Abstract
The actin cytoskeleton is critical for form and function of vascular cells, serving mechanical, organizational and signaling roles. Because many cytoskeletal proteins are sensitive to reactive oxygen species, redox regulation has emerged as a pivotal modulator of the actin cytoskeleton and its associated proteins. Here, we summarize work implicating oxidants in altering actin cytoskeletal proteins and focus on how these alterations affect cell migration, proliferation and contraction of vascular cells. Finally, we discuss the role of oxidative modification of the actin cytoskeleton in vivo and highlight its importance for vascular diseases.
Keywords: Redox regulation, Actin cytoskeleton, Vascular disease
INTRODUCTION
The cytoskeleton serves mechanical, organizational, and signaling functions within the cell [1, 2]. Because of its multiple roles, perturbation of the cytoskeleton is important in vascular physiology and pathophysiology [3–7]. Oxidation of cytoskeletal proteins has been observed in various cellular processes and vascular diseases [8–11], but our understanding of the role of these oxidative modifications is far from complete. Determining how oxidation of these proteins alters their function may provide a new perspective on vascular disease progression. Accordingly, this review presents an overview of our current knowledge of how actin cytoskeleton proteins are regulated by oxidative species and the potential role of cytosketetal oxidation in the vascular system.
ACTIN CYTOSKELETON STRUCTURE
Actin is a family of abundent and highly conserved cytoskeletal proteins that are expressed in nearly all eukaryotic cells and act as a scaffold to maintain cell shape and internal organization [1]. In humans, three classes of actin isoforms have been identified in different tissues: alpha (α)-actin (muscle variants, contractile structures), beta (β)-actin (nonmuscle variants, meshworks) and gamma (γ)-actin (smooth muscle and nonmuscle variants, stress fibers) [12, 13]. Monomeric actin has a globular shape (G-actin), which strongly binds to adenosine di- or tri-phosphate and divalent cations such as Mg2+ or Ca2+ (ATP/ADP-G-actin). In vivo, Mg2+ is usually bound to actin; however, Ca2+ binding is observed frequently upon actin isolation [14]. ATP-G-actin with concomitant ATP hydrolysis promotes filamentous-actin (F-actin) assembly [15] into microfilaments in the cytoskeleton and thin filaments in the contractile apparatus [13]. Actin filaments are dynamic, with nucleation, polymerization, branching, and crosslinking highly regulated by extra- and intracellular signal-mediated modification of actin itself and actin binding proteins. These processes play a crucial role in cell shape, movement, and proliferation as well as contraction [1, 16].
THE ACTIN CYTOSKELETON AS A TARGET OF OXIDANTS
Source and types of oxidative species in vascular cells
Redox signaling regulates physiological and pathological responses at different levels ranging from cells to tissues to biological systems. Many reactive oxygen species (ROS), including superoxide (O2•−), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•), as well as reactive nitrogen species (RNS) such as nitric oxide (NO) and peroxynitrite (ONOO-), are produced in biological systems [17]. Among these, O2•− and H2O2 are important signaling molecules in all kinds of vascular cells, including endothelial cells, smooth muscle cells, fibroblasts and perivascular adipocytes [10, 18].
Intracellular oxidative species can be formed by many different enzymes, of which mitochondrial respiratory enzymes and NADPH oxidases (NOXes) are major sources [19, 20]. Almost 95% of O2•− is generated from mitochondria by electron transport chain complexes [21]. This non-specific production of O2•− is generally scavenged by superoxide dismutase (SOD), which converts it to H2O2, a relatively stable molecule that reacts with proteins [21, 22] and contributes to the constitutive intracellular redox environment. In contrast, NOX family proteins are transmembrane enzymes whose major function is to transfer electrons from NADPH to oxygen to produce O2•− in localized intra- or extracellular compartments. Although O2•− does not penetrate the plasma membrane or the membrane of the intracellular organelle in which it is produced, once it is converted into H2O2 it can cross membranes and function as a signaling molecule [19]. The NOX family contains five NOXes (NOX1 to NOX5) and two dual oxidases (DUOX 1 and DUOX2). NOX1, NOX2, NOX4 and NOX5 are expressed and active in the vascular system. NOX1, NOX2 and NOX4 require a second transmembrane subunit, p22phox, and DUOXes require DUOX activator1/2, for activity [23, 24]. In addition, cytosolic regulatory partners are important in NOX maturation and expression. NOX1 interacts with NOXO1 or p47phox and NOXA1 as well as Rac; NOX2 is regulated by p47phox and p67phox; and NOX4 is constitutively active but can be regulated by poldip2 [19]. NOX5 and DUOXes are specifically activated by calcium [23, 25]. These regulatory mechanisms ensure tight temporal and spatial control of ROS release. While all NOX enzymes directly produce O2•−, O2•− is dismuated to H2O2 before release from NOX4 or by SOD in the case of the other NOXes. Degradation of H2O2 is controlled by peroxidases such as peroxiredoxins, catalase and glutathione peroxidase, an essential step in the regulation of signaling. Both O2•− and H2O2 have been implicated in oxidative modification of DNA, lipids and proteins [26], thus altering cellular functions in both physiological and pathological conditions [11, 17, 27].
Mechanisms of protein oxidation
Proteins are the most common signaling targets of ROS/RNS due to their many redox-sensitive sites [28, 29]. Oxidation of proteins can be classified into two types: reversible and irreversible [30, 31]. Cysteine (Cys) and methionine (Met) residues on proteins are typical targets of reversible oxidative modification because of their low pKa thiols that react with oxidative species [32–34]. Reversible oxidation occurs in the presence of ROS such as H2O2, O2•−, and RNS like peroxynitrite [35, 36], initially producing sulfenic acid residues (-SOH) that can then react with glutathione (GSH) or other sulfhydryl groups to form glutathione disulfide (GSSG), intra- or extra-molecular disulfide bonds (RS-SR′), and S-glutathionylated proteins (R-SSG) [36–39] (Figure 1). S-nitrosylation (SNO) is another important reversible oxidative modification of cysteines induced by nitric oxide, nitroxyl, and peroxynitrite [40] (Figure 1). S-nitrosylation in particular plays a role in integrin-dependent cell adhesion [41], cytoskeleton remodeling and cell migration [42, 43] as well as cardio protection [44–46]. These reversible modifications have been considered to play multiple beneficial roles in cellular physiological processes and may protect target proteins from further irreversible and perpetual damage [47–52].
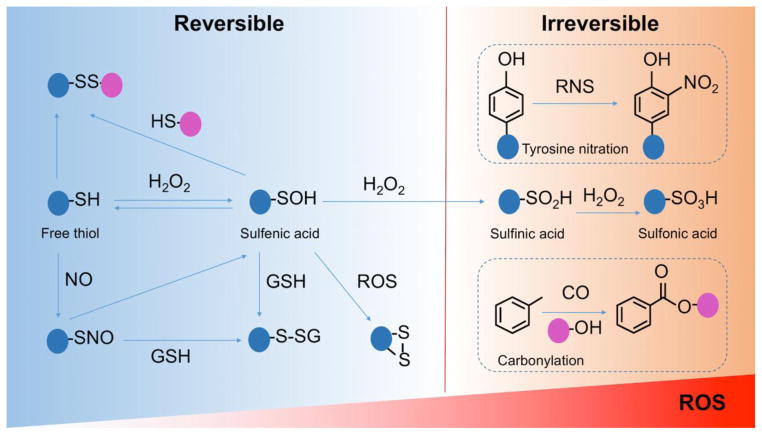
Figure 1. The major mechanisms of protein oxidative modifications.
Left panel indicates the conventional model of reversible oxidative modifications of protein on cysteine thiol groups. These reversible modifications of sulfenic acid residues (-SOH) include formation of glutathione disulfide (GSSG), intra- or extra-molecular disulfide bonds (RS-SR′), S-glutathionylated proteins (R-SSG) and S-nitrosylation (SNO). The modifications can be reversed by, for example, thioredoxin (Trx) and/or glutathione (GSH). When levels of ROS increase, sulfenic acids undergo further oxidation to sulfinic (SO2H) and/or sulfonic acid (SO3H), which are irreversible. These and two other major irreversible oxidative modifications of proteins (tyrosine nitration and carbonylation) are shown on the right panel.
However, excess production of ROS may convert -SOH to sulfinic acid (SO2H) and sulfonic acid (SO3H) derivatives, which are irreversible and may affect protein-protein interaction and function [48, 53–55] (Figure 1). Other irreversible oxidative modifications include carbonylation (aldehyde and ketone derivatives) and tyrosine nitration (3-nitrotyrosine) [56–59], which result in permanent configuration changes and affect functionality of target proteins. Irreversible modifications are generally associated with oxidative damage of proteins and are considered as biomarkers of oxidative stress in pathological processes [60–63].
A great number of signaling molecules, including receptor tyrosine kinases, phophatases, and transcription factors such as NF-κB (nuclear factor-κB), AP-1 (activator protein-1) and Hic-5 (hydrogen peroxide-inducible clone-5), have been shown to contain redox-sensitive cysteine residues [64–66]. Oxidative modification of these molecules alters activity, interaction with other proteins, and/or subcellular localization, which in turn affects downstream signaling. Since many cytoskeletal-associated proteins are particularly sensitive to redox regulation [8], in the following paragraphs we will focus on those cytoskeletal proteins that have been directly shown to be redox-regulated, and discuss their impact on basic cell behaviors such as adhesion, migration, proliferation and contraction.
Oxidation of actin
Actin itself is susceptible to oxidation and the effects of ROS/RNS on actin function have been extensively studied for almost 70 years [67–70]. As recently reviewed [67], the six cysteines in β/γ-actin and five cysteines in α-actin can all be oxidized, with Cys374 being the most redox-sensitive. Details on the oxidation of each residue are given in Table 1; however, in general, oxidation is thought to slow polymerization/elongation of G-actin [71, 72] and make F-actin more fragile [73]. Still, it should be noted that some of these studies were designed with high concentrations of oxidants (e.g., mM H2O2) instead of physiological levels (e.g., nM-μM H2O2 [74]). Recent work suggests that in some systems, low, physiologically relevant ROS production can promote actin polymerization and formation of stress fibers [69, 75, 76].
Table 1.
Summary of direct oxidation sites on actin, actin binding proteins, and actin regulatory proteins and the consequences of this oxidation.
| Site of oxidation | Oxidizing source/stimulus | Consequence | Refs. | ||
|---|---|---|---|---|---|
| ACTIN | Undetermined | Physiologic H2O2 generated in wound migration assay | Increased actin polymerization and endothelial cell migration | [76] | |
| Cys17* | Glutaredoxin 2 expression | Glutathiolyation of actin, correlating with cardiac neural crest cell migration and embryonic heart development | [414] | ||
| Cys217* | X-ray irradiation | Sulfenic, sulfinic, and sulfonic acid oxidation of actin, with decreased actin polymerization and decreased ability of actin to activate myosin II (also observed oxidation of Cys257, Met44, 47, 355, & Trp79, 86 so it is unclear which residue is responsible for functional consequences) | [415] | ||
| Diamide | S-glutathionylation of actin to undetermined endpoint | [416] | |||
| S-nitrosoglutathione | S-nitrosylation of actin to undetermined endpoint | [417] | |||
| Cys257* | X-ray irradiation | Sulfenic, sulfinic, and sulfonic acid oxidation of actin, with decreased actin polymerization and decreased ability of actin to activate myosin II (also observed oxidation of Cys217, Met44, 47, 355, & Trp79, 86 so it is unclear which residue is responsible for functional consequences) | [415] | ||
| 7-dimethylamino-4-methyl-(N-maleimidyl) coumarin (DACM) | G-actin is oxidized without affecting polymerization; F-actin is not oxidized | [80] | |||
| Glutaredoxin 2 expression | Glutathiolyation of actin, correlating with cardiac neural crest cell migration and embryonic heart development | [414] | |||
| S-nitrosoglutathione | S-nitrosylation of actin to undetermined endpoint | [417] | |||
| Treatment of neutrophils with excessive oxygen to generate physiologic amounts of NO | Nitrosylation of actin, with increased actin polymerization and impaired actin organization (also observed oxidation of Cys272, 285, 374, so it is unclear which residue is responsible for functional consequences); no observed effect on binding of other proteins to actin (mDia1, talin, profilin, α-actinin) | [41] | |||
| Cys272* (not in α-actin) | H2O2, 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), diamide | Cys272 and 374 oxidation; decreased polymerization and decreased profilin binding to G-actin; fragmentation and depolymerization of F-actin; functional effects are only observed in conditions where both Cys are oxidized | [70] | ||
| Treatment of neutrophils with excessive oxygen to generate physiologic amounts of NO | Nitrosylation of actin, with increased actin polymerization and impaired actin organization (also observed oxidation of Cys257, 285, 374, so it is unclear which residue is responsible for functional consequences); no observed effect on binding of other proteins to actin (mDia1, talin, profilin, α-actinin) | [41] | |||
| Cys285* | Excessive H2O2 | F-actin bundles; prevents proliferation at times of oxidative stress to protect yeast from oxidative damage | [9] | ||
| Treatment of neutrophils with excessive oxygen to generate physiologic amounts of NO | Nitrosylation of actin, with increased actin polymerization and impaired actin organization (also observed oxidation of Cys257, 272, 374, so it is unclear which residue is responsible for functional consequences); no observed effect on binding of other proteins to actin (mDia1, talin, profilin, α-actinin) | [41] | |||
| S-nitrosoglutathione | S-nitrosylation of actin to undetermined endpoint | [417] | |||
| S-nitrosocysteine | S-nitrosylation of actin correlates with enhanced relaxation and impaired contraction of cardiomyocytes | [82] | |||
| Hypoxia treatment of endothelial cells to generate physiologic amounts of NO | S-nitrosylation of Cys285 to undetermined endpoint | [418] | |||
| Sickle cell disease | Disulfide bond between Cys285 and Cys374 in actin correlates with delayed dissociation of actin and spectrin, impaired actin remodeling and retention of sickle shape | [419] | |||
| Cys374* | H2O2, 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), diamide | Cys272 and 374 oxidation; decreased polymerization and decreased profilin binding to G-actin; fragmentation and depolymerization of F-actin; functional effects are only observed in conditions where both Cys are oxidized | [70] | ||
| Excessive H2O2 | F-actin bundles; prevents proliferation at times of oxidative stress, to protect yeast from oxidative damage | [9] | |||
| tert-butyl hydroperoxide (t-BH) | Oxidation causes change in actin conformation and results in impaired polymerization | [71] | |||
| Physiologic levels of ROS generated during integrin-mediated cell adhesion | Glutathionylation of actin Cys374 leading to actin/non-muscle-myosin II disassembly and cell spreading | [98] | |||
| Glutathionylation in vitro | Decreased ability to polymerize (specifically filament elongation is inhibited); increased ATP exchange rate, change in actin conformation, decreased actomyosin ATPase activity | [420, 421] | |||
| EGF | De-glutathionylation promotes actin polymerization | [72] | |||
| Treatment of neutrophils with excessive oxygen to generate physiologic amounts of NO | Nitrosylation of actin, with increased actin polymerization and impaired actin organization (also observed oxidation of Cys257, 272, 285, so it is unclear which residue is responsible for functional consequences); no observed effect on binding of other proteins to actin (mDia1, talin, profilin, α-actinin) | [41] | |||
| S-nitrosoglutathione | S-nitrosylation impairs G-actin polymerization and acts as vasodilator (NO donor) | [417, 422] | |||
| Sickle cell disease | Disulfide bond between Cys285 and Cys374 in actin correlates with delayed dissociation of actin and spectrin, impairing actin remodeling and retention of sickle shape | [419] | |||
| Mutant forms of actin C374A/C374D/C374E | Oxidation-mimetic impaired actin organization, generated less stable actin filaments, promoted actin/non-muscle-myosin II disassembly and cell spreading | [98, 423] | |||
| Methionine | X-ray irradiation | Sulfenic, sulfinic, and sulfonic acid oxidation of actin, with decreased actin polymerization and decreased ability of actin to activate myosin II (observed oxidation of Cys217, 259, Met44, 47, 355, & Trp79, 86 so it is unclear which residue is responsible for functional consequences) | [415] | ||
| Chloramine-T (CT) | Met44, 47, 355 are most reactive, but Met176, 190, 227, 269 are also oxidized and Met176, 190, 269 oxidation causes F-actin depolymerization and inhibits G-actin polymerization | [424] | |||
| MICALs (Molecule Interacting with CasL) | Met44, 47 are both oxidized, but Met44 is responsible for decreases in inter-actin contacts leading to fragile F-actin that is quick to disassemble and slow to reassemble; enhances cofilin binding to F-actin; increases SRF/MRTF-A-driven gene expression including cardiac muscle specific genes | [77, 78, 83] | |||
| ACTIN BINDING PROTEINS | Myosin II | Undetermined site on non-muscle-myosin heavy chain | Integrin engagement (ROS produced by 5- lipoxygenase) | Decreased myosin II:actin binding and increased cell spreading | [98] |
| Met394 (Protista), equivalent of Cys400 muscle-myosin heavy chain | H2O2 (Protista) and oxidized glutathione for muscle-muscle myosin heavy chain | Decreased ATPase activity and actin interaction | [96, 97] | ||
| Cofilin | Cys39, Cys80, Cys139, & Cys147 | H2O2, oxidized glutathione, cell migration | Oxidation inhibits cofilin severing of actin to increase the F-actin:G-actin ratio and promote migration; cysteine oxidation can also generate rod-shaped actin/cofilin aggregates; cysteine oxidation can also induce apoptosis | [106–110] | |
| Met115 | Taurine chloramine | Oxidation increases the F-actin:G-actin ratio in the cell by preventing cofilin from binding to and severing actin | [106, 108] | ||
| ACTIN REGULATORY PROTEINS | Integrin | Cysteine in genu / calf-2 domain ( α7 integrin ) | H2O2 | Unlocking a disulfide bridge | [121] |
| RhoA | Cys16, Cys20 | O2•−, •NO2, or HO•, H2O2 and NO | Cys20 oxidation promotes nucleotide exchange, RhoA activation and stress fiber formation; Cys16 and Cys20 co-oxidation causes disulfide bond formation and RhoA inactivation; S-nitrosylation is inactivating | [138, 144, 146] | |
| Rac1 | Cys18 | O2•−, •NO2, or HO•, H2O2 and NO | Oxidation and glutathionylation promote nucleotide exchange and Rac1 activation and lamellipodia formation | [138–142] | |
| Cdc42 | Cys18 | O2•−, •NO2, or HO•, H2O2 and NO | Oxidation promotes nucleotide exchange and Cdc42 activation | [138] | |
| IQGAP | Unknown cysteine(s) | VEGF stimulation, hindlimb ischemia, H2O2 | Proposed to promote endothelial cell directed migration | [163] | |
| LMW-PTP | Cys12, Cys17 | H2O2 | Forms an inactivating disulfide bond | [176] | |
| Undetermined | ROS induced by VEGF | S-glutathionylation; inhibits its phosphorylation and activity | [177] | ||
| Src | Intramolecular sulfhydryl (SH) group | NO or N2O3 | Forms S-S bond; promotes trans-phosphorylation of Tyr416 | [186] | |
| SH2 domain (Cys245), kinase domain (Cys487) | H2O2 | Further activates an already active Src | [179] | ||
| PKC | N-terminal regulatory and the C-terminal catalytic domains | O2•− | Activation | [212, 213] | |
| Zinc finger motif | O2•− | Activation | [213] | ||
| LTCC | LTCC: α1C-subunit | HO•, thimerosal, p-chloromercuri-benzene sulphonic acid | Inactivation | [221–223] | |
| IP3R | Cys34, Cys42, Cys65 | GSH/GSSG oxidizing system | Reduces its activity in line with the conformational changes | [224, 225] | |
| SERCA | Cys674 | Nitric oxide, peroxynitrite | Reversible S-glutathiolation, enhances activity | [226–228] | |
| Cys674, Tyr294/295 | ROS | Sulfonylation of Cys674 or nitration of Tyr294/295 decreases activity | [229] | ||
| NCX | undetermined | H2O2 or that produced by xanthine oxidase with hypoxanthine | Activation | [232–234] | |
| undetermined | Hypochlorite | Disulfide bond formation, activation or inhibition | [235] | ||
| CaM | Met36, Met51, Met71, Met72 and Met145 | H2O2 | Degradation | [245, 246] | |
| Met144 and Met145 | Sulfoxides (CaMox), free-energy simulations, H2O2 | Tertiary structural rearrangements, destabilization and decreased affinity for substrates | [245, 247–249] | ||
| CaMK | Cys280/Met281 (α isoform) or Met281/282 (β, γ, and δ isoforms) | H2O2 | Activation | [256] | |
The human β-actin sequence was used for numbering actin residues.
