Abstract
OBJECTIVES:
The objective of this study is to evaluate the beneficial effect of karanjin for the treatment of experimental colitis.
METHODS:
Colitis was induced in the Balb/c mice by rectal administration of 2% solution of 2,4,6-trinitrobenzenesulfonic acid (TNBS) in 50% methanol. Karanjin (>98% pure) was administered in two different concentrations 100 and 200 mg/kg and sulfasalazine (100 mg/kg) as reference for 7 consecutive days to colitic mice. On the 8 day, mice were euthanized and degree of inflammation was assessed by macroscopic, microscopic, histology and biochemical estimation of myeloperoxidase (MPO), nitric oxide (NO), malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and reduced glutathione (GSH) level were measured.
RESULTS:
Karanjin significantly and dose dependently ameliorate the macroscopic damage, histological changes such as cellular infiltration, tissue necrosis, mucosal and submucosal damage as compared to the TNBS control group. Karanjin reduces the activity of MPO, depressed MDA, and NO level and helps in restoring the level of CAT, SOD, and GSH to normal when compared to the TNBS colitis group.
CONCLUSION:
Result of the present study indicates that karanjin has the potential to cure colitis induced by intracolonic administration of TNBS.
Keywords: 2,4,6-trinitrobenzenesulfonic acid induced colitis; furanoflavonoid; inflammatory bowel diseases; karanjin; Pongamia pinnata
Introduction
Inflammatory bowel disease (IBD) is a serious and major complication of colon. Ulcerative colitis (UC) affects the mucosal lining of the rectum and colon while Crohn's disease affects whole intestinal wall and may extend to whole gastrointestinal tract.[1] At present, drugs used for the treatment of IBD have serious side effects and do not completely cure IBD and prevent remission.
Mostly to produce Crohn's disease like conditions in animals, 2,,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis model is employed which include diarrhea, wasting, rectal prolapse and bleeding, histologically produce cellular infiltration, granuloma formation, and loss of normal colon architecture. Immunologically TNBS induces Th-1 response by increasing production of interleukin-12 (IL-12), IL-17, tumor necrosis factor-alpha, and interferon-gamma.[2]
Pongamia pinnata (L.) Pierre (Fabaceae is popularly known as Karanja). Seeds of karanja contains 33%–36% oil, which is known as Karanja oil, and are traditionally used for treating various disease conditions such as rheumatic pain, flatulence, and diarrhea.[3] Leaves reported to have anti-inflammatory.[4] Karanjin has gastroprotective activity by inhibiting H+, K+- ATPase and oxidation.[5] In our last paper, we evaluated the effect of karanjin on dextran sulfate sodium (DSS)-induced colitis which expresses the pathology of UC and found a significant effect in repairing colonic mucosal inflammation.[6]
To the best of our knowledge, the activity of karanjin has never been investigated in the treatment of TNBS induced colitis. Hence, to explore effects of Karanjin in treatment of TNBS-induced colitis and colonic enzyme activities which is imbalanced in intestinal inflammation, the present study was conducted.
Chemicals
TNBS and other chemicals were purchased from Sigma Chemicals, India. Sulfasalazine was provided by Zydus Cadila (Ahmedabad, India). All other chemicals purchased were of analytical grade.
Preparation Of Karanjin
Karanjin was isolated and analyzed according to the method presented by the Praful Patel and Trivedi and then used for the study.[7]
Animals And Grouping
Balb/c mice (25–30 g) were obtained from the animal house facility of the National Institute of Nutrition (Hyderabad, India). Animals were housed at temperature (22–25°C) and 60% ± 10% humidity with 12:12 h light-dark cycles. The animals were fed on standard pellets and water ad-libitum and fasted for 12 h before induction of colitis. The study protocol was approved by the Institutional Animal Ethics Committee (IAEC/09/2013-14) which follows the CPCSEA guidelines.
