Abstract
Background
Intraprostatic inflammation has been associated with lower urinary tract symptom (LUTS) progression. However, prior studies used tissue removed for clinical indications, potentially skewing inflammation extent or biasing the association. We, therefore, evaluated inflammation and LUTS incidence and progression in men who underwent biopsy of the prostate peripheral zone irrespective of indication.
Material and Methods
We developed nested case-control sets in men in the placebo arm of the Prostate Cancer Prevention Trial who were free of clinical BPH and had a protocol-directed year 7 biopsy. Cases had baseline IPSS < 15 and year 7 IPSS of 8-14 (low, N = 47), 15-19 (incident moderate, N = 42), or ≥20 (incident high, N = 44). Controls had baseline and year 7 IPSS < 8 (N = 41). For progression from IPSS<8, cases had baseline to year 7 IPSS slope > 75th percentile (N = 46) and controls had a slope < 25th percentile (N = 45). For progression from IPSS = 8-14, cases had a slope > 75th percentile (N = 46) and controls had a slope < 25th percentile (N = 46). We reviewed 3 H&E-stained biopsy cores per man to determine prevalence of ≥1 core with inflammation and mean extent (%) of tissue area with inflammation.
Results
Inflammation prevalence in low cases (64%) was similar to controls (66%), but higher in moderate (69%) and high (73%) cases (P-trend = 0.4). Extent did not differ across LUTS categories (P-trend = 0.5). For progression from IPSS < 8, prevalence (65%, P = 0.9) and extent (2.5%, P = 0.8) in cases did not differ from controls (64%, 2.7%). For progression from IPSS 8-14, prevalence in cases (52%) was lower than in controls (78%, P = 0.009), while extent was higher in cases (5.3%) than controls (3.6%), especially in men with ≥1 core with inflammation (10.1% versus 4.6%, P = 0.06).
Conclusion
Peripheral zone intraprostatic inflammation is not strongly associated with LUTS incidence or progression.
Keywords: lower urinary tract symptoms, inflammation, prostate, incidence, progression
Introduction
Benign prostatic hyperplasia (BPH) typically develops in the prostate transition zone. Studies in which transition zone tissue is removed for symptomatically enlarged prostate have documented the presence of chronic inflammatory infiltrates in and around nodules characteristic of histologic BPH [1-3]. Extent of inflammation in these tissues is associated with worsening of lower urinary tract symptoms (LUTS) in men who are symptomatic and with need for subsequent surgical intervention [4,5]. Whether inflammation elsewhere in the prostate is associated with LUTS incidence or progression in men without LUTS or limited symptoms is unknown. Inflammation elsewhere in the prostate may reflect a man's history of prostate infection, prior prostatic injury, or relative propensity to mount an inflammatory response to infection/insult, including in the region around BPH nodules. We hypothesize that inflammation, irrespective of prostate location, may influence LUTS via pro-inflammatory cytokines and/or reactive species elaborated by immune cells with resultant tissue damage.
We examined whether inflammation in the peripheral zone correlates with LUTS incidence and progression irrespective of transition zone hyperplasia. We used tissue from transrectal ultrasound guided biopsies targeting the peripheral zone, a region that does not develop BPH nodules. We tested this hypothesis in a setting in which the possible bias from any link between intraprostatic inflammation and reason for biopsy is minimized: men in the Prostate Cancer Prevention Trial (PCPT) placebo arm who underwent end-of-study biopsies per trial protocol, not based on PSA concentration or digital-rectal examination (DRE) status [6].
Materials and Methods
Study design and population
We developed case-control sets for LUTS incidence and progression nested in the PCPT [6] placebo arm. Trial inclusion criteria were: ≥55 years old, normal DRE, PSA ≤ 3 ng/mL, and International Prostate Symptom Score (IPSS) < 20. Exclusion criteria were transurethral resection of the prostate (TURP) in the past 6 months, finasteride use, or intention to seek BPH treatment. At trial start, demographics, lifestyle, and medical factors were collected by questionnaire. Weight and height were measured, from which body mass index (BMI; kg/m2) was calculated. IPSS and reported LUTS treatments were determined 3 months before randomization, at time of randomization, and at each annual visit. Participants were screened for prostate cancer at each annual visit and a biopsy was recommend if PSA > 4 ng/mL or DRE was abnormal. Men not diagnosed with prostate cancer during the trial were requested to undergo biopsy at the end of the trial 7 years post randomization irrespective of PSA concentration or DRE status. The Institutional Review Boards (IRB) at the participating trial sites approved the PCPT. This LUTS study was approved by the Johns Hopkins Bloomberg School of Public Health and the Colorado Multiple IRBs.