In addition to cysteine oxidation, actin is also directly oxidized on Met44 and Met47 [77]. This oxidation is mediated by a family of proteins called MICALs (Molecule Interacting with CasL), which include a monooxygenase domain to promote the conversion of methione to methionine sulfoxide [77]. Oxidation by MICAL causes decreased inter-actin contacts leading to F-actin disassembly. The resulting actin monomers are slower to reassemble and fragment more easily once reassembled. Additionally, MICAL oxidation of actin enhances cofilin binding to F-actin. The severing function of cofilin and modified actin interactions work synergistically to promote formation of monomeric actin upon MICAL-driven oxidation [78]. It appears that Met44 oxidation is the key residue for this actin disassembly, because Met44Leu actin mutants are resistant to MICAL-induced disassembly, while Met47Leu mutants are not [77].
Beyond the specific oxidized residues and oxidizing agents (Table 1), there are numerous other variables that determine how actin responds ROS/RNS and which residues are susceptible (Table 2). For example, although Cys17 is not very reactive, when actin is bound to myosin II it becomes more susceptible to oxidation [79]. Alternatively, G-actin contains sites of oxidation that are masked by polymerization of actin [80]. Cations (for example Ca2+ influx) can also determine if actin is oxidized and may modify the impact of this oxidation [67, 70, 81]. These complexities continue to be investigated, and suggest that the effect of actin oxidation is specific to the cell type, the intracellular redox environment and ongoing cellular processes.
Table 2.
Summary of variables that affect the susceptibility of actin to oxidation and the outcome of this oxidation.
| Variable | Susceptibility/Effect of oxidation | Notes | ||
|---|---|---|---|---|
| High | Medium | Low | ||
| Type of actin | α, β, γ | α-, β-, γ-actin are all readily oxidized, but β-, γ-actin have an additional cysteine (272) that can be oxidized which is lacking in α-actin. | ||
| Form of actin | Globular | Filamentous | Filamentous actin has been described as less accessible to oxidation [80] and greater amounts of oxidized G-actin have been observed compared to F-actin, yet this could be due to the fact that once actin is oxidized it promotes the globular form (F-actin depolymerizes and G-actin is slow to polymerize) [70]. | |
| Cysteine identity | Cys374 | Cys217, Cys257, Cys272, Cys285 | Cys17 | Cys374 is highly reactive and is even oxidized upon air exposure [67]. Cys17 is only oxidized in very specific conditions [70, 79, 414]. In conditions where cysteines other than Cys374 are highly susceptible to oxidation, the functional consequences are not as great. For example, when Cys272 is described to be oxidized before Cys374, it has no effect on polymerization until C374 is oxidized [70]. Similarly, Cys257 is highly reactive with 7-dimethylamino-4-methyl-(N-maleimidyl) coumarin, but this doesn’t affect polymerization [80]. |
| Methionine identity | Met44 | Met47 | Actin is oxidized at Met44 and Met47 by a family of proteins called MICALs (Molecule Interacting with CasL), but Met44 oxidation is the key residue for regulation of actin disassembly [77]. Other methionines can also be oxidized under extreme oxidative stress [415, 424]. | |
| Divalent ion bound | Ca2+ | Mg2+ | Unlike Ca2+-bound G-actin, Mg2+-bound G-actin is resistant to H2O2 [81]. | |
| Calcium concentration | Low Ca2+ | High Ca2+ | More residues become available for oxidation at low Ca2+, and high Ca2+ (>10μM) shields Cys374 from H2O2 oxidation [70]. | |
| Protein bound | Cys10 of Myosin bound-actin | Cys10 of Myosin free-actin | Actin Cys10 is more reactive when myosin II is bound [79], yet this effect may be specific to this cysteine as others have found that myosin II blocks reactivity of SH groups in actin [425]. | |
The consequences of actin oxidation vary by the oxidative modification and the cell type, but have not been studied extensively. In cardiomyocytes, S-nitrosylation of α-actin correlates with enhanced relaxation and impaired contraction [82]. In endothelial cells, oxidation of actin appears to be essential for proper cell migration, because pharmacologic reduction of ROS impairs endothelial cell migration and actin polymerization [76]. β-actin S-glutathionylation causes the disassembly of the actin-myosin complex, which is a necessary step for successful cell adhesion and migration [69]. Additionally, oxidation of β-actin via the formation of a mixed disulfide bond between Cys374 and glutathione is necessary for cell spreading in response to integrin engagement [69]. To our knowledge, the effect of actin oxidation on mitosis/cytokinesis has not been directly investigated. Depletion of MICAL in developing zebrafish embryos results in small heart size and cardiomyocytes with altered morphology and decreased expression of cardiac muscle specific genes [83]. Knockdown of MICAL can also lead to decreased cell viability [84] and increased apoptosis [85], suggesting that Met44 oxidation may regulate these functions as well. Indeed, Met44Cys actin is unable to form colonies in yeast [86] and, oxidation of actin on Cys374 (and Cys285) promotes actin aggregation, which delays yeast cell cycle reentry [9].
Oxidation of actin binding proteins
Actin binding proteins are not only involved in regulating polymerization and depolymerization of actin, but also mediate its physiological functions. While there are many actin binding proteins, to our knowledge only two have been shown to be directly oxidized in the context of the cardiovascular system: myosin II and cofilin.
Myosin II oxidation
Myosin II is a motor protein that generates force by stepping along actin as it hydrolyzes ATP to ADP [87]. It can be divided into two classes: non-muscle (NM) myosin II and muscle myosin II. Non-muscle (NM) myosin II is expressed in almost all cell types [88] and is involved in diverse cellular process such as cell adhesion, migration, and division. In the context of cell division, the force generated from filaments made of NM-myosin II multimers interacting with F-actin is required for contractile ring formation during cytokinesis [89, 90]. Additionally, NM-myosin II enables cell rounding at the onset of mitosis and correct mitotic spindle positioning [91, 92]. During migration, NM-myosin II helps establish cell polarity and is involved in focal adhesion maturation and disassembly [87]. NM-MyosinII-actin rearrangement is also associated with endothelial cell permeability and barrier dysfunction [93]. In muscle cells, such as striated, cardiomyocytes and vascular smooth muscle cells (VSMCs), both muscle- and NM-myosin II are expressed [87]. Muscle myosin II plays a critical role in cell contraction. In this process, muscle myosin II reversibly binds to actin filaments and converts chemical signals into mechanical force and movement [94, 95].
Both NM- and muscle- myosin II have been proposed to be redox sensors within cells, because both are reversibly oxidized and this oxidation inhibits their interaction with actin and their ATPase activity [96]. This regulatory oxidation occurs at Met394 of protist NM-myosin II [97], which is equivalent to a cysteine that is glutathyolated in mammalian cardiac and skeletal muscle myosin II [96]. However, Fiaschi and colleagues [98], who examined myosin oxidation in the context of cell spreading, suggested that only NM-MHC is under redox control. Using mass spectrometry, they showed that NM-myosin II is in a reduced form in detached rounded cells, while cells in the process of attaching and spreading contain oxidized NM-myosin II, which correlates with its interaction with actin. NM-myosin II and actin interact strongly in rounded cells or spread cells when ROS generation is inhibited, but not in control spread cells [98], suggesting that oxidation of NM-myosin II could be a physiologically relevant method of controlling the interaction of NM-myosin II and actin. Further proof of the consequence of direct NM-myosin II oxidation will require creation of oxidation-insensitive mutants of NM-myosin II.
It should be noted that myosin II is composed of 2 heavy chain molecules (MHC), each with a head that binds to actin and ATP, a neck that is wrapped in myosin light chains (MLC), and a tail where the heavy chain molecules bind to each other. The activity of myosin II is regulated by MLC and a variety of kinases and phosphatases that are also involved in cell division and migration; for example, Rho kinase (ROCK), citron kinase, and myosin light chain kinase (MLCK) phosphorylate MLC and activate myosin II, while myosin phosphatase dephosphorylates MLC and inactivates myosin II [91]. As discussed below, MLC is indirectly regulated by oxidation-sensitive signaling cascades; however, the direct oxidation described above only occurs on MHC.
Cofilin oxidation
Cofilin is an actin binding protein that regulates actin dynamics by severing F-actin filaments and promoting F-actin depolymerization [99]. This promotes actin rearrangement by breaking down old F-actin filaments and generating more G-actin and barbed ends, the ingredients necessary for polymerization of new actin filaments [100] during cell migration (reviewed in [101]) and contraction [102]. The reorganization of actin by cofilin is also important during cell proliferation, as overexpression/mutation of cofilin leads to accumulation of cells in the G1-phase of the cell cycle [103], incorrect mitotic spindle positioning [104], and cytokinesis defects [105]. As described above, actin oxidation by MICAL can enhance cofilin binding. However, cofilin itself can also be directly oxidized. Cofilin oxidation increases the F-actin:G-actin ratio in the cell by preventing cofilin from severing actin [106–109], but multiple mechanisms have been described for how this occurs. Some studies suggest that cofilin oxidation prevents cofilin binding to actin [106, 108, 109], while others indicate that cofilin still binds actin but does not induce depolymerization when oxidized [107] and can even cause formation of rod-shaped actin/cofilin aggregates [110]. Phosphorylation of serine 3 is known to inhibit the interaction of cofilin with actin as well [99], but it is currently under debate if this site is phosphorylated when cofilin is oxidized [108] or if oxidation promotes dephosphorylation [106, 107].
There are four cysteines (Cys39, Cys80, Cys139, Cys147) and a methionine (Met115), that can be oxidized in cofilin. However, there is disagreement about whether specific cysteines [107, 109, 110], all cysteines [108], or methione [106] residues are required for cofilin inhibition and if intra- [107, 108] or intermolecular [110] disulfide bonds are formed upon oxidation. These details likely vary based on stimulus and cellular conditions. Cofilin oxidation during cell division has not been studied; however, cofilin oxidation can induce apoptosis. Oxidized and Ser3-phosphorylated cofilin localizes to mitochondria where it promotes opening of the permeability transition pore to allow cytochrome C release and apoptosis onset [108]. It has also been proposed that ROS generated at the leading edge of a migrating cell cause cofilin oxidation on Cys139 and Cys147 to inhibit cofilin:actin binding and consequently promote actin polymerization toward the front of the cell to help it migrate [109].
Oxidation of proteins in signaling cascades that regulate the actin cytoskeleton
Actin cytoskeletal signaling networks are composed of a large group of proteins such as integrins, small GTPases, kinases, phosphatases, ion channels and transporters. These proteins not only directly regulate actin assembly, organization and function in response to different cellular stimuli, but also instigate signaling cascades and networks that influence cell growth, migration, contraction and survival. ROS can alter actin cytoskeletal signaling by directly oxidizing these regulatory proteins, by influencing upstream molecules that then affect these downstream targets, or, as with integrin regulation, by modulating protein or mRNA expression.
Integrin oxidation
Integrins are a family of cell-cell and cell-matrix binding transmembrane adhesion molecules that mediate firm contacts of cells with the extracellular matrix (ECM) [111, 112]. They are among the most abundant cell surface receptors and are expressed in all types of cells [113]. In mammalian cells, the integrin receptor family contains 18 α and 8 β subunits that link non-covalently in at least 24 known combinations of αβ subunit heterodimers [114, 115]. Integrin ligation induces promigratory intracellular signaling cascades from the “outside in”. On the other hand, activation from “inside out” also occurs by virtue of soluble signals promoting protein and inositol phosphorylation events that regulate formation of nascent focal contacts. Integrins cluster on the cell surface in focal adhesion complexes containing signaling adapter molecules and cytoskeletal structures. Through integrating the insoluble ECM with the intracellular cytoskeleton, integrins transduce mechanical signals that influence gene expression.
There is convincing evidence that integrins are redox-regulated [116–120]. Conformational changes in the integrin structure suggest a mechanism for this redox-regulation of integrin activity. Cysteine residues within the genu domain and calf-2 domain of the integrin α-subunit have been considered to be the redox-regulated sites within the integrin ectodomain [121]. For example, integrin α7β1 is oxidized by H2O2 derived from NOX4 at two specific cysteine residues [121]. The cysteine residues in each EGF-domain of the β integrin subunit, which stabilize the stalk domains [122–125], do not appear to be oxidized by H2O2 [121]. Overall, redox-dependent cleavage of the α-subunit influences the transition between a bent/inactive and extended/active conformation of the integrin ectodomain [121]. These conformational transitions induce the physical separation of the two stalks and lead to changes within the transmembrane and cytoplasmic domains [126, 127], which transduces a signal to integrin-associated molecules [112, 128, 129].
In addition to direct oxidative modifications, integrins are also indirectly affected by ROS-mediated regulation of their expression. In liver sinusoidal endothelial cells exposed to prolonged high glucose, integrin αvβ3 and laminin expression are upregulated, and this response is significantly inhibited by N-acetyl-cysteine (NAC), suggesting that the expression of αvβ3 is dependent on ROS levels in these cells [130]. In addition, ROS increase αv and decrease α5 expression in endothelial cells, affecting adhesion to vitronectin or fibronectin [131]. In VSMCs, downregulation of the Nox4 activator Poldip2 increases β1 integrin expression, leading to increased collagen I expression [132].
Rho family GTPase oxidation
Rho family GTPases act as molecular switches, “on” while GTP-bound and “off” while GDP-bound, whose primary function within the cell is to regulate the actin cytoskeleton [133, 134]. Of these GTPases, RhoA, Rac1, and Cdc42 are the best studied, and all three are required for cell migration and proliferation (Figures 2 and 4). During cell division, RhoA enhancement of linear actin polymerization and myosin II activation is essential for cell rounding and contractile ring ingression, and it is equally important that the opposing functions of Rac1 (actin branching and myosin II inactivation) are turned off during these processes (Figure 4) [92, 135]. Rho GTPases are also central regulators of cell migration. Their complex role in this process has been recently reviewed [136], but in brief, Rac1 and Cdc42 generate cell protrusions, while RhoA generates the force necessary to move. This activity is tightly regulated by a number of proteins. Guanine nucleotide exchange factors (GEFs) activate GTPases by promoting the release of nucleotide from GTPases. Since GTP is ten times more abundant than GDP within the cell, nucleotide release promotes GTP binding and enhances downstream effector binding. GTPase activating proteins (GAPs) catalyze GTP hydrolysis to GDP to turn the GTPase “off.” Post-translational modifications also regulate Rho GTPase activity. Prenylation of the C-terminus of the GTPase is required for membrane localization and Guanine Nucleotide Dissociation Inhibitors (GDIs) bind to the prenyl group to stabilize the GTPase and prevent membrane association. Phosphorylation can activate and inactivate specific Rho GTPases (reviewed in [137]). Similarly, oxidation can both enhance and inhibit Rho GTPase activity, as detailed below.