Groups
Animals were randomly divided into five groups (n = 6) as follows: (1) treated with ethanol enema and given saline only (20 ml/kg/day) (vehicle control); all other groups 2–5 were treated with TNBS enema and given (2) saline (20 ml/kg/day) (TNBS control); (3) Sulfasalazine (100 mg/kg); (4) Karanjin (100 mg/kg); and (5) Karanjin (200 mg/kg) All the drug treatments were given by gastric gavage.
Induction Of Colitis
Colitis was induced in Balb/c mice with TNBS according to a method described by Scheiffele.[2] Briefly, 24 h fasted mice were anesthetized, and then 0.1 ml of 20 mg/ml ethanolic (50%) solution of TNBS was slowly administered into the colon lumen via 3.5F polyethylene catheter up to 4 cm proximal to the anus. To distribute the TNBS within the entire colon and cecum, mice were carefully held in a vertical position for 30 s after the injection. The animals were excluded from the study if excreted TNBS solution. After TNBS instillation, administer test and standard drug for the next 7 days. On the 8th day, mice were euthanized under anesthesia, colon was excised and washed in ice-cold saline for the removal of adherent tissue and used for examination.
Evaluation Parameters
Measurement of body weight, diarrhea, colon length, and macroscopic scoring
Body weight, stool consistency was measured every day. Loss of body weight and percent weight loss was calculated. The fecal output was scored by criteria defined as: (1) formed stool, (2) loose stool, (3) diarrhea, and (4) bloody diarrhea.[8] At the end of the experiment, mice were euthanized and the colon length and weight was measured, colon edema was calculated by weight/length ratio. The spleens were also obtained and weighed.[9] Macroscopic damage score of colon was assessed blindly by the previously reported scoring system.[10]
Histology
Colons were fixed in 10% buffered formalin, paraffin-embedded, sectioned (4 μm thick), and stained with hematoxylin and eosin. All tissue sections were examined with a Trinocular microscope (S-500) fitted with CMOS camera and analyzed with Biowizard 3.5 image analysis software (Japan).
Biochemical analysis
Myeloperoxidase activity
Two hundred milligrams of quickly excised colon was homogenized in 2 ml of potassium phosphate-buffered saline (10% w/v) and centrifuged at 4°C for 20 min at 12,000 rpm. Pellets are resuspended in 2 ml 0.05 M potassium phosphate buffer containing 0.5% (w/v) hexadecyltrimethylammonium bromide (0.1 g/20 ml buffer) homogenate was frozen and thawed 3 times, centrifuged at 15,000 rpm for 15 min at 4°C. The supernatant obtained was used for estimation.[11] Hundred microliters of phosphate buffer containing 0.167 mg/ml O-dianisidine dihydrochloride was added to 100 μl of supernatant and 10 μl 0.05% H2O2. Change in optical density was measured at 460 nm and expressed as the amount of an enzyme necessary for the degradation of 1 μmol H2O2/min/100 mg tissue.[12]
Nitric oxide level
Nitric oxide (NO) level was estimated by the Griess reaction after the reduction of nitrate to nitrite by vanadium trichloride according to the method described by Miranda[13]0.1 ml supernatant of colon tissue homogenate is mixed with 0.1 ml of vanadium (III) chloride. Then, 50 μl of sulfanilamide (2%w/v) solution and 50 μl of NEDD (0.1% w/v) were added respectively, and incubated at 37°C for 30 min and measured at 540 nm against a blank and expressed as nmol/g wet tissue.
Estimation of superoxide dismutase, glutathione, catalase, malondialdehyde
Ten percent colon tissue homogenate was prepared in 0.1 Mol/l, ice-cold phosphate buffer saline (pH 7.4) homogenate was centrifuged at 15,000 rpm for 15 min at 4°C.[14] Supernatant obtained was used for the estimation of superoxide dismutase (SOD),[15] glutathione (GSH),[16] catalase (CAT),[17] and malondialdehyde.[18]
Statistical analysis
Data were expressed as means ± standard error of mean. Statistical analysis was performed using GraphPad prism-4 software. The statistical significance of the data was evaluated by one-way analysis of variance followed by Tukey's method. P < 0.05 was considered statistically significant.
