Abstract
Alzheimer's disease (AD) is a common, progressive, fatal neurodegenerative disorder, which will play an increasingly important role both socially and financially in the aging populations. Treatments for AD show modest improvements in cognition and global functioning among patients. Furthermore, the oral administration of treating AD has had some drawbacks that decrease the medication adherence and efficacy of the therapy. Transdermal drugs are proposed as an alternative remedy to overcome the disadvantages of current pharmaceutical dosage options for this chronic disorder. They could have different strengths, such as offering a stable diffusion of active substance, avoiding the first pass metabolism, and reducing system adverse reactions. This article reviews the technical principles, novel techniques of transdermal delivery drug, and prospects for future development for the management of cognitive and behavioral dysfunctions in AD patients.
Keywords: Alzheimer's disease, controlled release, patch, permeation flux, transdermal
Introduction
The first case of Alzheimer's disease (AD) was recorded in 1906 by Alois Alzheimer. Alzheimer causes mental and physical decline as a result of a progressive degeneration of neurological system, which progresses until death. While every part of cerebral cortex is involved, the occipital pole may be less affected in the great majority of patients. The cortical ribbon could be thinned, and the temporal horn shows obvious signs of ventricular dilatation due to atrophy of the amygdala and hippocampus. The main neuropathological hallmarks are the extraneuronal senile plaques and intraneuronal neurofibrillary tangles. Loss of neuronal synapses and neuronal death results due to decrease in acetylcholine and other neurotransmitters. Amyloid plaques and neurofibrillary tangles are both clearly visible under the microscope. AD can be characterized by gross diffuse atrophy of the brain, along with the loss of neurons, neuronal processes, and synapses in the cerebral cortex and certain subcortical regions, resulting in degeneration of the temporal lobes, parietal lobes, parts of the frontal cortex, and cingulated gyrus. Levels of some neurotransmitters, such as acetylcholine, serotonin, norepinephrine, somatostatin, and corticotropin-releasing factors, are reduced while the glutamate level is usually elevated. Accordingly, the affected people suffer from cognitive decline, memory impairment, difficulties in decision-making, and behavioral changes.[1] AD is one of the most common neurodegenerative disorders observed in more than 80% dementia patients in geriatric population.[2,3] Because of increasing of life standards over the next decades, the number of Alzheimer victims will grow up from 47 million patients now to 130 million by 2050.[4,5] Due to the report of World Alzheimer, $818 billion was the social and economic cost for dementia in 2015, and it was estimated to rise to 1 trillion by 2018. There are five drugs which were approved by the Food and Drug Administration (FDA) for of AD: Tacrine, rivastigmine, donepezil, and galantamine as cholinesterase inhibitors therapy and memantine as noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist.[6] These substances are mostly available in oral administration. Many disadvantages were reported, for example, the current dosage forms as high doses cause adverse reactions such as abdominal pain, nausea, vomiting, anorexia, and abdominal pain,[7] the poor medication adherence due to characteristics of disease with the age group,[8] low compliance because of high incidence of dysphagia and memory loss,[9,10] and variation of blood levels of drugs. Besides, other effects such as hepatotoxicity, renal failure, asthenia, or malaise often lead to discontinuation of treatment in affected people.[11] Fortunately, transdermal delivery systems could be the solution for these drawbacks, and it could be an advantage as a novel therapeutic approach. It bypasses the first-pass metabolism because drugs are absorbed directly into the blood through the skin to enable use at low doses[12] and circadian cholinergic rhythms would be unaffected.[13] Furthermore, transdermal drug delivery system could offer therapeutic levels of the drug in systemic circulation through a controlled drug delivery, while decreasing side effects by excluding the large fluctuations of plasma concentration of the drug,[14] and is particularly useful for patients with discomfort in swallowing. Transdermal patches would improve patient compliance as well as the benefit in prolonged use of drugs and preferred by caregiver during long-term treatment of disease.[15] The transdermal products in Alzheimer's disease known as a rivastigmine patch that product had an excess of US $400 million in sales in 2014, highlighting the commercial success of transdermal products for patients suffering from this condition. The purpose of this paper is to review recent the fabrication of transdermal pharmaceutical dosage form for treatment AD. In addition, the alternative technique will be discussed as improvement in the drug delivery for this neurological disorder condition.
Transdermal for Alzheimer's Disease
Cholinesterase inhibitors
The deficiency of acetylcholine was known as the oldest hypothesis of causing the AD. Inhibition of the enzymes which degenerate acetylcholine is an effective therapy for pharmacological treatment of this neurological impairment. This therapy is associated with adverse events in the gastrointestinal system, and fluctuation of plasma level causes the symptoms linked to cholinergic hyperstimulation, which reduces the medication adherence of patients.[16,17] Therefore, the research conducted to transdermal administration has been indispensable, essential for the cholinesterase treatment. One of the aims of the transdermal drug delivery is that it could facilitate the smooth and steady delivery of drugs through the skin as well as provide long-term efficacy with relatively low dose. The past and current transdermal delivery of acetylcholine inhibitions was evaluated as discussed below. More recently, the technical principles underlying the development of transdermal drug delivery systems are summarized in Table 1.
Table 1.
