Abstract
Maintenance dialysis patients experience a high burden of physical and emotional symptoms that directly affect their quality of life and health care utilization. In this review, we specifically highlight common troublesome symptoms affecting dialysis patients: insomnia, restless legs syndrome, and uremic pruritus. Epidemiology, pathophysiology, and evidence-based current treatment are reviewed with the goal of providing a guide for diagnosis and treatment. Finally, we identify multiple additional areas of further study needed to improve symptom management in dialysis patients.
INDEX WORDS: Sleep disorders, insomnia, restless legs syndrome, uremic pruritus, symptoms, dialysis, quality of life (QoL), kidney disease, chronic renal failure, end-stage renal disease (ESRD), symptom management, review
CASE PRESENTATION
A 78-year-old man with end-stage renal disease (ESRD) treated with hemodialysis (HD) for 8 years, diabetes, hypertension, and severe peripheral vascular disease is currently admitted to the hospital for gangrene of his left great toe. While being interviewed, he is restless and is furiously scratching his arms and trunk. He tells you, “If you can’t help me feel better, then I don’t want anything to do with you.” His wife pulls you aside and tells you that her husband is always angry, experiences constant itching, and never gets any sleep. She feels as if she has become his nurse and no longer is his wife. He has told his inpatient physicians that he no longer desires to undergo dialysis and that he is “done.” The team is considering a palliative care consult to discuss dialysis therapy withdrawal, believing that his quality of life (QoL) is no longer acceptable to him.
INTRODUCTION
Maintenance dialysis patients experience a high burden of physical and emotional symptoms that directly affect their QoL and health care utilization.1–4 Despite the high prevalence of these symptoms, evidence shows they are under-recognized and undertreated by dialysis providers.5,6 Given the direct impact of symptoms on patients’ well-being, symptom diagnosis and management is an important focus of patient-centered care. Table 1 illustrates common symptoms experienced by patients on dialysis therapy. Table 2 outlines commonly used symptom assessment tools for patients with ESRD. This review will serve as a guide for recognizing and treating the most common nonpain symptoms of maintenance dialysis patients.
Table 1.
Prevalence of Symptoms Associated With Chronic Kidney Disease
| Symptom | Prevalence |
|---|---|
| Uremic pruritus | 40.6% |
| Sleep disorders | 60.1% |
| Restless legs syndrome | 10%–20% |
| Anorexia | 56% |
| Nausea | 46% |
| Pain | 58% |
| Depression | 21%–23% |
Source: Davison et al.7
Table 2.
Examples of Validated Symptom Assessment Scores for Patients With Chronic Kidney Disease
SLEEP DISORDERS
Overview
The reported prevalence of sleep disorders (insomnia, restless legs syndrome [RLS], periodic limb movements [PLM] during sleep, and sleep apnea) ranges widely in patients with kidney disease, quoted from 20% to 70%.7–9 A large study (n = 11,351) from the DOPPS (Dialysis Outcomes and Practice Patterns Study) described a 49% self-reported prevalence of sleep disorders.8 In this study, common clinical characteristics of dialysis patients, such as higher body mass index, presence of pain, coronary artery disease, congestive heart failure, diabetes, lung disease, psychiatric disorders, peripheral artery disease, depression, and pruritus, all were significantly associated with poor sleep (P < 0.05).8 The associations of poor sleep with lower QoL scores (P < 0.0001) and increased risk for mortality (relative risk, 1.16; P < 0.03) demonstrate its clinical significance and the importance of its recognition and management.8
Insomnia
The International Classification of Sleep Disorders—Third Edition (ICSD-3) defines insomnia as: (1) difficulty initiating sleep, maintaining sleep, or waking up too early; (2) presence of these symptoms despite adequate opportunity for sleep; and (3) daytime deficits.10 Chronic insomnia occurs for 3 months with symptoms at least 3 times per week.10 Acute insomnia lasts less than 3 months and typically is linked to a life event or stressor.10 The clinical approach to diagnosis can be divided into 3 elements: (1) effects of non—kidney disease causes: comorbid conditions, medications, and lifestyle (Table 3); (2) consequences of kidney disease itself; and (3) adverse effects of dialysis. Diagnosis and assessment begin with a history that characterizes the patient’s sleep pattern with attention to its impact on daily function. Tools such as a sleep diary or a validated screening instrument, such as the Pittsburgh Sleep Quality Index (PSQI), can provide objective histories that help identify those at high risk for sleep disorders.9
Table 3.
Potential Causes of Insomnia Not Related to Kidney Failure Among Dialysis Patients
| Cause | Example of Sleep Disturbance |
|---|---|
| Comorbid condition: congestive heart failure | Orthopnea and nocturia for those who have residual kidney function |
| Comorbid condition: dementia | Nocturnal agitation |
| Comorbid condition: obstructive sleep apnea | Apneic spells and poor overall sleep |
| Medications | Stimulants |
| Lifestyle behavior | Caffeine late at night; daytime naps during dialysis |
Impact of Non—Kidney Disease Comorbid Conditions
Comorbid and non—kidney disease conditions should be considered in the differential diagnosis of insomnia in dialysis patients with multimorbidity. As shown in Table 3, examples include pulmonary disease such as sleep apnea, cardiac-related orthopnea, or nocturia in those who have residual kidney function and night-time agitated delirium in patients with dementia. If any of these are recognized and managed appropriately, poor sleep quality can be eliminated in many patients.
Physiologic Impact of Kidney Disease
Although the pathophysiology of insomnia remains elusive, it is considered a state of hyperarousal caused by an imbalance of processes that either promote or inhibit sleep or wakefulness.11 Specifically, the evening surge of melatonin that controls the circadian sleep-wake cycle is absent in dialysis patients.12 The association of other kidney disease–associated biochemical parameters with sleep (serum urea nitrogen, hemoglobin, or phosphorus) are variable.9,13,14 A small study (n = 156) showed that elevated phosphorus and serum urea nitrogen concentrations before dialysis were associated with a subjective decrease in sleep efficiency.15
Impact of Renal Replacement Therapies
There is a lack of data describing clinical differences in sleep between dialysis modalities.9 One study of HD (n = 48) versus peritoneal dialysis (n = 22) patients failed to show a difference in subjectively described sleep quality.16 The daytime sleepiness often experienced by dialysis patients may be linked to treatment-induced elevations in core body temperature that stimulate cooling mechanisms that promote daytime sleepiness.12,17 Morning dialysis patients have reported worse sleep-related symptoms and represent a group that might consider trying a different dialysis shift if symptoms are severe.9
Treatment
Although there is no consensus on a specific treatment for insomnia for patients with kidney disease, it is recommended to first use nonpharmacologic approaches. Initially, all potentially reversible uremic causes, such as RLS and pruritus (discussed later in this review), or reversible nonrenal causes should be explored and ruled out (Table 3). Treatment for all patients should begin with basic sleep hygiene. Examples include reviewing and modifying the patient’s sleep rituals and environment or identifying any caffeine or stimulant use prior to sleep. If symptoms persist, cognitive therapy is added to the sleep hygiene interventions as part of cognitive behavioral therapy for insomnia (CBT-I). Described in Table 4, CBT-I consists of 4 components that promote sleep hygiene and behaviors that improve sleep. It typically is done in 6 to 8 group or individual sessions. A trial of 103 maintenance HD patients randomly assigned to CBT-I (n = 52) versus controls (n = 51, conventional HD) showed improvement in depression, anxiety, and sleep quality in the CBT-I treatment group.18 Barriers to CBT-I include time investment on the part of patients and a lack of trained providers.19 Recently, a meta-analysis of 11 randomized controlled trials in the general population (n = 1,460) showed improved sleep characteristics with internet-based CBT-I, with results comparable to face-to-face CBT-I, representing a viable and easily delivered treatment option.20
Table 4.