The Rho family of GTPases consists of 20 proteins; about half of which contain a conserved redox sensitive motif GXXXXGK(S/T)C, including a cysteine that is solvent-exposed and in contact with bound nucleotide [138]. In vitro, O2•−, nitrogen dioxide (•NO2), or HO• treatment enhances GDP dissociation from RhoA, Rac1, and Cdc42 400- to 600-fold, while NO or H2O2 treatment enhances exchange approximately 5- and 10-fold, respectively [138]. Addition of radical scavengers allows re-binding of the nucleotide [138]. This nucleotide release is likely due to disruption of hydrogen bonds between the GTPase and nucleotide. Therefore, in cellulo, where the reducing potential is high and GTP is abundant, ROS/RNS should promote release of bound nucleotide, similar to a GEF, to activate Rho GTPases [139].
Such a scenario is the case for Rac1, which has been shown to be activated by ROS/RNS both in vitro [138, 140] and in cellulo [139–142]. Early work by Heo and colleages [138] showed that the dramatic increase in Rac1 nucleotide dissociation induced by O2•− in vitro has no effect on a Rac1 C18S mutant, suggesting that activation of Rac1 is due to direct oxidation at Cys18. Upon oxidation of cysteine thiols to sulfenic acid, cysteines become highly reactive with glutathione and S-glutathiolation is commonly observed [143]. Therefore, it is not surprising that in cellulo Rac1 is glutathiolated, and that mass spectrometry analysis of purified Rac1 in the presence of oxidized glutathione suggests that this occurs at Cys18 [140]. Purified glutathiolated Rac1 has a 200-fold enhanced rate of nucleotide exchange compared to non-oxidized Rac1 [140].
Unlike Rac1 and Cdc42, RhoA has an additional cysteine (Cys16) in its redox sensitive motif, GXXXCGK(S/T)C, that complicates GTPase activation [138, 144]. Upon •NO2 treatment (3–5 μM), oxidation of the GTPase can be observed by mass spectrometry as a disulfide bond formed between Cys16 and Cys20 [144]. This disulfide bond can only form when the GTPase is nucleotide-free; however, once the disulfide bond has formed, RhoA is unable to bind nucleotides or interact with the GEF, Vav2 [144]. Therefore, it has been proposed that at low levels of ROS, Cys20 is oxidized which promotes nucleotide exchange to activate RhoA; however, at higher levels of ROS disulfide bonds form between Cys16 and Cys20 to inactivate RhoA [144]. Consistent with the joint interaction of Cys16 and Cys20 reducing RhoA activity, treatment of RhoA with phenylarsine oxide (PAO), which crosslinks neighboring thiol groups, inactivates RhoA [145]. Further complicating the matter, NO treatment of recombinant RhoA has been described to cause S-nitrosylation and inactivation of RhoA, yet it is unclear on which cysteines this occurs [146].
Under normal cellular conditions, with a high reducing potential and abundant GTP, physiological levels of peroxide activate RhoA seemingly via direct oxidation [139]. Aghajanian and collegues [139] knocked down RhoA and then expressed wild type or C16/20A mutant RhoA in fibroblasts. Upon stimulation of these cells with various concentrations of H2O2 (0.1–10 μM), only WT RhoA was activated, suggesting that redox-sensitive Cys16 and/or Cys20 is required for H2O2-induced activation of RhoA. Importantly, the C16/20A mutant was still able to be activated by GEF-mediated stimulation and inhibited by C3 toxin, indicating that the ROS-induced activation of RhoA was driven by direct oxidation of these residues [139]. Despite this observation, controversy remains within in cellulo literature, some of which shows that RhoA is activated by ROS/RNS [139, 147–153] and others of which describe inhibition or reduced expression of RhoA by ROS/RNS [145, 146, 154, 155]. The controversy may be partially due to the fact that in addition to direct oxidation, ROS/RNS affect many regulators of Rho family GTPases. For example, p190RhoGAP is activated when low molecular weight PTP is oxidized [155], but p115RhoGEF is also activated indirectly by OONO- and H2O2–induced kinase activation [148], and ROS likely also cause release of RhoGDI from RhoA [156]; therefore, the outcome of redox signaling is hard to predict with respect to RhoA activity, and is likely specific to cellular conditions.
Although the effect of Rho family GTPase oxidation on migration and cell division has not been directly examined, it is clear that Rho family oxidation controls the cytoskeleton, because only cells expressing WT RhoA and not oxidation-resistant C16/20A RhoA showed increased stress fibers in response to H2O2 [139]. Similarly, expression of the oxidation-mimetic C18D Rac1 induces significantly more lamellipodia compared to wild type Rac1 [140]. Since ROS promote excessively high levels of Rac1 activity, one would predict that this should inhibit cell proliferation. RhoA oxidation is more complex, depending on the level of ROS and if both cysteines or just one is oxidized. Therefore, disentangling the redox regulation of RhoGTPases during migration and proliferation is an area ripe for investigation. Since RhoA activity [157], ROS production [158], and VSMC proliferation and migration [159] are all elevated in atherosclerotic plaques, this would be a particularly interesting system to use to determine the relevance of Rho GTPase oxidation.
IQGAP oxidation
IQGAP is a Rac1 and Cdc42 effector with numerous roles in actin and microtubule organization that is highly expressed in endothelial cells [160]. In brief, it can directly bind to actin to facilitate actin cross-linking and inhibit polymerization, and can also regulate many other cytoskeletal proteins. For example, it activates N-WASP to promote actin branching and binds mDia to promote actin elongation [160]. IQGAP1 localizes to the leading edge of migrating cells, where it enhances filopodia and lamellipodia formation and migration [161]. Of interest, IQGAP1/3 also appears to be required for proper localization of RhoA to the cleavage furrow during telophase, and its suppression leads to impaired cytokinesis [162].
Several years ago, Kaplan and colleagues [163] reported IQGAP oxidation following vascular endothelial growth factor (VEGF) treatment and hindlimb ischemia. Additionaly, using DCP-Bio1 (a compound that binds to cysteine sulfenic acid) for immunofluorescence, they found that cysteine sulfenic acid co-localized with IQGAP, p47phox (a regulatory component of NOX2), and F-actin at the leading edge of migrating cells, suggesting that these proteins are likely oxidized at this location [163]. Previous studies showed that in migrating cells, IQGAP recruits NOX2 to the leading edge to stimulate ROS production there [160, 164]. Therefore, it seems that IQGAP both enhances ROS production and is a target of ROS production during cell migration. However, it remains unclear exactly how IQGAP oxidation regulates its many functions.
Protein tyrosine phosphatase oxidation
One important class of cytoskeleton-related enzymes with extremely low pKa active-site catalytic cysteine residues is protein tyrosine phosphatases (PTPs), which have high sensitivity to redox inactivation. PTPs regulate cytoskeletal signal transduction pathways and participate in cell cycle control by dephosphorylating proteins [155, 165, 166]. Recently, oxidation has emerged as an important regulatory mechanism of PTPs. The oxidation of PTPs has been demonstrated for PTP1B [167], Src homology phosphatase (SHP)-2 [168], PTEN [169], receptor-PTPα [170], and low-molecular-weight protein tyrosine phosphatase (LMW-PTP) [171, 172]. LMW-PTP is involved in the control of mitogenic and adhesive signals [173, 174] and its redox regulation shows a peculiarity above all other PTPs. Two of the eight cysteine residues present in LMW-PTP are modified by H2O2 treatment, namely Cys12 and Cys17 [175]. Normally, activation of its receptor by the pro-migratory platelet-derived growth factor (PDGF) is limited by the LMW-PTP, but in the presence of ROS, LMW-PTP is modified by direct oxidation on Cys12 and Cys17 to form an inactivating disulfide bond, thus amplifying PDGF signaling [171, 176]. Another study showed that S-glutathionylation of LMW-PTP regulates VEGF-mediated focal adhesion kinase activation [177], an important step in focal adhesion formation. These examples suggest that oxidative regulation of LMW-PTP is necessary for efficient adhesion and migration.
Src oxidation
Src family kinases are critically involved in the control of cytoskeletal organization and in the generation of integrin-dependent signaling responses in fibroblasts, inducing tyrosine phosphorylation of many signaling and cytoskeletal proteins [178]. Redox regulation of Src is a central feature of cell adhesion, as the elimination of ROS by antioxidant treatment almost completely abolishes Src activation in this situation [179]. This observation is further supported by the ability of antioxidants to inhibit p125FAK/Src association and p125FAK phosphorylation and is in agreement with data showing that ROS are necessary for integrin signaling during fibroblast adhesion and spreading [180, 181]. Src itself is redox sensitive, since each Src domain contains cysteine residues with pKa’s low enough to be oxidized by physiological levels of ROS [182, 183]. Direct oxidative modifications on Src have been reported on, for instance, Cys277 [184] in the linker resion, Cys245 [179], Cys122 and Cys164 [185] in the SH2 domain, and Cys487 [179] in the kinase domain. Oxidation of cysteine residues in the SH2 domain causes inter-molecular disulfide bridge formation and disruption of internal Tyr527-SH2 domain interaction; this conformational change promotes auto phosphorylation at Tyr416 and leads to activation of Src [186]. Oxidation of Cys245 and Cys487 further activates an already active Src [179]. On the other hand, oxidative inhibition of the Src-specific PTP, which de-phosphorylates Tyr418 in the activation loop [187], and oxidative regulation of C-terminal Src-Kinase (CSK), which phosphorylates the auto-inhibitory C-teminal Tyr527 [185], also influences Src activity. In cell-based studies it is often difficult to differentiate the direct effect of redox regulation on Src activity from the indirect effects through Src regulators. Because both activation [179] and inactivation [188, 189] of Src in response to oxidative stress have been reported, is not clear whether direct oxidation of Src or simply inactivation of the PTPs that dephosphorylate Src predominates.
Focal adhesion kinase activation by oxidants
Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase important in integrin signaling pathways controlling VSMC migration and proliferation [190]. ROS induce activation of FAK and promote cell motility in endothelial cells in a dose- and time-dependent manner [191]. FAK consists of a central catalytic domain, which is flanked by large amino-and carboxy-terminal regions. The amino-terminal sequence binds the β1-integrin intracellular domain and the carboxy-terminus contains two proline-rich sequences that bind to SH2 domain-containing proteins, such as Src, paxillin or Graf [192, 193]. Activation of FAK is associated with its phosphorylation on several specific tyrosine residues, Tyr397, Tyr925, and Tyr577 [194]. FAK is autophosphorylated on Tyr397 when bound to activated β1-integrins, which is crucial in cell migration and cell cycle progression [192, 195, 196]. It has been shown that ROS induce tyrosine phosphorylation of FAK on Tyr397, Tyr925, and Tyr577, leading to increased kinase activity. The phosphorylation on Tyr397 was observed in both actin-rich and membrane-cytosol fractions, indicating that ROS-induced FAK phosphorylation is likely involved in adhesion [197]. This phosphorylation by ROS was suggested to occur through tyrosine phosphatase inhibition rather than activation of tyrosine kinases [191, 197]; however, ROS-induced phosphorylation of FAK on certain tyrosine residues is partly sensitive to tyrosine kinase inhibitors, suggesting that alternative mechanisms may contribute [191], such as ROS-induced Src activation [198]. The complexity of this pathway, with multiple redox-sensitive kinases and phosphatases but no known direct oxidation, makes it imperitive to study redox activation of FAK in different cells and cellular context.
Protein kinase C oxidation
The protein kinase C (PKC) family is composed of serine/threonine protein kinases that are involved in a variety of pathways to regulate cell growth, adhesion, migration, differentiation and gene transcription [199–201]. Traditionally, PKC isoforms are activated by Ca2+, which itself is regulated by ROS, and diacyglycerol (DAG), and require phosphorylation on serine/threonine for full activation [202]. In recent years, ROS, especially H2O2, have been proposed to be an upstream activator of PKC [203, 204]. For example, PKC can be activated by Ca2+ induced by ROS and mediates Rho GTPase activation during focal complex formation in the early stage of cell spreading [153, 201, 205]. In bovine pulmonary artery endothelial cells, cyclic strain stimulates mitochondrial ROS release, which induces PKC-dependent phosphorylation of FAK [206]. Conversely, many studies have found that PKCs regulate NOX1-3 by phosphorylating the indispensable subunit p47phox [207–209]. Taken together, these studies suggest that PKCs are both upstream and downstream of ROS.
Several reports indicate that PKC contains redox-sensitive cysteines [210, 211]. PKCs have two pairs of zinc fingers within their regulatory domains, the sites of DAG binding. Each zinc finger is composed of six cysteine residues and two zinc atoms, which render the regulatory domain susceptible to redox regulation [202, 210]. The C-terminal catalytic domains of PKCs also contain cysteine residues that are sensitive to oxidative modifications and affect kinase activity [212, 213]. For instance, thiol oxidation and release of zinc from zinc finger motifs are neccssary for O2•−-mediated activation of PKC [213]. However, the redox regulation of PKC is complex. While ROS such as O2•− [214, 215] and H2O2 [216] activate PKC, hydroxyl radical decreases PKC activity [217]. Furthermore, even when mild ROS activate PKC, prolonged exposure leads to further oxidation and inactivation [203]. Because activation of PKC is regulated at multiple levels, including phosphorylation, membrane insertion [218, 219], and proteolytic cleavage [220], the full range of effects of oxidation is unclear and remains to be further explored.
Oxidation of calcium channels and transporters
Cytosolic homeostasis of Ca2+ is essential for cytoskeletal-mediated cell behaviors, most notably cell contraction, migration and growth, and several ion channels and transporters that regulate Ca2+ flux have been shown to be responsive to oxidation. Cytosolic Ca2+ influx is regulated by L-type voltage-gated Ca2+ channels (LTCC), which contain an α1C-subunit that is sensitive to inactivation by oxidation [221–223]. Inositol 1,4,5-trisphosphate receptor (IP3R) is another major Ca2+ channel that releases Ca2+ from the sarcoplasmic reticulum (SR) into the cytosol and is conformationally regulated by oxidative species [224]. Oxidation of Cys34-Cys42 of the IP3R reduces its activity in line with the conformational changes, indicating that Cys34 and Cys42 may serve as reduction sensors. In addition, it has been suggested that Cys65 may act as an oxidation sensor [224, 225].
Calcium reuptake, and therefore reduction of cytosolic calcium, is also redox sensitive. Sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA), the major Ca2+ reuptake effector on SR, contains almost 25 cysteine residues that are easily oxidized, especially Cys674, which is the most redox-sensitive. Reversible S-glutathiolation of Cys674 enhances the SERCA activity [226–228]. However, irreversible oxidative modifications including sulfonylation of Cys674 and nitration of Tyr294/295 decrease SERCA activity [229]. The sodium-calcium exchanger (NCX) is another significant mechanism for cytosolic Ca2+ removal. NCX is mainly located on plasma membrane. It mediates efflux of one single Ca2+ ion in exchange for the import of three Na+ ions [230, 231]. NCX can be activated in the presence of ROS, such as H2O2 or that produced by xanthine oxidase with hypoxanthine in vitro [232–234]. However, strong ROS like hypochlorite inhibit NCX activity [235]. The potential mechanisms of these effects may involve disulfide bond formation by direct redox modification and phosphorylation by PKC [236, 237]. Moreover, the plasma membrane Ca2+-ATPase, which is responsible for a more modest cytosolic Ca2+ extrusion, is also suppressed by oxidative species [238, 239].
Although direct oxidative modification has effects on these Ca2+ channels and transporters, oxidative modification of upstream kinases plays a role in Ca2+ regulation as well. In particular, LTCC [240–242] and NCX [236, 237, 243] are substrates of protein kinases such as PKC (see above) and Ca2+/calmodulin-dependent protein kinase II (CAMKII, see below), which both can be regulated by ROS. The complexity of the intracellular Ca2+ system, together with multiple levels of redox sensitivity, makes it challenging to separate the effects of direct oxidation by ROS and redox-sensitive kinase activity on Ca2+ channels and transporters.
Calmodulin oxidation and calcium/calmodulin dependent protein kinase II activation by oxidants
Calmodulin (CaM) is a Ca2+ binding protein that mediates many effects of Ca2+. For example, the formation of the Ca2+/CaM complex activates MLCK, which in turn regulates actin-myosin interaction and VSM contraction [244]. CaM contains four EF-hand Ca2+ binding sites and at least six redox-sensitive methionines. While oxidation of Met36, Met51, Met71, Met72 and Met145 lead to CaM degradation [245, 246], oxidation of Met144 and Met145 affects the tertiary structure of the C-lobe, resulting in destabilization and decreasing substrate affinity [247–249].
CaMKII is a CaMK isoform with many functions, as it phosphorylates various proteins involved in migration [250], proliferation [251] and ion channel activity [252] in many vascular cells. CaMKII is activated by CaM binding to its autoinhibitory regulatory domain [253–255]. The methionines on CaMKII are also sensitive to oxidative species. Erickson et al. [256] reported that oxidative modification on Cys280/Met281 in the α isoform or Met281/282 in the β, γ, and δ isoforms leads to increased CaMKII activation. Methionine sulfoxide reductase A (MsrA) inactivates CaMKII by reducing oxidized methionine residues [256]. Moreover, it has been suggested that Ca2+ and CaM are required for oxidative modification on CaMKII [256].
Based on current knowledge, Ca2+ and CaM are more potent regulators of CaMKII activation than direct oxidative modification [257–260]. How CaM is oxidized in vivo and how oxidation might affect its interaction with CaMKII remains unclear. Since both CaM and CaMKII play important roles in calcium/CaM signaling and affect a wide range of Ca2+-mediated cellular functions [261, 262], further investigation of the interplay of oxidation with activation/inactivation of this complex is warranted.