Results
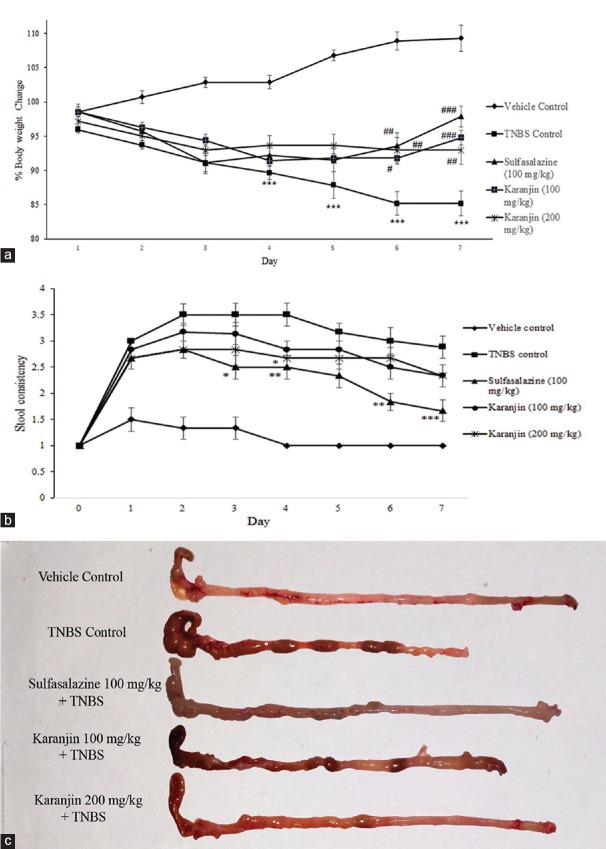
Karanjin attenuated body weight loss occurred by TNBS administration. All experimental animals at 24 h post-TNBS had diarrhea accompanied by piloerection, prostration, and hypomotility. As shown in Figure 1a and b, the body weights of vehicle control group treated with vehicle increasing gradually, however, the TNBS control group was progressively decreased as compared with vehicle controls animals. The body weight in mice treated with Karanjin (100 and 200 mg/kg) and Sulfasalazine significantly recovered at the end of the experiment compared with the TNBS control group. The increase in colonic edema was observed after TNBS instillation as compared to vehicle control (P < 0.001). Sulfasalazine and karanjin 200 mg/kg significantly (P < 0.001) inhibited colonic edema [Table 1]. In the colon of vehicle group, no inflammation was observed, whereas the TNBS control group exhibited significant hyperemia, erosion, and ulceration on 7 days after the induction of colitis.
Figure 1.
(a) Effect of karanjin (100 and 200 mg/kg) on body weight change in TNBS induced colitis. (b) Stool consistency score, (c) representative colon photos from each group. Data were expressed as the mean ± standard error of mean (n= 6). Significance was determined by one-way analysis of variance followed by post hoc Tukey's multiple comparison test. *P< 0.05, **P< 0.01, ***P< 0.001 compared with the vehicle control group; #P< 0.05, ##P< 0.01, ###P< 0.001 compared with 2,4,6-trinitrobenzenesulfonic acid control group
Table 1.
Parameters assessed in 2,4,6-trinitrobenzenesulfonic acid-induced colitis
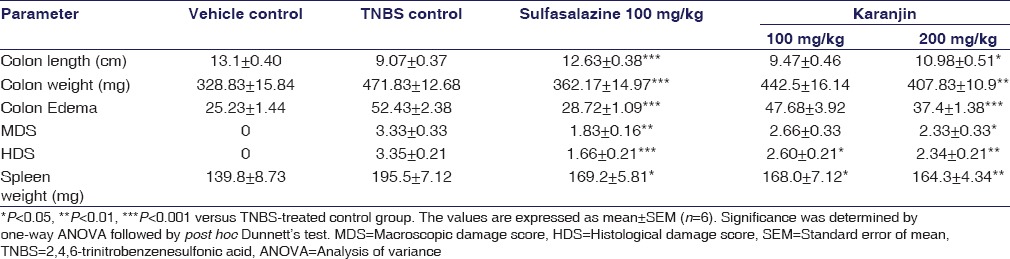
Karanjin treatments reduced the size of necrosis and ulceration; karanjin 200 mg/kg had significantly (P < 0.05) inhibited total macroscopic scores.