Components of Cognitive Behavioral Therapy-Insomnia
| Component | Goal | Examples |
|---|---|---|
| Sleep hygiene | Modifying behavior, environment, and lifestyle factors that inhibit sleep | Eliminate caffeine and alcohol; refrain from stimulating activities prior to sleep; maintain a dark and quiet bedroom; exercise more; avoid napping during day |
| Sleep restriction | Enhancing sleep drive and stabilizing CSWC | Estimate how many hours per night are actually spent sleeping and reduce time in bed to that number (no less than 5–6 h), then slowly add time in bed as sleep efficiency gets better |
| Stimulus control | Strengthening association between the bed and sleep | Refrain from napping; establish wake times; go to bed only when sleepy; if awake for ≥20 min, leave the bed; use the bed only for sleep and intimacy |
| Cognitive therapy | Correcting dysfunctional beliefs about sleep to help reduce sleep time anxiety | Meditation/mindfulness; muscle relaxation; breathing techniques; biofeedback; imagery |
Commonly used pharmacologic treatments for insomnia include the benzodiazepine receptor agonists of the nonbenzodiazepine class, known as “Z-drugs” (zolpidem, eszopiclone, zaleplon, and zopiclone; Table 5). Although there is limited evidence, very few of these medications require dose adjustment in kidney failure. Medications should be used for only a short time, starting with low doses, and titrated up carefully with close monitoring for adverse events. Among these agents, zaleplon has been studied in a randomized, double-blind, placebo-controlled trial of 14 maintenance HD patients in Italy. At a dose of 10 mg (5 mg if aged >65 years), there was significant improvement in the total score of sleep quality versus placebo (P < 0.03) without significant adverse effects.21
Table 5.
Pharmacologic Treatment of Insomnia in Kidney Failure
| Class | Drug | Adverse Effects | Kidney Failurea |
|---|---|---|---|
| Benzodiazepine-receptor agonists | Temezapam (Restoril), lorazepam (Ativan), triazolam (Halcion) | Sedation, ataxia, hypotension, dizziness, daytime impairment | No dose adjustment, no studies |
| Nonbenzodiazepine- benzodiazepine receptor agonists | Eszopiclone (Lunesta), zolpidem (Ambien), zaleplon (Sonata), zopicloneb | Sedation, ataxia, hypotension, unpleasant taste of eszopiclone | No dose adjustment; zaleplon and zolpidem have each been studied in small singular studies in hemodialysis patients |
| Antidepressants | Trazodone (Desyrel), mirtazapine (Remeron), doxepin (Sinequan, Silenor) | Sedation, anticholinergic effects, weight gain (mirtazapine) | No dose adjustment, no studies |
| Orexin antagonist | Suvorexant (Belsomra) | Drowsiness, headaches, dizziness | No dose adjustment, no studies |
| Melatonin agonist | Ramelteon (Rozerem) | Sedation | No dose adjustment, no studies |
| Melatonin | Melatonin | Drowsiness | No dose adjustments; improvement of sleep quality at 6–12 wk, lost by 12 mo |
Although no dose adjustments are needed, these drugs should be used with caution in hemodialysis patients given the serious adverse effects. Generally recommended use is for a short time, starting low and titrating slowly, and monitoring closely for adverse events and polypharmacy.
Not currently available in United States.
Given the aforementioned reported absence of melatonin surge in patients with kidney disease, exogenous melatonin has been studied as a treatment for insomnia. Although shown to be effective in dialysis patients in the short term, a study of 67 maintenance HD patients randomly assigned to receive melatonin (3 mg, immediate release) versus placebo for 1 year did not show an improvement in sleep parameters over the longer time frame.22
The effect of increased frequency of dialysis on sleep quality has also been explored in 2 studies in the United States. The FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) trial, which compared patients receiving short daily HD at home (6 times per week) with matched conventional HD patients identified through the US Renal Data System (USRDS), showed improvement in many sleep-related parameters, including sleep adequacy, sleep disturbances, and daytime sleepiness, but failed to show significant improvement in sleep quantity.23 The FHN (Frequent Hemodialysis Network) trial showed a small but significant improvement in sleep quality at 4 months in the group randomly assigned to more frequent dialysis (6 times per week) compared to 3-times-per-week conventional dialysis, although this benefit was lost at 12 months.24 Nocturnal HD (8–10 hours per night 7 days per week) has been shown to decrease sleep apnea episodes in a small number of patients (n = 15), but without changes in sleep fragmentation.25 Cooling dialysate (35°C) in a study of 7 patients has been shown to decrease sleep latency (P = 0.03), as well as a nonsignificant trend toward longer total sleep times.26
This overall lack of evidence-based treatment of insomnia despite its high prevalence makes this area of kidney disease rich in research opportunities. In general, nonpharmacologic methods, including identification of reversible causes and sleep hygiene exercises, should be used first. Pharmacologic treatment can be used for a short term with close follow-up. If insomnia becomes chronic, CBT-I is effective treatment.
RESTLESS LEGS SYNDROME
Overview
A neurologic sensorimotor disorder, RLS has a prevalence of 12% to 25% in dialysis patients.27 The syndrome is characterized as an uncontrollable urge to move one’s legs, with symptoms predominantly at night or at periods of rest. Clinical descriptions include achy, itchy, creeping, or crawling that is relieved with movement.27,28 The diagnostic criteria for RLS, as determined by the International RLS Study Group (IRLSSG), are outlined in Box 1.
Box 1. Diagnosis Criteria of Restless Legs Syndrome by International Restless Legs Syndrome Study Group.
An irresistible impulse to move one’s legs, often accompanied by unpleasant sensations in lower limbs
Such urges or sensations start or are made worse by periods of inactivity
Such urges or sensations are partly or completely relieved with movement
Such urges or sensations are more severe in the evening or at night than during daytime
The clinical criteria are not caused by any other medical or behavioral condition that can possibly mimic restless legs syndrome (myalgia, venous stasis, leg cramps)
Note: Supporting criteria: family history, responsiveness to dopaminergic therapy, and periodic limb movements during sleep.