Summary of transdermal drugs and techniques for cholinesterase inhibitors
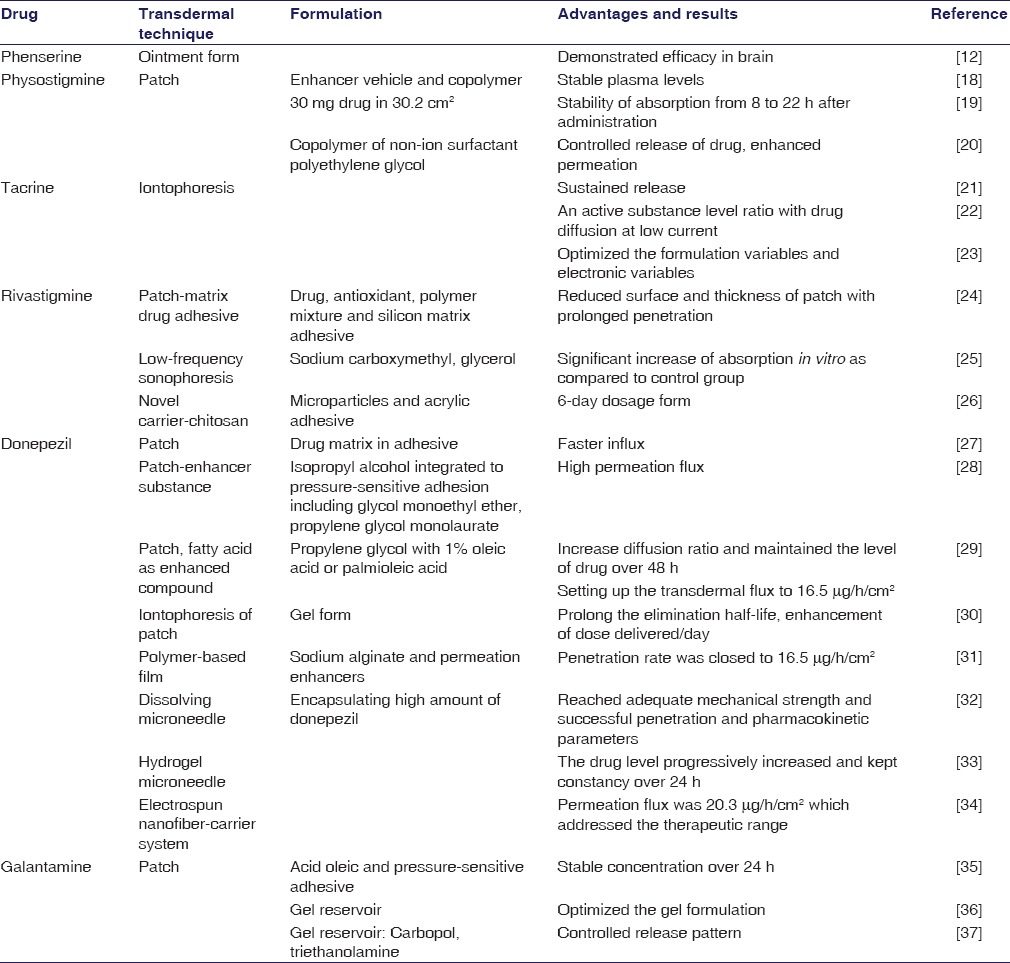
Physostigmine
Physostigmine was the first anticholinesterase documented as transdermal drug against treat AD. There may be some short-comings such as narrow therapeutic window, poor bioavailability, high first-past metabolic, and low user compliance of the oral and intravenous route of this drug that should be improved.[38,39] Levy et al. designed a formulation pad (3.6 cm × 3.6 cm) in 1986 which consisted of physostigmine in enhancer vehicle propionic acid and an ethylene/vinylacetate copolymer membrane with an adhesive on one side and an aluminum foil cover on its back.[40,41] The human study with this structure showed that a stable plasma concentration was recorded at 0.56 ± 0.1 ng/ml during the test and correlated well with blood acetyl-cholinesterase inhibition.[18] Consequently, physostigmine transdermal system was fabricated by Lohmann Therapie-Systeme GmbH that contained 30 mg active molecular in surface area 30.2 cm2. The in vitro release revealed a stability of absorption from 8 h after administration over 22 h. The human test corresponded with the previous result like the therapeutic range maintained for 18 h after a single dose.[19] A clinical study was carried out in a large number of participants (204 patients) with this patch; however, the plasma concentrations which were obtained (100 pg/ml) may not be efficient in the compensation for cholinergic deficiencies in affected brain areas and to produce benefits.[42] To enhance the permeation, a copolymer of non-ion surfactant polyethylene glycol was developed as a transdermal drug delivery for physostigmine with the 20% concentration of solution in the mixture of water/ethanol (80/20) and the amount of drug was 5.3 mg/cm2. The area under the curve (AUC) over 24 h experiment was 245.2 ± 337.2 h.ng/ml, and the mean patch flux reached 4.6 ± 6.3 μg/cm2 by rabbit test. This promising report enabled a possible carrier for functional agent on Alzheimer therapy.[20] There has a demand for further investigation of this innovative system for physostigmine.
Tacrine
In the early stage of transdermal drug fabrication for AD, tacrine was one of the experimental drugs. Tacrine is a reversible cholinesterase inhibitor; its passive diffusion was difficult because of the lipophilic and weak base property. Therefore, the transport was done with the utilization of ion-exchange fibers and iontophoresis. In an iontophoresis system, there were an anode with cationic or neutral therapeutic agents and a cathode with anionic therapeutic agents, which were utilized under an external electric field. The drugs had the same polarity with the electrode and were driven into the skin by electrorepulsion and electroosmosis. With electron repulsion, cationic drugs were driven into and through the skin by the anode (active electrode), which also extracted anions from the tissue underneath the skin into the anode. At the cathode (return electrode), anionic buffer ions were driven into the skin and cations from the tissues are extracted into the cathode.[43] Electroosmosis could act as current-induced convective solvent flow, while the skin could be buffered at a physiologic pH of 7.4, it acquired a net negative charge, causing electroosmotic flow to occur from anode to cathode.[44,45] In 2002, Kankkunen integrated tacrine in an ion exchange fiber to evaluate the permeation of this drug across the skin. A forming of drug reservoir was offered by the ion-exchange and was kept inside until they are released by the mobile ions which from the iontophoresis system.[21] The result showed that with the support from iontophoresis system, the delivery of active ingredients was well controlled to the steady state of about 14.9 ± 2.6 ng/ml in human beings until the device was turned off.[21] Subsequently, delivery parameters for iontophoresis were reported by Upasani and Banga in 2004. Iontophoresis is a complex process which is affected by many factors such as drug concentration, molecular size, and strength of the donor buffer and electrical factors such as current density and mode. At low current density, the permeation of substance was not influenced; however, with high current density, the concentration was in ratio with the drug diffusion.[22] Finally, the dependence of iontophoresis delivery of tacrine on electronic variables and formulation variables was demonstrated that helped the formulators to optimize the conditions for the absorbance of this drug through the skin.[23] In 2013, despite benefits on cognition, tacrine was withdrawn from the market because of the adverse effect related to the hepatotoxicity.[45,46]
Rivastigmine
Transdermal patches were developed in the 1970s and the first was approved by the FDA in 1979. In most patch designs, the drug is stored in a reservoir that is enclosed on one side with an impermeable backing and has an adhesive that contacts the skin on the other side.[47] A special membrane is used to control the rate, at which the liquid drug contained in the reservoir within the patch can pass through the skin and into the bloodstream. There are some methods to apply simultaneously with the patch to reach a diffusion of the active substance such as iontophoresis, electroporation, ultrasound, and microscopy projection.[48] The first generation of patch acted as a simple reservoir integrated a piece of plastic with an adhesive edge dipped into the drug solution in alcohol, this system caused irritation on the skin.[24] The development of matrix patch next overcame this drawback. This kind of system was designed in four layers [Figure 1], which help release the drug consistently, thus avoiding skin reaction and prolong the adhesion duration.[24]
Figure 1.
