Abstract
X-linked acro-gigantism (X-LAG) syndrome is a newly described disease caused by microduplications on chromosome Xq26.3 leading to copy number gain of GPR101. We describe the clinical progress of a sporadic male X-LAG syndrome patient with an Xq26.3 microduplication, highlighting the aggressive natural history of pituitary tumor growth in the absence of treatment. The patient first presented elsewhere aged 5 years 8 months with a history of excessive growth for >2 years. His height was 163 cm, his weight was 36 kg, and he had markedly elevated GH and IGF-1. MRI showed a non-invasive sellar mass measuring 32.5 × 23.9 × 29.1 mm. Treatment was declined and the family was lost to follow-up. At the age of 10 years and 7 months, he presented again with headaches, seizures, and visual disturbance. His height had increased to 197 cm. MRI showed an invasive mass measuring 56.2 × 58.1 × 45.0 mm, with compression of optic chiasma, bilateral cavernous sinus invasion, and hydrocephalus. His thyrotrope, corticotrope, and gonadotrope axes were deficient. Surgery, somatostatin analogs, and cabergoline did not control vertical growth and pegvisomant was added, although vertical growth continues (currently 207 cm at 11 years 7 months of age). X-LAG syndrome is a new genomic disorder in which early-onset pituitary tumorigenesis can lead to marked overgrowth and gigantism. This case illustrates the aggressive nature of tumor evolution and the challenging clinical management in X-LAG syndrome.
Keywords: Giant, Pituitary adenoma, X-linked acro-gigantism syndrome, FIPA, GPR101
Introduction
Increased stature in children is a complex clinical problem, running the gamut from the extremes of normal to a group of heterogeneous genetic disorders that require expert investigation [1, 2]. Some genetic overgrowth syndromes occur with features involving other organ systems (e.g., Beckwith-Wiedemann, Sotos, and Simpson-Golabi-Behmel syndromes). Classical pituitary gigantism due to GH hyper-secretion occurs either as an isolated condition, or in familial isolated pituitary adenomas (FIPA), often in association with AIP gene mutations [3–7]. Gigantism also occurs as a component of endocrine tumor syndromes [3, 4, 8, 9].
Recently, we reported a novel early-childhood onset form of pituitary gigantism, X-linked acro-gigantism (X-LAG) syndrome (MIM#300942) [10]. X-LAG syndrome is associated with a microduplication on chromosome Xq26.3 that includes GPR101 and can occur either sporadically or in the setting of FIPA (MIM#102200) [10, 11]. These cases become apparent usually within the first 12 months of life, when dramatically excessive physical growth is usually the first sign. Their management is complex as the anterior pituitary can be widely affected by adenomatous and hyperplastic changes and responses to traditional somatostatin analogs (e.g., octreotide) are poor [10, 11]. Hence, significant resection of the anterior pituitary is often undertaken, or debulking is combined with drugs such as pegvisomant [11].
The natural history of pituitary tumor evolution in X-LAG syndrome is not clear as patients are usually identified and operated on early. We report here a boy with pituitary gigantism due to X-LAG syndrome in whom treatment was refused by his family for a period of years, with dramatic consequences for his tumor progression.
Methods
Clinical, hormonal, and radiological data were collected from first symptoms to the most recent presentation. Brief details of the patient were reported in [11]. Height and weight z-scores were calculated from normative data from www.cdc.gov/growth charts/z-scores. Informed consent for clinical and genetic studies was provided by the patient’s legal guardian. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the University of Brasilia.
Array comparative genomic hybridization
Array comparative genome hybridization (aCGH) was performed using a standard clinical array, with further investigation using a customized, high-density 8 × 60 K array CGH (HD-aCGH) (Agilent Technologies) with high-density probes tiling the critical region inside Xq26.3 (ChrX: 135001882-136499429, hg19) to precisely determine the sizes, genomic boundaries, and gene contents of the rearrangements in the patient’s genomic DNA, as previously described [10].
Whole exome sequencing
Genomic DNA samples were fragmented, ligated to Illumina multiplexing paired-end adapters, amplified by a polymerase-chain-reaction assay with the use of primers, and hybridized to biotin-labeled VCRome, a solution-based exome capture reagent (Roche NimbleGen). Hybridization was performed at 7 °C for 64 to 72 h, and paired-end sequencing was performed on the Illumina NextSeq 500.