LUTS cases and controls
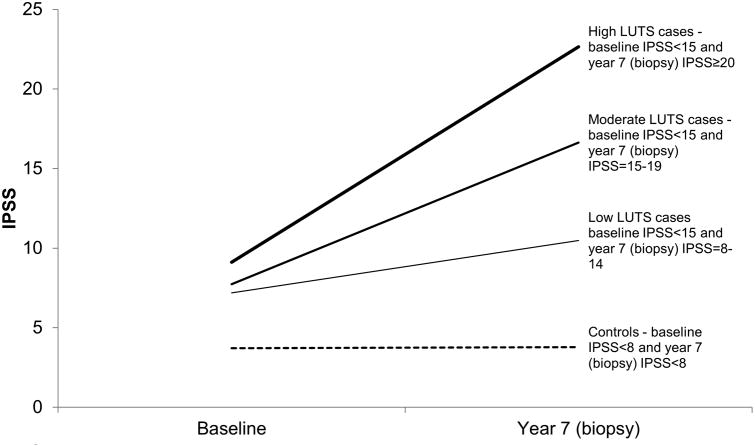
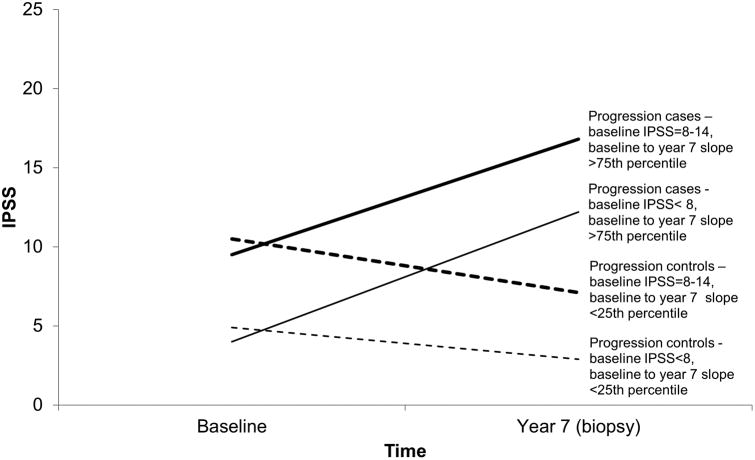
We used the IPSS [7] to ascertain cases and controls. We identified men who at baseline (mean of 3 months before and at randomization) had IPSS < 15, never had BPH surgery, were not taking BPH/LUTS medications, and did not report a physician diagnosis of BPH/LUTS. Men who received BPH/LUTS treatment during the trialor who did not have an end-of-study biopsy were excluded. Men who had prostate cancer detected on the end-of-study biopsy were not excluded to avoid selection bias. From the remaining men, we developed nested case-control sets for incidence (Figure 1) and progression (Figure 2). For incidence, all cases had a baseline IPSS < 15. We sampled as incident cases, men with a year 7 IPSS of 15-19 (moderate) and ≥20 (high). We also sampled men with a year 7 IPSS = 8-14 (low); this case group includes men whose IPSS increased from <8 to 8-14 (incident, N = 25) and stayed within 8-14 (prevalent, N = 22). Controls for low, moderate, and high cases, had an IPSS < 8 (no/very low LUTS) at baseline and year 7. For progression from IPSS < 8 at baseline, cases had baseline to year 7 IPSS slope > 75th percentile and controls had baseline to year 7 slope < 25th percentile. For progression from IPSS = 8-14 at baseline, cases had baseline to year 7 slope > 75th percentile and controls had baseline to year 7 slope < 25th percentile. Slopes were calculated based on all eligible men, including those in the finasteride arm (to be reported on separately). 50 men per case or control group were sampled. Controls were frequency matched to cases on age and race.
Figure 1. Definitions of Incident LUTS Cases and Controls, Placebo Arm, PCPT.
Note that of the men with low LUTS, 25 (53%) of them had a baseline IPSS < 8.
Figure 2. Definitions of Progression LUTS Cases and Controls, Placebo Arm, PCPT.
Inflammation in prostate biopsies
For the 400 cases and controls, we requested H&E-stained slides for 3 randomly selected biopsy cores (of 6-10 taken) from the PCPT repository. Most cores were of the apex or mid-region (instructions were for biopsies to be directed laterally to ensure maximal peripheral zone sampling). Tissue was available for 357 (89.3%).
H&E-stained slides were digitally imaged using the Aperio ScanScope slide scanner (Aperio, Vista, CA). Images were uploaded into the Aperio Spectrum Digital Pathology Information Management System and reviewed [8] online using the Aperio ImageScope Viewer Software package. When cancer was present, only benign areas were reviewed. Presence of any inflammatory cells was recorded. Percentage of total biopsy core area with inflammatory cell involvement was visually estimated. Two pathologists (IK, BG-O) blinded to case-control status reviewed the images.
Other factors
From the baseline questionnaire, we selected factors positively (obesity [9], inactivity [10-12], smoking [11-13], energy [14], polyunsaturated fatty acids [14]) or inversely (vegetables [15], alcohol [11,13]) associated with BPH/LUTS. General lifestyle indicators, including intake of total fat, total protein, and red meat were also selected. Finally, aspirin use, hypothesized to influence inflammation, and history of diabetes, a consequence of which (polyuria) may masquerade as LUTS, were selected.
Statistical analysis
We calculated means and/or prevalences of lifestyle and medical characteristics for cases and controls. For each man, 3 measures of inflammation were evaluated across his 3 biopsy cores: 1) having at least one biopsy core with inflammation (no/yes), 2) percentage of biopsy cores with inflammation, and 3) mean percentage of tissue area with inflammation. For each case or control group, prevalence or mean of the 3 inflammation measures was calculated. We determined whether percentage of tissue with inflammation was correlated with dietary and lifestyle factors (continuous variables) using the Spearman correlation coefficient and whether this percentage differed by anthropometric and other lifestyle factors (proportions) using the Wilcoxon rank sum test.