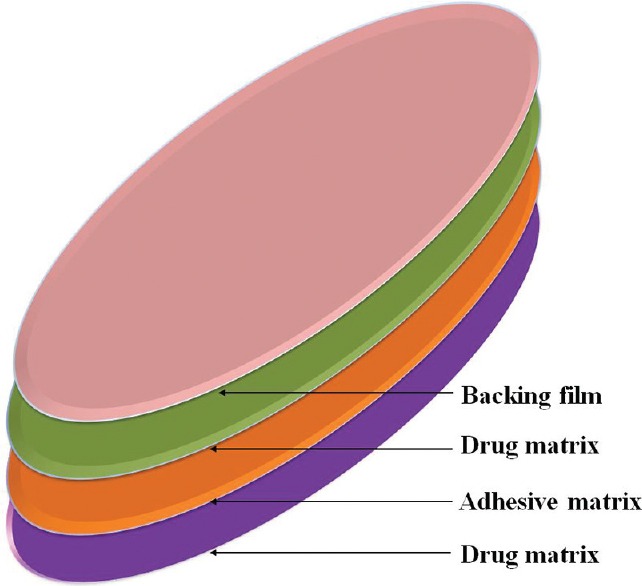
Structure of Rivastigmine transdermal patch
Rivastigmine appeared promising because of the suited chemical properties and the requirement for the small daily dose. The rivastigmine patch includes drug, antioxidants, polymer mixture, and silicon matrix adhesive into a single layer from which the drug would diffuse. With the unique technology, it could reduce the surface and thickness of the patch which making the convenient dosage form for administration. The surface of site patch application is proportional to the level of active substance that enter the bloodstream and it was formed in three sizes in the market 5 cm2(4.6 mg/24 h), 10 cm2(9.5 mg/24 h), and 15 cm2(13.3 mg/24 h).[49] In spite of these advantages, passive permeation is limited by the natural function of the skin acting as an barrier to prevent the diffusion of substances.[50] The loss of patch from the skin and the skin irritation has was observed during the treatment with this patch,[51] leading to the hypothesis that using low-frequency sonophoresis could improve these advantages. The ultrasound would create the localized transport pathways that permit the penetration of drug through the skin.[52] The formulation with rivastigmine, sodium carboxymethyl, and glycerol was formed and put under the low-frequency ultrasound. A dramatic increase in transdermal absorption for rivastigmine in vitro with Cmax of 0.8 of active group and 0.28 μg/ml of control group was observed; AUC value was 12.35 compared with 4.14, respectively.[25] A recent advance is the 6-day dosage form of transdermal rivastigmine. Chitosan, a N-deacetylated derivative of chitin, is a novel drug carrier or reservoir for controlled release because of its structural possibility for chemical and mechanical modifications.[53,54] Microparticles integrated into the acrylic adhesive provided the prolong release of active drug over almost 1 week. Significant improvement of diffusion in vitro was demonstrated. This is considered as the feasible replacement from 1 day dose to 6 days dose application of anti-Alzheimer pharmaceutical dosage form.[26]
Donepezil
In the late 2008s, transdermal donepezil pharmaceutical dosage form has been evaluated. Among the cholinesterase inhibitors, donepezil is the most superior due to its high potency and selectivity for the enzyme in the central nervous system.[55] Valia et al. proposed two types of patches: A drug reservoir-in-adhesive and a drug matrix-in-adhesive [Figure 2]. A faster influx of functional substance was achieved with the second variant because the drug would migrate from reservoir into and through the adhesive layer. This novel system facilitated the controlled release by the correction of active surface of the patch which contacts directly to the skin.[27]
Figure 2.
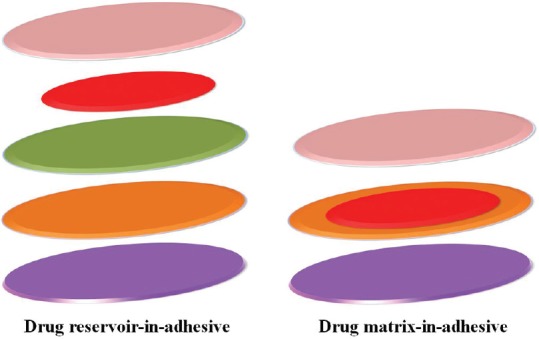
Structure of two types of patch for Alzheimer's disease
Subsequently, Kim and Gwak demonstrated that various vehicles as solvents and permeation enhancers as fatty acids could improve the diffusion of donepezil through the stratum corneum go into the blood. Excised hairless mouse model was used for penetration test, and it was reported that the formulations including isopropyl alcohol, ethyl alcohol, and water acted as co-solvents and increased the permeation fluxes. The most exciting result was the formulation that contained isopropyl alcohol integrated to pressure-sensitive adhesion including glycol monoethyl ether and propylene glycol monolaurate (40:60), which showed the highest permeation flux (0.1 ± 0.0024 μg/h/cm2). This was the most favorable candidate for donepezil transdermal delivery.[28] In tandem with previous reports that fatty acid could improve the permeation rate, the base or salt form of donepezil saturated in propylene glycol with 1% oleic acid and palmioleic acid increased the diffusion ratio up to 19.31 μg/h/cm2 from 1.63 μg/h/cm2 and 98.29 μg/h/cm2 from 0.9 μg/h/cm2, respectively, in rat model. Equally importantly, the Cmax was at steady state at 52.2 ng/ml and maintained for 48 h with donepezil formulation using oleic acid.[29] Iontophoresis system was applied for the penetration of a patch with gel formulation of donepezil across the skin, which by Saluja et al. The pharmacokinetic parameters showed a significant enhancement of dose delivered/day, Cmax, Tmax with the iontophoresis application groups compared to intravenously route. Besides, the apparent elimination half-life following transdermal administration was longer than vascular injection method with an average number of 13.4 h, 14.9 h, 29.2 h (0.13 mA, 0.26 mA, 0.39 mA, respectively) with 3.2 h.[56] Thus, iontophoresis could offer an attractive pharmacotherapy option in treating AD. Patches with different doses 8.75 mg/2.5 cm2, 17.5 mg/5 cm2, and 35 mg/10 cm2 donepezil were tested in the rats with the result cited that Cmax was dose proportional (36.5 ng/ml, 64.4 ng/ml, and 129.1 ng/ml, respectively) with Tmax values of 24–36 h compared to Cmax 41.4 ng/ml of oral single dose administration.[30]
Galipoglu et al. used the biopolymer from sodium alginate combined with the permeation enhancers in formulation to fabricate the transdermal films of donepezil. The test with pig skin showed that the penetration rate was 12.45 μg/h/cm2, which was similar to the previous study of 16.5/h/cm2.[29] Thus, transfer donepezil by novel polymer-based films seemed possible for management of neurological disorder.[31] Microneedles are tiny scale of single or array needle used to overcome the barrier characteristic of skin and to deliver the substances which have the specific physicochemical properties.[57] An optimal formulation for encapsulating high amount of donepezil on tips of dissolving microneedle was assessed to address the acceptable clinical application [Figure 3]. Approximately 78% w/w of active substance was encapsulated in the microneedle with enough mechanical strength, and 95% of this content was delivered through the porcine skin on 5 min. In addition, a satisfactory result of pharmacokinetics in rats was cited as evidence with the Cmax that was higher than 4 times compared to the oral administration at 9 ng/ml. This sophisticated technology could be the replaceable therapy for the prescription anti-AD therapy.