Abstract
Reactivation of hepatitis B in the context of immunosuppressive therapy may be severe and potentially fatal. The US Food and Drug Administration has recently drawn attention to the potentially fatal risk of hepatitis B reactivation in patients receiving the anti-CD20 agents ofatumumab or rituximab. This action focuses attention on the broader issue of hepatitis B virus reactivation, which may occur with a wide variety of immunosuppressive therapies in benign or malignant disease. This article summarizes the data behind this issue. These data support the recommendation that all patients undergoing chemotherapy, immunosuppressive therapy, hematopoietic stem cell transplantation, or solid organ transplantation be screened for active or prior hepatitis B viral infection by testing for hepatitis B surface antigen and the antibody to hepatitis B core antigen in serum. Those who are found to be hepatitis B surface antigen–positive should start appropriate antiviral therapy to prevent reactivation. Additionally, even those who have recovered from hepatitis B will benefit from antiviral therapy in certain circumstances because of the risks associated with a form of hepatitis B virus reactivation referred to as “reverse seroconversion.” There remain many uncertain areas that warrant further study, and further advances will benefit from close interactions between various medical specialties, regulatory agencies, and researchers.
Conclusions
There is good evidence to support routine screening of all patients for hepatitis B prior to undergoing chemotherapy or immunosuppressive treatment; use of prompt antiviral treatment appears to diminish the risk of severe or fatal reactivation of hepatitis B.
On September 25, 2013, the US Food and Drug Administration (FDA) issued a Drug Safety Communication entitled “Boxed Warning and New Recommendations to Decrease Risk of Hepatitis B Reactivation With the Immune-Suppressing and Anticancer Drugs Arzerra (Ofatumumab) and Rituxan (Rituximab).”1 This communiqué urges healthcare professionals to screen all patients for hepatitis B viral (HBV) infection before starting treatment with one of these agents. The recommendation is to test for hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc) and then to consult with hepatitis experts regarding monitoring and use of HBV antiviral therapy if either of these tests is positive. In patients who develop reactivation of HBV while on therapy with ofatumumab or rituximab, that treatment should be immediately discontinued and appropriate treatment for hepatitis B initiated.
This article describes the background to this new recommendation and suggests that the issue of HBV reactivation may be an underappreciated clinical challenge that extends well beyond the use of these two agents. This topic was explored in depth in a recent conference sponsored by the American Association for the Study of Liver Disease, with support from the American Academy of Dermatology, the American College of Rheumatology, and the American Society of Clinical Oncology. In addition, a review of the literature was done with the assistance of the National Library of Medicine, searching for all articles related to reactivation of hepatitis B. A total of 504 were identified through 2012. Each of these articles was reviewed by one of the authors (J.H.H.), and an annotated bibliography was produced and distributed to the other authors. Reactivation of HBV is known to occur with a wide variety of immune-suppressive therapies and may occur in the context of cancer treatment, immunosuppressive therapy for autoimmune disease, and transplantation. It is a potentially lethal condition and yet is preventable.
HBV Reactivation: Definition and Significance
Between 5% and 10% of the world’s population, or approximately 350 million persons, are chronically infected with HBV. In the United States, the prevalence of chronic HBV infection is far lower, approximately 0.5% in the general population; but the prevalence is as high as 10% in some immigrant populations.2 Infection with HBV is diagnosed by the presence of circulating HBsAg, and in most patients, HBV DNA is also detectable in serum with levels as low as 10 IU/mL and as high as several billion international units per milliliter. All patients with HBV infection develop anti-HBc, which persists after clearance of HBsAg. Thus, it is an accurate and reliable marker of current as well as previous infection with HBV. Antibody to HBsAg (anti-HBs), in contrast, is a marker for immunity to hepatitis B and can be present as a result of previous infection or successful vaccination. Thus, the presence of anti-HBc without HBsAg or anti-HBs indicates resolved HBV infection which still has the potential for reactivation (Table 1).
Table 1.