Source: Novak et al.27
The RLS-induced irresistible urge to move during periods of inactivity is associated with premature stopping of dialysis (adjusted odds ratio per 1-point increase in RLS severity, 1.59; 95% confidence interval [CI], 1.22–2.06).27,29 Patients with RLS have been reported to have decreased QoL (P < 0.001)30,31 and increased mortality when compared with those without RLS, in both prevalent (32.3% vs 14.5%; P < 0.04) and incident (adjusted hazard ratio, 1.39; 95% CI, 1.08–1.79) dialysis patients.30,32 Additionally, in a study of 246 prevalent HD patients, those with RLS had more anxiety, daytime sleepiness, and sexual dysfunction than those without RLS.33
Despite the significant clinical impact of RLS, it is largely under-recognized by dialysis providers. An analysis of claims data from the USRDS (n = 341,548) of incident dialysis patients implied a prevalence of 0.8% despite a higher prevalence documented in several epidemiologic studies.34 This discrepancy suggests that inadequacies in education underlie dialysis providers’ inability to recognize such a disturbing symptom.
Pathogenesis
The pathogenesis of RLS remains unclear. In the general population, a positive family history is present in 40% of cases (autosomal dominant inheritance pattern).28 A recent study of prevalent dialysis patients showed a genetic association with the BTBD9 gene, also implicated in idiopathic RLS.35 Other pathologic associations include cerebral iron deficiency, dopamine deficiency, and increased glutamate levels. Mechanistically, this is explained in autopsy studies of patients with RLS that show altered transferrin expression at the level of the choroid plexus, the location of iron transport to the brain.28 Although there is not a large body of literature examining this association in kidney disease, kidney-related RLS has been correlated with lower hemoglobin level, transferrin saturation < 20%, and poor response to epoetin alfa.36,37 Iron also is necessary for neurologic dopamine recycling and is a key cofactor for the rate-limiting enzyme of dopamine synthesis (ie, tyrosine hydroxylase).28 Glutamate levels have been shown to be increased in the thalamus of patients with RLS, and they may play a role pathologically.28 Opioid receptors, known to be decreased in dialysis patients, have also been associated with RLS.28 Several case reports have linked antidepressant medications such as selective serotonin reuptake inhibitors or serotonin/norepinephrine reuptake inhibitors to exacerbation of RLS symptoms. The mechanism is thought to be related to serotonin-mediated inhibition of dopamine transmission.38 Although the pathology is uncertain, kidney disease itself likely plays a role, illustrated by the decreased prevalence of RLS in patients who have received a transplant (4% vs 11%; P < 0.001).36
Treatment
Overview
As with most symptoms associated with kidney disease, the evidence base for treatment is small. A review from 2010 identified 17 studies on the management of RLS in kidney disease, with a total of 111 patients included in a randomized trial.39 Treatment can be divided into pharmacologic and non-pharmacologic approaches, with most effective drugs falling into the dopaminergic or gabapentinoid class.
Pharmacologic Treatment
The first category of pharmacologic treatment for RLS comprises medications that influence dopamine pathways. Levodopa has been studied in 2 small (n = 5 and n = 28), randomized, double-blind, placebo-controlled, crossover trials.40,41 Despite success in decreasing nocturnal movements, disrupted sleep was still reported in these studies.40 The nonergoline dopamine receptor agonists (ropinirole, pramipexole, and rotigotine) have shown promising results, although, again, in small studies. These drugs have a 90- to 120-minute onset of action, with the main adverse effects being nausea, lightheadedness, and fatigue, all of which are reversible with drug discontinuation.42 Long-term use of all dopaminergic therapies carries a risk for augmentation of symptoms, of which the prescribing clinician must be cognizant.43 Augmentation is a serious complication of dopaminergic therapy, defined as earlier onset of more severe symptoms (either by time, faster onset of symptoms, spreading of symptoms to other body parts, or shorter relief from treatment), which may require switching to a longer acting medication or discontinuation of this entire class of drugs.43
There are 4 main studies examining the use of nonergoline drugs in patients with kidney disease, with participant numbers ranging from 10 to 30. Pellecchia et al44 conducted a randomized, open-label, 14-week, crossover study of ropinirole versus levodopa in 11 maintenance dialysis patients. Patients were randomly assigned and received either drug for 6 weeks, then had a 1-week washout period before cross over to the alternative agent. Ropinirole produced better improvement in symptoms (74% vs 34%) and was more effective in improving total sleep time (P < 0.001) without significant adverse effects.44 In addition, Giannaki et al45 published a 6-month partially double-blind placebo-controlled trial of ropinirole (0.25 mg/d; n = 8) versus exercise therapy (n = 16) and placebo (n = 8). This study showed that both exercise and ropinirole decreased symptoms of RLS and depression scores, but only the ropinirole group reported improvement in sleep quality.45 Finally, Miranda et al46 examined pramipexole in 10 (6 female) dialysis patients with RLS after 1 month of treatment. Although there was no control or comparison group, this study showed improvement in the severity of RLS scores without significant adverse effects.46 A recent double-blind placebo-controlled study (n = 30) of the transdermal rotigotine patch (3 mg/24 h) showed improvement in PLMs over a 2-week period, although the clinical significance remains unclear because the frequency of movements was still severe.47 Overall, these drugs show encouraging results in dialysis patients, but the evidence base remains small and more studies are required to allow for stronger recommendations for their use.
The second class of drugs that can treat RLS is the gabapentinoids. These drugs, gabapentin and pre-gabalin, although promising because of their inhibition of glutamate release, must be used cautiously in the setting of kidney disease. Gabapentin can be given to dialysis patients (assuming a regular schedule with treatments of 4 hours) at doses of 100 to 300 mg after each dialysis session, with close observation for adverse events.27,42 In 2001, Thorp et al48 published a randomized, double-blind, placebo-controlled, crossover study (n = 16) that evaluated RLS severity in maintenance dialysis patients after 6 weeks of treatment with gabapentin (300 mg 3 times a week, after each dialysis session). This study showed that gabapentin significantly improved RLS symptoms (P < 0.01) when compared to placebo, with 2 patients dropping out due to adverse events.48 In 2004, Micozkadioglu et al49 published results of a randomized trial of 4 weeks of treatment with either levodopa (125 mg/d) or gabapentin (200 mg after each dialysis session) in 15 dialysis patients. Gabapentin significantly decreased RLS severity when compared to levodopa (P < 0.001) while also improving general health, body pain, and social function parameters (P < 0.01).49 Similar results have recently been reported in a randomized study of 87 dialysis patients treated with either gabapentin (200 mg after dialysis session) or levodopa/carbidopa (110 mg/d).50 In aggregate, these studies show that gabapentin is a safe and effective treatment for RLS in dialysis patients, but its use is limited somewhat by a lack of larger randomized controlled trials. If prescribed, the patient must be evaluated soon after use for adverse effects. There are no studies to date of pregabalin for RLS treatment in dialysis patients.