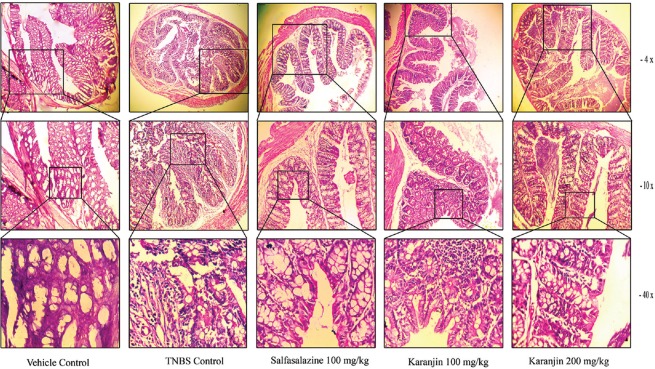
TNBS instillation produces intestinal inflammation. Treatment with sulfasalazine shows intact epithelial cell, mild edema, and necrosis, low dose of Karanjin (100 mg/kg) can restore the necrosis, edema, and inflammation but mild epithelial infiltration observed. High dose of karanjin (200 mg/kg) significantly ameliorated colitis and histological injury and restored the normal architecture [Figure 2]. Myeloperoxidase (MPO) activity [Table 2] was significantly increased in TNBS control as compared to vehicle control, Karanjin 100 mg/kg (P < 0.05) and Sulfasalazine (P < 0.001) produced significant reduction as compared to TNBS control. NO level [Table 2] was found to increase in TNBS control group as compared to vehicle control, administration of karanjin (100 and 200 mg/kg) significantly (P < 0.05 and P < 0.01, respectively) dose-dependently inhibited TNBS-induced NO production in mice. TNBS control group showed a significant (P < 0.01) increase in levels of thiobarbituric acid reactive substances (TBARS) in the colon as compared to vehicle control group [Table 2]. Karanjin 200 mg/kg significantly (P < 0.05) decreased TBARS levels as compared to TNBS group. TNBS instillation leads to a significant (P < 0.01) decrease in the level of CAT (U/mg protein), SOD (U/mg protein), and GSH (μg/g tissue) as compared to the vehicle group [Table 2]. Karanjin dose dependently (100 and 200 mg/kg,) counteracted the deleterious effect of TNBS by significantly increasing the content of these antioxidants.
Figure 2.
Representative section of colonic tissue stained with hematoxylin and eosin. Light micrograph of vehicle control mice shows normal architecture; 2,4,6-trinitrobenzenesulfonic acid-control group show destruction of epithelial architecture with loss of epithelial integrity, edema, and intense cellular infiltration; sulfasalazine shows intact epithelial cell, mild edema, and necrosis; Karanjin (100 mg/kg) + 2,4,6-trinitrobenzenesulfonic acid shows less epithelial infiltration, no edema, and necrosis; Karanjin (200 mg/kg) + 2,4,6-trinitrobenzenesulfonic acid shows marked restoration of normal epithelial structure
Table 2.
Effect of karanjin on myeloperoxidase, nitric oxide, catalase, lipid peroxidation, superoxide dismutase, and reduced glutathione in colonic tissues
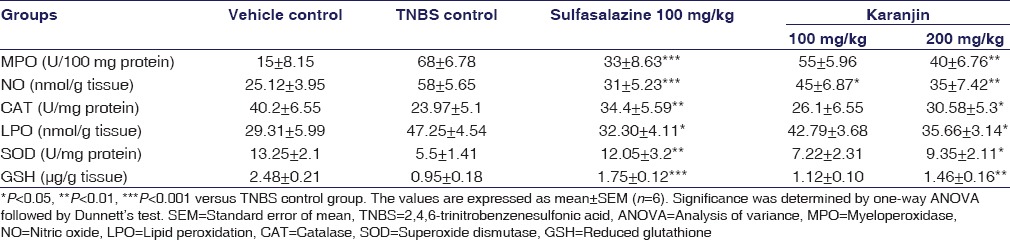
A significant increase in the levels of CAT, GSH and SOD (P < 0.05) was observed with 200 mg/kg karanjin dose. Significantly increased spleen weight was observed in TNBS group as compared to vehicle control group. Karanjin 100 mg/kg and Sulfasalazine decreases spleen enlargement but less significant and Karanjin 200 mg/kg significantly decreased splenic enlargement (P < 0.001) as compared to TNBS control group as shown in Table 1.