Definitions
| HBV reactivation | Abrupt, marked increase in HBV replication (HBV DNA levels) usually accompanied by elevations in serum aminotransferase levels |
| Reverse seroconversion | Reappearance of HBsAg in a person who was HBsAg-negative, anti-HBc-positive |
| Recovered hepatitis B | Seropositivity for anti-HBc without detectable HBsAg (with or without anti-HBs) |
| Current HBV infection | Seropositivity for HBsAg |
Reactivation of hepatitis B is a rare but distinctive syndrome defined by an abrupt, marked increase in HBV replication usually accompanied by elevations in serum aminotransferase levels and sometimes by jaundice. Reactivation of HBV can occur spontaneously but is more common in the setting of immune suppression or cancer chemotherapy. It typically occurs in individuals who have preexisting HBV infection with HBsAg in serum. More rarely, reactivation occurs in persons who have recovered from hepatitis B and have antibodies to HBV (anti-HBc with or without anti-HBs) and no detectable HBsAg in serum. The clinical challenge is that HBV reactivation is not just a serological or biochemical event. It is often associated with clinical symptoms consistent with acute viral hepatitis and jaundice. The illness may last for several weeks and delay further chemotherapy by several cycles. Reactivation may be so severe in some individuals as to cause acute liver failure and death. The mortality rate of reactivation of hepatitis B is reported to be as high as 25%.3
Mechanisms of HBV Reactivation
Although the precise mechanism by which HBV reactivation occurs is unclear, the initiating factor is thought to be loss of immune control over viral replication. This is best understood in the context of cancer chemotherapy. When these drugs are given, lymphocyte function is suppressed and many effector pathways, including the production of viral inhibitory cytokines such as gamma-interferon and tumor necrosis factor-alpha, are inhibited.3 This permits increased viral replication and viral protein expression on the surface of infected hepatocytes. After cancer chemotherapy is discontinued or in between cycles of administration, the immune system reconstitutes and lymphocyte function improves. Cytotoxic T cells recognize the viral peptide-expressing hepatocytes and cause variable degrees of liver cell injury and necrosis.4 The clinical and laboratory correlates of these events are depicted in Fig. 1.
Fig. 1.
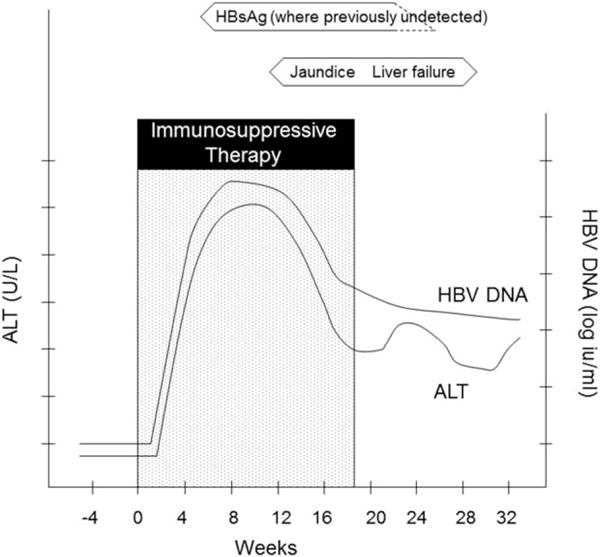
Pattern of typical serological and biochemical changes associated with HBV reactivation (based on examples in Hoofnagle5). Abbreviations: ALT, alanine aminotransferase.
Some immunosuppressive agents directly block B-cell function. Ofatumumab and rituximab are humanized antibodies to CD20, a cell-surface marker on B lymphocytes, that block humoral immunity; but drugs affecting other elements of the immune response may similarly cause HBV reactivation. This is possible because in chronic HBV infection HBV DNA persists within hepatocytes even in those with undetectable levels of circulating HBV DNA. In patients who are seronegative for HBsAg but have detectable anti-HBc, HBV DNA is rarely found in the circulation but trace amounts are often found within the liver and can be reactivated when the immune response is suppressed.
Historical Perspective
It was in the context of cancer chemotherapy that HBV reactivation was first appreciated. Early case reports demonstrated an increase in titer of HBsAg and loss of anti-HBs. Subsequently, studies described patients with elevations in serum aminotransferase levels, sometimes with features of liver failure (jaundice, hypoprothrombinemia, development of ascites, and even death) accompanied by an increase in HBV DNA levels in patients who were seropositive for HBsAg. The problem of HBV reactivation was best appreciated in Asia initially, presumably because of the endemicity of HBV infection increasing the likelihood of this complication. Several retrospective case series described the problem, but the first prospective study of HBV reactivation was done in Hong Kong.6 Among 100 patients undergoing chemotherapy for non-Hodgkin’s lymphoma with a variety of chemotherapy regimens in the pre-rituximab era, reactivation with hepatitis was noted in 13 of 27 (48%) HBsAg-positive individuals, in two of 45 (4%) anti-HBc-positive patients, but in none of 28 patients with no evidence of previous HBV infection (i.e., anti-HBc-negative). The onset of reactivation occurred after one to five courses of treatment. Three patients developed acute liver failure, and the overall mortality among those with HBV reactivation was 20%.
Subsequent reports described the phenomenon of HBV reactivation occurring after solid organ transplantation, bone marrow transplantation, and chemotherapy for other cancers including solid tumors. The introduction of infused biological agents with immunosuppressive properties led to reports of HBV reactivation in patients with benign autoimmune diseases receiving these treatments. Reactivation of HBV has been described in Western countries, although at a lower frequency, presumably because of the lower prevalence of HBV infection in Western countries.