REDOX REGULATION OF THE ACTIN CYTOSKELETON IN CELL PHYSIOLOGY
Cell adhesion and migration
Mounting evidence suggests that ROS, particularly H2O2 derived from NADPH oxidases, are necessary for cell movement [17, 263]. When cell surface receptors like receptor tyrosine kinases (RTKs) and G-protein-coupled receptors (GPCRs) bind to extracellular stimuli such as growth factors and chemoattractants, ROS are generated at the cell surface as well as within intracellular compartments and react with specific proteins to regulate their activity and function [17, 20]. Early studies showed simply that ROS promote cell migration, because treatment with ROS-degrading enzymes suppressed growth factor- and chemoattractant-induced migration in endothelial cells, neutrophils, fibroblasts and VSMCs [264–268]. Moreover, it has been shown that ROS are produced during migration in these cells [264, 265, 267, 268]. In particular, PDGF one of the most potent migratory factors for VSMCs, activates NOX1 to produce H2O2, which is required for PDGF-induced migration [269–272]. Migration in response to this peptide is attenuated by pre-treatment with antioxidants such as NAC, the glutathione peroxidase (GPx) mimetic, ebselen, and catalase [269]. VSMCs from NOX1 deficient mice display reduced PDGF- and basic fibroblast growth factor (bFGF)-induced migration [273, 274], while VSMCs from NOX1 transgenic mice exhibit enhanced migration [273]. NOX4 has also been shown to be critical for VSMC migration in response to PDGF [147], and both NOX1 and NOX4 play a role in VSMC migration in response to other agonists such as insulin-like growth factor-I [134], VEGF [275] and angiotensin II [266].
Migration begins when a cell is exposed to an environmental signal such as a chemoattractant or growth factor [276, 277]. Initially, cell membrane extensions protrude from the plasma membrane either as spike-like filopodia or broad lamellipodia [278]. Protrusive forces, generated by actin polymerization and increased actin branching, are needed for the formation of these structures at the leading edge of the cell [16]. Integrins on the tips of these lamellipodia mediate the first contacts with the substrate and thus stabilize the membrane extensions. If appropriate integrin ligands are sensed, signals are conveyed into the cells, resulting in recruitment of further integrins to these sites and the assembly of cytoskeletal and signaling molecules into focal contacts, which consequently mature into focal adhesions [279, 280]. Next, cell contraction via engagement of actin-myosin interactions occurs, facilitated by the Rho signaling pathway [156]. Focal adhesions mature and strengthen at the leading edge, while their dissolution in the rear allows the cell to contract and move forward [281]. Redox regulation of the actin cytoskeleton and its associated proteins is highly integrated into the entire cycle of migration, beginning with lamellipodium formation [282]. The growth factor or chemoattractant binds receptor tyrosine kinases or G protein-coupled receptors and activates a GEF and consequently Rac and CDC42 [276, 283, 284]. Rac and CDC42 separately activate WASP homologue (WH) domain-containing proteins neural WASP (NWASP) and WAVE, activating the ARP2/3 complex and resulting in extension and branching of F-actin [285–287] (Figure 2). Because both Rac and CDC42 are redox-sensitive, it is very likely that branched F-actin formation is controlled by ROS. Another potential point of redox regulation is the involvement of cofilin in F-actin dynamics. Extension of F-actin requires free-barbed ends [288, 289], which cofilin provides by its severing function [290, 291]. Thus, free-barbed ends formed by cofilin activation, along with the released G-actin, enable formation of new actin filaments [292]. As noted above, cofilin itself can be directly oxidized. It was reported recently that elevation of H2O2 in migrating cells results in cofilin oxidation on Cys139 and Cys147. Oxidation-resistant cofilin impedes cell spreading, adhesion and directional migration [109]. This evidence is in line with recent studies that showed physiological oxidative modifications on cytoskeletal proteins are required for cell motility [69, 75, 76]. Perhaps more importantly, Slingshot1L (SSH1L) phosphatase, which activates cofilin by dephosphorylating its inactive form, is also mediated by ROS. In PDGF-treated VSMCs, H2O2 derived from NOX1 oxidizes the scaffold protein 14-3-3, leading to disruption of the SSH1L/14-3-3 complex and activation of SSH1L, activating cofilin [293, 294]. Cofilin is returned to its inactive form by LIMK-mediated phosphorylation [295]. LIMKs, in turn, are phosphorylated and activated by Rho and its downstream kinase ROCK as well as Rac and its downstream kinase p21-activated kinase (PAK) [295, 296] (Figure 2). Because Rho and Rac are redox-sensitive, LIMK is also likely to be dependent upon ROS, although this possibility has not yet been studied in detail.
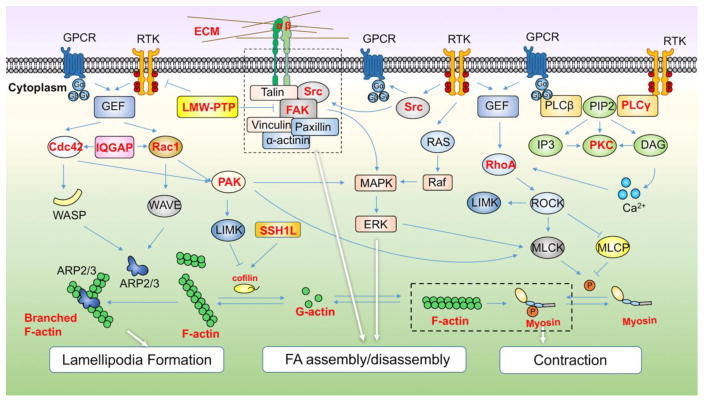
Figure 2. The actin cytoskeleton signaling network controlling cell motility and its redox regulation.
Cell migration consists of cycles of lamellipodia formation, focal adhesion assembly at the leading edge, contraction of the cell body and de-adhesion and retraction at the rear edge. The signaling pathways that have been implicated in cell adhesion and migration are shown., including cell division control protein 42 homolog, Cdc42; Wiskott-Aldrich syndrome protein, WASP; WASp family verprolin-homologous protein, WAVE; actin-related protein 2/3, ARP2/3; protein kinase C, PKC; Rho-associated protein kinase, ROCK; LIM domain kinase, LIMK; myosin light chain kinase, MLCK; p21-activated kinase, PAK; protein tyrosine phosphatases, slingshot-1L phosphatase, SSH1L, low molecular weight PTPs, LMW-PTPs, myosin light chain phosphatase, MLCP; Rho GTPases and guanine nucleotide exchange factors (GEFs); phospholipase C β/γ (PLC β/γ); phosphatidylinositol (4,5)-bisphosphate (PIP2); inositol 1,4,5-trisphosphate (IP3); diacylglycerol (DAG). In this diagram, directly oxidized proteins are indicated by bold in red.
The next step in migration—leading edge attachment to the substrate—is mediated by integrin binding, a phenomenon that has been closely linked to ROS [115]. Integrin engagement occurs upon binding with specific sequence motifs of extracellular matrix proteins [115, 281]. Subsequently, the activated integrins recruit actin binding proteins such as talin [297], paxillin [298], vinculin [299], and α-actinin [300], as well as signaling proteins such as Src, FAK and integrin-linked kinase [301], creating focal adhesions (Figure 2). In fibroblasts, ROS are dramatically increased due to NOX and 5-lipoxygenase activation during the adhesion process [181]. Because integrin signaling pathways contain a range of redox-sensitive kinases, phosphatases, GTPases and transcription factors, altered ROS levels likely affect focal adhesions at multiple levels. For example, inhibition of LMW-PTP by direct oxidation enhances FAK and focal adhesion maturation [181]. Two other important redox-sensitive protein kinases involved in integrin-mediated signaling are Src and FAK [302, 303] (Figure 2). PDGF activates Src during VSMC migration, and inhibition of Src attenuates PDGF-induced VSMC migration [302]. Integrin-mediated activation of FAK leads to phosphorylation of paxillin, thus regulating its translocation to focal adhesions and promoting focal adhesion formation [303]. At the same time, autophosphorylation of FAK on tyrosine 397promotes its association with Src [304, 305]. This phosphorylation is essential for FAK-induced focal adhesion disassembly, which is important as the cell begins to move [306]. Thus, it has been suggested that FAK–Src signaling interrupts focal adhesion maturation by promoting disassembly and in turn promoting adhesion turnover as the cell extends [307]. Since H2O2 induces FAK autophosphorylation and activates Src, ROS are almost certainly physiological mediators of focal adhesion turnover. Furthermore, FAK–Src signaling [308] leads to the activation of several downstream signaling cascades, including the mitogen-activated protein kinase (MAP kinase)/ERK pathway [309], which in turn phosphorylates and activates MLCK [310] and alters the rate constant of FA disassembly [311]. Although MAPK/ERK are not known to be directly modified by ROS in this context, they experience redox regulation by ROS-activated upstream molecules such as Src, PKC and PAK [312–314] (Figure 2).
In addition to influencing focal adhesion disassembly, actin-myosin interactions regulate contraction of the cell body to propel forward movement. Ca2+-dependent activation of MLCK is the major mechanism regulating VSMC contraction, but Rho GTPases also fine tune actin-myosin function [281] (see Contraction below for further details). ROS have been extensively linked to activation of RhoA/ROCK signaling in rat aorta [153] and in pulmonary VSMCs and endothelial cells [153, 315, 316]. ROCK phosphorylates the myosin binding subunit, myosin light chain phosphatase (MLCP), reducing its activity [317]. ROCK also acts in cooperation with MLCK to phosphorylate myosin II during migration [318]. PAKs, downstream of Rac, negatively regulate myosin II phosphorylation by phosphorylating MLCK, but also phosphorylates myosin II directly, leading to actomyosin interaction [319] (Figure 2). This mechanism may be ROS-regulated, because PAK activation in VSMCs is dependent on NOX1-generated ROS [273]. However, how ROS-specific modification of these proteins interacts with phosphorylation signals remains to be determined.
In summary, based on the known redox-sensitivity of many cytoskeleton-related signaling molecules, as well as whole cell studies using antioxidants to inhibit migration, a clear role for targeted, specific redox regulation of migration exists (Figure 2). It is likely that cell migration occurring during both normal and pathological processes is regulated by ROS via effects on actin dynamics [320–322]. Thus, further investigations of the specific targets of ROS and how they are modified during migration of all vascular cells types is in order.
Cell contraction
Contraction of VSMCs is integral to control of vessel tone and blood pressure, and there is increasing evidence that ROS are involved in cell contraction pathways. Since Heinle [323] showed that exogenous H2O2 application induces vasoconstriction of carotid artery, it has been demonstrated that both exposure to ROS and selective depletion of endogenous ROS alter cell contractility [324]. The specific roles of ROS in VSMC contraction remain unclear, although there are several likely molecular targets. Under oxidative conditions, ROS act both upstream and downstream of intracellular Ca2+ release and cytosolic Ca2+ influx. ROS increase the open probability of membrane Ca2+ channels and increase Ca2+ release [325–327] to promote contractile bundle formation. It should be noted that most studies report that higher concentrations of ROS suppress force [328, 329]; however, mounting evidence shows that low levels of ROS increase force [324, 325, 329].
Although contractile mechanisms differ among cells and tissues, the most well-established model of cell contraction relies on actin-myosin cross-bridge cycling driven by ATP hydrolysis (Figure 3). This pathway is present in striated muscle as well as in nonmuscle cells. The repeated cycles begin with myosin activation, which occurs via phosphorylation of the myosin light chain by MLCK, a Ca2+/calmodulin-dependent process [330]. As the myosin head crawls along actin filaments, ATP is hydrolyzed. The energy produced during this process induces a conformational change in myosin, leading to continued cycles of actin-myosin complex formation, ATP hydrolysis and muscle contraction [95]. Actin-myosin complex formation is regulated by two accessory proteins bound to actin filaments, tropomyosin and troponin. In non-muscle and smooth muscle cells, the actin contractile bundles are associated with tropomyosin [317]. Here, we mainly focus on redox regulation mechanism of contraction in VSMCs (Figure 3).
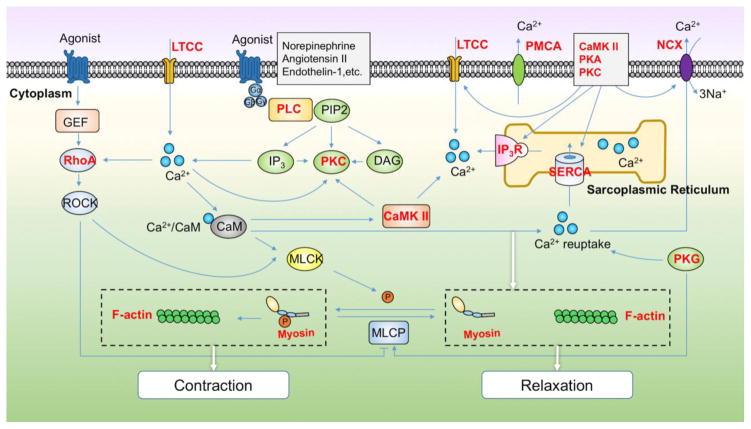
Figure 3. The actin cytoskeleton signaling network controlling cell contraction and its redox regulation.
Cell contraction is induced when agonists such as norepinephrine or angiotensin II bind to receptors and activate phosphoinositide-specific-phospholipase C (PLC) to catalyze the formation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol (4,5)-bisphosphate (PIP2). Meanwhile, Ca2+ influx induced by voltage-gated Ca2+ channels (LTCC) along with inositol 1,4,5-trisphosphate receptor (IP3R) activation inducing release of Ca2+ from the endoplasmic reticulum, promotes Ca2+ /calmodulin (CaM) activation of the actin-myosin complex. Decreased intracellular Ca2+ concentration achieved by inactivation of LTCC, activation of Ca2+ reuptake by the sarco-/endoplasmic reticulum Ca2+ -ATPase (SERCA), and activation of Ca2+ extrusion by the sodium-calcium exchanger (NCX) and plasma membrane Ca2+-ATPase (PMCA) results in cell relaxation by reducing Ca2+ and disrupting actin-myosin interaction. These processes are also regulated by kinases (calmodulin-dependent protein kinase II, CaMKII; Rho-associated protein kinase, ROCK; myosin light chain kinase, MLCK; protein kinase C, PKC; protein kinase A, PKA; protein kinase G, PKG) and phosphatases (myosin light chain phosphatase, MLCP), Rho GTPases and Guanine Nucleotide Exchange Factors (GEFs). In this diagram, directly oxidized proteins are indicated by bold in red.
Cell contraction is induced by multiple stimuli (Figure 3). When agonists such as norepinephrine and angiotensin II bind to G-protein coupled receptors, or growth factors bind to RTKs, phospholipase C (PLC) is activated. Phospholipase Cγ in particular is a redox-sensitive protein activated by recruitment of its Src homology domains to phosphotyrosine residues on activated RTKs [331]. In contrast, PLCβ isoforms, which are activated by GPCRs, do not have SH2 domains, are not regulated through tyrosine phosphorylation, and are not redox-sensitive enzymes [331]. Activation of PLCs catalyzes the formation of IP3 and DAG. IP3 binds to receptors in the SR to release Ca2+ into the cytosol. Of note, the IP3R is targeted to proteasome degradation by H2O2 [332], resulting in a decrease in Ca2+ efflux from the SR. DAG, along with Ca2+, activates the redox-sensitive PKC to promote downstream contractile signaling [95] (Figure 3). How oxidants interact with PKC during contraction is unknown. Increases in cytosolic Ca2+ promote the binding of CaM to MLCK, resulting in phosphorylation of MLCK and actin-mysoin assembly. The small G protein RhoA and its downstream effector ROCK can also regulate the activity of MLC by promoting MLCK and inhibiting MLCP, as noted above [317] (Figure 3). ROS-induced vascular smooth muscle cell contraction mediated via activation of Rho/ROCK has been demonstrated by several experimental approaches [139, 153, 315, 333, 334].
Redox regulation of ion channels also has a well-established role in contraction. LTCCs open, Ca2+ influx occurs, which, together with IP3R activation, induces Ca2+ release from the SR [330] (Figure 3). It has been shown that oxidative modifications suppress the activity of these channels [335], while ROS-regulated activation of CaMKII [336], PKA [337], and PKC [240] lead to an increase in Ca2+ current by phosphorylation of these channels.
Cell relaxation occurs as a result of decreased intracellular Ca2+ due to inactivation of LTCC and activation of SERCA and NCX as well as PMCA to re-uptake Ca2+ into the SR, and increased MLCP activity [330] (Figure 3). This complicated process is mediated by a variety of redox-sensitive protein kinases and phosphatases such as CaMKII, PKA, PKC, among others [255, 338, 339]. As with cell migration, establishing the relationship between regulation of these molecules by direct oxidative modification and phosphorylation is challenging.
Finally, it should be noted that redox regulation of NO-mediated vasodilation is well established. Aside from O2•−-mediated inactivation of NO itself, which inhibits vasodilation, activation of protein kinase G (PKG) to enhance relaxation can be achieved by S-nitrosylation of Cys42 [340] (Figure 3). This cGMP-independent mechanism of activation facilitates Ca2+ extrusion, MLCP activation and consequently relaxation [341].
Cell division and proliferation
Depending on the concentration and duration of ROS exposure, ROS are widely acknowledged to both promote and inhibit cell proliferation [342–344]. It appears that low levels of transient ROS promote proliferation, while higher levels and/or sustained ROS result in cell cycle arrest [344], and very high levels induce apoptosis [345, 346]. Indeed, depletion of the ROS generator NOX1 reduces cell proliferation, while NOX1 overexpression enhances proliferation [273] in an H2O2-dependent manner [347]. Similarly, treatment with ROS scavengers or knockdown of NOX2 or NOX4 to decrease endogenous ROS decreases endothelial cell proliferation [348], yet NOX4 can also induce VSMC differentiation via H2O2 to have the opposite effect [349, 350].