Discussion
The present study was particularly focused on the effect of Karanjin on TNBS-induced colitis in mice; this model was widely used to screen potential drugs for the treatment of colitis because of its similarity with the Crohn's disease. Karanjin significantly attenuated shortening of colon, colon edema, and percent body weight loss in a dose-dependent manner. Karanjin (200 and 100 mg/kg) shows a decrease in body weight up to day 3; after day 3 no further loss in body weight was observed; as per data obtained, karanjin is more efficient in attenuating body weight in the early stage of colitis as compared to sulfasalazine. Karanjin treatment decreased stool consistency score and bleeding. This observation implies that karanjin stop leakage of blood into intestinal lumen by decreasing vascular permeability and healing of ulcers.
Activated neutrophils produce reactive oxygen and nitrogen species in the intestinal mucosa and induce inflammatory response which could directly or indirectly cause damage to the intestinal epithelial cells which eventually leads to severe impairment in experimental colitis.[19] Two important enzymes present in the cell to combat with the oxidants are SOD which convert superoxide radical into hydrogen peroxide and CAT which convert hydrogen peroxide into water. The decrease in the cellular level of these enzymes results cellular injury and contributes to the inflammation.[20] Karanjin administration dose-dependently restore the level of CAT and SOD. GSH, the most abundant cytosolic thiol synthesized by GSH peroxidase which provides protection against oxidative damage by reaction with free radicals and form reduced GSH which is nontoxic. Decreased GSH level has been observed in a chronic and acute inflammation[21] Karanjin significantly prevent loss of GSH.
MPO is an enzyme exist in neutrophils, which catalyzes the formation of hypochlorous acid a potent cytotoxic oxidants and its activity, reflects the degree of neutrophil infiltration[22] and reduction of MPO activity can be interpreted as a manifestation of the anticolitic effect. Our result demonstrated that karanjin inhibited the increase in MPO activity in a dose-dependent manner but comparatively less effective than sulfasalazine. NO is a potent inflammatory mediator because of its strong reactivity with superoxide leading to tissue damage by the production of peroxynitrite (ONOO−).[23] Karanjin significantly decreased NO production. The spleen plays an important role in modulation of the immune system by clearance of circulating apoptotic cells, differentiation, and activation of T- and B-cells.[24] Spleen enlarged may be due to increase in the number of apoptotic cells in response to the intestinal inflammation. In this study, we observed a significant decrease in the spleen enlargement in sulfasalazine and karanjin (200 mg/kg) treated groups.
TNBS colitis histologically shows mucosal and submucosal lymphocyte, macrophages, polymorphonuclear leukocyte, mast cell infiltration, destruction of epithelial architecture with loss of epithelial integrity, edema, segmental ulcer, and granuloma.[25] Histological examination of Karanjin (200 mg/kg) significantly ameliorated inflammatory infiltration, edema, necrosis, epithelial destruction, segmental ulceration, and restores the normal architecture [Figure 2]. In our last paper, we evaluated the effect of karanjin on the DSS-induced colitis which is closely related to the UC pathology,[6] in this study, we found that karanjin significantly restored mucosal inflammation. In the present study, we evaluated the effect on transmural inflammation, which is produced by TNBS administration and we found that karanjin is also able to restore transmural inflammation by inhibiting oxidation and inflammatory cell migration in the inflamed colon.
This is the first study which demonstrates the healing effect of karanjin on TNBS induced colitis. There is further need to elaborate and confirm the anticolitic effect of karanjin using other experimental in vivo and in vitro models.