Scenarios in Which HBV Reactivation May Occur (see Table 2)
Table 2.
Common Clinical Scenarios and Drugs Associated With Reactivation of Hepatitis B in Patients With Detectable HBsAg or Anti-HBc
| Clinical scenarios |
| Cancer chemotherapy |
| Solid organ transplantation |
| Bone marrow or stem cell transplantation |
| Immunosuppressive therapy for benign conditions (e.g., rheumatoid arthritis, psoriasis, and inflammatory bowel disease) |
| Drugs associated with HBV reactivation |
| Corticosteroids |
| Conventional chemotherapeutic agents |
| Injectable or infused biological (e.g., anti-CD20, anti-tumor necrosis factor) |
Cancer Chemotherapy
In a systematic review of prevention of HBV reactivation during chemotherapy of HBsAg-positive patients with the antiviral agent lamivudine, 14 studies were summarized, with 12 from China or Southeast Asia, and a total of 485 control patients with HBsAg not given prophylaxis. In this untreated group, reactivation defined as an increase in serum HBV DNA by at least 10-fold with elevations in serum aminotransferase levels was noted in 32%, liver failure in 13%, and death in 7%, giving the best current estimate of the incidence and outcome of HBV reactivation.3
Organ and Tissue Transplantation
Issues of hepatitis B reactivation associated with liver transplantation have been extensively studied. Thus, there is an 80% risk of recurrent hepatitis B following liver transplantation for hepatitis B-associated liver disease, unless prophylaxis is used with oral antiviral agents with or without hepatitis B immunoglobulin. In addition, use of a liver from a donor who has recovered from hepatitis B (HBsAg-negative, anti-HBc-positive) is associated with a 50%–75% risk of infection for the recipient, a dramatic example of reverse seroconversion, which can also be prevented by antiviral prophylaxis (see Table 2). Recipients of kidney or heart transplants who have preexisting HBsAg also commonly experience reactivation of hepatitis B associated with their posttransplant immunosuppression, which can be severe and result in acute liver failure or severe, progressive chronic hepatitis B unless antiviral agents are used.
Hematopoietic stem cell transplantation (HSCT) poses a special set of issues. This procedure is associated with very profound immunosuppression during the conditioning regimen and after transplantation for those receiving allogeneic transplants. Hepatitis B reactivation is very frequent in this setting, and the rate of reverse seroconversion (reappearance of HBsAg in a person who was HBsAg-negative, anti-HBc-positive prior to HSCT) is high. Reverse seroconversion is probably the result of both profound immunosuppression as well as ablation of the preexisting immune system and its replacement by an immune system that is naive to hepatitis B.
The greatest risk is among patients undergoing allogeneic HSCT (whereas the risks in autologous transplantation are similar to those in patients undergoing intensive chemotherapy). Interestingly, it has also been observed in several studies that HBsAg-positive recipients have spontaneously reverted to HBsAg-negative status after transplantation from an HBV immune donor and that this may be enhanced with HBV antiviral therapy.7,8
In a study from the Brigham and Women’s Hospital in Boston, 61 patients were identified with resolved HBV infection before transplantation (HBsAg-negative, anti-HBc-positive). Of these, 12 (20%) developed reverse seroconversion. Reverse seroconversion often occurred many months or years after transplantation. The cumulative probability of reverse seroconversion at 1, 2, and 4 years after HSCT was 9.0%, 21.7%, and 42.9%, respectively.9
Biological Agents to Treat Autoimmune Diseases
The use of biological therapies targeting tumor necrosis factor-alpha has highlighted the problem of HBV reactivation in patients with autoimmune diseases. Anti–tumor necrosis factor agents are used to treat rheumatologic, digestive, and dermatologic conditions, particularly rheumatoid arthritis, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and psoriasis. A recent systematic literature review identified reports of 257 patients with active or recovered HBV infection treated with anti-tumor necrosis factor.10 Elevations in serum aminotransferase levels were noted in 42%, signs and symptoms of liver disease in 16%, reappearance of HBV DNA in 39%, and death related to liver failure in 5%. Reactivation was more frequent among patients receiving infliximab compared to etanercept, and reactivation was seven-fold higher among patients who were HBsAg-positive (38%) compared to those who were HBsAg-negative but anti-HBc-positive (5%).