[32] In addition to the state-of-art of microneedle pharmaceutical dosage form, the hydrogel microneedle integrated with transdermal patch donepezil film was prepared to add more proof-of-concepts for microneedle in transdermal delivery of anticholinesterase drugs [Figure 4]. It was observed that the dose of 2.5 mg and 5 mg resulted in plasma level of 35.6 ng/ml for the first dose and 51.8 ng/ml for the latter dose. Furthermore, the concentration progressively increased and was constant over 24 h and experiment due to the microneedle swelling during the treatment time.[33] Gencturk et al. made the polyurethane hydroxyl propyl cellulose electrospun nanofiber mats as a potential carrier system for transfer of donepezil across the skin. In vitro permeation study showed that the flux of functional molecule was 20.3 μg/h/cm2, which addressed the requisite therapeutic range. Because of the mechanism of the diffusion, drug in the surface of nanofiber would dissolve first after that the drugs inside the matrix would release slowly in sustained manner; this is suitable for the controlled release of anticholinesterase for management AD.[34]
Figure 3.
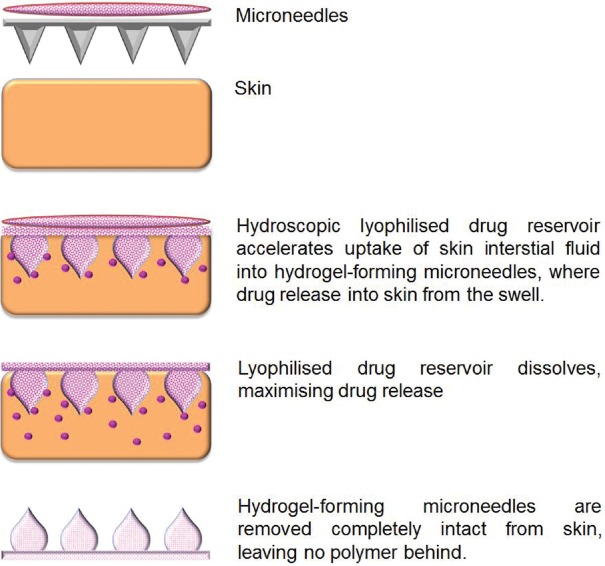
Illustration of the diffusion of drug from hydrogel microneedles
Figure 4.
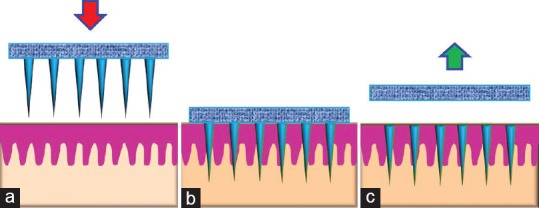
Schematic depiction of dissolving microneedles in which drug is embedded in the matrix of dissolving microneedles. (a) Hydroscopic lyophilized drug reservoir accelerates uptake of skin interstial fluid into hydrogel-forming microneedles, where drug release into skin from the swell; (b) lyophilized drug reservoir dissolves, maximizing drug release; (c) microneedles are removed completely intact from skin, leaving no polymer behind
Phenserine
Phenserine has the same disadvantages as an anticholinesterase like first-past hepatic and digestion metabolism. To minimize this problem, the ointment formulation of phenserine was developed and tested in vitro, in vivo. The in vivo result indicated that this drug induced the efficacy, with both plasma (30% decrease at 8 h) and brain (60% inhibition), resulting in improved cognitive function in the animal model.[10] This finding offered the clinical benefit of transdermal phenserine delivery; however, more research are needed before marketing the drug.
Galantamine
Unlike other anticholinesterase substances, galantamine has dual action as it inhibits the cholinergic system and modulates allosterically nicotinic acetylcholine receptors.[58] The oral administration of this active molecular was presently approved for use in the market.[59] A drug-in-adhesive transdermal form of galantamine was designed to offer a novel pathway for treating Alzheimer's patients. The effect of formulation factors such as pressure-sensitive adhesive, enhancers, and drug concentration was evaluated. It is documented that the optimal patch contains 8% galantamine, 3% acid oleic in DT-2510 (a –OH group functional pressure-sensitive adhesive) and has a high bioavailability about 80%, with a stable concentration of active compound over a long period; being 24 h in animal test of rabbit model.[35] In 2015, Fong Yen et al. fabricated the gel reservoir in a patch for this substance and observed its effect. The highest drug release was achieved with lower amount of polymer, cross-linker, higher amount of drug, chemical permeation enhancer. The gel had suitable characteristics that could be used to produced transdermal system for anti-AD.[36] Accordingly, an optimal formulation of gel reservoir was optimized which contained 0.89% w/w carbopol, 1.16% w/w triethanolamine, and 4.19% w/w galantamine. This patch had amount of substance extraction at 8 h at 16.93 mg/cm2 and actual permeation flux of 2.32 mg/h/cm2, thus allowing high drug content and controlled drug release pattern for transdermal delivery of galantamine.[37]
Noncompetitive N-Methyl-D-Aspartate: Memantine
There is a hypothesis that excessive activation of NMDA receptors may underlie the degeneration of cholinergic cells. Memantine blocks the NMDA receptors during the sustained release of low concentrations of glutamate and therefore reduce the NMDA receptor's function.[60,61] Recently, the transdermal dosage form of this active compound has been formulated for an alternative strategy for the management of AD. del Rio-Sancho et al. evaluated some factors which could provide an increase the permeability of memantine across the outermost layer of the skin. It is recorded that pretreatment with oleic acid, decenoic acid, or laurocapram gave a statistically dramatic increment of the transdermal flux of memantine (P < 0.05), providing a flux value of 24.5 ± 3.2, 22.6 ± 2.3, and 38.4 ± 4.7 lg/h/cm2, respectively. The highest flux value was cited when treated with R-(+)-limonene. Besides that, the most commonly used physical enhancer is iontophoresis which was much more efficient than all chemical enhancers tested, and it increases 22.3 folds the memantine flux with passive diffusion.[62] Consequently, the patch of memantine was prepared with specific polymer for controlling release as Platoid B and this system was assessed for pharmacokinetic in rats. In single doses, the transdermal patch had the AUC value higher 4.3 times than oral doses; meanwhile, the Cmax was similarly compared with oral route. In addition, the multiple dose study showed that AUC0-24 of patch administration corresponded with AUC0-12 of oral method. In addition, the memantine patch formulation displayed a smaller inter-individual variability and lower accumulation than the oral formulation.[63] This report established the feasibility of applying memantine delivery through the skin for treating Alzheimer's patients.
