio in both the groups did not vary significantly (48/52% vs. 45/55%; P = 0.714).
The eradication rate of H. pylori in peptic ulcer disease patients treated with HT and ST was 88.3% versus 73.3% (P = 0.037) by intention-to-treat (ITT) analysis. By modified ITT, the eradication rate with HT and ST was 91.4% versus 77.2% (P = 0.036), and by per-protocol (PP) analysis, it was 93% versus 81.5% (P = 0.068) [Table 1].
Table 1.
Comparing the Helicobacter pylori eradication rates with the hybrid therapy and the sequential therapy
Compliance in HT and ST groups was 96.7% and 95% (P = 0.648), respectively [Table 2].
Table 2.
Comparison of the compliance between the hybrid therapy and the sequential therapy

The most common side effect in HT and ST by PP analysis was metallic taste. Other side effects in HT groups included epigastric pain (8.8%), nausea/vomiting (3.5%), diarrhea (3.5%), and bloating (1.8%). ST group also demonstrated epigastric pain (12.96%), nausea/vomiting (7.4%), diarrhea (5.6%), bloating (1.9%), and rashes (1.9%) as the other side effects.
The cost of a complete course of HT was $10.77 and $6.347 for ST.
DISCUSSION
Increasing antibiotic resistance has become a global threat. Resistance develops partly due to irrational antibiotic use and in part by increased virulence of the microorganism. H. pylori not being an exception has shown an increasing resistance not only for monomicrobial therapy but also for dual therapy in the past two decades. To overcome this shortfall, combinations of antibiotics have been introduced with each targeting a particular function to eradicate the pathogen. Undesirably, resistance to the commonly used STT by H. pylori has also been shown to be increasing by many studies from the various regions. ST and concomitant therapy with four drugs were introduced in the recent years to overcome the unacceptable eradication rate by STT.
Jafri et al. in their meta-analysis have proved that ST has superior eradication rate compared to STT.[20] Although ST achieves higher eradication than STT, the target eradication rate has not been demonstrated by other studies. Recently, a novel concept with HT has been shown to have promising results by few trials. However, the superiority of the HT over ST in achieving target eradication rate needs to be evaluated. The present study has tried to assess the efficacy of HT in achieving the target eradication rate and compared the eradication rate with that of the ST.
The prevalence of H. pylori varies with different geographical locations, with high infection rates in the population living in developing countries, in India being over 80% in rural areas.[21] The H. pylori positivity in the present study population was 61.85%. Majority of the study population was young, being < 40 years. The mean age of the patients did not differ significantly between HT and ST groups. More than half the patients in both groups were females; however, the gender distribution did not show any significant difference.
In the present study, ST group had H. pylori eradication rate of 73.3% and 81.5% by ITT and PP analysis, respectively. A better eradication rate was shown by Eisig et al. where the rate was 86% and 89.6% by ITT and PP analysis, respectively.[22] A study from Taiwan also demonstrated a higher eradication of 92.3% by ITT and 93.1% by PP analysis with ST.[14] A meta-analysis consisting of ten RCTs with 3006 enrolled patients showed superior eradication rate with ST compared to STT (with an odds ratio of 2.99 [95% CI 2.47–3.62] resulting a number need to treat of 6 [95% CI 5–7]).[23] The present study achieved a lower eradication rate that may be attributed to the increasing antibiotic resistance of the population. Previous studies carried out in the present institute also showed a similar rate of eradication, necessitating the use of a newer regimen.[9]
In the present study, HT achieved an eradication rate of H. pylori of 88.3% and 93% by ITT and PP analysis respectively in patients suffering from peptic ulcer disease and gastritis which was in concordance with a study by Sardarian et al. from Iran, where HT achieved an eradication rate of 89.5% and 92.9% ITT and PP analysis respectively.[5] De Francesco et al. also showed the eradication rate of 82.7% by ITT and 95.7% by PP analysis.[24] A recent study by Chen et al. showed a better eradication rate of HT with 92% and 96.4% by ITT and PP analysis respectively[25] as compared to ST which was 78.2% by ITT and 81.9% by PP analysis that was significantly lower than HT. Few studies have shown lower eradication of HT as well. A study from Korea by Oh et al. showed eradication rate by HT to be only 81.1% by ITT and 85.9% by PP analysis.[26] A meta-analysis of six studies with 1985 participants by Hsu et al. demonstrated the higher efficacy of HT in a non-Italian population that showed eradication rate of HT significantly better with 88.6% and 92.1% by ITT and PP analysis respectively as compared to ST.[27]
In the present study, HT achieved better eradication rate compared to ST, especially in the setting of dual resistance to clarithromycin and metronidazole. The higher eradication rate in HT in the presence of dual resistance could be attributed to longer duration of HT compared to ST (14 days vs. 10 days) and also continuation of amoxicillin throughout the coarse which has low antibiotic resistance (ranging from 0% to 1.6%)[25,28] compared to other drugs in the treatment regimen. Chen et al. found in their study that dual resistance has not been found as a predictor of eradication in ST or HT group.[25] A previous study demonstrated the failure of ST in the eradication of H. pylori in areas of dual antibiotic resistance.[14] Due to irrational use of antibiotics, the antibiotic resistance rate is increasing all over the world. In India, the resistance of H. pylori to clarithromycin and metronidazole was 33% and 78%, respectively.[29] Both HT and ST used metronidazole as one of the components of their regimens. Although in vitro metronidazole resistance is found very often, it has very little impact on the clinical result.[19,30] HT in this study achieved grade B level (>90%) eradication by PP analysis, which though not the target eradication rate, can be recommended in clinical practice and may be due to the dual antibiotic resistance in India.