Evidence supporting the use of iron supplementation as treatment for RLS is lacking. One randomized double-blind placebo-controlled study compared use of 1,000 mg of intravenous iron dextran (n = 11) to placebo (normal saline solution; n = 14) in patients with ESRD and showed significant improvement in RLS symptoms at 1 and 2 weeks, although this statistical significance did not persist at 4 weeks.51 Opioids have also shown promising results in the treatment of refractory idiopathic RLS, but there have been no studies in dialysis patients to date.28,42
Nonpharmacologic Treatment
During periods of inactivity, RLS is exacerbated, meaning that dialysis sessions themselves represent approximately 4 hours in which the risk for RLS and PLMs is increased. A few small studies have shown that aerobic exercise during dialysis sessions can decrease the severity of RLS or PLMs.52–54 With participant numbers ranging from 14 to 26 patients, as little as 30 minutes of intradialytic pedaling improved the severity of RLS, with mixed results on QoL.54,55 Most recently, a randomized double-blind placebo-controlled trial of 14 dialysis patients compared intradialytic aerobic exercise treatment and placebo to exercise treatment and low-dose ropinirole (0.25 mg/d) for 6 months.56 This study showed nearly equal effectiveness in reducing RLS symptoms (57% and 61%, respectively), but did not assess sleep quality parameters, suggesting that a lower dose of dopamine agonist with exercise may be effective for the treatment of RLS.45,56 Dialysis schedule and session length may also be therapy targets because earlier dialysis and short daily HD sessions have been associated with decreased RLS symptoms and show promise for further research.27 Figure 1 summarizes an approach to the treatment of RLS in a dialysis patient.
Figure 1.
Approach to diagnosis and management of restless legs syndrome (RLS). *Drugs should be used with careful monitoring for signs of augmentation, as described in text. Drug treatment recommendations based on Molnar et al.42
UREMIC PRURITUS
Epidemiology
Uremic pruritus is a common symptom in patients with progressive kidney disease. The most comprehensive epidemiologic data are from DOPPS,57 in which 41.7% of patients reported moderate to extreme pruritus. The most recent sizeable study of the prevalence of uremic pruritus included the DOPPS III data specifically from Japan, which reported an overall incidence of moderate to extreme pruritus of 44%.58 The weighted mean prevalence of severe pruritus (including “very much” or “extreme” pruritus) was 24.5% among 16,672 dialysis patients in 7 studies assessing severity.57,59–64
An assortment of patient characteristics and dialysis parameters have been associated, albeit somewhat inconsistently, with the prevalence of uremic pruritus. These include lower dialysis adequacy,65 use of low-flux (vs high-flux) dialyzers,65 hepatitis C virus positivity,57,63 higher serum C-reactive protein levels,63 higher serum calcium and/or phosphorus levels,57,64,66 low serum albumin levels,57,67 elevated ferritin levels,32 current or recent smoking,57 older age,57 male sex,57,64 and underlying depression.57,68
Significance
Uremic pruritus is associated with decreased QoL and depression, is an independent predictor of mortality, and amplifies other symptoms (especially poor sleep) that also impair QoL and can lead to other poor patient outcomes.57,62,64,68–70 Mathur et al70 were able to demonstrate a statistically significant relationship between the intensity of uremic pruritus and health-related QoL, particularly with regard to mood, social relations, and sleep. They noted that a decrease in uremic pruritus intensity of 20% was sufficient to produce a significant improvement in health-related QoL.
Clinical Presentation
The clinical presentation of pruritus in chronic kidney disease (CKD) varies greatly. A prospective observational study of 103 HD patients followed up over 12 weeks found that 84% of patients had itching daily or nearly daily, and most patients had itching that affected large discontinuous but bilateral and symmetric skin areas. Itching was worse at night and, unfortunately, was likely to persist for months to years from patient to patient.70
In general, nonuremic causes for pruritus should be considered in patients who are refractory to a reasonable trial of treatment, whose symptoms are largely asymmetric, with bullous or ulcerating lesions, or who manifest with clinical findings characteristic of other systemic diseases. Additionally, pruritus may represent a drug reaction from recently initiated or long-standing pharmacologic treatment. Box 2 outlines a differential diagnosis of nonuremic and treatable causes of itch in patients with CKD.
Box 2. Nonuremic Causes of Itch.
Primary Dermatologic Conditions
Drug-induced hypersensitivity and other allergies
Contact dermatitis
Psoriasis
Xerosis
Urticaria
Dermatophytosis (tinea cruris, tinea pedis, tinea corporis)
Bullous pemphigoid
Kyrle disease (acquired perforating dermatitis)
Lichen simplex chronicus
-
Infestations
Bed bugs
Scabies
Lice
Systemic Conditions
Hypercalcemic states
-
Cholestasis
Viral hepatitis
Primary biliary cirrhosis
Drug-induced cholestasis
-
Hematologic malignancy
Hodgkin lymphoma
Cutaneous T-cell lymphoma
Multiple myeloma
Polycythemia vera
Solid tumors with paraneoplastic syndrome
Postherpetic neuralgia
Human immunodeficiency virus
Treatment
Overview
Treatments for uremic pruritus have been inadequately studied. Treatment regimens are variable and largely reflect our limited understanding of the pathophysiology of uremic pruritus. Hypotheses of uremic pruritus pathophysiology are detailed further in other recent reviews.71,72
Topical Treatments
Xerosis (dry scaly skin) is very common in patients with kidney disease. Therefore, the cornerstone of uremic pruritus therapy is adequate skin hydration. Skin hydration with aqueous cream emollient73 and baby oil74,75 have both been demonstrated to effectively reduce skin dryness and the severity of uremic pruritus and improve patient QoL when applied 2 to 4 times daily. There are several small placebo-controlled trials that have shown a significant benefit in uremic pruritus with capsaicin cream, 0.025% to 0.03%, applied 2 to 4 times per day to affected areas.76–78 Unfortunately, capsaicin is not well tolerated due to a transient burning feeling on the skin and its use is likely best limited to patients who have small areas of itching.
Systemic Treatments
Of all the systemic therapies currently used for the treatment of uremic pruritus, gabapentin has been the most consistently successful in clinical trials. Gunal et al79 randomly assigned 25 HD patients with uremic pruritus to receive 300 mg of gabapentin after dialysis. A significant response was observed over placebo (P < 0.001), with no participants dropping out because of adverse effects.79 Naini et al80 enrolled 34 HD patients who were given gabapentin (400 mg) versus placebo post-HD twice weekly for 4 weeks. A significant response was observed (P < 0.001).80 Finally, Razeghi et al81 enrolled 34 HD patients who were given gabapentin (100 mg) post-HD for 4 weeks, with a 1-week washout period and then 4 weeks of placebo. Again, a significant response was observed (P < 0.001).81 One recent qualitative systematic review summarizes these data by stating that they recommend gabapentin use for patients not responding to emollients, but that the “results should be interpreted cautiously due to the lower quality of included studies.”82p9 The authors recommended starting at a dose of 100 mg orally after HD 3 times weekly. In 2 small single-center prospective studies of patients receiving HD, pregabalin has been demonstrated to be similarly effective for the treatment of pruritus at doses of 25 or 75 mg at night-time and could be considered for those who do not tolerate gabapentin.83,84
Antihistamines have not been shown to be effective in CKD pruritus. They are not recommended in recent reviews.71,72 Crucially, a recent functional magnetic resonance imaging study revealed that in uremic pruritis, the central transmission of itch is via a non-histaminergic pruritus pathway and not a histaminergic pathway.85 These medications have sedating effects, which are especially risky in the older and frailer ESRD population.