We tested for trends in inflammation prevalences or means across controls and 3 incidence case groups using logistic or linear regression, respectively. Differences in inflammation prevalences or means between the two progression case groups and their controls were tested using the chi-square test (or Fisher's exact test when expected counts < 5) or t-test, respectively. Multivariable adjustment was performed for factors appearing to differ across cases and controls and/or were associated with inflammation in controls. Analyses were performed using SAS release 9.3 (SAS Institute, Cary, NC).Tests were 2-sided with P < 0.05 considered to be statistically significant.
Results
LUTS incidence
Age, race, and education did not differ among controls (N = 41) and cases with low (N = 47), moderate (N = 42), or high (N = 44) LUTS at year 7 (Table 1). PSA concentration measured immediately prior to biopsy did not differ among cases and controls (P-trend = 0.5). Diabetes history (P-trend = 0.02), intake of energy (P-trend = 0.02), total fat (P-trend = 0.03), polyunsaturated fat (P-trend = 0.07), and total protein (P-trend = 0.02) increased across control and case groups, whereas smoking status, pack-years, BMI, waist circumference, physical activity, aspirin use, and intake of vegetables, and red meat did not change across case and control groups. While not statistically significant, alcohol intake decreased across groups.
Table 1. Characteristics* of Incident LUTS** Cases and Controls, Placebo Arm, PCPT.
| Controls | Cases | P-Trend | |||
|---|---|---|---|---|---|
|
| |||||
| No or very low LUTS | Low LUTS | Moderate LUTS | High LUTS | ||
| N | 41 | 47 | 42 | 44 | |
| Age at end of study biopsy in years (mean) | 69.3 | 69.4 | 70.9 | 70.3 | *** |
| White (%) | 95.1 | 95.7 | 95.2 | 97.7 | *** |
| College education or higher (%) | 63.4 | 53.2 | 50.0 | 54.6 | 0.4 |
| BMI in kg/m2(mean) | 27.0 | 27.2 | 27.6 | 27.6 | 0.9 |
| Waist circumference in cm (mean) | 100.1 | 99.9 | 102.6 | 100.9 | 0.7 |
| Moderate or higher physical activity (%) | 68.3 | 57.4 | 64.3 | 56.8 | 0.4 |
| Ever smoked cigarettes (%) | 68.3 | 70.2 | 52.4 | 79.6 | 0.6 |
| Pack years among those who ever smoked (mean) | 21.3 | 24.3 | 25.9 | 26.4 | 0.6 |
| History of diabetes (%) | 2.4 | 2.1 | 9.5 | 13.6 | 0.02 |
| Aspirin use (%) | 53.7 | 46.8 | 52.4 | 47.7 | 0.7 |
| Serum PSA just prior to end of study biopsy in ng/mL (mean) | 1.9 | 3.5 | 2.00 | 2.00 | 0.5 |
| Prostate cancer diagnosis on end of study biopsy (%) | 17.1 | 10.6 | 14.3 | 11.4 | 0.6 |
| Daily intake (mean) | |||||
| Energy in kcal | 1,984 | 2,042 | 2,429 | 2,422 | 0.02 |
| Vegetables in servings, 5-A-Day Method | 2.2 | 2.0 | 2.3 | 2.1 | 0.7 |
| Total fat in g | 70.2 | 78.6 | 90.8 | 96.5 | 0.03 |
| Polyunsaturated fatty acids in g | 14.9 | 16.0 | 18.6 | 19.6 | 0.07 |
| Total protein in g | 82.1 | 84.3 | 100.5 | 104.4 | 0.02 |
| Red meat in servings | 4.1 | 4.4 | 4.8 | 5.1 | 0.6 |
| Alcoholic beverages in number of drinks | 1.3 | 1.1 | 1.0 | 0.6 | 0.2 |
| IPSS (mean) | |||||
| Baseline | 3.7 | 7.2 | 7.7 | 9.1 | <0.0001 |
| Year 1 | 4.6 | 7.6 | 10.1 | 11.7 | <0.0001 |
| Year 2 | 4.4 | 8.5 | 11.6 | 12.7 | <0.0001 |
| Year 3 | 5.4 | 9.1 | 13.1 | 12.7 | <0.0001 |
| Year 4 | 4.2 | 9.5 | 12.9 | 14.0 | <0.0001 |
| Year 5 | 4.3 | 9.0 | 13.2 | 15.8 | <0.0001 |
| Year 6 | 4.2 | 9.6 | 13.1 | 18.9 | <0.0001 |
| Year 7 | 3.8 | 10.5 | 16.6 | 22.7 | <0.0001 |
| Mean change in IPSS from baseline to year 7 | 0.07 | 3.3 | 8.9 | 13.6 | <0.0001 |
At baseline unless otherwise specified.
Controls had IPSS < 8 at baseline and year 7. Cases had an IPSS < 15 at baseline and IPSS at year 7 of 8-14 (low), 15-19 (moderate), and ≥20 (high).Of the men with low LUTS, 25 (53%) of them had a baseline IPSS < 8.
Frequency matched.