Integrin activation (necessary for anchorage-dependent growth) and growth factor binding both stimulate ROS production that is thought to act as a signaling molecule in a variety of pathways to regulate cell division [342–344]. Indeed, ROS levels are highest during mitosis and lowest during the G1 phase of the cell cycle [351–353]. ROS levels are especially high and diffuse in prophase, and while levels of ROS drop during cytokinesis, there appear to be discrete areas of high ROS. For example, a methodology paper by Hsiegh and colleagues [352] shows high ROS levels in what appears to be the intercellular bridge during late stages of cytokinesis.
While we have not been able to identify any reports of the actin cytoskeleton or its regulatory proteins being oxidized during proliferation, the actin cytoskeleton is intimately involved in cell division, as are many of the proteins discussed above that are known to be oxidized. With this in mind, we summarize here the indirect evidence for cytoskeletal oxidation in proliferation (Figure 4).
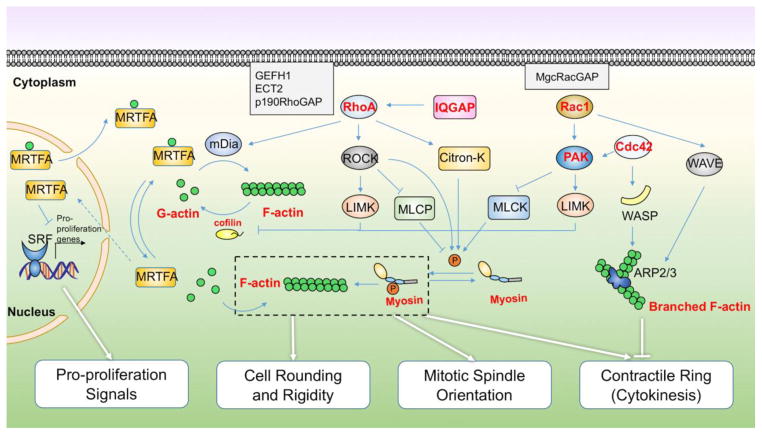
Figure 4. The actin cytoskeleton signaling network controlling cell division and its redox regulation.
The ratio of globular to filamentous actin within a cell regulates transcription of anti-proliferative genes; cell rounding at mitosis onset; mitotic spindle orientation and function; and contractile ring formation/cytokinesis completion. These processes are further regulated by transcriptional regulators (serum response factor, SRF; myocardin-related transcription factor, MRTFA), actin regulatory proteins (diaphanous-related formin-1, mDia; Cofilin; cell division control protein 42 homolog, Cdc42; Wiskott-Aldrich syndrome protein, WASP; WASp family verprolin-homologous protein, WAVE; actin-related protein 2/3, ARP2/3), kinases (Rho-associated protein kinase, ROCK; LIM domain kinase, LIMK; myosin light chain kinase, MLCK; citron kinase, Citron-K; p21-activated kinase, PAK), phosphatases (myosin light chain phosphatase, MLCP), Rho GTPases, guanine nucleotide exchange factors (Rho guanine nucleotide exchange factor 2, GEFH1; epithelial cell transforming sequence #2, Ect2), and GTPase activating proteins, of which many can be directly oxidized to regulate their function. In this diagram, directly oxidized proteins are indicated by bold in red.
The role of the actin cytoskeleton in cell division has been recently reviewed in detail [92]. The dynamic ratio of G- and F-actin within a cell regulates four main aspects of cell division: 1) transcription of pro-proliferative genes; 2) cell rounding at mitosis onset; 3) mitotic spindle orientation and function; and 4) contractile ring formation/cytokinesis completion (Figure 4). The first of these functions is related to the transcriptional co-factor myocardin-related transcription factor (MRTF-A). When actin is in its monomeric (globular) form it prevents MRTF-A nuclear localization via multiple mechanisms. Nuclear G-actin binds MRTF-A and enhances its nuclear export [354]. Cytoplasmic G-actin binds MRTF-A and sequesters it in the cytoplasm. However, polymerization of actin allows the release of MRTF-A, which then translocates to the nucleus to enhance transcription of serum response factor (SRF)-target genes [355]. Depending on a variety of factors (SRF phosphorylation, cell type, etc. [356, 357]), SRF can either enhance proliferation or promote VSMC differentiation. In general, MRTF-A/SRF signaling promotes the latter. This effectively inhibits pro-growth signaling of SRF, because MRTF-A competes for a common binding surface on SRF with co-activators that promote proliferation [358]. While there are exceptions, in general oxidation of actin on cysteine or methionines (by MICALs) causes the balance between G- and F- actin to be shifted towards G-actin [70, 71, 77, 78]. However, cofilin oxidation has the opposite effect, impairing cofilin’s severing functions and promoting F-actin [106–109]. Therefore, one would predict that actin oxidation will enhance cell proliferation, but cofilin oxidation will inhibit proliferation by promoting MRTF-A/SRF complex formation. However, a recent report suggests that it is more complicated than this. As expected, MICAL-2 oxidation of nuclear actin on Met44 promoted F-actin disassembly, yet surprisingly this promoted MRTF-A/SRF-driven gene expression, including cardiac muscle specific genes in developing zebrafish embryos [83]. Since differentiation and proliferation are antagonistic in muscle cells, this suggests that oxidation of nuclear actin by MICAL-2 may impair proliferation in these conditions. To complicate matters further, F-actin promotes nuclear entry of pro-proliferative transcriptional co-activators (e.g.YAP and TAZ), as well [359].
Separate from this probability, myosin II recruitment to the cell cortex generates intracellular tension that is required for cell rounding during mitosis [360]. This rounding is important for proper spindle assembly and efficient, error-free chromosome separation [361]. When myosin II is directly oxidized, its activity is inhibited [96]. Therefore, for proper cell division to occur, myosin II must be protected from the elevated levels of ROS during mitosis to enable cell rounding. Additionally, the interaction of F-actin with myosin II helps generate the forces necessary to promote contractile ring ingression and centrosome separation [92, 362]. Opposing this, branching of actin seems to inhibit contractile ring formation and thus cytokinesis [92]. Cdc42 and IQGAP, which promote actin branching, are both directly oxidized [138, 163], yet it is unclear how this affects their roles in cell division.
All of these functions of actin are tightly regulated spatiotemporally by numerous proteins (Figure 4). Cofilin binds to F-actin and severs it to shift the balance towards G-actin [359], while formins (such as mDia) speed the assembly of F-actin [134]. Cofilin and myosin II activities are regulated by kinases, which in turn are regulated by Rho family GTPases, which are activated by specific GEFs and turned “off” by GAPs at discrete locations during mitosis and cytokinesis. For example, during anaphase, the RhoGEF Ect2 is recruited to the mitotic spindle (and held in an active conformation) through binding MgcRacGAP [363]. This causes RhoA activation directly above the central spindle, which leads to formin-induced actin polymerization to generate the contractile ring. RhoA also activates ROCK to stimulate myosin II to pull on the actin filaments and cause furrow ingression [134]. Too wide an area of RhoA activation and/or Rac1 activation at the cleavage furrow leads to cytokinesis defects, demonstrating how important it is that these GTPases are tightly regulated spatiotemporally during this process [364]. As described above, both RhoA and Rac1 can be directly oxidized to activate these GTPases [92, 139] and RhoA can also be inhibited by high levels of ROS [144].
Unfortunately, the role of ROS in cell division is difficult to predict, since different concentrations and durations of ROS signaling can have very different effects [342–344], and ROS are transient signaling molecules that must be generated at the correct time and place to regulate cellular processes. The known role of ROS in growth, coupled with the established regulation of the cytoskeleton by ROS, clearly indicate the need for further investigation into how ROS regulate the cytoskeleton in the context of cell division.
REDOX REGULATION OF THE ACTIN CYTOSKELETON IN VASCULAR DISEASE
Vascular disease results in part from impaired vascular function caused by several vessel wall pathologies such as hypertensive vascular remodeling, atherosclerosis, restenosis and thrombosis [365–367]. A considerable amount of in vivo evidence suggests that ROS are involved in both hypertension and proatherogenic mechanisms and contribute to vascular dysfunction [368, 369]. Activation of NOXes has been demonstrated in a variety of animal models of vascular disease such as spontaneous hypertension [370], angiotensin-II-induced hypertension [371, 372], hypercholesterolemia [373] and atherosclerosis [374–376]. Many vasoactive agonists, such as angiotensin II, VEGF, PDGF, and thrombin [377–381] generate ROS. As discussed above, ROS regulate the actin cytoskeleton at multiple levels, impacting cell migration, proliferation and contraction [382]. All these process have distinct repercussions on the structure of vascular wall, suggesting that redox regulation of actin cytoskeletal signaling may play important roles in vascular remodeling and dysfunction.
In hypertension, vascular remodeling is characterized by rearrangement of vascular wall components like the actin cytoskeleton [383]. A decline in G-actin content, conforming to increased actin polymerization and F-actin formation was described [384, 385]. Actin polymerization is also believed to contribute to vascular constriction and resistance artery remodeling in hypertension [386, 387]. Disruption of actin filaments inhibits Ca2+ channels in VSMC from spontaneously hypertensive rats, resulting in dysfunction of vasodilation [388]. Several redox-senstive signaling pathways have also been suggested to link the cytoskeleton and vascular disease. For example, PKC-mediated vasoconstriction in cerebral arteries is highly dependent on cytoskeletal actin polymerization for force generation [389]. Although only a few studies have directly explored the relationship of ROS-induced cytoskeleton modification to vascular remodeling, most of the regulatory mechanisms have yet to be elucidated. The role of redox modification of cytoskeleton signaling must be considered in this context.
Studies in atherosclerosis showed that ROS modulate cytoskeletal changes in VSMCs [390, 391]. A reduction of actin binding proteins such as gelsolin and vinculin associated with actin disorganization was reported in the medial layer of human atherosclerotic coronary arteries [390, 391]. In animal models of vascular injury, superoxide dismutase attenuated neointima formation by inhibiting VSMC migration and proliferation [392, 393], potentially through down-regulation of the actin polymerization protein, mDia1 [392]. In a hypercholesterolemic rabbit model, changes in actin microfilaments initially promoted endothelial–substratum adhesion, but eventually led to a reduction of stress fibers and dysfunctional adhesion, with accumulation of macrophages and atherosclerotic plaque formation [394]. Several studies indicated that inhibition of actin filament polymerization interferes with vasodilator signaling in human coronary arterioles and the pulmonary circulation, also suggesting a pivotal role for endothelial cytoskeleton integrity in modulating endothelial-dependent vasodilator signal transduction [206, 395, 396]. However, none of these studies considered the potential role of redox regulation of the actin cytoskeleton in these outcomes, even though in every case ROS have been implicated in the response.
The redox-sensitive Rho family GTPases also play an important role in hypertension and atherosclerosis. Alterations in activity of RhoA/ROCK signaling have been proposed to contribute to the increased peripheral vascular resistance in hypertension [397–402]. Uehata et al [403] first reported a reduction of smooth muscle contraction and correction of hypertension in several hypertensive rat models by the specific ROCK inhibitor Y-27632. Recent studies showed that ROCK inhibition also prevented hypertrophic remodeling of coronary arterioles in spontaneously hypertensive rats [400, 404]. In addition, ROCK has been shown to participate in the pathogenesis of atherosclerosis [405], since long-term inhibition of ROCK can significantly reduce atherosclerotic lesion both in LDLr−/− mice and porcine models [406–408]. However, these studies did not explore the role of ROS in ROCK activation. Only Jin et al. [153] reported that Y-27632 relaxed rat aortic segments made to contract in response to experimental protocols that generate ROS. Because oxidative stress characterizes many models of vascular diseases [26, 409, 410], the intersection between ROS and the Rho/ROCK pathway should be further explored.
CONCLUSIONS AND FUTURE DIRECTIONS
While ROS regulate cell motility and proliferation, and direct oxidation of many of the actin/actin-regulatory proteins involved in these responses has been described, the precise role of oxidation of specific proteins in these critical cellular functions largely remains to be determined. Measurement of oxidation of specific cytoskeletal proteins during migration, proliferation and contraction is warranted. As mentioned above, different concentrations and durations of ROS signaling can have very different effects [342–344], and ROS are transient signaling molecules that must be generated at the correct time and place to regulate cellular processes. This suggests that measurement of oxidation of cytoskeletal proteins must be considered over time and in specific subcellular localizations. Tools are just now being developed that allow us to determine the subcellular localization of specific ROS generated throughout the cell cycle and during migration (for example organelle-targeted HyPer for H2O2 [411] or DCP-Bio to detect oxidized proteins in situ [412]). Additionally, it will be necessary to synchronize cells or use single cells to determine if each of the proteins described above is oxidized at specific times during migration, contraction and cell division. Oxidation-resistant mutants of these proteins should be tested for their ability to impair these processes in order to confirm a role for oxidation. Although many contributing factors lead to the development of vascular diseases [413], the potential role of oxidative modification of actin/actin-regulatory proteins appears to be a ripe area for investigation.
Highlights.
The actin cytoskeleton serves structural and signaling functions in vascular cells.
Actin, its associated proteins and upstream signaling molecules can be oxidized by reactive oxygen species induced by physiological or pathophysiological stimuli.
Redox-regulation of the actin signaling network is involved in cell migration, contraction and proliferation.
Redox modification of actin cytoskeletal proteins may be important in the development of vascular diseases
Acknowledgments
This work was supported by National Institutes of Health grants HL38206 and HL095070.