Other Drugs
Allopregnanolone
A recent advance in AD is the preservation of neurological function and prevention of neurogenerative disease by targeting the regenerative neurogenic capacity of the brain as a promising, alternative remedy.[64] The triple transgenic AD mouse was the model for the effect of allopregnanolone in increasing the neurogenesis within the hippocampus and sustaining the memory function to normal before and following the onset of AD pathology.[65,66] Notwithstanding the well-suited properties for targeting the brain bases such as small molecular weight, low number of hydrogen bond donors and receptors, and alogp 5.042, this active substance is difficult to formulate in aqueous form.[67] Therefore, transdermal would be the novel choice for applying this molecular in curing AD. A gel solution of allopregnanolone was tested in the pharmacokinetic animal models. The result in rabbit experiment showed that Cmax was 9.6 ng/ml following 15 min application and the concentration of compound in brain was higher than intravascular allopregnanolone after 8 h. Interestingly, this drug level was maintained in the brain for 24 h during the study period, resulting in neurological system exposure with the transdermal therapy that was 36% of the IV group.[68]
Vaccine therapy
The amyloid β (Aβ) oligomers vaccine therapy is based on the hypothesis that one of characteristics of the AD pathology such as neuronal cell death and the cognitive decline is the deposition of Aβ in the brain parenchyma.[69] The Th2-dominant immune responses have been the target of the researches. Skin is the best candidate for the vaccine therapy due to the abundance of T-cell in the dermis.[70] Matsuo et al. prepared a dissolving microneedle system containing Aβ1-42 (a synthetic Aβ consisting of 1–42 amino acid residues) and adjuvant (cholera toxin) and the vaccine efficacy was tested in in mice. Although this system just offered a little improvement in cognitive function and Th2-dominant immune responses, it provide the novel, alternative therapy for treating AD and would be further investigated.[71]
Alkaloid from herbal medicine
Huperzine A
Among the alkaloids that are extracted from the herbal sources, huperzine demonstrates cholinesterase inhibitor property across the blood–brain barrier.[72] It has been approved as a supplement for treatment of AD in oral pharmaceutical dosage form administered 2–3 times per day in the USA and as a drug in China.[73] Transdermal form of huperzine A has been evaluated. Ye et al. fabricated formulated a patch which comprises three layers: A drug-free backing layer, a layer of adhesive containing drug and skin penetration enhancers, and a protective release liner. The active ingredient was dissolved in ethyl acetate and formulation had a 20 cm2 surface area and contained 4 mg of huperzine A. The sustained release property of this system was evaluated in dog model. With the dose of 500 μg a day, the patch was applied for 84 h. The pharmacokinetic observations were Tmax value (24 h vs. 3 h), Cmax 3.4 ng/ml; AUC during the treatment was 325.6 ng/h/ml. Interestingly, the lowest drug concentration was 2.1 ng/ml and was maintained through 84 h. The patch exhibited a good controlled release characteristics and would be applied twice a week.[74] Consequently, a microemulsion-based patch of huperzine and ligustrazine was tested the permeation across the skin. It is reported that the microemulsion system increased the permeation rate of both drugs; thus, this might provide a feasible strategy for prevention of AD.[75] Recently, microemulsion, solid lipid nanoparticles, and nanostructure lipid carriers were formulated in gel form and assessed for permeation simultaneously. The microemulsion showed a superior penetration than nanostructure lipid carriers, followed by solid lipid nanoparticles (147.68 μg/cm2, 129 μg/cm2, 10.74 μg/cm2, respectively).[76]
Vinpocetine
Vinpocetine is an alkaloid derived from the common periwinkle plant that has a potential for use to improve memory function.[77] The transdermal formulation of vinpocetine using ethosome as a novel lipid carrier is under evaluation. Compared to vinpocetine solution, the nanoethosomal form increased the flux and entrapment efficiency and has a potential for transdermal use in AD.[78]
Ligustrazine
Ligustrazine is an alkaloid extracted from Chinese herbal medicine. It is hypothesized that this could be a novel drug candidate for treating AD due to the results from recent studies that demonstrated its role in improvement in hippocampal cholinergic system function.[79] It has certain disadvantages, such as short half-life, first pass metabolism, and low bioavailability. The transdermal route has been provided more opportunities for use of this drug in AD. Ethosome is an innovative liposome system that contains soft phospholipid vesicles in the presence of high concentrations of ethanol that enables penetration into the deep layers of the skin, both in terms of both quantity and depth.[80,81] Ethosomal system offers a significant improvement in permeation and drug delivery. The improved behavioral performance of tested rats tested are a proof-of-concept that ethosome containing ligustrazine could be an alternative choice for transdermal delivery of this drug for AD.[82]
Conclusions and Future Perspectives
A wide range of active compounds and therapies such as cholinesterase inhibitors, NMDA, vaccine, and alkaloid from herbal medicine have been under investigation in transdermal form for management of neurological disorders. The increase in permeability of these substances through the skin was demonstrated in vitro, in vivo as also their efficacy and lesser of adverse effects have been cited as the best evidence for the advantages of this route of drug delivery.
Although there is just one marketed product is in use, the results of these researches are promising. The Phase I trial for the donepezil patch has been completed and further studies are ongoing. Various transdermal drug delivery technologies are described including the use of suitable formulations, carriers and penetration enhancers. The particular success of rivastigmine patch offers opportunities for development of similar formulations for patients with AD.