In controls (IPSS < 8), percentage of tissue area with inflammation was correlated with PSA measured just prior to biopsy (Spearman correlation coefficient=0.19, P = 0.007). Percentage of tissue area with inflammation differed by diabetes status (yes [N = 13]: 0% [interquartile range: 0.0-0.5%]; no [N = 185]: 2.5% [0.0-5.0]; P = 0.03). Percentage of tissue area with inflammation was not correlated with BMI, waist circumference, packyears, intake of energy, total fat, polyunsaturated fatty acids, total protein, vegetables, red meat, and alcohol (all Spearman < ±0.10, P > 0.15), and did not differ by race, education, physical activity, ever smoking, or aspirin use (all P > 0.5) in controls (data not shown).
Prevalence of at least one biopsy core with inflammation was similar in low LUTS cases and controls, but higher in moderate and high LUTS cases (Table 2). However, the increase in prevalence across these groups was not statistically significant. Results were similar after multivariable adjustment (controls 66%, LUTS cases –low 63%, moderate 70%, and high 72%; P-trend = 0.4).Percentage of biopsy cores with inflammation was about a third in each group. Percentage of tissue area with inflammation did not change across groups, including when restricting to men who had at least one core with inflammation.
Table 2. Prevalence and Extent of Intraprostatic Inflammation* in Incident LUTS** Cases and Controls, Placebo Arm, PCPT.
| Controls | Cases | P-Trend | |||
|---|---|---|---|---|---|
|
| |||||
| No or very low LUTS | Low LUTS | Moderate LUTS | High LUTS | ||
| All men | 41 | 47 | 42 | 44 | |
| At least one biopsy core with inflammation*** | 66 | 64 | 69 | 73 | 0.4 |
| Mean of the percentage of biopsy cores with inflammation | 31 | 32 | 35 | 32 | 1.0 |
| Mean of the mean of percentage of tissue area with inflammation | |||||
| Overall | 2.2 | 3.7 | 3.8 | 2.5 | 0.5 |
| In men with at least one biopsy core with inflammation | 3.3 | 5.8 | 5.5 | 3.4 | 0.4 |
| In men without prostate cancer | 34 | 42 | 36 | 39 | |
| At least one biopsy core with inflammation | 65 | 67 | 67 | 77 | 0.3 |
| Mean of the percentage of biopsy cores with inflammation | 27 | 34 | 35 | 35 | 0.7 |
| Mean of the mean of percentage of tissue area with inflammation | |||||
| Overall | 1.6 | 4.0 | 3.9 | 2.7 | 0.4 |
| In men with at least one biopsy core with inflammation | 2.5 | 6.0 | 5.8 | 3.5 | 0.3 |
| In men without diabetes | 40 | 46 | 38 | 38 | |
| At least one biopsy core with inflammation | 65 | 65 | 68 | 74 | 0.4 |
| Mean of the percentage of biopsy cores with inflammation | 32 | 32 | 35 | 32 | 0.9 |
| Mean of the mean of percentage of tissue area with inflammation | |||||
| Overall | 2.2 | 3.8 | 3.8 | 2.4 | 0.6 |
| In men with at least one biopsy core with inflammation | 3.4 | 5.8 | 5.5 | 3.3 | 0.4 |
Assessed in 3 cores of 6-10 obtained largely from the peripheral zone from transrectal ultrasound guided biopsies.
Controls had IPSS < 8 at baseline and year 7. Cases had an IPSS < 15 at baseline and IPSS at year 7 of 8-14 (low), 15-19 (moderate), and ≥20 (high).Of the men with low LUTS, 25 (53%) of them had a baseline IPSS < 8.
After multivariable adjustment for age; race; diabetes history; daily intake (continuous) of energy, polyunsaturated fat, total fat, total protein, red meat; number of alcoholic beverages per day(continuous); aspirin use: controls 66%, low LUTS 63%, moderate LUTS 70%, and high LUTS72%; P-trend = 0.4.
All patterns were similar to overall in younger (≤62 years; N = 270) and older (>62 years; N = 264) men, although older cases and controls had higher measures of inflammation than younger cases and controls (data not shown). Results were similar excluding 13.2% of men with a prostate cancer diagnosis or 6.9% of men with a diabetes history (Table 2).Results were similar excluding the 11.5% of men with a prior negative biopsy during the trial, although prevalence and extent of inflammation were generally slightly lower than overall (Supplemental Table 1).
LUTS progression
Age, race, and education did not differ between cases with progression from no/very low LUTS (N = 46) and their controls (N = 45), or cases with progression from low LUTS (N-46) and their controls (N = 46) (Table 3). PSA just prior to biopsy did not differ between cases and controls in either progression group. BMI (P = 0.07) and waist circumference (P = 0.02) were higher in cases with progression from no/low LUTS than their controls. No difference was observed between cases with progression from low LUTS and their controls (Table 3). Differences between cases and controls for other dietary and lifestyle were not statistically significant, and when possible patterns of difference were observed, they were not the same in the two progression groups. In general, risk factors were more common in cases with progression from low LUTS relative to their controls, whereas the opposite was observed for cases with progression from no/very low LUTS relative to their controls.