Given clinical observations that μ-receptor agonists can cause or worsen pruritus, a variety of studies have investigated opioid receptor modulators for the treatment of uremic pruritus. Naltrexone, a μ-opioid receptor antagonist, was tested in 2 studies of the treatment of uremic pruritus in patients with ESRD at a dose of 50 mg/d by mouth, with widely contradicting results.86,87 κ-Opioid receptor agonists have been tested in oral and intravenous form for the treatment of uremic pruritus with some success.88 Currently, there are several ongoing trials of κ-receptor agonists worldwide for the treatment of uremic pruritus. γ-Linolenic acid (an active constituent of evening primrose oil),89 sertraline (50 mg/d),90 and doxepin91 (10 mg twice daily) have all been tested in very small trials with positive effect.
Phototherapy
Following several small studies in the 1970s that showed an improvement in CKD pruritus with type B UV light (UV-B),92–94 Tan et al95 published a meta-analysis of randomized controlled trials of uremic pruritus treatments in 1991 and identified UV-B phototherapy as the only treatment that successfully fulfilled the criteria for clinical significance. However, the most recent randomized controlled trial testing UV-B therapy efficacy, published in 2011, did not demonstrate a significant result.96 Much is still unknown about the long-term effects of UV-B therapy, and its use should be carefully considered prior to initiation, particularly in patients who are chronically immunosuppressed or in those who may soon undergo kidney transplantation.
Acupuncture
Kim et al97 published a meta-analysis of prospective clinical studies of needle acupuncture for uremic pruritus in patients with ESRD and concluded that despite 6 separate trials reporting the beneficial effects of acupuncture, most of the trials demonstrated high risk of bias and therefore current evidence is insufficient to support its efficacy. However, acupuncture has virtually no lasting adverse effects and therefore could be very reasonably offered as an alternative therapy to interested patients with uremic pruritus who do not respond to first-line treatments, who are unwilling to take systemic medications, or who have a particular interest in acupuncture.
Approach to Therapy
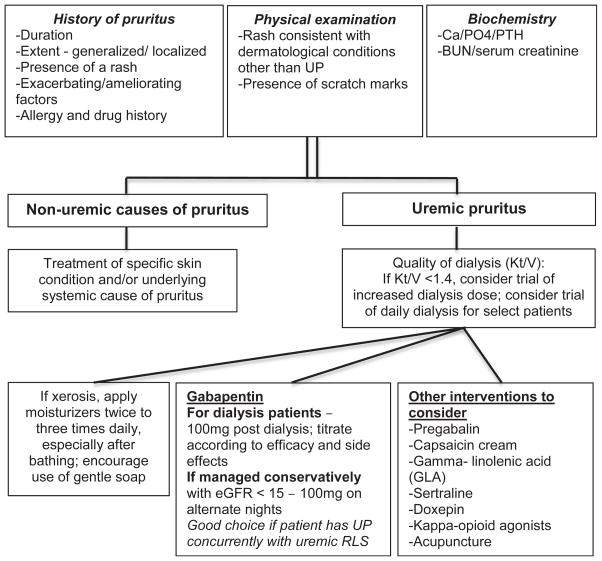
Most experts recommend taking a stepwise approach to the treatment of uremic pruritus, beginning with optimization of dialysis adequacy, calcium and phosphorus levels, skin hydration, and nutrition and with patient education on the importance of avoiding or minimizing scratching. If symptoms persist, providers may offer pharmacologic and/or nonpharmacologic therapy (Fig 2). Providers should approach treatment according to patient preference and with consideration of available resources, potential drug-drug interactions and adverse drug reactions, and their own comfort level in prescribing the various therapies.
Figure 2.
Management of pruritus in patients with chronic kidney disease.72–74,77–80,82,85–93,95,96 BUN, blood urea nitrogen; GFR, glomerular filtration rate; PTH, parathyroid hormone; UP, uremic pruritus.
CONCLUSION
Patients with ESRD commonly have a multitude of symptoms and syndromes that affect sleep quality, including insomnia, RLS, and uremic pruritus. The syndromes and associations often overlap; therefore, obtaining a careful history is essential to identifying and treating the condition. Treatment regimens as outlined in this review vary from interventions to encouraging behavior changes, nonpharmacologic regimens, topical treatments, and pharmacologic remedies. These remedies often can treat more than one symptom simultaneously if several are present. If symptoms are properly identified and treated, patients’ QoL can be greatly improved.
CASE REVIEW
A palliative care physician met with the patient and his wife. A careful history of his symptoms revealed that he experienced severe uremic pruritus and RLS, both of which were preventing him from sleeping. The physicians recommended a regimen of aggressive skin hydration 3 times daily with an emollient and prescribed gabapentin, 100 mg 3 times weekly after HD, to help with both symptoms. Two weeks later, the patient reported improvement in symptoms and sleep quality. Four weeks later, his wife reported that he seemed like a “changed man” —he seemed happier and no longer was talking about stopping dialysis therapy.
Acknowledgments
Support: None.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Peer Review: Evaluated by 3 external peer reviewers, Deputy Editor Weiner, and Editor-in-Chief Levey.