Data analysis and interpretation
The output data from the Illumina NextSeq 500 were converted from a bcl to a FastQ file, by means of Illumina Consensus Assessment of Sequence and Variation software, version 1.8, and mapped into the reference haploid human-genome sequence (Genome Reference Consortium Human Genome), with the BWA program.
Immunohistochemistry (IHC)
Specimens were fixed in 10 % formalin, and submitted to embedding in paraffin according to standard histological procedures. Hematoxylin and eosin staining was performed in all sections. Immunohistochemical evaluation (using Streptavidin–Biotin systems) included hormonal (GH, PRL, FSH, LH, TSH, ACTH), as well as prognostic marker profiles (Ki67, p53, c-erb). The antibodies used were anti-GH (polyclonal 1:2000), anti-PRL (polyclonal 1:2000), p-53 (DO7 1:100), Ki-67 (Mib 1 1:100), and c-erb B2 (oncoprotein C 1:400). Reactions were developed with diaminobenzidine and counter-stained with hematoxylin. Stainings for somatostatin receptor (SSTR) 2 and 5, GHRH-receptor, and AIP were performed as described previously [11].
Results
Clinical case
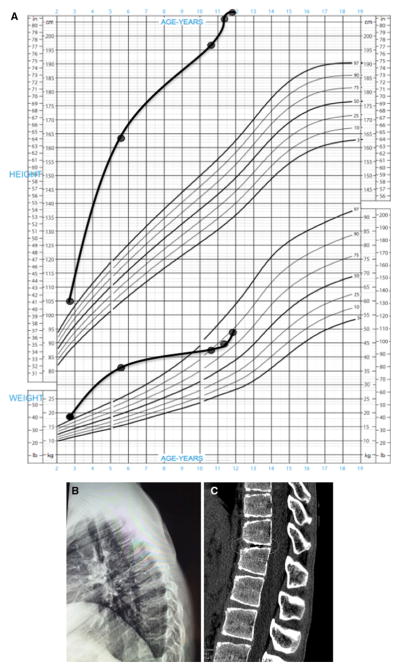
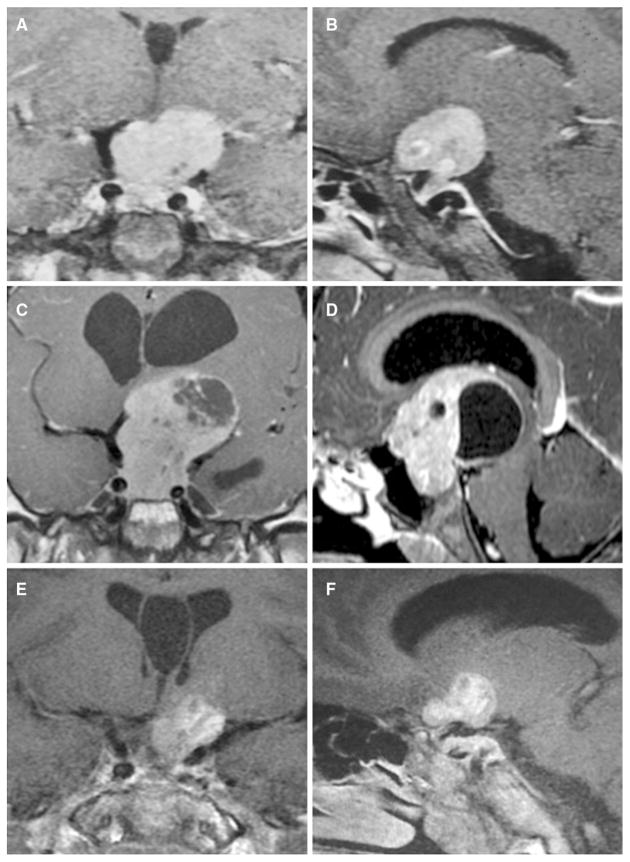
The patient was born by elective Caesarian section at 38 weeks; his birth length and weight were 2250 g and 48 cm, respectively. His family history was negative for growth disorders or pituitary disease. His mid-parental height was 176.5 cm. On chart review, overgrowth was already established by the age of 2 years 7 months (Fig. 1a). The patient first presented symptomatically at another center aged 5 years and 8 months, at a height of 163 cm (+10.0 SD), and he was Tanner stage G1 P1. At that time his bone age was 4 years, and hormonal testing revealed a random GH of 835 ng/ml (normal range <7) and an IGF-1 of 1035 ng/ml (normal range 84–235). MRI showed a heterogeneous intra- and suprasellar mass measuring 32.5 × 23.9 × 29.1 mm, with compression of the chiasma but no cavernous sinus invasion (Fig. 2a, b). The diagnosis of pituitary gigantism was made and the treatment recommendations were outlined, but the family declined treatment and was then lost to follow-up.