ABBREVIATIONS
- α
alpha
- β
beta
- γ
gamma
- G-actin
globular actin
- F-actin
filamentous actin
- ROS
reactive oxygen species
- O2•−
superoxide
- H2O2
hydrogen peroxide
- HO•
hydroxyl radical
- RNS
reactive nitrogen species
- NO
nitric oxide
- •NO2
nitrogen dioxide
- ONOO-
peroxynitrite
- NOXes
NADPH oxidases
- SOD
superoxide dismutase
- Cys
cysteine
- Met
methionine
- -SOH
sulfenic acid
- GSH
glutathione
- GSSG
glutathione disulfide
- RS-SR′
disulfide bond
- SNO
S-nitrosylation
- SO2H
sulfinic acid
- SO3H
sulfonic acid
- NF-κB
nuclear factor-κB
- AP-1
activator protein-1
- Hic-5
hydrogen peroxide-inducible clone-5
- MICALs
molecule interacting with CasL
- NM myosin II
non-muscle myosin II
- VSMCs
vascular smooth muscle cells
- MHC
myosin heavy chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- ROCK
Rho kinase
- ECM
extracellular matrix
- NAC
N-acetyl-cysteine
- GEFs
guanine nucleotide exchange factors
- GAPs
GTPase activating proteins
- GDIs
guanine nucleotide dissociation inhibitors
- PAO
phenylarsine oxide
- PTPs
protein tyrosine phosphatases
- LMW-PTP
low-molecular-weight protein tyrosine phosphatase
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- FAK
focal adhesion kinase
- CSK
C-terminal Src-kinase
- PKC
protein kinase C
- DAG
diacyglycerol
- LTCC
L-type voltage-gated Ca2+ channels
- IP3R
inositol 1,4,5-trisphosphate receptor
- SR
sarcoplasmic reticulum
- SERCA
sarco-/endoplasmic reticulum Ca2+-ATPase
- NCX
sodium-calcium exchanger
- CaM
calmodulin
- CAMKII
calmodulin-dependent protein kinase II
- RTKs
receptor tyrosine kinases
- GPCRs
G-protein-coupled receptors
- GPx
glutathione peroxidase
- bFGF
basic fibroblast growth factor
- WASP
Wiskott–Aldrich Syndrome protein
- SSH1L
slingshot1L
- PAK
p21-activated kinase
- MAPK
mitogen-activated protein kinase
- PLC
phospholipase C
- PKG
protein kinase G
- SRF
serum response factor
- MRTF-A
myocardin-related transcription factor
- YAP
yes-associated protein
- TAZ
transcriptional co-activator with PDZ-binding motif
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–92. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S, Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat Rev Mol Cell Biol. 2010;11:75–81. doi: 10.1038/nrm2818. [DOI] [PubMed] [Google Scholar]
- 3.Thomas TH, Advani A. Inflammation in cardiovascular disease and regulation of the actin cytoskeleton in inflammatory cells: the actin cytoskeleton as a target. Cardiovasc Hematol Agents Med Chem. 2006;4:165–82. doi: 10.2174/187152506776369926. [DOI] [PubMed] [Google Scholar]
- 4.McCain ML, Parker KK. Mechanotransduction: The role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch Eur J Physiol. 2011;462:89–104. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- 5.Meininger Ga. The central importance of the cytoskeleton for increased cell stiffness in cardiovascular disease. Focus on “Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation”. AJP Cell Physiol. 2014;307:C908–C909. doi: 10.1152/ajpcell.00279.2014. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Gupta S, Young D, Das B, McMahon J, Sen S. Impairment of ultrastructure and cytoskeleton during progression of cardiac hypertrophy to heart failure. Lab Invest. 2010;90:520–530. doi: 10.1038/labinvest.2010.43. [DOI] [PubMed] [Google Scholar]
- 7.Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45:273–278. doi: 10.1016/S0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 8.Wilson C, González-Billault C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front Cell Neurosci. 2015;9:381. doi: 10.3389/fncel.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farah ME, Sirotkin V, Haarer B, Kakhniashvili D, Amberg DC. Diverse protective roles of the actin cytoskeleton during oxidative stress. Cytoskeleton. 2011;68:340–354. doi: 10.1002/cm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vara D, Pula G. Reactive oxygen species: physiological roles in the regulation of vascular cells. Curr Mol Med. 2014;14:1103–25. doi: 10.2174/1566524014666140603114010#sthash.hhrzxJXX.dpuf. [DOI] [PubMed] [Google Scholar]
- 11.Förstermann U. Oxidative stress in vascular disease_causes, defense mechanisms and potential therapies. Nat Rev Cardiol. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 12.Mochida J, Yamamoto T, Fujimura-Kamada K, Tanaka K. The novel adaptor protein, Mti1p, and Vrp1p, a homolog of Wiskott-Aldrich syndrome protein-interacting protein (WIP), may antagonistically regulate type I myosins in Saccharomyces cerevisiae. Genetics. 2002;160:923–934. doi: 10.1152/physrev.00018.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–86. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estes JE, Selden LA, Kinosian HJ, Gershman LC. Tightly-bound divalent cation of actin. J Muscle Res Cell Motil. 1992;13:272–84. doi: 10.1007/BF01766455. [DOI] [PubMed] [Google Scholar]
- 15.Carlier M-F, Pantaloni D. Control of actin dynamics in cell motility. J Mol Biol. 1997;269:459–467. doi: 10.1006/jmbi.1997.1062. [DOI] [PubMed] [Google Scholar]
- 16.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 17.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016;2016:1–44. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taverne YJHJ, Bogers AJJC, Duncker DJ, Merkus D. Reactive oxygen species and the cardiovascular system. Oxid Med Cell Longev. 2013;2013:862423. doi: 10.1155/2013/862423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassègue B, Griendling KK. NADPH oxidases: Functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 21.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. \njphysiol.2003.049478 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veal EA, Day AM, Morgan BA. Hydrogen Peroxide Sensing and Signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 24.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90:491–493. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H Oxidase. Circ Res. 2000;86 doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 28.Stadtman ER, Levine RL. Protein Oxidation. Ann New York Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 29.Cai Z, Yan L-J. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J Biochem Pharmacol Res. 2013;1:15–26. doi: 10.1016/j.surg.2006.10.010.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 31.Fedorova M, Kuleva N, Hoffmann R. Reversible and irreversible modifications of skeletal muscle proteins in a rat model of acute oxidative stress. Biochim Biophys Acta. 2009;1792:1185–93. doi: 10.1016/j.bbadis.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Riederer BM. Oxidation proteomics: The role of thiol modifications. Curr Proteomics. 2009;6:51–62. doi: 10.2174/157016409787847448. [DOI] [Google Scholar]
- 33.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Murphy MP. Mitochondrial Thiols in Antioxidant Protection and Redox Signaling: Distinct Roles for Glutathionylation and Other Thiol Modifications. Antioxid Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 35.Charles RL, Schroder E, May G, Free P, Gaffney PRJ, Wait R, Begum S, Heads RJ, Eaton P. Protein Sulfenation as a Redox Sensor: Proteomics Studies Using a Novel Biotinylated Dimedone Analogue. Mol Cell Proteomics. 2007;6:1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A. 2008;105:8197–202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Janssen-Heininger YMW, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thom SR, Bhopale VM, Mancini DJ, Milovanova TN. Actin S-nitrosylation inhibits neutrophil beta2 integrin function. J Biol Chem. 2008;283:10822–10834. doi: 10.1074/jbc.M709200200. [DOI] [PubMed] [Google Scholar]
- 42.hai Zhang H, Wang W, Feng L, Yang Y, Zheng J, Huang L, bao Chen D. S-nitrosylation of cofilin-1 serves as a novel pathway for VEGF-stimulated endothelial cell migration. J Cell Physiol. 2015;230:406–417. doi: 10.1002/jcp.24724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Lechuga TJ, Tith T, Wang W, Wing DA, Chen D. S-Nitrosylation of Cofilin-1 Mediates Estradiol-17β-Stimulated Endothelial Cytoskeleton Remodeling. Mol Endocrinol. 2015;29:434–444. doi: 10.1210/me.2014-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy E, Kohr M, Sun J, Nguyen T, Steenbergen C. S-nitrosylation: A radical way to protect the heart. J Mol Cell Cardiol. 2012;52:568–577. doi: 10.1016/j.yjmcc.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohr MJ, Sun J, Aponte A, Wang G, Gucek M, Murphy E, Steenbergen C. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ Res. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Requejo R, Hurd TR, Costa NJ, Murphy MP. Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J. 2010;277:1465–1480. doi: 10.1111/j.1742-4658.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: Emerging roles in cell signaling. 2006 doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 49.Cooper AJ, Pinto JT, Callery PS. Reversible and irreversible protein glutathionylation: biological and clinical aspects. Expert Opin Drug Metab Toxicol. 2011;7:891–910. doi: 10.1517/17425255.2011.577738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coan C, Ji JY, Hideg K, Mehlhorn RJ. Protein sulfhydryls are protected from irreversible oxidation by conversion to mixed disulfides. Arch Biochem Biophys. 1992;295:369–78. doi: 10.1016/0003-9861(92)90530-a. [DOI] [PubMed] [Google Scholar]
- 51.Dalle-Donne I, Colombo G, Gagliano N, Colombo R, Giustarini D, Rossi R, Milzani A. S-glutathiolation in life and death decisions of the cell. Free Radic Res. 2011;45:3–15. doi: 10.3109/10715762.2010.515217. [DOI] [PubMed] [Google Scholar]
- 52.Xiong Y, Uys JD, Tew KD, Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–70. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- 54.Jacob C, Giles GI, Giles NM, Sies H. Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function. Angew Chemie - Int Ed. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 55.Lo Conte M, Carroll KS. The redox biochemistry of protein sulfenylation and sulfinylation. J Biol Chem. 2013;288:26480–26488. doi: 10.1074/jbc.R113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor SW, Fahy E, Murray J, Capaldi RA, Ghosh SS. Oxidative post-translational modification of tryptophan residues in cardiac mitochondrial proteins. J Biol Chem. 2003;278:19587–19590. doi: 10.1074/jbc.C300135200. [DOI] [PubMed] [Google Scholar]
- 58.Giulivi C, Traaseth NJ, Davies KJA. Tyrosine oxidation products: Analysis and biological relevance. Amino Acids. 2003;25:227–232. doi: 10.1007/s00726-003-0013-0. [DOI] [PubMed] [Google Scholar]
- 59.Warren JJ, Winkler JR, Gray HB. Redox properties of tyrosine and related molecules. FEBS Lett. 2012;586:596–602. doi: 10.1016/j.febslet.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prokai L, Yan LJ, Vera-Serrano JL, Stevens SM, Forster MJ. Mass spectrometry-based survey of age-associated protein carbonylation in rat brain mitochondria. J Mass Spectrom. 2007;42:1583–1589. doi: 10.1002/jms.1345. [DOI] [PubMed] [Google Scholar]
- 61.Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21:5164–5172. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci U S A. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stadtman ER. Protein oxidation in aging and age-related diseases. AnnN Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 64.Mohora M, Greabu M, Totan A, Mitrea N, Battino M. Redox-sensitive signaling factors and antioxidants. Farmacia. 2009;57:399–411. [Google Scholar]
- 65.De Nigris F, Lerman LO, Condorelli M, Lerman A, Napoli C. Oxidation-sensitive transcription factors and molecular mechanisms in the arterial wall. Antioxid Redox Signal. 2001;3:1119–1130. doi: 10.1089/152308601317203620. [DOI] [PubMed] [Google Scholar]
- 66.Brigelius-Flohé R, Flohé L. Basic Principles and Emerging Concepts in the Redox Control of Transcription Factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson C, Terman JR, González-Billault C, Ahmed G. Actin filaments-A target for redox regulation. Cytoskeleton. 2016;73:577–595. doi: 10.1002/cm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milzani a, DalleDonne I, Colombo R. Prolonged oxidative stress on actin. Arch Biochem Biophys. 1997;339:267–74. doi: 10.1006/abbi.1996.9847. [DOI] [PubMed] [Google Scholar]
- 69.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox Regulation of β-Actin during Integrin-mediated Cell Adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 70.Lassing I, Schmitzberger F, Björnstedt M, Holmgren A, Nordlund P, Schutt CE, Lindberg U. Molecular and Structural Basis for Redox Regulation of β-Actin. J Mol Biol. 2007;370:331–348. doi: 10.1016/j.jmb.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 71.DalleDonne I, Milzani A, Colombo R. The tert-butyl hydroperoxide-induced oxidation of actin Cys-374 is coupled with structural changes in distant regions of the protein. Biochemistry. 1999;38:12471–12480. doi: 10.1021/bi990367k. [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible Glutathionylation Regulates Actin Polymerization in A431 Cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 73.Stournaras C, Drewes G, Blackholm H, Merkler I, Faulstich H. Glutathionyl(cysteine-374) actin forms filaments of low mechanical stability. Biochim Biophys Acta (BBA)/Protein Struct Mol. 1990;1037:86–91. doi: 10.1016/0167-4838(90)90105-O. [DOI] [PubMed] [Google Scholar]
- 74.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–70. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 75.Munnamalai V, Suter DM. Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. J Neurochem. 2009;108:644–661. doi: 10.1111/j.1471-4159.2008.05787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moldovan L, Moldovan NI, Sohn RH, Parikh Sa, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557. doi: 10.1161/01.RES.86.5.549. [DOI] [PubMed] [Google Scholar]
- 77.Ruei-Jiun Hung JRT, Pak Chi W. Direct Redox Regulation of F-Actin. Science (80- ) 2011;832:1710–1713. doi: 10.1126/science.1211956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grintsevich EE, Yesilyurt HG, Rich SK, Hung R-J, Terman JR, Reisler E. F-actin dismantling through a redox-driven synergy between Mical and cofilin. Nat Cell Biol. 2016;18:876–85. doi: 10.1038/ncb3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duke J, Takashi R, Ue K, Morales MF. Reciprocal reactivities of specific thiols when actin binds to myosin. [accessed February 3, 2017];Proc Natl Acad Sci U S A. 1976 73:302–6. doi: 10.1073/pnas.73.2.302. http://www.ncbi.nlm.nih.gov/pubmed/1061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu DF, Wang D, Stracher a. The accessibility of the thiol groups on G- and F-actin of rabbit muscle. Biochem J. 1990;266:453–459. doi: 10.1042/bj2660453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terman JR, Kashina A. Post-translational modification and regulation of actin. Curr Opin Cell Biol. 2013;25:30–38. doi: 10.1016/j.ceb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Figueiredo-Freitas C, Dulce Ra, Foster MW, Liang J, Yamashita AMS, Lima-Rosa FL, Thompson JW, Moseley MA, Hare JM, Nogueira L, Sorenson MM, Pinto JR. S -Nitrosylation of Sarcomeric Proteins Depresses Myofilament Ca 2+ Sensitivity in Intact Cardiomyocytes. Antioxid Redox Signal. 2015;23:1017–1034. doi: 10.1089/ars.2015.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lundquist MR, Storaska AJ, Liu T-C, Larsen SD, Evans T, Neubig RR, Jaffrey SR. Redox modification of nuclear actin by MICAL-2 regulates SRF signaling. Cell. 2014;156:563–76. doi: 10.1016/j.cell.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ashida S, Furihata M, Katagiri T, Tamura K, Anazawa Y, Yoshioka H, Miki T, Fujioka T, Shuin T, Nakamura Y, Nakagawa H. Expression of novel molecules, MICAL2-PV (MICAL2 prostate cancer variants), increases with high gleason score and prostate cancer progression. Clin Cancer Res. 2006;12:2767–2773. doi: 10.1158/1078-0432.CCR-05-1995. [DOI] [PubMed] [Google Scholar]
- 85.Loria R, Bon G, Perotti V, Gallo E, Bersani I, Baldassari P, Porru M, Leonetti C, Di Carlo S, Visca P, Brizzi MF, Anichini A, Mortarini R, Falcioni R, Loria R, Bon G, Perotti V, Gallo E, Bersani I, Baldassari P, Porru M, Leonetti C, Di Carlo S, Visca P, Felice Brizzi M, Anichini A, Mortarini R, Falcioni R. Sema6A and Mical1 control cell growth and survival of BRAF V600E human melanoma cells. Oncotarget. 2014;6:2779–2793. doi: 10.18632/oncotarget.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oztug Durer ZA, Diraviyam K, Sept D, Kudryashov DS, Reisler E. F-Actin Structure Destabilization and DNase I Binding Loop Fluctuations. Mutational Cross-Linking and Electron Microscopy Analysis of Loop States and Effects on F-Actin. J Mol Biol. 2010;395:544–557. doi: 10.1016/j.jmb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Section 18.3 Myosin: The Actin Motor Protein. Mol Cell Biol. 2000 doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- 89.Hartman MA, Spudich JA. The myosin superfamily at a glance. J Cell Sci. 2012;125:1627–1632. doi: 10.1242/jcs.094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller AL. The contractile ring. Curr Biol. 2011;21:R976–8. doi: 10.1016/j.cub.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsumura F, Yamakita Y, Yamashiro S. Myosin light chain kinases and phosphatase in mitosis and cytokinesis. Arch Biochem Biophys. 2011;510:76–82. doi: 10.1016/j.abb.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heng Y-W, Koh C-G. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell Biol. 2010;42:1622–33. doi: 10.1016/j.biocel.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochem. 2002;67:75–84. doi: 10.1023/a:1013904231324. BCM67010088 [pii] [DOI] [PubMed] [Google Scholar]
- 94.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981;211:495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- 95.Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem. 1998;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- 96.Moen RJ, Cornea S, Oseid DE, Binder BP, Klein JC, Thomas DD. Redox-sensitive residue in the actin-binding interface of myosin. Biochem Biophys Res Commun. 2014;453:345–349. doi: 10.1016/j.bbrc.2014.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klein JC, Moen RJ, Smith EA, Titus MA, Thomas DD. Structural and functional impact of site-directed methionine oxidation in myosin. Biochemistry. 2011;50:10318–10327. doi: 10.1021/bi201279u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fiaschi T, Cozzi G, Chiarugi P. Redox Regulation of Nonmuscle Myosin Heavy Chain during Integrin Engagement. J Signal Transduct. 2012;2012:1–9. doi: 10.1155/2012/754964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bernstein BW, Bamburg JR. ADF/Cofilin: A functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–87. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bravo-Cordero JJ, Magalhaes MAO, Eddy RJ, Hodgson L, Condeelis J. Functions of cofilin in cell locomotion and invasion. Nat Rev Mol Cell Biol. 2013;14:405–417. doi: 10.1038/nrm3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem. 2008;283:36522–36531. doi: 10.1074/jbc.M805294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee Y-J, Keng PC. Studying the effects of actin cytoskeletal destabilization on cell cycle by cofilin overexpression. Mol Biotechnol. 2005;31:1–10. doi: 10.1385/MB:31:1:001. [DOI] [PubMed] [Google Scholar]
- 104.Kaji N, Muramoto A, Mizuno K. LIM kinase-mediated cofilin phosphorylation during mitosis is required for precise spindle positioning. J Biol Chem. 2008;283:4983–4992. doi: 10.1074/jbc.M708644200. [DOI] [PubMed] [Google Scholar]