Financial support and sponsorship
This work was supported by the Vietnam Education Foundation for funding (VEF Fellowship to Thuy Trang Nguyen and Vo Van Giau).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dong S, Duan Y, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer's disease: A re-evaluation of amyloid cascade hypothesis. Transl Neurodegener. 2012;1:18. doi: 10.1186/2047-9158-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giau VV, Bagyinszky E, An SS, Kim SY. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat. 2015;11:1723–37. doi: 10.2147/NDT.S84266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giau VV, An SS, Bagyinszky E, Kim SY. Next generation sequencing (NGS) Gene panels and primers for studies on neurodegenerative disorders. Toxicol Environ Health Sci. 2015;7:S32. [Google Scholar]
- 4.Thies W, Bleiler L Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8:131–68. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Thies W, Bleiler L. Alzheimer's Disease. Facts and figures. Alzheimers Dement. 2013;9:208–15. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Potyk D. Treatments for Alzheimer disease. South Med J. 2005;98:628–35. doi: 10.1097/01.SMJ.0000166671.86815.C1. [DOI] [PubMed] [Google Scholar]
- 7.Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002;127:45–63. [PubMed] [Google Scholar]
- 8.Singh G, Thomas SK, Arcona S, Lingala V, Mithal A. Treatment persistency with rivastigmine and donepezil in a large state medicaid program. J Am Geriatr Soc. 2005;53:1269–70. doi: 10.1111/j.1532-5415.2005.53384_9.x. [DOI] [PubMed] [Google Scholar]
- 9.Mauskopf JA, Paramore C, Lee WC, Snyder EH. Drug persistency patterns for patients treated with rivastigmine or donepezil in usual care settings. J Manag Care Pharm. 2005;11:231–51. doi: 10.18553/jmcp.2005.11.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small G, Dubois B. A review of compliance to treatment in Alzheimer's disease: Potential benefits of a transdermal patch. Curr Med Res Opin. 2007;23:2705–13. doi: 10.1185/030079907x233403. [DOI] [PubMed] [Google Scholar]
- 11.Winblad B, Cummings J, Andreasen N, Grossberg G, Onofrj M, Sadowsky C, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer's disease – Rivastigmine patch versus capsule. Int J Geriatr Psychiatry. 2007;22:456–67. doi: 10.1002/gps.1788. [DOI] [PubMed] [Google Scholar]
- 12.Utsuki T, Uchimura N, Irikura M, Moriuchi H, Holloway HW, Yu QS, et al. Preclinical investigation of the topical administration of phenserine: Transdermal flux, cholinesterase inhibition, and cognitive efficacy. J Pharmacol Exp Ther. 2007;321:353–61. doi: 10.1124/jpet.106.118000. [DOI] [PubMed] [Google Scholar]
- 13.Davis B, Sadik K. Circadian cholinergic rhythms: Implications for cholinesterase inhibitor therapy. Dement Geriatr Cogn Disord. 2006;21:120–9. doi: 10.1159/000090630. [DOI] [PubMed] [Google Scholar]
- 14.Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease. Expert Opin Drug Deliv. 2008;5:1377–86. doi: 10.1517/17425240802542690. [DOI] [PubMed] [Google Scholar]
- 15.Blesa R, Ballard C, Orgogozo JM, Lane R, Thomas SK. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer disease. Neurology. 2007;69(4 Suppl 1):S23–8. doi: 10.1212/01.wnl.0000281848.25142.11. [DOI] [PubMed] [Google Scholar]
- 16.Imbimbo BP. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer's disease. CNS Drugs. 2001;15:375–90. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 17.Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41:719–39. doi: 10.2165/00003088-200241100-00003. [DOI] [PubMed] [Google Scholar]
- 18.Levy A, Brandeis R, Treves TA, Meshulam Y, Mawassi F, Feiler D, et al. Transdermal physostigmine in the treatment of Alzheimer's disease. Alzheimer Dis Assoc Disord. 1994;8:15–21. doi: 10.1097/00002093-199408010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Walter K, Müller M, Barkworth MF, Nieciecki AV, Stanislaus F. Pharmacokinetics of physostigmine in man following a single application of a transdermal system. Br J Clin Pharmacol. 1995;39:59–63. doi: 10.1111/j.1365-2125.1995.tb04410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benech H, Vincenti M, Fouchart F, Pruvost A, Vienet R, Istin M, et al. Development and in vivo assessment of a transdermal system for physostigmine. Methods Find Exp Clin Pharmacol. 1998;20:489–98. doi: 10.1358/mf.1998.20.6.485712. [DOI] [PubMed] [Google Scholar]
- 21.Kankkunen T, Sulkava R, Vuorio M, Kontturi K, Hirvonen J. Transdermal iontophoresis of tacrine in vivo. Pharm Res. 2002;19:704–7. doi: 10.1023/a:1015374600683. [DOI] [PubMed] [Google Scholar]
- 22.Upasani RS, Banga AK. Response surface methodology to investigate the iontophoretic delivery of tacrine hydrochloride. Pharm Res. 2004;21:2293–9. doi: 10.1007/s11095-004-7682-6. [DOI] [PubMed] [Google Scholar]
- 23.Patel N, Jain S, Madan P, Lin S. Influence of electronic and formulation variables on transdermal iontophoresis of tacrine hydrochloride. Pharm Dev Technol. 2015;20:442–57. doi: 10.3109/10837450.2013.879886. [DOI] [PubMed] [Google Scholar]
- 24.Petersen TA. Transdermal drug formulation and process development. Pharm Technol 2003. 2003;27(6 Suppl):18–21. [Google Scholar]
- 25.Yu ZW, Liang Y, Liang WQ. Low-frequency sonophoresis enhances rivastigmine permeation in vitro and in vivo. Pharmazie. 2015;70:379–80. [PubMed] [Google Scholar]
- 26.Sadeghi M, Ganji F, Taghizadeh SM, Daraei B. Preparation and characterization of rivastigmine transdermal patch based on chitosan microparticles. Iran J Pharm Res. 2016;15:283–94. [PMC free article] [PubMed] [Google Scholar]
- 27.Valia KH, Ramaraju VS. inventors. Core Tech Solutions, assignee. Transdermal methods and systems for treating Alzheimer's disease. US 20080044461A1. United States patent. 2008 Feb 21; [Google Scholar]
- 28.Kim KH, Gwak HS. Effects of vehicles on the percutaneous absorption of donepezil hydrochloride across the excised hairless mouse skin. Drug Dev Ind Pharm. 2011;37:1125–30. doi: 10.3109/03639045.2011.561352. [DOI] [PubMed] [Google Scholar]
- 29.Choi J, Choi MK, Chong S, Chung SJ, Shim CK, Kim DD. Effect of fatty acids on the transdermal delivery of donepezil: In vitro and in vivo evaluation. Int J Pharm. 2012;422:83–90. doi: 10.1016/j.ijpharm.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 30.iCure. (Donepezil Patch) [Investigator's Brochure] iCure Pharmaceutical; Version 02. [release date: 24 March, 2014].