Fig. 1.
a Growth chart of the patient, with excess height and weight already being established at the age of 2 years 7 months. The patient’s height has remained excessive throughout this period, but his weight fell back into the normal range (90th percentile) due to poor nutrition, which has been addressed. b Lateral radiograph of the thoracic spine illustrating kyphosis. c Helical CT scan of the thoraco-lumbar region illustrating irregularities of the vertebral plateau and an intervertebral vacuum phenomenon (circled). Such vacuum phenomena are due to gas (nitrogen) accumulation from sources such as Schmorl node formation in juvenile kyphosis or osteoarthritis [19]
Fig. 2.
a, b Coronal and sagittal T1-weighted MRI taken at the first presentation (age of 5 years and 8 months) images showing large homogeneously enhanced pituitary macroadenoma, without cavernous sinus invasion or encasement of internal carotids. Coronal and sagittal T1-weighted MRI taken at age of 10 years. c, d Showing a marked increase in the size of the pituitary mass. The top of the tumor reaches the floor of the lateral ventricles, which are dilated. A heterogeneous region of necrotic/degenerative change is seen principally in the upper part of the tumor. Coronal and sagittal T1-weighted MRI with contrast taken three months after surgical debulking. e, f Showing pituitary macroadenoma remnant with cystic degeneration and resolution of the hydrocephalus. An incidental cyst of the septum pellucidum is seen on multiple images
He presented again at the age of 10 years and 6 months. His growth had continued unabated and in the interim no medical attention was sought. He complained of headaches, seizures, and visual disturbance. On examination, his height had increased to 197 cm (+8.3 SD); he had kyphosis and evidence of intervertebral disc disease (Fig. 1b, c). His weight had, in contrast, fallen to the 90th percentile in association with malnutrition. His Tanner stage was G1 P2, and there was no axillary hair. On examination he had excessive perspiration, acral enlargement, and facial changes (an enlarged, widened nose but no prognathism). Non-specific intellectual impairment had been noted in school. Visual examination showed bitemporal hemianopsia. Laboratory investigation confirmed GH/IGF-1 hypersecretion, and marked hyperprolactinemia was present. In addition, he had hypopituitarism of his thyrotrope, corticotrope, and gonadotrope axes. A repeat MRI showed an invasive intra- and suprasellar mass that had grown markedly to measure 56.2 × 58.1 × 45.0 mm, with compression of optic chiasma, cystic degeneration of suprasellar portion, bilateral cavernous sinus invasion, and encasement of internal carotids, with hydrocephalus (Fig. 2c, d).
The patient underwent transsphenoidal surgery to debulk his tumor mass (Fig. 2e, f). Postoperatively, he developed diabetes insipidus and his hypopituitarism persisted but the GH levels fell to 185 ng/ml; IGF-1 was 301.5 % of the upper limit of normal. In the post-operative period he continued to grow and he received lanreotide Autogel 120 mg/month, in combination with cabergoline. After 5 months of treatment, however, his GH levels remained elevated. Although his IGF-1 had decreased to nearly 79 % of the upper limit of normal, his vertical growth continued (205 cm; +8.7 SD). For this reason, it was determined that more profound IGF-1 suppression would be needed, and pegvisomant was added. This led to a further reduction in IGF-1 to <70 % of the upper limit of normal but by early October 2015 his height had continued to increase to 207 cm and again neither GH nor IGF-1 were controlled. As strict compliance with current medical therapy (lanreotide Autogel 120 mg/month; cabergoline 2 mg/week; and pegvisomant 10 mg/day) could not be assured; further debulking surgery and radiotherapy are planned.