Table 3. Characteristics* of Cases with Progression from No/Very Low or Low LUTS and Controls, Placebo Arm, PCPT.
| Progression from no/very low LUTS** | Progression from low LUTS*** | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Controls | Cases | P | Controls | Cases | P | |
| N | 45 | 46 | 46 | 46 | ||
| Age at end of study biopsy in years (mean) | 69.4 | 69.6 | **** | 70.1 | 69.8 | **** |
| White (%) | 95.6 | 95.7 | **** | 95.7 | 95.7 | **** |
| College education or higher (%) | 44.4 | 47.8 | 0.7 | 50 | 58.7 | 0.4 |
| BMI in kg/m2 (mean) | 26.8 | 28.0 | 0.07 | 28.2 | 27.6 | 0.5 |
| Waist circumference in cm (mean) | 96.7 | 102.5 | 0.02 | 103.0 | 100.7 | 0.3 |
| Moderate or higher physical activity (%) | 26.8 | 28.0 | 0.07 | 28.2 | 27.6 | 0.5 |
| Ever smoked cigarettes (%) | 53.3 | 54.4 | 0.9 | 82.6 | 71.7 | 0.2 |
| Pack years among those who ever smoked (mean) | 20.7 | 23.0 | 0.6 | 22.9 | 27.1 | 0.3 |
| History of diabetes (%) | 8.9 | 0 | 0.06 | 4.4 | 4.4 | 1.0 |
| Aspirin use (%) | 42.2 | 26.1 | 0.1 | 50.0 | 30.4 | 0.05 |
| Serum PSA just prior to the end of study biopsy in ng/mL (mean) | 1.7 | 1.8 | 0.7 | 1.8 | 2.0 | 0.5 |
| Prostate cancer diagnosis on end of study biopsy (%) | 20.0 | 21.7 | 0.8 | 23.9 | 19.6 | 0.6 |
| Daily intake (mean) | ||||||
| Energy in kcal | 2,106 | 1,897 | 0.2 | 2,119 | 2,449 | 0.09 |
| Vegetables in servings, 5-A-Day Method | 2.3 | 2.1 | 0.6 | 2.2 | 2.7 | 0.2 |
| Total fat in g | 75.2 | 67.1 | 0.2 | 76.1 | 90.3 | 0.1 |
| Polyunsaturated fatty acids in g | 16.0 | 13.4 | 0.09 | 15.8 | 18.7 | 0.1 |
| Total protein in g | 90.5 | 80.0 | 0.1 | 88.6 | 104.4 | 0.07 |
| Red meat in servings | 4.5 | 3.6 | 0.09 | 3.8 | 5.1 | 0.05 |
| Alcoholic beverages in number of drinks | 1.0 | 0.7 | 0.4 | 0.7 | 1.0 | 0.3 |
| IPSS (mean) | ||||||
| Baseline | 4.9 | 4.0 | 0.03 | 10.5 | 9.5 | 0.003 |
| Year 1 | 3.9 | 4.9 | 0.1 | 8.9 | 10.9 | 0.01 |
| Year 2 | 4.3 | 6.8 | 0.002 | 8.2 | 12.2 | <0.0001 |
| Year 3 | 4.3 | 7.6 | 0.0001 | 9.4 | 11.9 | 0.005 |
| Year 4 | 4.2 | 8.2 | <0.0001 | 8.7 | 13 | <0.0001 |
| Year 5 | 5.1 | 8.6 | <0.0001 | 8.5 | 13.3 | <0.0001 |
| Year 6 | 3.9 | 9.5 | <0.0001 | 8.6 | 15.1 | <0.0001 |
| Year 7 | 2.9 | 12.2 | <0.0001 | 7.1 | 16.8 | <0.0001 |
| Mean change in IPSS from baseline to year 7 | -2.0 | 8.3 | <0.0001 | -3.4 | 7.3 | <0.0001 |
Baseline unless otherwise specified.
Controls had IPSS < 8 at baseline and baseline to year 7 slope < 25th percentile. Cases had IPSS < 8 at baseline and baseline to year 7 slope >75th percentile.
Controls had IPSS = 8-14 at baseline and baseline to year 7 slope < 25th percentile. Cases had IPSS = 8-14 at baseline and baseline to year 7 slope >75th percentile.
Frequency matched.
Prevalence of at least one biopsy core with inflammation did not differ between cases with progression from no/very low LUTS and their controls, whereas prevalence was lower (P = 0.009) in cases with progression from low LUTS than their controls (Table 4). Results were similar after multivariable adjustment (progression from no/very low to higher LUTS: controls 64%, cases 66%; P = 0.9; progression from low to higher LUTS: controls 78%, cases 53%, P = 0.01). Percentage of biopsy cores with inflammation was about a third in each group. Percentage of tissue area with inflammation did not differ between cases with progression from no/low LUTS and their controls, including when restricting to men who had at least one core with inflammation. Percentage of tissue area with inflammation was higher in cases with progression from low LUTS than their controls, especially when restricting to men with at least one biopsy core with inflammation (cases 10.1%, controls 4.6%, P = 0.06; Table 4). Results were similar excluding 17.6% and 21.7%of men with a diagnosis of prostate cancer or 1.1% and 7.6% of men with a diabetes history from the progression groups that at baseline had no/very low or low LUTS, respectively (Table 4). Results were also similar excluding 11.0% and 20.7% of men with a prior negative biopsy from the progression groups that at baseline had no/very low or low LUTS, respectively (Supplemental Table 2).