Other Conditions and Drugs Associated With HBV Reactivation
The use of high-dose corticosteroids carries a significant risk of HBV reactivation.11 High-dose corticosteroids are a part of the combination treatment regimen for many malignancies, particularly lymphoma; but corticosteroids alone may result in HBV reactivation even when used in benign conditions. Reactivation of HBV has been noted to occur after transarterial chemoembolization, a procedure used to treat localized hepatocellular carcinoma.12 In this approach the chemotherapeutic agents are directed at the tumor, so the doses used are typically much smaller than when administered systematically. Finally, HBV reactivation has been observed with antitumor agents not thought to be particularly immunosuppressive. Thus, there are isolated reports of reactivation with imatinib and thalidomide, raising questions regarding mechanisms by which other immunosuppressive agents cause HBV reactivation.13
Table 3 shows risk stratification for HBV reactivation based on intensity and type of immunosuppressive therapy and baseline HBsAg and anti-HBc status.
Table 3.
Risk Stratification for HBV Reactivation
| Therapy | HBsAg-Positive | HBsAg-Negative, Anti-HBc-Positive |
|---|---|---|
| Anti-CD20 Hematopoietic stem cell transplantation |
Very high‡ | Moderate |
| High-dose corticosteroids* Other cytokine inhibitors (e.g., anti-CD52) |
High | Low |
| Combination cytotoxic chemotherapy† (without corticosteroids) Anti-tumor necrosis factor Anti-rejection therapy for solid organ transplant recipients |
Moderate | Rare |
| Methotrexate Azathioprine |
Low | Rare |
| Androgen deprivation therapy Estrogen and progesterone blockers |
No known effect | No known effect |
Doses of corticosteroids in excess of 20 mg of prednisone (or equivalent) have been reported to have a high risk of HBV reactivation.
Examples of combinations of cytotoxic therapy that have been associated with HBV reactivation include cisplatin-based chemotherapy for squamous cell carcinoma and CHOP (cyclophosphamide [Cytoxan], hydroxydoxorubicin [Adriamycin], vincristine [Oncovin], and prednisone) for lymphoma.
Although reported rates of HBV reactivation vary considerably, rough estimates of very high risk could be considered to be in excess of 20%, high in the 11%–20% range, moderate somewhere between 1% and 10%, and low less than 1%.
Assessing the Severity of HBV Reactivation
The diagnosis of HBV reactivation is made based upon whether there is a marked rise or de novo appearance of HBV DNA in serum. Different levels of change are used in describing reactivation; a rise of more than 2 log10 IU/mL (100-fold) is an appropriate criterion. Difficulties arise if there is no documentation of HBV DNA levels before the cancer or immunosuppressive therapy and if the previous HBsAg or antibody status is unknown. Reactivation can be suspected in the appropriate clinical setting—elevated serum aminotransferase levels in the setting of positive HBsAg and serum levels of HBV DNA more than 5 log10 IU/mL (after initiation of cancer chemotherapy or immunosuppressive therapy), particularly if there is no obvious recent source of de novo hepatitis B infection such as sexual exposure or injection drug use. It is important to recognize that varying degrees of HBV reactivation may occur. Particularly in discussions of its frequency, it is important to distinguish subclinical from clinically apparent episodes. Thus, “silent” HBV reactivation with an increase in HBV DNA levels without an increase in aminotransferase levels or the appearance of symptoms is more common (but usually missed) than overt reactivation. Mild reactivation can be defined as a rise in serum aminotransferase levels without jaundice or symptoms, moderate by the appearance of jaundice or clear symptoms of liver injury such as fatigue or dark urine, and severe by the appearance of features of liver failure (coagulopathy, hepatic encephalopathy, or appearance of ascites). Severe cases may lead to death. Therefore, prompt recognition of HBV reactivation and institution of antiviral therapy are warranted.
Approach to Screening
The Centers for Disease Control and Prevention have long recommended HBV screening for at-risk persons. However, large numbers of individuals with hepatitis B remain undiagnosed. In 2008, the Centers for Disease Control and Prevention updated their recommendations to include screening of immigrants to the United States from intermediate and high endemic areas.2 These recommendations have been endorsed by the Institute of Medicine and by the US Preventive Services Task Force.14 The Centers for Disease Control and Prevention also calls for hepatitis B testing for all patients needing immunosuppressive therapy (see Table 4), and this recommendation has been endorsed by several professional societies. Patients at risk of HBV reactivation are readily identified by testing for HBsAg and anti-HBc, which is both widely available and inexpensive.
Table 4.