Immunohistochemistry
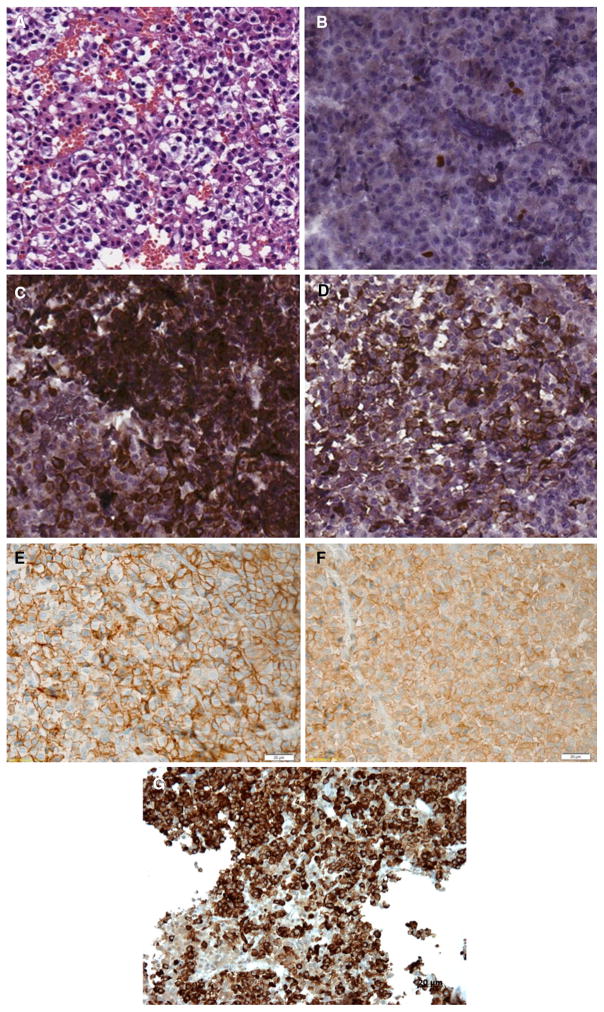
Immunohistochemistry confirmed strong (+++) staining of the adenoma for GH and prolactin, and negative staining for FSH, LH, GH, TSH, and ACTH (Fig. 3). The Ki-67 labeling index was 3.5 %. p53 staining was positive in 10 % of cells and no membrane immunoreactivity for c-erbB2 was observed; AIP staining was strongly positive (data not shown). GHRH-R staining was also strongly positive, as was SSTR2 and SSTR5 immunostaining (immunoreactive score 10/10 for both; Fig. 3).
Fig. 3.
Immunohistochemical (IHC) analysis showing a pituitary adenoma (a) with a Ki-67 index of 3.5 % (b) and with strong positivity for both GH (c) and prolactin (d). There was positivity for SSTR2 (e) and SSTR5 (f). The tumor was also strongly positive for the GHRH-receptor (g)
Genetic analyses
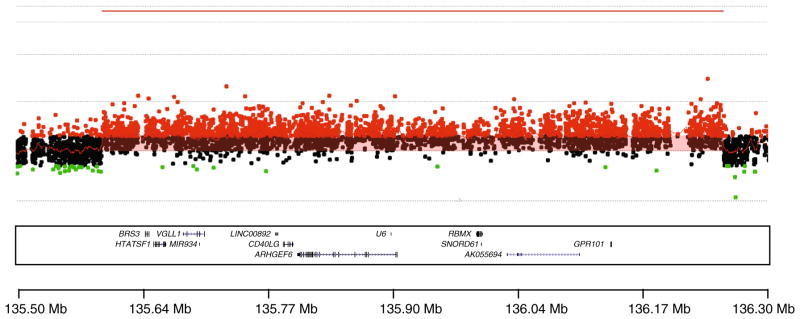
The karyotype was normal (46XY). Using HD-aCGH, a copy number gain at Xq26.3 spanning approximately 0.656 Mb was observed. The genes included within the duplication copy number variant (CNV) include VGLL1, MIR934, LOC100128420, CD40LG, ARHGEF6, RBMX, SNORD61, and GPR101. On HD-aCGH and breakpoint junction sequencing, the microduplication was refined to chrX:135602028–136259908 (Fig. 4).