Patient compliance in both groups was comparable with a compliance rate of 96.7% in HT group and 95% in ST group. Sardarian et al. observed a comparable high compliance in both HT and ST groups (96.7% and 98.6%, respectively).[5] Chen et al. reported a 97.6% adherence rate in HT group and 97.5% in ST group.[25] Oh et al. also showed a higher compliance in both the groups with no significant difference (97.3% vs. 96.3%, respectively, with P = 0.824).[26] Antibiotics used in both the HT and ST groups were same with similar dosage and frequency schedule except for the duration of treatment which could be the reason for the similar compliance in both the groups.
The most common side effect of both HT and ST was metallic taste (24.6% vs. 14.8%, respectively, by PP analysis). Other major side effects observed in HT group including diarrhea, nausea/vomiting, bloating, and epigastric pain were similar to that of ST. Both Sardarian et al. and Chen et al. in their study found bitter taste as a major side effect in both the groups.[5,25] The study done by De Francesco et al. showed diarrhea as the most common side effect in both the groups.[24] A Korean study demonstrated bitter taste, epigastric discomfort, and diarrhea as the most commonly found adverse effects in both the groups.[25] In all these studies, the adverse effects did not significantly vary between HT and ST.
In the developing countries like India, cost of therapy plays a vital role as the expenses toward the treatment is borne by the patient, and health insurance coverage is not widely available to all the population. In the present study, eradication of H. pylori depends on the completion of full course of HT or ST. Hence, the cost of therapy was calculated which could possibly play an important role in the implementation of treatment for H. pylori eradication. The price of a complete course of HT was $10.77 whereas it was half the cost ($6.347) for ST, indicating ST to be economically better than HT. Increased total duration of therapy in the HT group, i.e. 14-day course with the addition of amoxicillin in the second phase of 7 days compared to that of 10-day course with less number of drugs in ST justifies the higher cost for the HT course in the present study. Although HT achieves superior eradication rate than ST, this difference in the cost may play an important role in the acceptance and wide utility of the HT.
CONCLUSIONS
In the present study, it was found that HT showed a higher eradication rate for H. pylori infection when compared to that of ST. The side effects and the compliance were comparable between the two groups. Both the groups had no serious side effects that led to any interruption of treatment; however, due to the longer duration of multiple drugs, HT is costlier than ST. However, the cost of retreatment was not evaluated in this study.
Limitations
Antibiotic sensitivity and culture of H. pylori could not be done in the study due to unavailability of resources.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Roby Das, senior resident at the department of surgery, for his priceless support during the conduction of the study.