Recommendations of Various Authoritative Bodies Regarding Screening for Hepatitis B to Mitigate the Risk of HBV Reactivation
| Organization | Recommendation | Tests to Be Done |
|---|---|---|
| Centers for Disease Control and Prevention2 | Persons needing immunosuppressive therapy, including chemotherapy, immunosuppression related to organ transplantation, and immunosuppression for rheumatologic or gastroenterologic disorders | HBsAg, anti-HBc, anti-HBs |
| American Academy of Dermatology15 | Hepatitis B reactivation after treatment with tumor necrosis factor inhibitors has been reported; in the appropriate clinical setting, patients should be screened for hepatitis B infection. | Not stated |
| American Association for the Study of Liver Diseases16,17 | All patients before beginning immunosuppressive therapy | HBsAg, anti-HBc |
| Asian Pacific Association for the Study of the Liver18 | Before receiving immunosuppression or chemotherapy, patients should be screened for HBsAg. Patients who are going to receive biologic agents such as anti-CD20 or anti-tumor necrosis factor-α should be screened for anti-HBc. | HBsAg, anti-HBc |
| European Association for the Study of the Liver19 | All candidates for chemotherapy and immunosuppressive therapy should be screened. | HBsAg, anti-HBc |
| American Society of Clinical Oncology20 | Physicians may consider screening patients belonging to groups at heightened risk for chronic HBV infection or if highly immunosuppressive therapy is recommended. | Consider HBsAg, consider anti-HBc |
| US Preventive Services Task Force13 | Screen persons who are immunosuppressed. | HBsAg |
In the case of ofatumumab and rituximab, the FDA has recommended in a boxed warning that all patients should be screened for HBV infection with these two serological tests prior to using these agents. While similar recommendations have appeared within the package inserts for these and other immunosuppressive agents in the past, this is the first time they have been given this prominence. The American Association for the Study of Liver Diseases has for several years recommended routine screening for hepatitis B in patients who are at risk of HBV infection prior to initiation of chemotherapy or immunosuppressive treatment—this includes persons born in areas of high or intermediate HBV prevalence (particularly east Asia, sub-Saharan Africa, and eastern Europe) and those with high-risk behaviors as recommended by the Centers for Disease Control and Prevention.2,16,17 This strategy, though more cost-effective than universal screening, has not been effective in clinical practice because risk factors for HBV infection are not always assessed by providers or recognized by patients.21
Large cancer centers in the United States have moved to screening all patients newly diagnosed with cancer prior to therapy. A retrospective study of patients with cancer at The University of Texas MD Anderson Cancer Center in 2004–2007 found that the HBV screening rate before chemotherapy was low (17%). Among those who were screened, the prevalence of HBsAg was 1.5%, while 7.4% had anti-HBc without HBsAg. The key predictors of HBV screening were having a history of HBV infection (odds ratio = 10.2, confidence interval 5.9–17.6), hematologic malignancy (odds ratio = 21.5, confidence interval 18.3–25.2), and rituximab treatment (odds ratio = 4.2, confidence interval 3.4–5.1).21
A similar retrospective analysis done at Memorial Sloan-Kettering Cancer Center led to the institution of universal HBV screening before immunosuppressive therapy and an algorithm to guide appropriate prophylaxis in those who test positive. This became a hospital-mandated screening program in March 2009 (E. Ludwig, personal communication, 2013). Since then, 12,688 patients have been screened for HBV prior to starting chemotherapy; 78 (0.61%) were HBsAg-positive and 1039 (8.2%) were anti-HBc-positive. At Memorial Sloan-Kettering Cancer Center HBV screening rates have increased over time from approximately 15% before the implementation of mandatory screening to approaching 90% at present. Strikingly, no cases of HBV reactivation were observed after the implementation of that requirement.
The present authors and the American Association for the Study of Liver Disease strongly recommend that all patients undergoing chemotherapy, immunosuppressive therapy, HSCT, or solid organ transplantation be screened for active or prior HBV infection by testing for HBsAg and anti-HBc in serum. The American Society of Clinical Oncology has issued a provisional clinical opinion supporting screening patients with risk factors for HBV infection prior to highly immunosuppressive therapy.20 Interestingly, the MD Anderson study cited above found that case identification was substantially improved through a policy of universal, rather than high-risk, screening.21 In addition, it is recommended that screening of patients with inflammatory bowel disease or other benign medical conditions necessitating long-term immunosuppressive therapy for hepatitis B be incorporated into their routine care.
Prophylactic Antiviral Therapy
Once a patient is identified as being seropositive for HBsAg, there is good evidence that he or she would benefit from receiving antiviral treatment for hepatitis B before starting chemotherapy or receiving immunosuppressive agents. With the availability of well-tolerated orally administered antiviral agents active against hepatitis B, treatment or even prevention of HBV reactivation has been possible. Lamivudine was the first such agent to become available and, in fact, has been the agent used in most of the clinical studies on HBV reactivation. Lamivudine is a nucleoside analogue with potent antiviral activity against HBV as well as being safe, well-tolerated, and inexpensive; the main problem with it is the frequent emergence of resistant viral variants after prolonged use (typically for more than 6 months).16
There have been several prospective, randomized controlled trials (see Table 5) and a very large number of case series published, demonstrating the effectiveness of lamivudine in preventing HBV reactivation. A systematic review of prevention of reactivation during chemotherapy of HBsAg-positive patients by lamivudine3 included 14 studies with 275 patients receiving lamivudine for prophylaxis and 485 control patients not given lamivudine. Lamivudine prophylaxis decreased HBV reactivation and HBV-hepatitis by 80%–100% and eliminated HBV-liver failure. Lamivudine prophylaxis also decreased cancer-related mortality, probably by reducing the need for delay or interruption of chemotherapy.