Fig. 4.
Chromosome Xq26.3 microduplication of the X-LAG syndrome patient. The region with the microduplication is shown by red probes and indicated by a continuous red line above the area denoting increased copy number. The duplicated genes are shown at the bottom of the image alongside the location indicator
Whole exome capture using leukocyte DNA did not demonstrate relevant germline mutations in genes related to familial tumor pituitary syndromes (MEN1, CDKN1B, AIP, or PRKAR1A) or of genes in the GH/IGF-1 signaling pathways. A heterozygous missense change in the RET gene and heterozygous missense variant in the SSTR5 gene were observed.
Discussion
Pituitary gigantism is a rare condition associated with excessive stature for age according to population normative values and is usually caused by GH excess from a pituitary GH secreting adenoma occurring during childhood and adolescence. A number of gene mutations have been shown to cause pituitary adenomas in childhood and pituitary gigantism [1]. AIP mutations are the most common known genetic cause in pituitary gigantism [4]. Gigantism patients with AIP mutations can present sporadically or within the setting of FIPA [5, 6, 12–16].
Recently, we described a new cause of pituitary gigantism, X-LAG syndrome, due to microduplications on chromosome Xq26.3 including the GPR101 gene [10]. X-LAG syndrome has a well-defined phenotype of early-onset overgrowth due to mixed GH and prolactin-secreting pituitary adenomas/hyperplasia that have a median age of diagnosis of about 36 months [11]. Up until now, the behavior of such tumors has been uncertain, as patients frequently underwent gross total resection or anterior hypophysectomy at an early age. In the current report, due to an initial refusal of the recommended treatment options by the patient’s family, we can confirm that tumor growth in X-LAG syndrome is aggressive and relentless.
The patient had a very large macroadenoma by the time of the first MRI, when overgrowth had been documented since before the age of 3 years. The overgrowth as shown in Fig. 1 to the current height of 207 cm at the age of 11 years and 7 months was fuelled by the aggressive growth of underlying pituitary disease that secreted increasing levels of GH and IGF-1 over time. Immunohistochemical analyses confirmed the case as a mixed GH and PRL secreting adenoma (no hyperplasia was reported), as is typical in X-LAG syndrome. However, the Ki-67 was 3.5 % and >2 mitoses/high-power field were seen, both indicative of higher proliferation than in other X-LAG syndrome cases [11]. Despite the incomplete GH response to lanreotide, the SSTR2 staining was intact. The tumor was strongly positive for SSTR5 at a higher level than that seen previously in X-LAG syndrome cases. The high affinity of pasireotide for the SSTR5 receptor suggests that it might have clinical utility in such cases.
The behavior of the tumor and its profound effect on growth in this case underlines the importance of early intervention in X-LAG syndrome. Early effective treatment using surgery either alone or with pegvisomant can reduce GH/IGF-1 hypersecretion and arrest overgrowth in X-LAG syndrome and other forms of gigantism [11, 17, 18]. In this case, halting more vertical growth is vital given the history of unrestrained GH action on the skeleton allied with the fact that the patient is pre-pubertal and has panhypopituitarism. The increased stature is accompanied by a postural kyphosis and on helical CT scanning of the spine an intervertebral vacuum phenomenon was seen at the level of T11/T12. This finding, which is due to gas around the intervertebral discs and surroundings, is infrequently seen in children and can indicate underlying pathology such as osteoarthritis or spinal deformity formation [19]. The current combination medical therapy has not proven effective in halting vertical growth and treatment is complicated by poor adherence to daily pegvisomant injections and cabergoline. A further neurosurgical intervention to grossly resect accessible tumor (in combination with radiotherapy) is envisaged, which may permit greater hormonal reductions and growth control with subsequent medical therapy. At that stage, efforts to induce epiphyseal closure could halt further worsening of his gigantism.