References
- 1.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–1064. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14:82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage. 2010;39:477–485. doi: 10.1016/j.jpainsymman.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Lowney AC, Myles HT, Bristowe K, et al. Understanding what influences the health-related quality of life of hemodialysis patients: a collaborative study in England and Ireland. J Pain Symptom Manage. 2015;50:778–785. doi: 10.1016/j.jpainsymman.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemo-dialysis. J Pain Symptom Manage. 2010;39:211–218. doi: 10.1016/j.jpainsymman.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2:960–967. doi: 10.2215/CJN.00990207. [DOI] [PubMed] [Google Scholar]
- 7.Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88:447–459. doi: 10.1038/ki.2015.110. [DOI] [PubMed] [Google Scholar]
- 8.Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 9.Lindner AV, Novak M, Bohra M, Mucsi I. Insomnia in patients with chronic kidney disease. Semin Nephrol. 2015;35:359–372. doi: 10.1016/j.semnephrol.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Sateia MJ. International Classification of Sleep Disorders-Third Edition: highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 11.Levenson JC, Kay DB, Buysse DJ. The pathophysiology of insomnia. Chest. 2015;147:1179–1192. doi: 10.1378/chest.14-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch BC, Nagtegaal JE, Kerkhof GA, ter Wee PM. Circadian sleep-wake rhythm disturbances in end-stage renal disease. Nat Rev Nephrol. 2009;5:407–416. doi: 10.1038/nrneph.2009.88. [DOI] [PubMed] [Google Scholar]
- 13.Iliescu EA, Yeates KE, Holland DC. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant. 2004;19:95–99. doi: 10.1093/ndt/gfg423. [DOI] [PubMed] [Google Scholar]
- 14.Unruh M, Kurella Tamura M, Larive B, et al. Impact of sleep quality on cardiovascular outcomes in hemodialysis patients: results from the Frequent Hemodialysis Network Study. Am J Nephrol. 2011;33:398–406. doi: 10.1159/000326343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch BC, Nagtegaal JE, Hagen EC, et al. Subjective sleep efficiency of hemodialysis patients. Clin Nephrol. 2008;70:411–416. doi: 10.5414/cnp70411. [DOI] [PubMed] [Google Scholar]
- 16.Holley JL, Nespor S, Rault R. A comparison of reported sleep disorders in patients on chronic hemodialysis and continuous peritoneal dialysis. Am J Kidney Dis. 1992;19:156–161. doi: 10.1016/s0272-6386(12)70125-7. [DOI] [PubMed] [Google Scholar]
- 17.Parker KP, Bliwise DL, Rye DB. Hemodialysis disrupts basic sleep regulatory mechanisms: building hypotheses. Nurs Res. 2000;49:327–332. doi: 10.1097/00006199-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hou Y, Hu P, Liang Y, Mo Z. Effects of cognitive behavioral therapy on insomnia of maintenance hemodialysis patients. Cell Biochem Biophys. 2014;69:531–537. doi: 10.1007/s12013-014-9828-4. [DOI] [PubMed] [Google Scholar]
- 19.Winkelman JW. Clinical practice. Insomnia disorder. N Engl J Med. 2015;373:1437–1444. doi: 10.1056/NEJMcp1412740. [DOI] [PubMed] [Google Scholar]
- 20.Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2015;30:1–10. doi: 10.1016/j.smrv.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Sabbatini M, Crispo A, Pisani A, et al. Zaleplon improves sleep quality in maintenance hemodialysis patients. Nephron Clin Pract. 2003;94:c99–c103. doi: 10.1159/000072493. [DOI] [PubMed] [Google Scholar]
- 22.Russcher M, Koch BC, Nagtegaal JE, et al. Long-term effects of melatonin on quality of life and sleep in haemodialysis patients (Melody study): a randomized controlled trial. Br J Clin Pharmacol. 2013;76:668–679. doi: 10.1111/bcp.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaber BL, Schiller B, Burkart JM, et al. Impact of short daily hemodialysis on restless legs symptoms and sleep disturbances. Clin J Am Soc Nephrol. 2011;6:1049–1056. doi: 10.2215/CJN.10451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unruh ML, Larive B, Eggers PW, et al. The effect of frequent hemodialysis on self-reported sleep quality: Frequent Hemodialysis Network Trials. Nephrol Dial Transplant. 2016;31(6):984–991. doi: 10.1093/ndt/gfw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanly PJ, Gabor JY, Chan C, Pierratos A. Daytime sleepiness in patients with CRF: impact of nocturnal hemodialysis. Am J Kidney Dis. 2003;41:403–410. doi: 10.1053/ajkd.2003.50066. [DOI] [PubMed] [Google Scholar]
- 26.Toth-Manikowski SM, Sozio SM. Cooling dialysate during in-center hemodialysis: beneficial and deleterious effects. World J Nephrol. 2016;5:166–171. doi: 10.5527/wjn.v5.i2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novak M, Winkelman JW, Unruh M. Restless legs syndrome in patients with chronic kidney disease. Semin Nephrol. 2015;35:347–358. doi: 10.1016/j.semnephrol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Venkateshiah SB, Ioachimescu OC. Restless legs syndrome. Crit Care Clin. 2015;31:459–472. doi: 10.1016/j.ccc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Winkelman JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis. 1996;28:372–378. doi: 10.1016/s0272-6386(96)90494-1. [DOI] [PubMed] [Google Scholar]
- 30.Unruh ML, Levey AS, D’Ambrosio C, Fink NE, Powe NR, Meyer KB. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43:900–909. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Tekdos Demircioglu D, Kavadar G, Esen Ore O, Emre TY, Yaka U. Relationship between restless leg syndrome and quality of life in uremic patients. Agri. 2015;27:73–78. doi: 10.5505/agri.2015.19327. [DOI] [PubMed] [Google Scholar]
- 32.La Manna G, Pizza F, Persici E, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26:1976–1983. doi: 10.1093/ndt/gfq681. [DOI] [PubMed] [Google Scholar]
- 33.Dikici S, Bahadir A, Baltaci D, et al. Association of anxiety, sleepiness, and sexual dysfunction with restless legs syndrome in hemodialysis patients. Hemodial Int. 2014;18:809–818. doi: 10.1111/hdi.12175. [DOI] [PubMed] [Google Scholar]
- 34.Kutner NG, Zhang R, Bliwise DL. Restless legs syndrome is underdiagnosed in the US Renal Data System. QJM. 2013;106:487. doi: 10.1093/qjmed/hct014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schormair B, Plag J, Kaffe M, et al. MEIS1 and BTBD9: genetic association with restless leg syndrome in end stage renal disease. J Med Genet. 2011;48:462–466. doi: 10.1136/jmg.2010.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molnar MZ, Novak M, Ambrus C, et al. Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis. 2005;45:388–396. doi: 10.1053/j.ajkd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Roger SD, Harris DC, Stewart JH. Possible relation between restless legs and anaemia in renal dialysis patients. Lancet. 1991;337:1551. doi: 10.1016/0140-6736(91)93248-8. [DOI] [PubMed] [Google Scholar]