Table 5.
Prospective, Randomized Controlled Trials of Antiviral Therapy to Prevent HBV Reactivation
| Author | No. of Patients | Disease and Treatment | Treatment | Antiviral Agent | Rate of HBV Reactivation (%) | P Value | |
|---|---|---|---|---|---|---|---|
| Prophylactic treatment | Control** | ||||||
| Hwang et al.21 | 42 | Breast | Chemotherapy | Lamivudine | 0.0 | 28.6 | 0.021 |
| Huang et al.22 | 80 | Lymphoma | Rituximab-containing chemotherapy | Entecavir | 2.4 | 17.9 | 0.027 |
| Lau et al.23 | 30 | Lymphoma | Chemotherapy | Lamivudine | 0.0 | 53 | 0.002 |
| Hsu et al.24 | 52 | Lymphoma | Chemotherapy | Lamivudine | 11.5 | 56 | 0.001 |
| Jang et al.25 | 76 | HCC | TACE | Lamivudine | 2.8 | 40.5 | <0.001 |
Control patients either remained untreated or had delayed treatment that began only after HBV reactivation had occurred. Abbreviations: HCC, hepatocellular carcinoma; TACE, trans-arterial chemoembolization.
More recently, other antiviral agents with a lower risk of antiviral drug resistance, notably tenofovir and entecavir, have replaced lamivudine as first-line agents to treat chronic hepatitis B.16 Emerging data show that entecavir is safe and effective in preventing HBV reactivation in patients receiving cancer chemotherapy and in recipients of solid organ or stem cell transplantation. A recent randomized controlled trial of HBsAg-negative, anti-HBc-positive patients receiving chemotherapy that included an anti-CD20 agent found that HBV reactivation occurred in 2% of the group that received entecavir prophylaxis compared to 18% of the control group (P < 0.05).23
Despite the availability of safe and effective antiviral agents, several questions remain, mostly about the timing of starting therapy and the appropriate duration of therapy. If antiviral therapy is initiated after a flare of hepatitis has already begun, it may take several months to reduce viral levels and to control the disease. Several randomized trials have demonstrated that prophylactic (starting antiviral therapy prior to or at the same time as starting chemotherapy) is more effective than preemptive (starting when viral levels begin to rise) antiviral therapy in preventing HBV reactivation (see Table 5) and that these preventative approaches are in turn more effective than deferring antiviral treatment until there is clinical evidence of hepatitis or liver failure. Most experts agree that antiviral therapy should be continued for the duration of chemotherapy, but there is debate about how long to continue thereafter because of the paucity of data on optimal duration of therapy. Most studies have continued therapy for 3 to 6 months after the last cycle of chemotherapy, though many experts favor continuing for up to 12 months, particularly when anti-CD20 is used; reactivation after stopping therapy remains a possibility, particularly in patients who started out with high levels of viremia. In patients with active hepatitis (elevated HBV DNA and aminotransferase levels) prior to starting chemotherapy, antiviral therapy may need to be administered longer term or indefinitely. In patients who receive a solid organ transplant or HSCT, antiviral prophylaxis may be required long term, if not for life. Approaches using vaccination against HBV to induce immunity and allow discontinuation of antiviral therapy are now being evaluated in prospective studies after HSCT.
Appropriate recommendations concerning antiviral prophylaxis of patients who are seronegative for HBsAg but positive for anti-HBc (i.e., recovered from hepatitis B) are not quite as clear as those for patients with HBsAg. Reactivation of HBV is well documented among this category of patients. For example, the recent Drug Safety Communication from the FDA described reverse seroconversion (that is, reappearance of HBsAg in a patient who had previously recovered from hepatitis B) among 22 of the 109 cases identified with HBV reactivation associated with use of rituxan or ofatumumab.1 Reverse seroconversion is less frequent than HBV reactivation in HBsAg-positive persons and appears to occur mostly with more potent forms of immunosuppression. Nevertheless, reverse seroconversion may still result in severe or even fatal liver injury. Given that HBV reactivation is less common in this situation and many more patients would be identified by screening (approximately 4% of the general population in the United States and >60% in east Asia), more restricted recommendations for prophylactic antiviral treatment are perhaps appropriate.