We studied the exome of the subject’s germline DNA to screen for other genetic variants that could modify the phenotype (analogous work has been recently been shown for neuropathies [20]). Three variants were seen, one a heterozygotic missense change in RET (p.V648I; dbSNP rs77711105), which has been reported accompanying a pathological mutation (p.634R) in a MEN2A kindred [21]. This change is classified as benign using standard in silico software (e.g., SIFT), but as it is very rare (ExAC browser allele frequency: 9.076 × 10−05) and has been reported in various MEN2 cases, its status remains uncertain. The patient and family had no evidence of MEN2. However, he had suffered from lifelong severe constipation and subacute large intestine obstruction and has a working diagnosis of Hirschsprung disease (MIM#142623). There was a probably benign heterozygous missense variant (p.T9 M) in the SSTR5 gene (rs143790659; ExAC browser allele frequency: 0.001259) that did not alter SSTR5 protein expression in the pituitary adenoma. Finally, a rare heterozygotic missense variant in the PEPD gene (p.G448R; dbSNP rs121917724) that encodes peptidase D could have contributed to his intellectual disability [22].
In conclusion, X-LAG syndrome is a novel disorder of early-childhood pituitary gigantism due to duplications on chromosome Xq26.3 involving the GPR101 gene. This case describes for the first time the natural aggressive behavior of pituitary disease in a patient with X-LAG syndrome, underlining the importance of early, effective diagnosis, and intervention.
Acknowledgments
We thank Núcleo de Apoio a Pesquisa and Instituto Sabin in Brasilia-Brazil, for sample preparations and for performing hormonal studies; Drs. Luiz Augusto Casulari and Dr. Roberpaulo Barboza Filho, for clinical assistance; Ms Doralice Rabello from Pathology Department of University of Brasilia, for preparing tissue samples; Prof Jean Francois Bonneville for discussions on the neuroradiological findings; Dr. Misu Lee for discussion and analysis of somatostatin receptor studies. We thank the CNPq for support. This study was assisted in part by a grant from Fonds d’Investissement de Recherche Scientifique (FIRS) of the Centre Hospitalier Universitaire de Liège to Prof. Albert Beckers. This work was supported in part by grants from the US National Institute of Neurological Disorders and Stroke (NINDS; R01NS058529) to JRL and National Human Genome Research Institute (NHGRI)/National Heart Lung and Blood Institute (NHLBI) (U54 HG006542) to the Baylor-Hopkins Center for Mendelian Genomics.
Footnotes
Compliance with ethical standards
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Davies JH, Cheetham T. Investigation and management of tall stature. Arch Dis Child. 2014;99(8):772–777. doi: 10.1136/archdischild-2013-304830. [DOI] [PubMed] [Google Scholar]
- 2.Verge CF, Mowat D. Overgrowth Arch Dis Child. 2010;95(6):458–463. doi: 10.1136/adc.2009.157693. [DOI] [PubMed] [Google Scholar]
- 3.Stratakis CA. A giant? Think of genetics: growth hormone-producing adenomas in the young are almost always the result of genetic defects. Endocrine. 2015 doi: 10.1007/s12020-015-0645-3. [DOI] [PubMed] [Google Scholar]