REFERENCES
- 1.Kate V, Ananthakrishnan N, Badrinath S, Ratnakar C. Prevalence of Helicobacter pylori infection in disorders of the upper gastrointestinal tract in South India. Natl Med J India. 1998;11:5–8. [PubMed] [Google Scholar]
- 2.Tomita T, Fukuda Y, Tamura K, Tanaka J, Hida N, Kosaka T, et al. Successful eradication of Helicobacter pylori prevents relapse of peptic ulcer disease. Aliment Pharmacol Ther. 2002;16(Suppl 2):204–9. doi: 10.1046/j.1365-2036.16.s2.24.x. [DOI] [PubMed] [Google Scholar]
- 3.Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: Systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833–55. doi: 10.1111/j.1572-0241.2004.40014.x. [DOI] [PubMed] [Google Scholar]
- 4.Ermis F, Senocak Tasci E. Current Helicobacter pylori treatment in 2014. World J Methodol. 2015;5:101–7. doi: 10.5662/wjm.v5.i2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: A prospective randomized trial. Helicobacter. 2013;18:129–34. doi: 10.1111/hel.12017. [DOI] [PubMed] [Google Scholar]
- 6.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 7.Huang YK, Wu MC, Wang SS, Kuo CH, Lee YC, Chang LL, et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis. 2012;13:232–8. doi: 10.1111/j.1751-2980.2012.00575.x. [DOI] [PubMed] [Google Scholar]
- 8.Mégraud F. The challenge of Helicobacter pylori resistance to antibiotics: The comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012;5:103–9. doi: 10.1177/1756283X11432492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valooran GJ, Kate V, Jagdish S, Basu D. Sequential therapy versus standard triple drug therapy for eradication of Helicobacter pylori in patients with perforated duodenal ulcer following simple closure. Scand J Gastroenterol. 2011;46:1045–50. doi: 10.3109/00365521.2011.584894. [DOI] [PubMed] [Google Scholar]
- 10.Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: Proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139–45. doi: 10.1111/j.1523-5378.2011.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham DY. Helicobacter pylori eradication therapy research: Ethical issues and description of results. Clin Gastroenterol Hepatol. 2010;8:1032–6. doi: 10.1016/j.cgh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Wang YH, Lv ZF, Xiong HF, Wang H, Yang Y, et al. Review: Efficacy and safety of hybrid therapy for Helicobacter pylori infection: A systematic review and meta-analysis. Helicobacter. 2015;20:79–88. doi: 10.1111/hel.12180. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–31. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.e1. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol. 2014;20:1438–49. doi: 10.3748/wjg.v20.i6.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allahverdiyev AM, Bagirova M, Caliskan R, Tokman HB, Aliyeva H, Unal G, et al. Isolation and diagnosis of Helicobacter pylori by a new method: Microcapillary culture. World J Gastroenterol. 2015;21:2622–8. doi: 10.3748/wjg.v21.i9.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015;3:9. doi: 10.3978/j.issn.2305-5839.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segamwenge IL, Kagimu M, Ocama P, Opio K. The utility of the Helicobacter pylori stool antigen test in managing dyspepsia: An experience from a low resource setting. Afr Health Sci. 2014;14:829–34. doi: 10.4314/ahs.v14i4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das R, Sureshkumar S, Sreenath GS, Kate V. Sequential versus concomitant therapy for eradication of Helicobacter pylori in patients with perforated duodenal ulcer: A randomized trial. Saudi J Gastroenterol. 2016;22:309–15. doi: 10.4103/1319-3767.187605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafri NS, Hornung CA, Howden CW. Meta-analysis: Sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 21.Thirumurthi S, Graham DY. Helicobacter pylori infection in India from a western perspective. Indian J Med Res. 2012;136:549–62. [PMC free article] [PubMed] [Google Scholar]
- 22.Eisig JN, Navarro-Rodriguez T, Teixeira AC, Silva FM, Mattar R, Chinzon D, et al. Standard triple therapy versus sequential therapy in Helicobacter pylori eradication: A double-blind, randomized, and controlled trial. Gastroenterol Res Pract. 2015;2015:818043. doi: 10.1155/2015/818043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: Systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–79. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 24.De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: A prospective randomized study. J Med Microbiol. 2014;63(Pt 5):748–52. doi: 10.1099/jmm.0.072322-0. [DOI] [PubMed] [Google Scholar]
- 25.Chen KY, Lin TJ, Lin CL, Lee HC, Wang CK, Wu DC. Hybrid vs. sequential therapy for eradication of Helicobacter pylori in Taiwan: A prospective randomized trial. World J Gastroenterol. 2015;21:10435–42. doi: 10.3748/wjg.v21.i36.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh DH, Lee DH, Kang KK, Park YS, Shin CM, Kim N, et al. Efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol. 2014;29:1171–6. doi: 10.1111/jgh.12518. [DOI] [PubMed] [Google Scholar]
- 27.Hsu PI, Lin PC, Graham DY. Hybrid therapy for Helicobacter pylori infection: A systemic review and meta-analysis. World J Gastroenterol. 2015;21:12954–62. doi: 10.3748/wjg.v21.i45.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miftahussurur M, Cruz M, Subsomwong P, Jiménez Abreu JA, Hosking C, Nagashima H, et al. Clarithromycin-based triple therapy is still useful as an initial treatment for Helicobacter pylori infection in the Dominican Republic. Am J Trop Med Hyg. 2017:pii: 16-0729. doi: 10.4269/ajtmh.16-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thyagarajan SP, Ray P, Das BK, Ayyagari A, Khan AA, Dharmalingam S, et al. Geographical difference in antimicrobial resistance pattern of Helicobacter pylori clinical isolates from Indian patients: Multicentric study. J Gastroenterol Hepatol. 2003;18:1373–8. doi: 10.1046/j.1440-1746.2003.03174.x. [DOI] [PubMed] [Google Scholar]
- 30.Mégraud F. Failed eradication for Helicobacter pylori. What should be done? Dig Dis. 2016;34:505–9. doi: 10.1159/000445230. [DOI] [PubMed] [Google Scholar]