- 38.Hening WA, Buchfuhrer MJ, Lee HB. Clinical Management of Restless Legs Syndrome. West Islip, NY: Professional Communications, Inc; 2007. [Google Scholar]
- 39.de Oliveira MM, Conti CF, Valbuza JS, de Carvalho LB, do Prado GF. The pharmacological treatment for uremic restless legs syndrome: evidence–based review. Mov Disord. 2010;25:1335–1342. doi: 10.1002/mds.22955. [DOI] [PubMed] [Google Scholar]
- 40.Walker SL, Fine A, Kryger MH. l-DOPA/carbidopa for nocturnal movement disorders in uremia. Sleep. 1996;19:214–218. [PubMed] [Google Scholar]
- 41.Trenkwalder C, Stiasny K, Pollmacher T, et al. l-Dopa therapy of uremic and idiopathic restless legs syndrome: a double-blind, crossover trial. Sleep. 1995;18:681–688. doi: 10.1093/sleep/18.8.681. [DOI] [PubMed] [Google Scholar]
- 42.Molnar MZ, Novak M, Mucsi I. Management of restless legs syndrome in patients on dialysis. Drugs. 2006;66:607–624. doi: 10.2165/00003495-200666050-00003. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Borreguero D, Benitez A, Kohnen R, Allen R. Augmentation of restless leg syndrome (Willis-Ekbom disease) during long-term dopaminergic treatment. Postgrad Med. 2015;127:716–725. doi: 10.1080/00325481.2015.1058140. [DOI] [PubMed] [Google Scholar]
- 44.Pellecchia MT, Vitale C, Sabatini M, et al. Ropinirole as a treatment of restless legs syndrome in patients on chronic hemo-dialysis: an open randomized crossover trial versus levodopa sustained release. Clin Neuropharmacol. 2004;27:178–181. doi: 10.1097/01.wnf.0000135480.78529.06. [DOI] [PubMed] [Google Scholar]
- 45.Giannaki CD, Sakkas GK, Karatzaferi C, et al. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 2013;14:194. doi: 10.1186/1471-2369-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miranda M, Kagi M, Fabres L, et al. Pramipexole for the treatment of uremic restless legs in patients undergoing hemodialysis. Neurology. 2004;62:831–832. doi: 10.1212/01.wnl.0000113752.14744.15. [DOI] [PubMed] [Google Scholar]
- 47.Dauvilliers Y, Benes H, Partinen M, et al. Rotigotine in hemodialysis-associated restless legs syndrome: a randomized controlled trial. Am J Kidney Dis. 2016;68(3):434–443. doi: 10.1053/j.ajkd.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 48.Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemo-dialysis patients. Am J Kidney Dis. 2001;38:104–108. doi: 10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- 49.Micozkadioglu H, Ozdemir FN, Kut A, Sezer S, Saatci U, Haberal M. Gabapentin versus levodopa for the treatment of restless legs syndrome in hemodialysis patients: an open-label study. Ren Fail. 2004;26:393–397. doi: 10.1081/jdi-120039823. [DOI] [PubMed] [Google Scholar]
- 50.Razazian N, Azimi H, Heidarnejadian J, Afshari D, Ghadami MR. Gabapentin versus levodopa-c for the treatment of restless legs syndrome in hemodialysis patients: a randomized clinical trial. Saudi J Kidney Dis Transpl. 2015;26:271–278. doi: 10.4103/1319-2442.152417. [DOI] [PubMed] [Google Scholar]
- 51.Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis. 2004;43:663–670. doi: 10.1053/j.ajkd.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 52.Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, et al. A single-blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome. Nephrol Dial Transplant. 2013;28:2834–2840. doi: 10.1093/ndt/gft288. [DOI] [PubMed] [Google Scholar]
- 53.Giannaki CD, Sakkas GK, Hadjigeorgiou GM, et al. Non-pharmacological management of periodic limb movements during hemodialysis session in patients with uremic restless legs syndrome. ASAIO J. 2010;56:538–542. doi: 10.1097/MAT.0b013e3181f1cc04. [DOI] [PubMed] [Google Scholar]
- 54.Mortazavi M, Vahdatpour B, Ghasempour A, et al. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis. Sci World J. 2013;2013:628142. doi: 10.1155/2013/628142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakkas GK, Hadjigeorgiou GM, Karatzaferi C, et al. Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis: a pilot study. ASAIO J. 2008;54:185–190. doi: 10.1097/MAT.0b013e3181641b07. [DOI] [PubMed] [Google Scholar]
- 56.Giannaki CD, Sakkas GK, Karatzaferi C, et al. Combination of exercise training and dopamine agonists in patients with RLS on dialysis: a randomized, double-blind placebo-controlled study. ASAIO J. 2015;61:738–741. doi: 10.1097/MAT.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 57.Pisoni RL, Wikstrom B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21:3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 58.Kimata N, Fuller DS, Saito A, et al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS) Hemodial Int. 2014;18:657–667. doi: 10.1111/hdi.12158. [DOI] [PubMed] [Google Scholar]
- 59.de Welter EQ, Frainer RH, Maldotti A, Losekann A, Weber MB. Evaluating the association between alterations in mineral metabolism and pruritus in hemodialysis patients. Anais Bras Dermatol. 2011;86:31–36. doi: 10.1590/s0365-05962011000100003. [DOI] [PubMed] [Google Scholar]
- 60.Stahle–Backdahl M, Hagermark O, Lins LE. Pruritus in patients on maintenance hemodialysis. Acta Med Scand. 1988;224:55–60. doi: 10.1111/j.0954-6820.1988.tb16738.x. [DOI] [PubMed] [Google Scholar]
- 61.Razeghi E, Tavakolizadeh S, Ahmadi F. Inflammation and pruritus in hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19:62–66. [PubMed] [Google Scholar]
- 62.Lopes GB, Nogueira FC, de Souza MR, et al. Assessment of the psychological burden associated with pruritus in hemodialysis patients using the kidney disease quality of life short form. Qual Life Res. 2012;21:603–612. doi: 10.1007/s11136-011-9964-x. [DOI] [PubMed] [Google Scholar]
- 63.Chiu YL, Chen HY, Chuang YF, et al. Association of uraemic pruritus with inflammation and hepatitis infection in haemodialysis patients. Nephrol Dial Transplant. 2008;23:3685–3689. doi: 10.1093/ndt/gfn303. [DOI] [PubMed] [Google Scholar]
- 64.Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69:1626–1632. doi: 10.1038/sj.ki.5000251. [DOI] [PubMed] [Google Scholar]
- 65.Ko MJ, Wu HY, Chen HY, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PloS One. 2013;8:e71404. doi: 10.1371/journal.pone.0071404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duque MI, Thevarajah S, Chan YH, Tuttle AB, Freedman BI, Yosipovitch G. Uremic pruritus is associated with higher Kt/V and serum calcium concentration. Clin Nephrol. 2006;66:184–191. doi: 10.5414/cnp66184. [DOI] [PubMed] [Google Scholar]
- 67.Virga G, Visentin I, La Milia V, Bonadonna A. Inflammation and pruritus in haemodialysis patients. Nephrol Dial Transplant. 2002;17:2164–2169. doi: 10.1093/ndt/17.12.2164. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto Y, Hayashino Y, Yamazaki S, et al. Depressive symptoms predict the future risk of severe pruritus in haemodialysis patients: Japan Dialysis Outcomes and Practice Patterns Study. Br J Dermatol. 2009;161:384–389. doi: 10.1111/j.1365-2133.2009.09088.x. [DOI] [PubMed] [Google Scholar]