Table 4. Prevalence and Extent of Intraprostatic Inflammation* in Cases with Progression from No/Very Low or Low LUTS and Controls, Placebo Arm, PCPT.
| Progression from no/very low LUTS** | Progression from low LUTS*** | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Controls | Cases | P | Controls | Cases | P | |
| All men | 45 | 46 | 46 | 46 | ||
| At least one biopsy core with inflammation**** | 64 | 65 | 0.9 | 78 | 52 | 0.009 |
| Mean of the percentage of biopsy cores with inflammation | 32 | 30 | 0.8 | 37 | 33 | 0.6 |
| Mean of the mean of percentage of tissue area with inflammation | ||||||
| Overall | 2.7 | 2.5 | 0.8 | 3.6 | 5.3 | 0.3 |
| In men with at least one biopsy core with inflammation | 4.1 | 3.9 | 0.8 | 4.6 | 10.1 | 0.06 |
| In men without prostate cancer | ||||||
| At least one biopsy core with inflammation | 61 | 67 | 0.6 | 77 | 54 | 0.04 |
| Mean of the percentage of biopsy cores with inflammation | 29 | 30 | 0.9 | 35 | 35 | 0.9 |
| Mean of the mean of percentage of tissue area with inflammation | ||||||
| Overall | 2.4 | 2.3 | 0.9 | 2.9 | 5.8 | 0.2 |
| In men with at least one biopsy core with inflammation | 3.9 | 3.5 | 0.7 | 3.8 | 10.7 | 0.05 |
| In men without diabetes | ||||||
| At least one biopsy core with inflammation | 68 | 65 | 0.8 | 80 | 55 | 0.01 |
| Mean of the percentage of biopsy cores with inflammation | 34 | 30 | 0.5 | 39 | 35 | 0.6 |
| Mean of the mean of percentage of tissue area with inflammation | ||||||
| Overall | 2.9 | 2.5 | 0.6 | 3.7 | 5.5 | 0.3 |
| In men with at least one biopsy core with inflammation | 4.3 | 3.9 | 0.6 | 4.6 | 10.1 | 0.06 |
Assessed in 3 cores of 6-10 obtained largely from the peripheral zone from transrectal ultrasound guided biopsies.
Controls had IPSS < 8 at baseline and baseline to biopsy slope < 25th percentile. Cases had IPSS < 8 at baseline and baseline to biopsy slope >75th percentile.
Controls had IPSS = 8-14 at baseline and baseline to biopsy slope < 25th percentile. Cases had IPSS = 8-14 at baseline and baseline to biopsy slope >75th percentile.
After multivariable adjustment for age; race; diabetes history; daily intake (continuous) of energy, polyunsaturated fat, total fat, total protein, red meat; number of alcoholic beverages per day (continuous); aspirin use: progression from no/very low to higher LUTS: controls 64%, cases 66%; P = 0.9; progression from low to higher LUTS: controls 78%, cases 53%, P = 0.01).
Discussion
In these nested case-control analyses in the PCPT placebo arm, we tested whether prevalence and extent of intraprostatic inflammatory infiltrates from non-BPH tissue obtained by transrectal ultrasound guided biopsies in the peripheral zone were associated with LUTS incidence and progression. We hypothesized that prevalence and extent of inflammation would be greater in men with incident LUTS and with a steeper slope of progression of LUTS over time. However, we did not observe a strong association between inflammation in these prostate biopsy tissues and LUTS incidence or progression. Given that a prospective study of transition zone tissue showed an association between inflammation and progression in men with established symptomatic BPH [5], we conclude that inflammation in the peripheral zone, while potentially linked to cancer [8], is not strongly associated with LUTS.
Several groups have reviewed the literature on inflammation and BPH/LUTS [16-19]. Studies suggest that men with inflamed BPH have larger prostates than men with non-inflamed BPH [20], and that men who undergo TURP for acute urinary retention have a greater extent of prostate inflammation than men who undergo TURP for LUTS [21]. Thus, men with worse inflammation are more likely to develop the worst consequences of BPH. In REDUCE, chronic inflammation measured in baseline biopsies was weakly correlated with mean (r=0.057) and maximum (r=0.036) IPSS (P < 0.001). The authors noted that participants were enriched with BPH due to trial entry criteria of an elevated PSA and a biopsy negative for prostate cancer in the past 6 months [22], which may explain why the correlation was not stronger [23]. Despite these positive associations, most studies were conducted cross-sectionally; therefore, it is unknown whether inflammation extent was a cause or consequence of BPH. An additional study reported that the magnitude of IPSS decline following prostatectomy for prostate cancer increased with increasing periurethral inflammation [24]. Taken together, that study and the others mentioned above support a potential role of inflammation in the etiology of LUTS and suggest that periurethral, and/or transition zone inflammation may be of primary importance.
Only one study, conducted in the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, investigated the link between inflammation in transition zone biopsies and BPH/LUTS prospectively. In that study, the outcome was progression from established symptomatic BPH; participants had a median symptom score of 16 [5]. Using immune cell markers (CD45, CD4, CD8, and CD68) as the measure of inflammation, investigators observed that men with moderate or severe inflammation in transition zone biopsies had a higher risk of progression, especially acute urinary retention or incontinence, over a median of 4.8 years. A strength of their study is clear temporality: inflammation preceded BPH progression [25]. In our study, intraprostatic inflammation in peripheral zone biopsies was not strongly associated with LUTS incidence and progression from no/limited LUTS. However, in PCPT we could not study progression of moderate LUTS to a higher IPSS or other BPH-associated outcomes (trial enrollment criteria precluded this) and could not evaluate transition zone inflammation (trial biopsies targeted the peripheral zone).