- 105.Gunsalus KC, Bonaccorsi S, Williams E, Verni F, Gatti M, Goldberg ML. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J Cell Biol. 1995;131:1243–1259. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo S, Uehara H, Shacter E. Taurine chloramine-induced inactivation of cofilin protein through methionine oxidation. Free Radic Biol Med. 2014;75:84–94. doi: 10.1016/j.freeradbiomed.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 107.Klemke M, Wabnitz GH, Funke F, Funk B, Kirchgessner H, Samstag Y. Oxidation of Cofilin Mediates T Cell Hyporesponsiveness under Oxidative Stress Conditions. Immunity. 2008;29:404–413. doi: 10.1016/j.immuni.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 108.Klamt F, Zdanov S, Levine RL, Pariser A, Zhang Y, Zhang B, Yu LR, Veenstra TD, Shacter E. Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat Cell Biol. 2009;11:1241–1246. doi: 10.1038/ncb1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cameron JM, Gabrielsen M, Chim YH, Munro J, McGhee EJ, Sumpton D, Eaton P, Anderson KI, Yin H, Olson MF. Polarized cell motility induces hydrogen peroxide to inhibit cofilin via cysteine oxidation. Curr Biol. 2015;25:1520–1525. doi: 10.1016/j.cub.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bernstein BW, Shaw AE, Minamide LS, Pak CW, Bamburg JR. Incorporation of cofilin into rods depends on disulfide intermolecular bonds: implications for actin regulation and neurodegenerative disease. J Neurosci. 2012;32:6670–6681. doi: 10.1523/JNEUROSCI.6020-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arnaout MA, Mahalingam B, Xiong J-P. Integrin Structure, Allostery, and Bidirectional Signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 112.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. Integrin traffic - the update. J Cell Sci. 2015;128:839–52. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bazan-Socha S, Bukiej a, Marcinkiewicz C, Musial J. Integrins in pulmonary inflammatory diseases. Curr Pharm Des. 2005;11:893–901. doi: 10.2174/1381612053381710. [DOI] [PubMed] [Google Scholar]
- 115.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 116.Chuang KP, Tsai WS, Wang YJ, Shieh CC. Superoxide activates very late antigen-4 on an eosinophil cell line and increases cellular binding to vascular cell adhesion molecule-1. Eur J Immunol. 2003;33:645–655. doi: 10.1002/eji.200323446. [DOI] [PubMed] [Google Scholar]
- 117.Eble JA, de Rezende FF. Redox-Relevant Aspects of the Extracellular Matrix and Its Cellular Contacts via Integrins. Antioxid Redox Signal. 2014;20:1977–1993. doi: 10.1089/ars.2013.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mou Y, Ni H, Wilkins Ja. The selective inhibition of beta 1 and beta 7 integrin-mediated lymphocyte adhesion by bacitracin. J Immunol. 1998;161:6323–9. [PubMed] [Google Scholar]
- 119.Liu CY, Sun QH, Wang R, Paddock CM, Newman PJ. Disruption of the long-range GPIIIa Cys5-Cys435 disulfide bond results in the production of a constitutively active GPIIb-IIIa integrin complex. Blood. 1997;90:573a. doi: 10.1182/blood-2002-02-0418. abstr. [DOI] [PubMed] [Google Scholar]
- 120.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Rezende FF, Martins Lima A, Niland S, Wittig I, Heide H, Schröder K, Eble JA. Integrin α7β1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free Radic Biol Med. 2012;53:521–531. doi: 10.1016/j.freeradbiomed.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 122.Kamata T, Ambo H, Puzon-McLaughlin W, Tieu KK, Handa M, Ikeda Y, Takada Y. Critical cysteine residues for regulation of integrin alphaIIbbeta3 are clustered in the epidermal growth factor domains of the beta3 subunit. Biochem J. 2004;378:1079–1082. doi: 10.1042/BJ20031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mor-Cohen R, Rosenberg N, Landau M, Lahav J, Seligsohn U. Specific cysteines in beta3 are involved in disulfide bond exchange-dependent and -independent activation of alphaIIbbeta3. J Biol Chem. 2008;283:19235–19244. doi: 10.1074/jbc.M802399200. [DOI] [PubMed] [Google Scholar]
- 124.Shi M, Sundramurthy K, Liu B, Tan SM, Law SKA, Lescar J. The crystal structure of the plexin-semaphorin-integrin domain/hybrid domain/I-EGF1 segment from the human integrin β2 subunit at 1.8-Å resolution. J Biol Chem. 2005;280:30586–30593. doi: 10.1074/jbc.M502525200. [DOI] [PubMed] [Google Scholar]
- 125.Takagi J, Beglova N, Yalamanchili P, Blacklow SC, Springer Ta. Definition of EGF-like, closely interacting modules that bear activation epitopes in integrin beta subunits. Proc Natl Acad Sci U S A. 2001;98:11175–80. doi: 10.1073/pnas.201420198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang W, Luo BH. Structural basis of integrin transmembrane activation. J Cell Biochem. 2010;109:447–452. doi: 10.1002/jcb.22427. [DOI] [PubMed] [Google Scholar]
- 127.Zhu J, Carman CV, Kim M, Shimaoka M, Springer TA, Luo BH. Requirement of alpha and beta subunit transmembrane helix separation for integrin outside-in signaling. Blood. 2007;110:2475–2483. doi: 10.1182/blood-2007-03-080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luo BH, Springer TA, Takagi J. A specific interface between integrin transmembrane helices and affinity for ligand. PLoS Biol. 2004;2 doi: 10.1371/journal.pbio.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Metcalf D, Moore D, Wu Y, Kielec J, Molnar K, Valentine K, Wand A, Bennett J, DeGrado W. NMR analysis of the alphaIIb beta3 cytoplasmic interaction suggests a mechanism for integrin regulation. Proc Natl Acad Sci U S A. 2010;107:22481–6. doi: 10.1073/pnas.1015545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu J, Quan J, Feng J, Zhang Q, Xu Y, Liu J, Huang W, Liu J, Tian L. High glucose regulates LN expression in human liver sinusoidal endothelial cells through ROS/integrin αvβ3 pathway. Environ Toxicol Pharmacol. 2016;42:231–236. doi: 10.1016/j.etap.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 131.Lamari F, Braut-Boucher F, Pongnimitprasert N, Bernard M, Foglietti M-J, Derappe C, Aubery M. Cell adhesion and integrin expression are modulated by oxidative stress in EA.hy 926 cells. Free Radic Res. 2007;41:812–22. doi: 10.1080/10715760701390027. [DOI] [PubMed] [Google Scholar]
- 132.Fujii M, Amanso A, Abrahão TB, Lassègue B, Griendling KK. Polymerase delta-interacting protein 2 regulates collagen accumulation via activation of the Akt/mTOR pathway in vascular smooth muscle cells. J Mol Cell Cardiol. 2016;92:21–9. doi: 10.1016/j.yjmcc.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burridge K, Wennerberg K. Rho and Rac Take Center Stage. Cell. 2004;116:167–179. doi: 10.1016/S0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 134.Piekny A, Werner M, Glotzer M. Cytokinesis: Welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 135.Chircop M. Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells. LANDES Biosci. 2014;5:37–41. doi: 10.4161/sgtp.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–112. doi: 10.1016/j.ceb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hodges-Loaiza HB, Parker LE, Cox AD. Prenylation and Phosphorylation of Ras Superfamily Small GTPases. Enzymes. 2011:43–69. doi: 10.1016/B978-0-12-415922-8.00003-3. [DOI] [Google Scholar]
- 138.Heo J, Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active Rho GTPases. J Biol Chem. 2005;280:31003–31010. doi: 10.1074/jbc.M504768200. [DOI] [PubMed] [Google Scholar]
- 139.Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct Activation of RhoA by Reactive Oxygen Species Requires a Redox-Sensitive Motif. PLoS One. 2009;4:e8045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hobbs GA, Mitchell LE, Arrington ME, Gunawardena HP, Decristo MJ, Loeser RF, Chen X, Cox AD, Campbell SL. Redox regulation of Rac1 by thiol oxidation. Free Radic Biol Med. 2015;79:237–250. doi: 10.1016/j.freeradbiomed.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 2004;64:7464–7472. doi: 10.1158/0008-5472.CAN-04-1725. [DOI] [PubMed] [Google Scholar]
- 142.Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, Fujita T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: Role of small GTPase Rac1. Hypertension. 2012;59:500–506. doi: 10.1161/HYPERTENSIONAHA.111.185520. [DOI] [PubMed] [Google Scholar]
- 143.Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine s-glutathionylation. J Biol Chem. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heo J, Raines KW, Mocanu V, Campbell SL. Redox regulation of RhoA. Biochemistry. 2006;45:14481–14489. doi: 10.1021/bi0610101. [DOI] [PubMed] [Google Scholar]
- 145.Gerhard R, Nottrott S, Schoentaube J, Tatge H, Oiling A, Just I. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J Med Microbiol. 2008;57:765–770. doi: 10.1099/jmm.0.47769-0. [DOI] [PubMed] [Google Scholar]
- 146.Wink DA, Hines HB, Cheng RYS, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassègue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol. 2012;165:506–519. doi: 10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu W, Beaudry S, Negoro H, Boucher I, Tran M, Kong T, Denker BM. H2O2 activates G protein, 12 to disrupt the junctional complex and enhance ischemia reperfusion injury. Proc Natl Acad Sci. 2012;109:6680–6685. doi: 10.1073/pnas.1116800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lopez-Haber C, Kazanietz MG. Cucurbitacin I inhibits Rac1 activation in breast cancer cells by a reactive oxygen species-mediated mechanism and independently of Janus tyrosine kinase 2 and P-Rex1. Mol Pharmacol. 2013;83:1141–54. doi: 10.1124/mol.112.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW. Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation. Exp Diabetes Res. 2010;2010:247861. doi: 10.1155/2010/247861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dada La, Novoa E, Lecuona E, Sun H, Sznajder JI. Role of the small GTPase RhoA in the hypoxia-induced decrease of plasma membrane Na,K-ATPase in A549 cells. J Cell Sci. 2007;120:2214–22. doi: 10.1242/jcs.003038. [DOI] [PubMed] [Google Scholar]
- 153.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 154.Sun S, Wong S, Mak A, Cho M. Impact of oxidative stress on cellular biomechanics and rho signaling in C2C12 myoblasts. J Biomech. 2014;47:3650–3656. doi: 10.1016/j.jbiomech.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 155.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 156.Kondrikov D, Caldwell RB, Dong Z, Su Y. Reactive oxygen species-dependent RhoA activation mediates collagen synthesis in hyperoxic lung fibrosis. Free Radic Biol Med. 2011;50:1689–1698. doi: 10.1016/j.freeradbiomed.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Loirand G, Sauzeau V, Pacaud P. Small G Proteins in the Cardiovascular System: Physiological and Pathological Aspects. Physiol Rev. 2013;93:1659–1720. doi: 10.1152/physrev.00021.2012. [DOI] [PubMed] [Google Scholar]
- 158.Manea A, Simionescu M. Nox enzymes and oxidative stress in atherosclerosis. Front Biosci (Schol Ed) 2012;4:651–70. doi: 10.2741/s291. [DOI] [PubMed] [Google Scholar]
- 159.Louis SF, Zahradka P. Vascular smooth muscle cell motility: From migration to invasion. [accessed February 3, 2017];Exp Clin Cardiol. 2010 15:e75–85. http://www.ncbi.nlm.nih.gov/pubmed/21264073. [PMC free article] [PubMed] [Google Scholar]
- 160.Watanabe T, Wang S, Kaibuchi K. IQGAPs as Key Regulators of Actin-cytoskeleton Dynamics. Cell Struct Funct. 2015;40:69–77. doi: 10.1247/csf.15003. [DOI] [PubMed] [Google Scholar]
- 161.Mataraza JM, Briggs MW, Li Z, Entwistle A, Ridley AJ, Sacks DB. IQGAP1 Promotes Cell Motility and Invasion. J Biol Chem. 2003;278:41237–41245. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- 162.Adachi M, Kawasaki A, Nojima H, Nishida E, Tsukita S. Involvement of IQGAP family proteins in the regulation of mammalian cell cytokinesis. Genes to Cells. 2014;19:803–820. doi: 10.1111/gtc.12179. [DOI] [PubMed] [Google Scholar]
- 163.Kaplan N, Urao N, Furuta E, Kim S-J, Razvi M, Nakamura Y, McKinney RD, Poole LB, Fukai T, Ushio-Fukai M. Localized cysteine sulfenic acid formation by vascular endothelial growth factor: role in endothelial cell migration and angiogenesis. Free Radic Res. 2011;45:1124–35. doi: 10.3109/10715762.2011.602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ikeda S, Yamaoka-tojo M, Hilenski L, Patrushev NA, Anwar M, Quinn MT, Ushio-fukai M, Anwar GM. IQGAP1 Regulates Reactive Oxygen Species – Dependent Endothelial Cell Migration Through Interacting With Nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 165.Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, Fukai T, Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee S-R, Kwon KS, Kim SR, Rhee SG. Reversible Inactivation of Protein-tyrosine Phosphatase 1B in A431 Cells Stimulated with Epidermal Growth Factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 168.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/S1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 169.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 170.Blanchetot C, Tertoolen LGJ, Den Hertog J. Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress. EMBO J. 2002;21:493–503. doi: 10.1093/emboj/21.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chiarugi P. The redox regulation of LMW-PTP during cell proliferation or growth inhibition. IUBMB Life. 2001;52:55–9. doi: 10.1080/15216540252774775. [DOI] [PubMed] [Google Scholar]
- 172.Chiarugi P, Biochimiche S. Reactive oxygen species as mediators of cell adhesion. J Cell Biol. 2003;52:28–32. [PubMed] [Google Scholar]
- 173.Cirri P, Chiarugi P, Taddei L, Raugei G, Camici G, Manao G, Ramponi G. Low molecular weight protein-tyrosine phosphatase tyrosine phosphorylation by c-Src during platelet-derived growth factor-induced mitogenesis correlates with its subcellular targeting. J Biol Chem. 1998;273:32522–32527. doi: 10.1074/jbc.273.49.32522. [DOI] [PubMed] [Google Scholar]
- 174.Chiarugi P, Cirri P, Taddei L, Giannoni E, Camici G, Manao G, Raugei G, Ramponi G. The low M(r) protein-tyrosine phosphatase is involved in Rho-mediated cytoskeleton rearrangement after integrin and platelet-derived growth factor stimulation. J Biol Chem. 2000;275:4640–4646. doi: 10.1074/jbc.275.7.4640. [DOI] [PubMed] [Google Scholar]
- 175.Chiarugi P, Cirri P, Taddei ML, Giannoni E, Fiaschi T, Buricchi F, Camici G, Raugei G, Ramponi G. Insight into the role of low molecular weight phosphotyrosine phosphatase (LMW-PTP) on platelet-derived growth factor receptor (PDGF-r) signaling: LMW-PTP controls PDGF-r kinase activity through TYR-857 dephosphorylation. J Biol Chem. 2002;277:37331–37338. doi: 10.1074/jbc.M205203200. [DOI] [PubMed] [Google Scholar]
- 176.Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–60. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 177.Abdelsaid MA, El-Remessy AB. S-glutathionylation of LMW-PTP regulates VEGF-mediated FAK activation and endothelial cell migration. J Cell Sci. 2012;125:4751–60. doi: 10.1242/jcs.103481. [DOI] [PubMed] [Google Scholar]
- 178.Tatosyan aG, Mizenina Oa. Kinases of the Src family: structure and functions. [accessed November 27, 2016];Biochemistry. 2000 65:49–58. http://www.ncbi.nlm.nih.gov/pubmed/10702640. [PubMed] [Google Scholar]
- 179.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Werner E, Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J Cell Biol. 2002;158:357–368. doi: 10.1083/jcb.200111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Knock GA, Ward JP. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal. 2011;15:1531–1547. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 183.MacKay CE, Knock Ga. Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease. J Physiol. 2014;0:1–14. doi: 10.1113/jphysiol.2014.285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang H, Davies KJA, Forman HJ. TGFβ1 rapidly activates Src through a non-canonical redox signaling mechanism. Arch Biochem Biophys. 2015;568:1–7. doi: 10.1016/j.abb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mills JE, Whitford PC, Shaffer J, Onuchic JN, Adams JA, Jennings PA. A Novel Disulfide Bond in the SH2 Domain of the C-terminal Src Kinase Controls Catalytic Activity. J Mol Biol. 2007;365:1460–1468. doi: 10.1016/j.jmb.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Akhand AA, Pu M, Senga T, Kato M, Suzuki H, Miyata T, Hamaguchi M, Nakashima I. Nitric oxide controls Src kinase activity through a sulfhydryl group modification-mediated Tyr-527-independent and Tyr-416-linked mechanism. J Biol Chem. 1999;274:25821–25826. doi: 10.1074/jbc.274.36.25821. [DOI] [PubMed] [Google Scholar]
- 187.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/S1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 188.Cunnick JM, Dorsey JF, Standley T, Turkson J, Kraker aJ, Fry DW, Jove R, Wu J. Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:14468–75. doi: 10.1074/jbc.273.23.14468. [DOI] [PubMed] [Google Scholar]
- 189.Tang H, Hao Q, Rutherford SA, Low B, Zhao ZJ. Inactivation of SRC family tyrosine kinases by reactive oxygen species in vivo. J Biol Chem. 2005;280:23918–23925. doi: 10.1074/jbc.M503498200. M503498200 [pii]\r. [DOI] [PubMed] [Google Scholar]
- 190.Morla AO, Mogford JE. Control of smooth muscle cell proliferation and phenotype by integrin signaling through focal adhesion kinase. Biochem Biophys Res Commun. 2000;272:298–302. doi: 10.1006/bbrc.2000.2769. [DOI] [PubMed] [Google Scholar]
- 191.Vepa S, Scribner WM, Parinandi NL, English D, Garcia JG, Natarajan V. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am J Physiol. 1999;277:L150–L158. doi: 10.1152/ajplung.1999.277.1.L150. [DOI] [PubMed] [Google Scholar]
- 192.Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145:1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the Focal Adhesion Kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 195.Zhao X, Guan J-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610–5. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ben Mahdi MH, Andrieu V, Pasquier C. Focal adhesion kinase regulation by oxidative stress in different cell types. IUBMB Life. 2001;50:291–299. doi: 10.1080/713803721. [DOI] [PubMed] [Google Scholar]
- 197.Basuroy S, Dunagan M, Sheth P, Seth A, Rao RK. Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers. AJP Gastrointest Liver Physiol. 2010;299:G186–95. doi: 10.1152/ajpgi.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thomas JW, Ellis B, Boerner RJ, Knight WB, White GC, Schaller MD. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- 199.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 200.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 201.Dovas A, Yoneda A, Couchman JR. PKCbeta-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J Cell Sci. 2006;119:2837–46. doi: 10.1242/jcs.03020. [DOI] [PubMed] [Google Scholar]
- 202.Giorgi C, Agnoletto C, Baldini C, Bononi A, Bonora M, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A, Zavan B, Pinton P. Redox control of protein kinase C: cell- and disease-specific aspects. Antioxid Redox Signal. 2010;13:1051–1085. doi: 10.1089/ars.2009.2825. [DOI] [PubMed] [Google Scholar]
- 203.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–62. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Taher MM, Garcia JG, Natarajan V. Hydroperoxide-induced diacylglycerol formation and protein kinase C activation in vascular endothelial cells. Arch Biochem Biophys. 1993;303:260–266. doi: 10.1006/abbi.1993.1281. [DOI] [PubMed] [Google Scholar]
- 205.Dovas A, Choi Y, Yoneda A, Multhaupt HAB, Kwon SH, Kang D, Oh ES, Couchman JR. Serine 34 phosphorylation of rho guanine dissociation inhibitor (RhoGDIalpha) links signaling from conventional protein kinase C to RhoGTPase in cell adhesion. J Biol Chem. 2010;285:23296–23308. doi: 10.1074/jbc.M109.098129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ali MH, Mungai PT, Schumacker PT. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol. 2006;291:L38–L45. doi: 10.1152/ajplung.00287.2004. [DOI] [PubMed] [Google Scholar]