- 69.Tessari G, Dalle Vedove C, Loschiavo C, et al. The impact of pruritus on the quality of life of patients undergoing dialysis: a single centre cohort study. J Nephrol. 2009;22:241–248. [PubMed] [Google Scholar]
- 70.Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1410–1419. doi: 10.2215/CJN.00100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87(4):685–691. doi: 10.1038/ki.2013.454. [DOI] [PubMed] [Google Scholar]
- 72.Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383–391. doi: 10.1016/j.semnephrol.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morton CA, Lafferty M, Hau C, Henderson I, Jones M, Lowe JG. Pruritus and skin hydration during dialysis. Nephrol Dial Transplant. 1996;11:2031–2036. doi: 10.1093/oxfordjournals.ndt.a027092. [DOI] [PubMed] [Google Scholar]
- 74.Karadag E, Kilic SP, Karatay G, Metin O. Effect of baby oil on pruritus, sleep quality, and quality of life in hemodialysis patients: pretest-post-test model with control groups. Jpn J Nurs Sci. 2014;11:180–189. doi: 10.1111/jjns.12019. [DOI] [PubMed] [Google Scholar]
- 75.Lin TC, Lai YH, Guo SE, et al. Baby oil therapy for uremic pruritus in haemodialysis patients. J Clin Nurs. 2012;21:139–148. doi: 10.1111/j.1365-2702.2011.03906.x. [DOI] [PubMed] [Google Scholar]
- 76.Breneman DL, Cardone JS, Blumsack RF, Lather RM, Searle EA, Pollack VE. Topical capsaicin for treatment of hemodialysis-related pruritus. J Am Acad Dermatol. 1992;26:91–94. doi: 10.1016/0190-9622(92)70013-6. [DOI] [PubMed] [Google Scholar]
- 77.Makhlough A, Ala S, Haj-Heydari Z, Kashi Z, Bari A. Topical capsaicin therapy for uremic pruritus in patients on hemodialysis. Iran J Kidney Dis. 2010;4:137–140. [PubMed] [Google Scholar]
- 78.Tarng DC, Cho YL, Liu HN, Huang TP. Hemodialysis-related pruritus: a double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron. 1996;72:617–622. doi: 10.1159/000188949. [DOI] [PubMed] [Google Scholar]
- 79.Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19:3137–3139. doi: 10.1093/ndt/gfh496. [DOI] [PubMed] [Google Scholar]
- 80.Naini AE, Harandi AA, Khanbabapour S, Shahidi S, Seirafiyan S, Mohseni M. Gabapentin: a promising drug for the treatment of uremic pruritus. Saudi J Kidney Dis Transpl. 2007;18:378–381. [PubMed] [Google Scholar]
- 81.Razeghi E, Eskandari D, Ganji MR, Meysamie AP, Togha M, Khashayar P. Gabapentin and uremic pruritus in hemodialysis patients. Ren Fail. 2009;31:85–90. doi: 10.1080/08860220802595476. [DOI] [PubMed] [Google Scholar]
- 82.Lau T, Leung S, Lau W. Gabapentin for uremic pruritus in hemodialysis patients: a qualitative systematic review. Can J Kidney Health Dis. 2016;3:1–14. doi: 10.1186/s40697-016-0107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aperis G, Paliouras C, Zervos A, Arvanitis A, Alivanis P. The use of pregabalin in the treatment of uraemic pruritus in haemodialysis patients. J Renal Care. 2010;36:180–185. doi: 10.1111/j.1755-6686.2010.00190.x. [DOI] [PubMed] [Google Scholar]
- 84.Solak Y, Biyik Z, Atalay H, et al. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: a prospective, crossover study. Nephrology (Carlton) 2012;17:710–717. doi: 10.1111/j.1440-1797.2012.01655.x. [DOI] [PubMed] [Google Scholar]
- 85.Papoiu AD, Emerson NM, Patel TS, et al. Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol. 2014;112:1729–1738. doi: 10.1152/jn.00827.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peer G, Kivity S, Agami O, et al. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet. 1996;348:1552–1554. doi: 10.1016/s0140-6736(96)04176-1. [DOI] [PubMed] [Google Scholar]
- 87.Pauli-Magnus C, Mikus G, Alscher DM, et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J Am Soc Nephrol. 2000;11:514–519. doi: 10.1681/ASN.V113514. [DOI] [PubMed] [Google Scholar]
- 88.Kumagai H, Ebata T, Takamori K, et al. Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175–183. doi: 10.1159/000341268. [DOI] [PubMed] [Google Scholar]
- 89.Yoshimoto-Furuie K, Yoshimoto K, Tanaka T, et al. Effects of oral supplementation with evening primrose oil for six weeks on plasma essential fatty acids and uremic skin symptoms in hemodialysis patients. Nephron. 1999;81:151–159. doi: 10.1159/000045271. [DOI] [PubMed] [Google Scholar]
- 90.Shakiba M, Sanadgol H, Azmoude HR, Mashhadi MA, Sharifi H. Effect of sertraline on uremic pruritus improvement in ESRD patients. Int J Nephrol. 2012;2012:363901. doi: 10.1155/2012/363901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pour-Reza-Gholi F, Nasrollahi A, Firouzan A, Nasli Esfahani E, Farrokhi F. Low-dose doxepin for treatment of pruritus in patients on hemodialysis. Iran J Kidney Dis. 2007;1:34–37. [PubMed] [Google Scholar]
- 92.Gilchrest BA. Ultraviolet phototherapy of uremic pruritus. Int J Dermatol. 1979;18:741–748. doi: 10.1111/j.1365-4362.1979.tb05011.x. [DOI] [PubMed] [Google Scholar]
- 93.Gilchrest BA, Rowe JW, Brown RS, Steinman TI, Arndt KA. Relief of uremic pruritus with ultraviolet phototherapy. N Engl J Med. 1977;297:136–138. doi: 10.1056/NEJM197707212970304. [DOI] [PubMed] [Google Scholar]
- 94.Gilchrest BA, Rowe JW, Brown RS, Steinman TI, Arndt KA. Ultraviolet phototherapy of uremic pruritus. Long-term results and possible mechanism of action. Ann Intern Med. 1979;91:17–21. doi: 10.7326/0003-4819-91-1-17. [DOI] [PubMed] [Google Scholar]
- 95.Tan JK, Haberman HF, Coldman AJ. Identifying effective treatments for uremic pruritus. J Am Acad Dermatol. 1991;25:811–818. doi: 10.1016/s0190-9622(08)80975-9. [DOI] [PubMed] [Google Scholar]
- 96.Ko MJ, Yang JY, Wu HY, et al. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. Br J Dermatol. 2011;165:633–639. doi: 10.1111/j.1365-2133.2011.10448.x. [DOI] [PubMed] [Google Scholar]
- 97.Kim KH, Lee MS, Choi SM. Acupuncture for treating uremic pruritus in patients with end-stage renal disease: a systematic review. J Pain Symptom Manage. 2010;40:117–125. doi: 10.1016/j.jpainsymman.2009.11.325. [DOI] [PubMed] [Google Scholar]
- 98.Davison SN, Jhangri GS, Johnson JA. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69:1621–1625. doi: 10.1038/sj.ki.5000184. [DOI] [PubMed] [Google Scholar]
- 99.Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3189–3195. doi: 10.1093/ndt/gfl380. [DOI] [PubMed] [Google Scholar]
- 100.Murphy EL, Murtagh FE, Carey I, Sheerin NS. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract. 2009;111:c74–c80. doi: 10.1159/000183177. [DOI] [PubMed] [Google Scholar]
- 101.Weisbord SD, Fried LF, Arnold RM, et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage. 2004;27:226–240. doi: 10.1016/j.jpainsymman.2003.07.004. [DOI] [PubMed] [Google Scholar]