The American Association for the Study of Liver Diseases recommends routine use of appropriate antiviral therapy in all patients who are seropositive for HBsAg prior to or at the initiation of chemotherapy, immunosuppressive therapy, HSCT, or solid organ transplantation (Fig. 2). For patients who are seronegative for HBsAg but have anti-HBc in serum, we recommend routine antiviral prophylaxis in those receiving anti-CD20 therapies and in those undergoing HSCT or solid organ transplantation. Monitoring and initiation of antiviral therapy only if HBV DNA becomes detectable in serum may be appropriate for lesser degrees of immunosuppression in patients who have recovered from hepatitis B (i.e., HBsAg-negative, anti-HBc-positive). While the optimal monitoring interval is not clear, this might range from as short as 1 month to as long as 6 months. Because of the risk of delayed reactivation, monitoring should continue for 6 months after cessation of immunosuppressive therapy and longer in those who underwent HSCT or received rituximab.
Fig. 2.
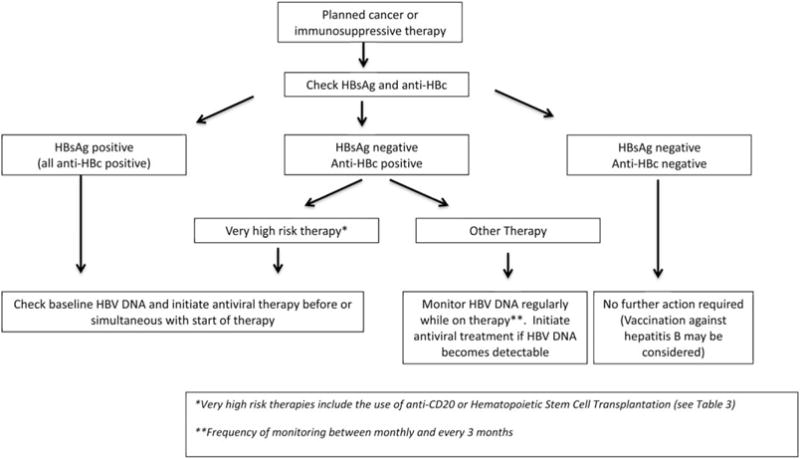
Recommended algorithm for HBV testing and treatment in patients undergoing immunosuppressive therapy.
Summary and Conclusions
In summary, HBV reactivation is clearly an underappreciated clinical challenge. Recent FDA action to recommend screening of all patients for hepatitis B prior to receiving ofatumumab or rituximab has focused attention on this issue. All patients with active or previous HBV infection face some risk of HBV reactivation when receiving cancer chemotherapy or immunosuppressive therapy, even for benign conditions. Reactivation of HBV can readily be prevented by initiating prophylactic antiviral therapy before starting the immunosuppressive regimen, and safe and effective antiviral agents are now widely available for this purpose.
We recommend that all patients undergoing chemotherapy, immunosuppressive therapy, HSCT, or solid organ transplantation be screened for active or prior HBV infection by testing for HBsAg and anti-HBc in serum. Those who are found to be HBsAg-positive should start appropriate antiviral therapy with a first-line agent prior to or at the start of the planned therapy (Fig. 2). Because of the risk of reverse seroconversion among those who have recovered from hepatitis B (i.e., those who are seronegative for HBsAg but have anti-HBc in serum), we further recommend routine antiviral prophylaxis if they are to be profoundly immunosuppressed by receiving anti-CD20 therapies or undergoing HSCT or solid organ transplantation.
While further study will be necessary to completely define the risks, benefits, and costs of universal screening, we feel that the evidence as it stands now is sufficiently compelling to make routine screening for hepatitis B a standard practice. More information is certainly needed with the use of minimally immunosuppressive therapies such as hormonal therapy or newer targeted treatments. Further research is also needed into the details of how best to deal with the risk of reverse seroconversion.
Recent events as described above draw attention to the need for further study of HBV reactivation. Algorithms to manage those who are HBsAg-negative but anti-HBc-positive need to be validated on a large scale. It is likely that the number of patients treated with immunosuppressive drug regimens will increase in the future because novel cytokine inhibitors are already in advanced clinical development to treat a variety of dermatologic, rheumatologic, and other autoimmune conditions.26,27 It is clearly preferable for all medical specialties to develop uniform protocols and algorithms together in consultation with regulatory agencies and to identify areas of uncertainty for collaborative research. Standardized protocols and algorithms should be communicated by the specialty societies to their members as well as other physicians involved in the care of patients who require immunosuppressive therapies for cancer or benign diseases.
Acknowledgments
This article was the outcome of an Emerging Trends Conference, entitled “Reactivation of Hepatitis B,” held March 21–22, 2013, in Crystal City, Arlington, VA, funded by the American Association for the Study of Liver Diseases. The authors did not receive salary or honoraria for attending this meeting or preparing this article. The manuscript benefited from input from representatives of the American Academy of Dermatology, the American College of Rheumatology, and the American Society of Clinical Oncology, although these associations do not necessarily endorse its full content.