We conducted this work in the PCPT, an exceptional resource for studies on inflammation and LUTS, because the men were not enriched for prevalent BPH at trial entry, completed the IPSS annually, had a PSA test and DRE annually, and underwent biopsy 7 years after randomization irrespective of indication. We restricted to men without clinical BPH at baseline, thus, we studied incident moderate and high LUTS rather than prevalent. To our knowledge, ours is the first study examining inflammation in relation to LUTS early in its natural history and irrespective of the usual reasons why tissue is available – TURP, biopsy for elevated PSA, prostatectomy for prostate cancer – conditions that may be driven by underlying inflammation. We used a standardized method for determining prevalence and extent of inflammation[8].
This study has some points for discussion. First, while the moderate and high LUTS cases were incident, we assessed inflammation in biopsies from the end of the trial. Baseline biopsies were not performed. Thus, we cannot determine whether the weak, non-statistically significant associations that we observed are causal, and if causal, whether we underestimated the association because we did not measure inflammation at the most etiologically relevant time point. Second, we visually assessed inflammation based on cell morphology rather than by image analysis, and for feasibility, we evaluated inflammation in only 3 of the 6-10 biopsy cores obtained per man. Nevertheless, men who tend to have more inflammation would, on average, be more likely to have a greater extent of inflammation in a given biopsy core selected than men with less inflammation. Further, any inaccuracy in quantifying extent of inflammation should be similar between cases and controls because the pathologists were blinded. While in theory these two possible sources of imperfect measurement could explain our weak associations, we used the same method to evaluate the association between inflammation and prostate cancer and observed moderate strength associations [8]. Further, we showed that PSA measured before but close in time to the biopsies was positively correlated with percentage of tissue area with inflammation in controls, as expected [26]. Third, unlike prostate cancer, it is possible that inflammation generally is not related to LUTS, but that an over or under abundance of specific immune cells contributes to LUTS. Fourth, the prostate biopsies we used were taken primarily from the peripheral zone, rather than the transition zone (which is closer in proximity and anatomically related to the periurethral region), where BPH nodules tend to occur. We hypothesized that cytokines and consequent damage and morphologic changes, irrespective of prostate location, could contribute to LUTS. That we observed only weak, non-statistically significant associations may indicate that inflammation in general, or that inflammation in the peripheral zone specifically, does not notably influence LUTS risk. Nevertheless, our study is perhaps the best that could be performed; it is unlikely that a large prospective study in which the transition zone/periurethral region is biopsied in healthy men followed for the development of LUTS could ever be performed.
We do not know whether the PCPT eligibility criteria restricted inflammation present in the biopsies, which would reduce the ability to detect associations. While we are uncertain to what extent a prior biopsy may elicit inflammation and regeneration that may have persisted, we did not exclude men who had a negative biopsy during the trial. The final sample size was smaller than planned because not all tissue was available, reducing precision and power to detect modest differences, and precluding obstructive and irritative symptom subanalyses.
Conclusions
In summary, we observed weak, non-statistically significant evidence that inflammation in peripheral zone prostate biopsy tissue is associated with LUTS incidence and progression. Given that MTOPS observed an association between transition zone inflammation and worsening of BPH/LUTS [5], our results support the hypothesis that inflammation in the prostate per se may not be associated with LUTS; rather to influence LUTS, inflammation may need to be present in close proximity to the urethra and transition zone.
Supplementary Material
Acknowledgments
Grant support: This work was funded by the National Institute of Diabetes and Digestive, and Kidney Diseases (P50 DK082998, WB Isaacs) and the National Cancer Institute U10 CA37429 (CD Blanke), UM1 CA182883 (IM Thompson/CM Tangen), and P50 CA58236 (WG Nelson)].
Abbreviations
- BMI
Body mass index
- BPH
Benign prostatic hyperplasia
- DRE
Digital-rectal examination
- IPSS
International Prostate Symptom Score
- IRB
Institutional Review Board
- LUTS
Lower urinary tract symptoms
- MTOPS
Medical Therapy of Prostatic Symptoms
- PCPT
Prostate Cancer Prevention Trial
- PSA
Prostate specific antigen
- TURP
Transurethral resection of the prostate
Footnotes
A SWOG-Coordinated Study S9217
Disclosure of potential conflicts of interest: The authors declare that they have no other competing financial interests related to this paper.