- 31.Galipoglu M, Erdal MS, Güngör S. Biopolymer-based transdermal films of donepezil as an alternative delivery approach in Alzheimer's disease treatment. AAPS PharmSciTech. 2015;16:284–92. doi: 10.1208/s12249-014-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Han MR, Kim YH, Shin SW, Nam SY, Park JH. Tip-loaded dissolving microneedles for transdermal delivery of donepezil hydrochloride for treatment of Alzheimer's disease. Eur J Pharm Biopharm. 2016;105:148–55. doi: 10.1016/j.ejpb.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Kearney MC, Caffarel-Salvador E, Fallows SJ, McCarthy HO, Donnelly RF. Microneedle-mediated delivery of donepezil: Potential for improved treatment options in Alzheimer's disease. Eur J Pharm Biopharm. 2016;103:43–50. doi: 10.1016/j.ejpb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Gencturk A, Kahraman E, Güngör S, ízhan G, ízsoy Y, Sarac AS. Polyurethane/hydroxypropyl cellulose electrospun nanofiber mats as potential transdermal drug delivery system: Characterization studies and in vitro assays. Artif Cells Nanomed Biotechnol. 2016;22:1–10. doi: 10.3109/21691401.2016.1173047. [DOI] [PubMed] [Google Scholar]
- 35.Park CW, Son DD, Kim JY, Oh TO, Ha JM, Rhee YS, et al. Investigation of formulation factors affecting in vitro and in vivo characteristics of a galantamine transdermal system. Int J Pharm. 2012;436:32–40. doi: 10.1016/j.ijpharm.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 36.Fong Yen W, Basri M, Ahmad M, Ismail M. Formulation and evaluation of galantamine gel as drug reservoir in transdermal patch delivery system. ScientificWorldJournal. 2015;2015:495271. doi: 10.1155/2015/495271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo FY, Basri M, Masoumi HR, Ahmad MB, Ismail M. Formulation optimization of galantamine hydrobromide loaded gel drug reservoirs in transdermal patch for Alzheimer's disease. Int J Nanomedicine. 2015;10:3879–86. doi: 10.2147/IJN.S80253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whelpton R, Hurst P. Bioavailability of oral physostigmine. N Engl J Med. 1985;313:1293–4. doi: 10.1056/NEJM198511143132016. [DOI] [PubMed] [Google Scholar]
- 39.Somani SM, Dube SN. Physostigmine – An overview as pretreatment drug for organophosphate intoxication. Int J Clin Pharmacol Ther Toxicol. 1989;27:367–87. [PubMed] [Google Scholar]
- 40.Levy D, Glikfeld P. A novel percutaneous device as a potential treatment of Alzheimer's disease. In: Fisher A, Hanin I, Lachman C, editors. Alzheimer's and Parkinson's Disease: Strategies for Research and Development. New York: Plenum Press; 1986. pp. 557–63. [Google Scholar]
- 41.Levy D, Meshulam Y, Grunwald J, Brucstein R. Proceeding of the 3rd International Symposium, Protection Against Chemical Warfare Agents, Umea, Sweden. Umea, Sweden: National Defense Research Establishment; 1989. A long-acting transdermal systems for the treatment of organophosphate poisoning; pp. 151–6. [Google Scholar]
- 42.Moller HJ, Hampel H, Hegerl U, Schmitt W, Walter K. Double-blind, randomized, placebo-controlled clinical trial on the efficacy and tolerability of a physostigmined path in patients with senile dementia of the Alzheimer type. Pharmacopsychiatry. 1999;32:99–106. doi: 10.1055/s-2007-979202. [DOI] [PubMed] [Google Scholar]
- 43.Riviere JE, Sage B, Williams PL. Effects of vasoactive drugs on transdermal lidocaine iontophoresis. J Pharm Sci. 1991;80:615–20. doi: 10.1002/jps.2600800702. [DOI] [PubMed] [Google Scholar]
- 44.Guy RH, Kalia YN, Delgado-Charro MB, Merino V, López A, Marro D. Iontophoresis: Electrorepulsion and electroosmosis. J Control Release. 2000;64:129–32. doi: 10.1016/s0168-3659(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 45.Jaskari T, Vuorio M, Kontturi K, Urtti A, Manzanares JA, Hirvonen J. Controlled transdermal iontophoresis by ion-exchange fiber. J Control Release. 2000;67:179–90. doi: 10.1016/s0168-3659(00)00204-2. [DOI] [PubMed] [Google Scholar]
- 46.Qizilbash N, Whitehead A, Higgins J, Wilcock G, Schneider L, Farlow M. Cholinesterase inhibition for Alzheimer disease: A meta-analysis of the tacrine trials. Dementia Trialists' Collaboration. JAMA. 1998;280:1777–82. doi: 10.1001/jama.280.20.1777. [DOI] [PubMed] [Google Scholar]
- 47.Venkatraman S, Gale R. Skin adhesives and skin adhesion 1. Transdermal drug delivery systems. Biomaterials. 1998;19:1119–36. doi: 10.1016/s0142-9612(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 48.Patel D, Chaudhary SA, Parmar B, Bhura N. Transdermal Drug delivery system: A review. Pharma Innov. 2012;1:66–75. [Google Scholar]
- 49.Patch E. Full Prescribing Information. East Hanover, NJ. Novartis Pharmaceuticals Corporation. 2016. [Last accessed on 2016 Jan 27]. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/exelonpatch.pdf .