Abbreviations
- anti-HBc
antibody to hepatitis B core antigen
- anti-HBs
antibody to hepatitis B surface antigen
- FDA
US Food and Drug Administration
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HSCT
hematopoietic stem cell transplantation
Footnotes
View this article online at wileyonlinelibrary.com.
Potential conflicts of interest: Dr. Di Bisceglie consults for and received grants from Gilead and Bristol-Myers Squibb; Dr. Martin consults for Gilead and Bristol-Myers Squibb; Dr. Lok consults and received grants from Gilead, advises GlaxoSmithKline, and received grants from Bristol-Myers Squibb; Dr. Perrillo advises Novartis and is on the speakers’ bureau for Gilead and Bristol-Myers Squibb; Dr. Terrault advises Bristol-Myers Squibb and received grants from Gilead.
References
- 1.US Food and Drug Administration. Boxed warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab) Drug Safety Communications. http://www.fda.-gov/downloads/Drugs/DrugSafety/UCM369436.pdf. Published September 25, 2013. Accessed October 17, 2013.
- 2.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 3.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, et al. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, et al. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci USA. 2010;107:798–802. doi: 10.1073/pnas.0913498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoofnagle JH. Reactivation of hepatitis B. HEPATOLOGY. 2009;49:S156–S165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 6.Lok ASF, Liang RHS, Chiu EKW, Wong K-L, Chan T-K, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 7.Hui C-K, Lie A, Au W-Y, Leung Y-H, Ma S-Y, Cheung WWW, et al. A long-term follow-up study on hepatitis B surface antigen positive patients undergoing allogeneic hematopoietic stem cell transplantation. Blood. 2005;106:464–469. doi: 10.1182/blood-2005-02-0698. [DOI] [PubMed] [Google Scholar]
- 8.Lau GKK, Suri D, Rigopoulou EI, Thomas MG, Mullerova I, Yuen S-T, et al. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002;122:614–624. doi: 10.1053/gast.2002.31887. [DOI] [PubMed] [Google Scholar]
- 9.Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1049–1059. doi: 10.1016/j.bbmt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Alvarez R, Diaz-Lagares C, García-Hernández F, Lopez-Roses L, Brito-Zerón P, Pérez-de-Lis M, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy. Medicine (Baltimore) 2011;90:359–371. doi: 10.1097/MD.0b013e3182380a76. [DOI] [PubMed] [Google Scholar]
- 11.Hoofnagle JH, Davis GL, Pappas SC, Hanson RG, Peters M, Avigan MI, et al. A short course of prednisolone in chronic type B hepatitis. Report of a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1986;104:12–17. doi: 10.7326/0003-4819-104-1-12. [DOI] [PubMed] [Google Scholar]
- 12.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemolipiodolization. HEPATOLOGY. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 13.Kim SG, Chun JM, Jin R, Kim JY, Won DI, Hwang YJ. Living donor liver transplantation for acute hepatic failure caused by reactivation of hepatitis B virus infection after chemotherapy for hematologic malignancy: case reports. Transplant Proc. 2010;42:843–845. doi: 10.1016/j.transproceed.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 14.LeFevre ML, US Preventive Services Task Force Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:58–66. doi: 10.7326/M14-1018. [DOI] [PubMed] [Google Scholar]
- 15.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Lok ASF, McMahon BJ. Chronic hepatitis B: update 2009. HEPATOLOGY. 2009;50:661–662. doi: 10.1002/hep.23190. Available at http://www.aasld.org/practiceguidelines/Documents/Bookmarked%20Practice%20Guidelines/Chronic_Hep_B_Update_2009%208_24_2009.pdf. [DOI] [PubMed] [Google Scholar]
- 17.Lok ASF, Ward JW, Perrillo RP, McMahon BJ, Liang TJ. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743–745. doi: 10.1059/0003-4819-156-10-201205150-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liaw Y-F, Kao J-H, Piratvisuth T, Chan HLY, Chien R-N, Liu C-J, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Artz AS, Somerfield MR, Feld JJ, Giusti AF, Kramer BS, Sabichi AL, et al. American Society of Clinical oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin oncol. 2010;28:3199–3202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 21.Hwang JP, Fisch MJ, Zhang H, Kallen MA, Routbort MJ, Lal MS, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J oncol Pract. 2012;8:e32–e39. doi: 10.1200/JOP.2011.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YH, Hsiao LT, Hong YC, Chious TJ, Yu YB, Gau JP, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2014;31:2765–2772. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 23.Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit for prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. HEPATOLOGY. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 25.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemolipiodolization. HEPATOLOGY. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 26.Toussirot E. the IL23/Th 17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets. 2012;11:159–168. doi: 10.2174/187152812800392805. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]