Note: The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Steiner GE, Djavan B, Kramer G, Handisurya A, Newman M, Lee C, Marberger M. The picture of the prostatic lymphokine network is becoming increasingly complex. Reviews in urology. 2002;4:171–177. [PMC free article] [PubMed] [Google Scholar]
- 2.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 3.Morote J, Lopez M, Encabo G, de Torres IM. Effect of inflammation and benign prostatic enlargement on total and percent free serum prostatic specific antigen. Eur Urol. 2000;37:537–540. doi: 10.1159/000020190. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Yang JR, Yang LY, Liu ZT. Chronic inflammation in benign prostatic hyperplasia: Implications for therapy. Med Hypotheses. 2008;70:1021–1023. doi: 10.1016/j.mehy.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Torkko KC, Wilson RS, Smith EE, Kusek JW, van Bokhoven A, Lucia MS. Prostate Biopsy Markers of Inflammation are Associated with Risk of Clinical Progression of Benign Prostatic Hyperplasia: Findings from the MTOPS Study. The Journal of urology. 2015;194:454–461. doi: 10.1016/j.juro.2015.03.103. [DOI] [PubMed] [Google Scholar]
- 6.Thompson I, Goodman P, Tangen C, Lucia M, Miller G, Ford L, Lieber M, Cespedes R, Atkins J, Lippman S, Carlin S, Ryan A, Szczepanek C, Crowley J, Coltman C. The influence of finasteride on the development of prostate cancer. The New England journal of medicine. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 7.Barry MJ, Fowler FJ, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett ATK The Measurement Committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. The Journal of urology. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 8.Gurel B, Lucia MS, Thompson IM, Jr, Goodman PJ, Tangen CM, Kristal AR, Parnes HL, Hoque A, Lippman SM, Sutcliffe S, Peskoe SB, Drake CG, Nelson WG, De Marzo AM, Platz EA. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23:847–856. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondul AM, Giovannucci E, Platz EA. A prospective study of obesity, and the incidence and progression of lower urinary tract symptoms. The Journal of urology. 2014;191:715–721. doi: 10.1016/j.juro.2013.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platz EA, Kawachi I, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, Giovannucci E. Physical activity and benign prostatic hyperplasia. Arch Intern Med. 1998;158:2349–2356. doi: 10.1001/archinte.158.21.2349. [DOI] [PubMed] [Google Scholar]
- 11.Rohrmann S, Crespo CJ, Weber JR, Smit E, Giovannucci E, Platz EA. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: findings from the third National Health And Nutrition Examination Survey. BJU Int. 2005;96:77–82. doi: 10.1111/j.1464-410X.2005.05571.x. [DOI] [PubMed] [Google Scholar]
- 12.Rohrmann S, Fallin MD, Page WF, Reed T, Partin AW, Walsh PC, Platz EA. Concordance rates and modifiable risk factors for lower urinary tract symptoms in twins. Epidemiology. 2006;17:419–427. doi: 10.1097/01.ede.0000219723.14476.28. [DOI] [PubMed] [Google Scholar]
- 13.Platz EA, Rimm EB, Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Giovannucci E. Alcohol consumption, cigarette smoking, and risk of benign prostatic hyperplasia. Am J Epidemiol. 1999;149:106–115. doi: 10.1093/oxfordjournals.aje.a009775. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Platz EA, Kawachi I, Willett WC, Giovannucci E. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr. 2002;75:689–697. doi: 10.1093/ajcn/75.4.689. [DOI] [PubMed] [Google Scholar]
- 15.Rohrmann S, Giovannucci E, Willett WC, Platz EA. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr. 2007;85:523–529. doi: 10.1093/ajcn/85.2.523. [DOI] [PubMed] [Google Scholar]
- 16.Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Current opinion in urology. 2006;16:25–29. [PubMed] [Google Scholar]
- 17.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Current opinion in urology. 2013;23:5–10. doi: 10.1097/MOU.0b013e32835abd4a. [DOI] [PubMed] [Google Scholar]
- 19.Gandaglia G, Briganti A, Gontero P, Mondaini N, Novara G, Salonia A, Sciarra A, Montorsi F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH) BJU Int. 2013;112:432–441. doi: 10.1111/bju.12118. [DOI] [PubMed] [Google Scholar]
- 20.Gerstenbluth RE, Seftel AD, MacLennan GT, Rao RN, Corty EW, Ferguson K, Resnick MI. Distribution of chronic prostatitis in radical prostatectomy specimens with up-regulation of bcl-2 in areas of inflammation. The Journal of urology. 2002;167:2267–2270. [PubMed] [Google Scholar]
- 21.Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, Motiwala HG, Laniado ME. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100:327–331. doi: 10.1111/j.1464-410X.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- 22.Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE Trial. Eur Urol. 2008;54:1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karazanashvili G. Editorial comment on The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE Trial. Eur Urol. 2008;54:1383–1384. doi: 10.1016/j.eururo.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Burris MB, Cathro HP, Kowalik CG, Jensen D, Culp SH, Steers WD, Krupski TL. Lower urinary tract symptom improvement after radical prostatectomy correlates with degree of prostatic inflammation. Urology. 2014;83:186–190. doi: 10.1016/j.urology.2013.07.080. [DOI] [PubMed] [Google Scholar]
- 25.Armitage J, Emberton M. Is it time to reconsider the role of prostatic inflammation in the pathogenesis of lower urinary tract symptoms? BJU Int. 2005;96:745–746. doi: 10.1111/j.1464-410X.2005.05761.x. [DOI] [PubMed] [Google Scholar]
- 26.Umbehr MH, Gurel B, Murtola TJ, Sutcliffe S, Peskoe SB, Tangen CM, Goodman PJ, Thompson IM, Lippman SM, Lucia MS, Parnes HL, Drake CG, Nelson WG, De Marzo AM, Platz EA. Intraprostatic inflammation is positively associated with serum PSA in men with PSA < 4 ng ml(-1), normal DRE and negative for prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:264–269. doi: 10.1038/pcan.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.