Conclusion
Karanjin isolated from Karanja oil prevented progression and induction of TNBS colitis in mice probably by inhibiting inflammatory cell recruitment and production of oxidant. Thus, these findings suggest that karanjin may be a promising alternative agent for treatment of colitic inflammation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors are thankful to Zydus Cadila, Ahmedabad, for supplying standard drugs and valuable support. We are also thankful to Dr. Chandragauda R. Patil, R. C. Patel College of Pharmacy, Shirpur, for guidance on designing a study.
References
- 1.Sartor RB. Mechanisms of disease: Pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 2.Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002;49(15.19):15.19.1–15.19.14. doi: 10.1002/0471142735.im1519s49. [DOI] [PubMed] [Google Scholar]
- 3.Khare CP. Indian Medicinal Plants. Springer-Verlag Berlin; 2007. Pongamia pinnata; pp. 511–2. [Google Scholar]
- 4.Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Prakash VR. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol. 2001;78:151–7. doi: 10.1016/s0378-8741(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 5.Dharmesh SM, Vismaya BS, Rajashekhar S, Jayaram VB, Kanya S, Thirumakudalu C. Gastroprotective properties of karanjin from karanja (Pongamia pinnata) seeds; role as antioxidant and H +, K + - ATPase inhibitor. Evid Based Complement Altern Med. 2011:1–10. doi: 10.1093/ecam/neq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel PP, Trivedi ND. Karanjin ameliorates DSS induced colitis in C57BL/6 mice. Int J Pharm Sci Res. 2012;6:4866–74. [Google Scholar]
- 7.Patel PP, Trivedi ND. Simple, effective and economic method forisolation and analysis of karanjin and pongamol from Karanja seed oil and screening of antimicrobial potential. Int J Pharm Pharm Sci. 2015;7:248–52. [Google Scholar]
- 8.Motavallian-Naeini A, Andalib S, Rabbani M, Mahzouni P, Afsharipour M, Minaiyan M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res Pharm Sci. 2012;7:159–69. [PMC free article] [PubMed] [Google Scholar]
- 9.Cho EJ, Shin JS, Noh YS, Cho YW, Hong SJ, Park JH, et al. Anti-inflammatory effects of methanol extract of Patrinia scabiosifolia in mice with ulcerative colitis. J Ethnopharmacol. 2011;136:428–35. doi: 10.1016/j.jep.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, et al. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–15. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Queiroz-Junior CM, Pacheco CM, Fonseca AH, Klein A, Caliari MV, de Francischi JN. Myeloperoxidase content is a marker of systemic inflammation in a chronic condition: The example given by the periodontal disease in rats. Mediators Inflamm. 2009;2009:760837. doi: 10.1155/2009/760837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jawhara S, Thuru X, Standaert-Vitse A, Jouault T, Mordon S, Sendid B, Desreumaux P, Poulain D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008;197:972–80. doi: 10.1086/528990. [DOI] [PubMed] [Google Scholar]
- 13.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33:940–5. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 15.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 16.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Schellhorn HE. Rapid kinetic microassay for catalase activity. J Biomol Tech. 2007;18:185–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2002;47:1447–57. doi: 10.1023/a:1015931128583. [DOI] [PubMed] [Google Scholar]
- 20.Seguí J, Gironella M, Sans M, Granell S, Gil F, Gimeno M, et al. Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J Leukoc Biol. 2004;76:537–44. doi: 10.1189/jlb.0304196. [DOI] [PubMed] [Google Scholar]
- 21.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–55. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 23.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 24.Tarantino G, Scalera A, Finelli C. Liver-spleen axis: Intersection between immunity, infections and metabolism. World J Gastroenterol. 2013;19:3534–42. doi: 10.3748/wjg.v19.i23.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coskun ZK, Kerem M, Gurbuz N, Omeroglu S, Pasaoglu H, Demirtas C, et al. The study of biochemical and histopathological effects of spirulina in rats with TNBS-induced colitis. Bratisl Lek Listy. 2011;112:235–43. [PubMed] [Google Scholar]