- 4.Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, Holdaway I, Kranenburg-van Klaveren DJ, Chiara Zatelli M, Palacios N, Nozieres C, Zacharin M, Ebeling T, Ojaniemi M, Rozhinskaya L, Verrua E, Jaffrain-Rea ML, Filipponi S, Gusakova D, Pronin V, Bertherat J, Belaya Z, Ilovayskaya I, Sahnoun-Fathallah M, Sievers C, Stalla GK, Castermans E, Caberg JH, Sorkina E, Auriemma RS, Mittal S, Kareva M, Lysy PA, Emy P, De Menis E, Choong CS, Mantovani G, Bours V, De Herder W, Brue T, Barlier A, Neggers SJ, Zacharieva S, Chanson P, Shah NS, Stratakis CA, Naves LA, Beckers A. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. 2015;22(5):745–757. doi: 10.1530/ERC-15-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly AF, Beckers A. Familial isolated pituitary adenomas (FIPA) and mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrinol Metab Clin North Am. 2015;44(1):19–25. doi: 10.1016/j.ecl.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez LC, Gabrovska P, Denes J, Stals K, Trivellin G, Tilley D, Ferrau F, Evanson J, Ellard S, Grossman AB, Roncaroli F, Gadelha MR, Korbonits M. Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J Clin Endocrinol Metab. 2015 doi: 10.1210/jc.2015-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chahal HS, Stals K, Unterlander M, Balding DJ, Thomas MG, Kumar AV, Besser GM, Atkinson AB, Morrison PJ, Howlett TA, Levy MJ, Orme SM, Akker SA, Abel RL, Grossman AB, Burger J, Ellard S, Korbonits M. AIP mutation in pituitary adenomas in the 18th century and today. New Engl J Med. 2011;364(1):43–50. doi: 10.1056/NEJMoa1008020. [DOI] [PubMed] [Google Scholar]
- 8.Sambugaro S, Di Ruvo M, Ambrosio MR, Pellegata NS, Bellio M, Guerra A, Buratto M, Foschini MP, Tagliati F, DegliUberti E, Zatelli MC. Early onset acromegaly associated with a novel deletion in CDKN1B 5′UTR region. Endocrine. 2015;49(1):58–64. doi: 10.1007/s12020-015-0540-y. [DOI] [PubMed] [Google Scholar]
- 9.Vasilev V, Daly AF, Thiry A, Petrossians P, Fina F, Rostomyan L, Silvy M, Enjalbert A, Barlier A, Beckers A. McCune-Albright syndrome: a detailed pathological and genetic analysis of disease effects in an adult patient. J Clin Endocrinol Metab. 2014;99(10):E2029–E2038. doi: 10.1210/jc.2014-1291. [DOI] [PubMed] [Google Scholar]
- 10.Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg JH, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy PA, Ferrante E, Strebkova N, Mazerkina N, Zatelli MC, Lodish M, Horvath A, de Alexandre RB, Manning AD, Levy I, Keil MF, SierraMde L, Palmeira L, Coppieters W, Georges M, Naves LA, Jamar M, Bours V, Wu TJ, Choong CS, Bertherat J, Chanson P, Kamenicky P, Farrell WE, Barlier A, Quezado M, Bjelobaba I, Stojilkovic SS, Wess J, Costanzi S, Liu P, Lupski JR, Beckers A, Stratakis CA. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371(25):2363–2374. doi: 10.1056/NEJMoa1408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckers A, Lodish MB, Trivellin G, Rostomyan L, Lee M, Faucz FR, Yuan B, Choong CS, Caberg JH, Verrua E, Naves LA, Cheetham TD, Young J, Lysy PA, Petrossians P, Cotterill A, Shah NS, Metzger D, Castermans E, Ambrosio MR, Villa C, Strebkova N, Mazerkina N, Gaillard S, Barra GB, Casulari LA, Neggers SJ, Salvatori R, Jaffrain-Rea ML, Zacharin M, Santamaria BL, Zacharieva S, Lim EM, Mantovani G, Zatelli MC, Collins MT, Bonneville JF, Quezado M, Chittiboina P, Oldfield EH, Bours V, Liu P, WdH W, Pellegata N, Lupski JR, Daly AF, Stratakis CA. X-linked acro-gigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22(3):353–367. doi: 10.1530/ERC-15-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly AF, Tichomirowa MA, Petrossians P, Heliovaara E, Jaffrain-Rea ML, Barlier A, Naves LA, Ebeling T, Karhu A, Raappana A, Cazabat L, De Menis E, Montanana CF, Raverot G, Weil RJ, Sane T, Maiter D, Neggers S, Yaneva M, Tabarin A, Verrua E, Eloranta E, Murat A, Vierimaa O, Salmela PI, Emy P, Toledo RA, Sabate MI, Villa C, Popelier M, Salvatori R, Jennings J, Longas AF, LabartaAizpun JI, Georgitsi M, Paschke R, Ronchi C, Valimaki M, Saloranta C, De Herder W, Cozzi R, Guitelman M, Magri F, Lagonigro MS, Halaby G, Corman V, Hagelstein MT, Vanbellinghen JF, Barra GB, Gimenez-Roqueplo AP, Cameron FJ, Borson-Chazot F, Holdaway I, Toledo SP, Stalla GK, Spada A, Zacharieva S, Bertherat J, Brue T, Bours V, Chanson P, Aaltonen LA, Beckers a. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010;95(11):E373–E383. doi: 10.1210/jc.2009-2556. [DOI] [PubMed] [Google Scholar]