- 50.Wong TW, Nor Khaizan A. Physicochemical modulation of skin barrier by microwave for transdermal drug delivery. Pharm Res. 2013;30:90–103. doi: 10.1007/s11095-012-0852-z. [DOI] [PubMed] [Google Scholar]
- 51.Greenspoon J, Herrmann N, Adam DN. Transdermal rivastigmine: Management of cutaneous adverse events and review of the literature. CNS Drugs. 2011;25:575–83. doi: 10.2165/11592230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Tezel A, Sens A, Mitragotri S. A theoretical analysis of low-frequency sonophoresis: Dependence of transdermal transport pathways on frequency and energy density. Pharm Res. 2002;19:1841–6. doi: 10.1023/a:1021493424737. [DOI] [PubMed] [Google Scholar]
- 53.Khanmohammadi M, Elmizadeh H, Ghasemi K. Inveatigation of size and morphology of chitosan nanoparticles used in drug delivery system employing chemometric technique. Iran J Pharm Res. 2015;14:665–75. [PMC free article] [PubMed] [Google Scholar]
- 54.He P, Davis SS, Illum L. Chitosan microspheres prepared by spray drying. Int J Pharm. 1999;187:53–65. doi: 10.1016/s0378-5173(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 55.Heydorn WE. Donepezil (E2020): A new acetylcholinesterase inhibitor. Review of its pharmacology, pharmacokinetics, and utility in the treatment of Alzheimer's disease. Expert Opin Investig Drugs. 1997;6:1527–35. doi: 10.1517/13543784.6.10.1527. [DOI] [PubMed] [Google Scholar]
- 56.Saluja S, Kasha PC, Paturi J, Anderson C, Morris R, Banga AK. A novel electronic skin patch for delivery and pharmacokinetic evaluation of donepezil following transdermal iontophoresis. Int J Pharm. 2013;453:395–9. doi: 10.1016/j.ijpharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Al-Qallaf B, Das DB. Optimizing microneedle arrays to increase skin permeability for transdermal drug delivery. Ann N Y Acad Sci. 2009;1161:83–94. doi: 10.1111/j.1749-6632.2009.04083.x. [DOI] [PubMed] [Google Scholar]
- 58.Lilienfeld S. Galantamine – A novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer's disease. CNS Drug Rev. 2002;8:159–76. doi: 10.1111/j.1527-3458.2002.tb00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prvulovic D, Hampel H, Pantel J. Galantamine for Alzheimer's disease. Expert Opin Drug Metab Toxicol. 2010;6:345–54. doi: 10.1517/17425251003592137. [DOI] [PubMed] [Google Scholar]
- 60.McKeage K. Spotlight on memantine in moderate to severe Alzheimer's disease. Drugs Aging. 2010;27:177–9. doi: 10.2165/11204670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.van Marum RJ. Update on the use of memantine in Alzheimer's disease. Neuropsychiatr Dis Treat. 2009;5:237–47. doi: 10.2147/ndt.s4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Rio-Sancho S, Serna-Jiménez CE, Calatayud-Pascual MA, Balaguer-Fernández C, Femenía-Font A, Merino V, et al. Transdermal absorption of memantin – Effect of chemical enhancers, iontophoresis, and role of enhancer lipophilicity. Eur J Pharm Biopharm. 2012;82:164–70. doi: 10.1016/j.ejpb.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Lee SH, Kim SH, Noh YH, Choi BM, Noh GJ, Park WD, et al. Pharmacokinetics of memantine after a single and multiple dose of oral and patch administration in rats. Basic Clin Pharmacol Toxicol. 2016;118:122–7. doi: 10.1111/bcpt.12479. [DOI] [PubMed] [Google Scholar]
- 64.Brinton RD. Neurosteroids as regenerative agents in the brain: Therapeutic implications. Nat Rev Endocrinol. 2013;9:241–50. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:6498–503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S, Wang JM, Irwin RW, Yao J, Liu L, Brinton RD. Allopregnanolone promotes regeneration and reduces ß-amyloid burden in a preclinical model of Alzheimer's disease. PLoS One. 2011;6:e24293. doi: 10.1371/journal.pone.0024293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–49. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 68.Irwin RW, Solinsky CM, Loya CM, Salituro FG, Rodgers KE, Bauer G, et al. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer's disease. PLoS One. 2015;10:e0128313. doi: 10.1371/journal.pone.0128313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 70.Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–8. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 71.Matsuo K, Okamoto H, Kawai Y, Quan YS, Kamiyama F, Hirobe S, et al. Vaccine efficacy of transcutaneous immunization with amyloid ß using a dissolving microneedle array in a mouse model of Alzheimer's disease. J Neuroimmunol. 2014;266:1–11. doi: 10.1016/j.jneuroim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Wang R, Tang XC. Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer's disease. Neurosignals. 2005;14:71–82. doi: 10.1159/000085387. [DOI] [PubMed] [Google Scholar]
- 73.Jiang H, Luo X, Bai D. Progress in clinical, pharmacological, chemical and structural biological studies of huperzine A: A drug of traditional chinese medicine origin for the treatment of Alzheimer's disease. Curr Med Chem. 2003;10:2231–52. doi: 10.2174/0929867033456747. [DOI] [PubMed] [Google Scholar]
- 74.Ye JC, Zeng S, Zheng GL, Chen GS. Pharmacokinetics of Huperzine A after transdermal and oral administration in beagle dogs. Int J Pharm. 2008;356:187–92. doi: 10.1016/j.ijpharm.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Shi J, Cong W, Wang Y, Liu Q, Luo G. Microemulsion-based patch for transdermal delivery of huperzine A and ligustrazine phosphate in treatment of Alzheimer's disease. Drug Dev Ind Pharm. 2012;38:752–61. doi: 10.3109/03639045.2011.625031. [DOI] [PubMed] [Google Scholar]
- 76.Patel PA, Patil SC, Kalaria DR, Kalia YN, Patravale VB. Comparative in vitro and in vivo evaluation of lipid based nanocarriers of Huperzine A. Int J Pharm. 2013;446:16–23. doi: 10.1016/j.ijpharm.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Khulbe P, Juyal V. Vinpocetine: A step towards memory enhancement. Int J Pharm Res Dev. 2011;2:99–108. [Google Scholar]
- 78.Moghaddam AA, Aqil M, Ahmad FJ, Ali MM, Sultana Y, Ali A. Nanoethosomes mediated transdermal delivery of vinpocetine for management of Alzheimer's disease. Drug Deliv. 2015;22:1018–1026. doi: 10.3109/10717544.2013.846433. [DOI] [PubMed] [Google Scholar]
- 79.Zhao L, Wei MJ, He M, Jin WB, Zhao HS, Yao WF. The effects of tetramethylpyrazine on learning and memory abilities of mice with Alzheimer disease and its possible mechanism. Chin Pharmacol Bull. 2008;24:1088–92. [Google Scholar]
- 80.Fang YP, Tsai YH, Wu PC, Huang YB. Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int J Pharm. 2008;356:144–52. doi: 10.1016/j.ijpharm.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 81.Mishra D, Mishra PK, Dabadghao S, Dubey V, Nahar M, Jain NK. Comparative evaluation of hepatitis B surface antigen-loaded elastic liposomes and ethosomes for human dendritic cell uptake and immune response. Nanomedicine. 2010;6:110–8. doi: 10.1016/j.nano.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 82.Shi J, Wang Y, Luo G. Ligustrazine phosphate ethosomes for treatment of Alzheimer's disease, in vitro and in animal model studies. AAPS PharmSciTech. 2012;13:485–92. doi: 10.1208/s12249-012-9767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]