- 13.Jennings JE, Georgitsi M, Holdaway I, Daly AF, Tichomirowa M, Beckers A, Aaltonen LA, Karhu A, Cameron FJ. Aggressive pituitary adenomas occurring in young patients in a large Polynesian kindred with a germline R271 W mutation in the AIP gene. Eur J Endocrinol/Eur Fed Endocr Soc. 2009;161(5):799–804. doi: 10.1530/EJE-09-0406. [DOI] [PubMed] [Google Scholar]
- 14.Naves LA, Daly AF, Vanbellinghen JF, Casulari LA, Spilioti C, Magalhaes AV, Azevedo MF, Giacomini LA, Nascimento PP, Nunes RO, Rosa JW, Jaffrain-Rea ML, Bours V, Beckers A. Variable pathological and clinical features of a large Brazilian family harboring a mutation in the aryl hydrocarbon receptor-interacting protein gene. Eur J Endocrinol/Eur Fed Endocr Soc. 2007;157(4):383–391. doi: 10.1530/EJE-07-0533. [DOI] [PubMed] [Google Scholar]
- 15.Personnier C, Cazabat L, Bertherat J, Gaillard S, Souberbielle JC, Habrand JL, Dufour C, Clauser E, SainteRose C, Polak M. Clinical features and treatment of pediatric somatotropinoma: case study of an aggressive tumor due to a new AIP mutation and extensive literature review. Horm Res paediatr. 2011;75(6):392–402. doi: 10.1159/000327831. [DOI] [PubMed] [Google Scholar]
- 16.Williams F, Hunter S, Bradley L, Chahal HS, Storr HL, Akker SA, Kumar AV, Orme SM, Evanson J, Abid N, Morrison PJ, Korbonits M, Atkinson AB. Clinical experience in the screening and management of a large kindred with familial isolated pituitary adenoma due to an aryl hydrocarbon receptor interacting protein (AIP) mutation. J Clin Endocrinol Metab. 2014;99(4):1122–1131. doi: 10.1210/jc.2013-2868. [DOI] [PubMed] [Google Scholar]
- 17.Rix M, Laurberg P, Hoejberg AS, Brock-Jacobsen B. Pegvisomant therapy in pituitary gigantism: successful treatment in a 12-year-old girl. Eur J Endocrinol/Eur Fed Endocr Soc. 2005;153(2):195–201. doi: 10.1530/eje.1.01956. [DOI] [PubMed] [Google Scholar]
- 18.Mussig K, Gallwitz B, Honegger J, Strasburger CJ, Bidlingmaier M, Machicao F, Bornemann A, Ranke MB, Haring HU, Petersenn S. Pegvisomant treatment in gigantism caused by a growth hormone-secreting giant pituitary adenoma. Exp Clin Endocrinol Diabet. 2007;115(3):198–202. doi: 10.1055/s-2007-956172. [DOI] [PubMed] [Google Scholar]
- 19.Coulier B. The spectrum of vacuum phenomenon and gas in spine. JBR-BTR. 2004;87(1):9–16. [PubMed] [Google Scholar]
- 20.Gonzaga-Jauregui C, Harel T, Gambin T, Kousi M, Griffin LB, Francescatto L, Ozes B, Karaca E, Jhangiani SN, Bainbridge MN, Lawson KS, Pehlivan D, Okamoto Y, Withers M, Mancias P, Slavotinek A, Reitnauer PJ, Goksungur MT, Shy M, Crawford TO, Koenig M, Willer J, Flores BN, Pediaditrakis I, Us O, Wiszniewski W, Parman Y, Antonellis A, Muzny DM, Katsanis N, Battaloglu E, Boerwinkle E, Gibbs RA, Lupski JR. Exome sequence analysis suggests that genetic burden contributes to phenotypic variability and complex neuropathy. Cell Rep. 2015;12:1169–1183. doi: 10.1016/j.celrep.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunes AB, Ezabella MC, Pereira AC, Krieger JE, Toledo SP Baylor-Hopkins Center for Mendelian Genomics, International FIPA Consortium. A novel Val648Ile substitution in RET protooncogene observed in a Cys634Arg multiple endocrine neoplasia type 2A kindred presenting with an adrenocorticotropin-producing pheochromocytoma. J Clin Endocrinol Metab. 2002;87(12):5658–5661. doi: 10.1210/jc.2002-020345. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira C, Wang H. Prolidase deficiency. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) University of Washington; Seattle: 1993. [Google Scholar]