- 207.Siow YL, Au-Yeung KKW, Woo CWHKO. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem J. 2006;398:73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Herrera M, Silva GB, Garvin JL. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(α)-dependent NADPH oxidase activation. J Biol Chem. 2010;285:21323–8. doi: 10.1074/jbc.M110.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dekker LV, Leitges M, Altschuler G, Mistry N, McDermott A, Roes J, Segal AW. Protein kinase C-beta contributes to NADPH oxidase activation in neutrophils. Biochem J. 2000;347(Pt 1):285–9. [PMC free article] [PubMed] [Google Scholar]
- 210.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/S0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 211.DelCarlo M, Loeser RF. Chondrocyte cell death mediated by reactive oxygen species-dependent activation of PKC-betaI. Am J Physiol Cell Physiol. 2006;290:C802–11. doi: 10.1152/ajpcell.00214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275:24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 213.Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277:44327–44331. doi: 10.1074/jbc.M205634200. [DOI] [PubMed] [Google Scholar]
- 214.Kass GE, Duddy SK, Orrenius S. Activation of hepatocyte protein kinase C by redox-cycling quinones. Biochem J. 1989;260:499–507. doi: 10.1042/bj2600499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Larsson R, Cerutti P. Translocation and enhacement of phosphotransferase activity of protein kinase C following exposure in mouse epidermal cells to oxidants. Cancer Res. 1989;49:5627–5632. [PubMed] [Google Scholar]
- 216.Whisler RL, Goyette MA, Grants IS, Newhouse YG. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Arch Biochem Biophys. 1995;319:23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]
- 217.Gopalakrishna R, Anderson WB. Susceptibility of protein kinase C to oxidative inactivation: Loss of both phosphotransferase activity and phorbol diester binding. FEBS Lett. 1987;225:233–237. doi: 10.1016/0014-5793(87)81164-X. [DOI] [PubMed] [Google Scholar]
- 218.Wolf M, LeVine H, III, May WS, Cuatrecasas P, Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca{+2+} and phorbol esters. Nature. 1985;317:546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]
- 219.Kraft AS, Anderson WB. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983;301:621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- 220.Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- 221.Goldhaber JI, Ji S, Lamp ST, Weiss JN. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricle. Implications for ischemia and reperfusion injury. J Clin Invest. 1989;83:1800–1809. doi: 10.1172/JCI114085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gill JS, McKenna WJ, Camm aJ. Free radicals irreversibly decrease Ca2+ currents in isolated guinea-pig ventricular myocytes. Elsevier; 1995. [DOI] [PubMed] [Google Scholar]
- 223.Fearon IM, Palmer ACV, Balmforth AJ, Ball SG, Varadi G, Peers C. Modulation of recombinant human cardiac L-type Ca2+ channel α(1C) subunits by redox agents and hypoxia. J Physiol. 1999;514:629–637. doi: 10.1111/j.1469-7793.1999.629ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kang S, Kang J, Kwon H, Frueh D, Seung HY, Wagner G, Park S. Effects of redox potential and Ca2+ on the inositol 1,4,5-trisphosphate receptor L3-1 loop region: Implications for receptor regulation. J Biol Chem. 2008;283:25567–25575. doi: 10.1074/jbc.M803321200. [DOI] [PubMed] [Google Scholar]
- 225.Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 226.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44:361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–7. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 229.Lancel S, Qin F, Lennon SL, Zhang J, Tong X, Mazzini MJ, Kang YJ, Siwik DA, Cohen RA, Colucci WS. Oxidative Post-Translational Modifications Mediate Decreased SERCA Activity and Myocyte Dysfunction in Gαq-Overexpressing Mice. Circ Res. 2010;107:228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.ZHENG Y-M, WANG Y-X. Sodium-Calcium Exchanger in Pulmonary Artery Smooth Muscle Cells. Ann N Y Acad Sci. 2007;1099:427–435. doi: 10.1196/annals.1387.017. [DOI] [PubMed] [Google Scholar]
- 231.Syyong HT, Poburko D, Fameli N, van Breemen C. ATP promotes NCX-reversal in aortic smooth muscle cells by DAG-activated Na+ entry. Biochem Biophys Res Commun. 2007;357:1177–1182. doi: 10.1016/j.bbrc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 232.Yang Z, Pascarel C, Steele DS, Komukai K, Brette F, Orchard CH. Na+-Ca2+ exchange activity is localized in the t-tubules of rat ventricular myocytes. Circ Res. 2002;91:315–322. doi: 10.1161/01.RES.0000030180.06028.23. [DOI] [PubMed] [Google Scholar]
- 233.Teubl M. Na+/Ca2+ Exchange Facilitates Ca2+-dependent Activation of Endothelial Nitric-oxide Synthase. J Biol Chem. 1999;274:29529–29535. doi: 10.1074/jbc.274.41.29529. [DOI] [PubMed] [Google Scholar]
- 234.Babsky A, Doliba N, Doliba N, Savchenko A, Wehrli S, Osbakken M. Na+ effects on mitochondrial respiration and oxidative phosphorylation in diabetic hearts. ExpBiolMed(Maywood) 2001;226:543–551. doi: 10.1177/153537020122600606. [DOI] [PubMed] [Google Scholar]
- 235.Kato M, Kako KJ. Na+/Ca2+ exchange of isolated sarcolemmal membrane: effects of insulin, oxidants and insulin deficiency. Mol Cell Biochem. 1988;83:15–25. doi: 10.1007/BF00223194. [DOI] [PubMed] [Google Scholar]
- 236.Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, Shigekawa M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J Biol Chem. 1996;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- 237.Santacruz-Toloza L, Ottolia M, Nicoll DA, Philipson KD. Functional analysis of a disulfide bond in the cardiac Na+-Ca2+ exchanger. J Biol Chem. 2000;275:182–188. doi: 10.1074/jbc.275.1.182. [DOI] [PubMed] [Google Scholar]
- 238.Zaidi A, Barŕon L, Sharov VS, Schöneich C, Michaelis EK, Michaelis ML. Oxidative Inactivation of Purified Plasma Membrane Ca2+-ATPase by Hydrogen Peroxide and Protection by Calmodulin. Biochemistry. 2003;42:12001–12010. doi: 10.1021/bi034565u. [DOI] [PubMed] [Google Scholar]
- 239.Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca2+-ATPase. Free Radic Biol Med. 1999;27:810–821. doi: 10.1016/S0891-5849(99)00128-8. [DOI] [PubMed] [Google Scholar]
- 240.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 241.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci U S A. 2000;97:12334–8. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shigekawa M, Katanosaka Y, Wakabayashi S. Regulation of the Cardiac Na+/Ca2+ Exchanger by Calcineurin and Protein Kinase C. Ann N Y Acad Sci. 2007;1099:53–63. doi: 10.1196/annals.1387.059. [DOI] [PubMed] [Google Scholar]
- 244.Van Lierop JE, Wilson DP, Davis JP, Tikunova S, Sutherland C, Walsh MP, Johnson JD. Activation of smooth muscle myosin light chain kinase by calmodulin. Role of LYS(30) and GLY(40) J Biol Chem. 2002;277:6550–8. doi: 10.1074/jbc.M111404200. [DOI] [PubMed] [Google Scholar]
- 245.Sacksteder Ca, Whittier JE, Xiong Y, Li J, Galeva Na, Jacoby ME, Purvine SO, Williams TD, Rechsteiner MC, Bigelow DJ, Squier TC. Tertiary structural rearrangements upon oxidation of Methionine145 in calmodulin promotes targeted proteasomal degradation. Biophys J. 2006;91:1480–1493. doi: 10.1529/biophysj.106.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Balog EM, Lockamy EL, Thomas DD, Ferrington DA. Site-specific methionine oxidation initiates calmodulin degradation by the 20S proteasome. Biochemistry. 2009;48:3005–3016. doi: 10.1021/bi802117k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Robison AJ, Winder DG, Colbran RJ, Bartlett RK. Oxidation of calmodulin alters activation and regulation of CaMKII. Biochem Biophys Res Commun. 2007;356:97–101. doi: 10.1016/j.bbrc.2007.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jas GS, Kuczera K. Free-energy simulations of the oxidation of C-terminal methionines in calmodulin. Proteins Struct Funct Genet. 2002;48:257–268. doi: 10.1002/prot.10133. [DOI] [PubMed] [Google Scholar]
- 249.Bartlett RK, Urbauer RJB, Anbanandam A, Smallwood HS, Urbauer JL, Squier TC. Oxidation of Met144 and Met145 in calmodulin blocks calmodulin dependent activation of the plasma membrane Ca-ATPase. Biochemistry. 2003;42:3231–3238. doi: 10.1021/bi026956z. [DOI] [PubMed] [Google Scholar]
- 250.Hanson PI, Schulman H. Neuronal Ca 2+ /Calmodulin-Dependent Protein Kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 251.Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J Biol Chem. 2011;286:7990–7999. doi: 10.1074/jbc.M110.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Prasad AM, Nuno DW, Koval OM, Ketsawatsomkron P, Li W, Li H, Shen FY, Joiner MLA, Kutschke W, Weiss RM, Sigmund CD, Anderson ME, Lamping KG, Grumbach IM. Differential control of calcium homeostasis and vascular reactivity by Ca2+/calmodulin-dependent kinase II. Hypertension. 2013;62:434–441. doi: 10.1161/HYPERTENSIONAHA.113.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 254.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: From Form to Function. Annu Rev Physiol. 1995;4:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 255.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 256.Erickson JR, ling M, Joiner A, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Trottier H, Franco EL. Human papillomavirus and cervical cancer: burden of illness and basis for prevention. Am J Manag Care. 2006;12:S462–72. 3257 [pii] [PubMed] [Google Scholar]
- 258.Erickson JR. Mechanisms of CaMKII activation in the heart. Front Pharmacol. 2014 Apr;5:59. doi: 10.3389/fphar.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sossalla S, Maurer U, Schotola H, Hartmann N, Didié M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIδC can be reversed by inhibition of late Na+ current. Basic Res Cardiol. 2011;106:263–272. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kim I, Je HD, Gallant C, Zhan Q, Riper DV, Badwey Ja, Singer Ha, Morgan KG. Ca2+-calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. J Physiol. 2000;526(Pt 2):367–374. doi: 10.1111/j.1469-7793.2000.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhou ZH, Ando S, Furutsuka D, Ikebe M. Characterization of Ca2+/calmodulin-dependent protein kinase II from smooth muscle. Biochem J. 1995;310(Pt 2):517–525. doi: 10.1042/bj3100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bhattacharjee S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plants. Curr Sci. 2005;89:1113–1121. doi: 10.1080/15216540252774694. [DOI] [Google Scholar]
- 264.Wach F, Hein R, Adelmann-Grill BC, Krieg T. Inhibition of fibroblast chemotaxis by superoxide dismutase. Eur J Cell Biol. 1987;44:124–7. [PubMed] [Google Scholar]
- 265.Kato T, Terui T, Iizawa O, Tagami H. Lucigenin-induced chemiluminescence in human neutrophils in the process of chemotactic migration measured in a modified Boyden chamber. Dermatologica. 1989:113–5. doi: 10.1159/000248460. [DOI] [PubMed] [Google Scholar]
- 266.Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 Oxidase Overexpression Specifically Decreases Endogenous Nox4 mRNA and Inhibits Angiotensin II-Induced Adventitial Myofibroblast Migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Choi MH, Lee IK, Kim GW, Kim BU, Han Y-H, Yu D-Y, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 268.Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, Hussain SNa. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19:1728–1730. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- 269.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 270.Brandes RP, Viedt C, Nguyen K, Beer S, Kreuzer J, Busse R, Görlach A. Thrombin-induced MCP-1 expression involves activation of the P22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb Haemost. 2001;85:1104–1110. 01061104 [pii] [PubMed] [Google Scholar]
- 271.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–9. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 272.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Bäumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 273.Lee MY, Martin AS, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassègue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schroeder K, Keller A, Busse R, Brandes R. Nox1 mediates basic fibroblast growth factor-induced vascular smooth muscle cell migration. Circulation. 2005;112:U232–U232. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 275.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 276.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/S0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 277.Li S, Huang NF, Hsu S. Mechanotransduction in endothelial cell migration. J Cell Biochem. 2005;96:1110–1126. doi: 10.1002/jcb.20614. [DOI] [PubMed] [Google Scholar]
- 278.Nambiar R, McConnell RE, Tyska MJ. Myosin motor function: The ins and outs of actin-based membrane protrusions. Cell Mol Life Sci. 2010;67:1239–1254. doi: 10.1007/s00018-009-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–4. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Worth DC, Parsons M. Advances in imaging cell–matrix adhesions. J Cell Sci. 2010;123 doi: 10.1242/jcs.064485. [DOI] [PubMed] [Google Scholar]
- 281.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Taulet N, Delorme-Walker VD, DerMardirossian C. Reactive Oxygen species regulate protrusion efficiency by controlling actin dynamics. PLoS One. 2012;7:e41342. doi: 10.1371/journal.pone.0041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bailly M, Condeelis JS, Segall JE. Chemoattractant-induced lamellipod extension. Microsc Res Tech. 1998;43:433–443. doi: 10.1002/(SICI)1097-0029(19981201)43:5<433::AID-JEMT9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 284.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/S0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 285.Machesky LM, Insall RH. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1356. doi: 10.1016/S0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 286.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wear MA, Schafer DA, Cooper JA. Actin dynamics: Assembly and disassembly of actin networks. Curr Biol. 2000;10:R891–5. doi: 10.1016/S0960-9822(00)00845-9. [DOI] [PubMed] [Google Scholar]
- 288.Carlier M-F, Pantaloni D. Control of actin assembly dynamics in cell motility. J Biol Chem. 2007;282:23005–23009. doi: 10.1074/jbc.R700020200. [DOI] [PubMed] [Google Scholar]
- 289.Falet H, Hoffmeister KM, Neujahr R, Italiano JE, Stossel TP, Southwick FS, Hartwig JH. Importance of free actin filament barbed ends for Arp2/3 complex function in platelets and fibroblasts. Proc Natl Acad Sci U S A. 2002;99:16782–7. doi: 10.1073/pnas.222652499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 291.Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999;9:364–370. doi: 10.1016/S0962-8924(99)01619-0. [DOI] [PubMed] [Google Scholar]
- 292.Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci. 2000;25:19–23. doi: 10.1016/S0968-0004(99)01511-X. [DOI] [PubMed] [Google Scholar]
- 293.Maheswaranathan M, Gole HKA, Fernandez I, Lassègue B, Griendling KK, San Martín A. Platelet-derived growth factor (PDGF) regulates slingshot phosphatase activity via Nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J Biol Chem. 2011;286:35430–35437. doi: 10.1074/jbc.M111.268284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ohashi K. Roles of cofilin in development and its mechanisms of regulation. Dev Growth Differ. 2015;57:275–290. doi: 10.1111/dgd.12213. [DOI] [PubMed] [Google Scholar]
- 295.Yang N, Higuchi O, Ohashi K, Nagata K, Wada a, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 296.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 297.Calderwood Da, Ginsberg MHMH. Talin forges the links between integrins and actin. Nat Cell Biol. 2003;5:694–697. doi: 10.1038/ncb0803-694. [DOI] [PubMed] [Google Scholar]
- 298.Brown MC, Turner CE. Paxillin: Adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 299.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 300.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990;111:721–9. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- 302.Mureebe L, Nelson PR, Yamamura S, Lawitts J, Kent KC. Activation of pp60c-src is necessary for human vascular smooth muscle cell migration. Surgery. 1997;122:135–138. doi: 10.1016/s0039-6060(97)90002-7. S0039-6060(97)90002-7 [pii] [DOI] [PubMed] [Google Scholar]
- 303.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta - Mol Cell Res. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 304.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 305.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/MCB.14.3.1680.Updated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Rondé P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci. 2005;118:4415–4425. doi: 10.1242/jcs.02565. [DOI] [PubMed] [Google Scholar]
- 307.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 308.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/S0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 309.Yamboliev IA, Gerthoffer WT. Modulatory role of ERK MAPK-caldesmon pathway in PDGF-stimulated migration of cultured pulmonary artery SMCs. [accessed November 7, 2016];Am J Physiol Cell Physiol. 2001 280:C1680–8. doi: 10.1152/ajpcell.2001.280.6.C1680. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11350764. [DOI] [PubMed] [Google Scholar]
- 310.Klemke RL, Cai S, Giannini AL, Gallagher PJ, De Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lee SH, Hollingsworth R, Kwon HY, Lee N, Chung CY. β-arrestin 2-dependent activation of ERK1/2 is required for ADP-induced paxillin phosphorylation at Ser83 and microglia chemotaxis. Glia. 2012;60:1366–1377. doi: 10.1002/glia.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Blanc A, Pandey NR, Srivastava AK. Distinct Roles of Ca 2+, Calmodulin, and Protein Kinase C in H2O2-Induced Activation of ERK1/2, p38 MAPK, and Protein Kinase B Signaling in Vascular Smooth Muscle Cells. Antioxid Redox Signal. 2004;6:353–366. doi: 10.1089/152308604322899422. [DOI] [PubMed] [Google Scholar]
- 313.Tabet F, Schiffrin EL, Touyz RM. Mitogen-activated protein kinase activation by hydrogen peroxide is mediated through tyrosine kinase-dependent, protein kinase C-independent pathways in vascular smooth muscle cells: upregulation in spontaneously hypertensive rats. J Hypertens. 2005;23:2005–12. doi: 10.1097/01.hjh.0000185715.60788.1b. [DOI] [PubMed] [Google Scholar]
- 314.King CC, Gardiner EMM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J Biol Chem. 2000;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- 315.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–29. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chi AY, Waypa GB, Mungai PT, Schumacker PT. Prolonged hypoxia increases ROS signaling and RhoA activation in pulmonary artery smooth muscle and endothelial cells. Antioxid Redox Signal. 2010;12:603–610. doi: 10.1089/ars.2009.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Fukata Y, Kaibuchi K, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/S0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 318.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho- kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 319.Wirth A, Schroeter M, Kock-Hauser C, Manser E, Chalovich JM, de Lanerolle P, Pfitzer G. Inhibition of contraction and myosin light chain phosphorylation in guinea-pig smooth muscle by p21-activated kinase 1. J Physiol. 2003;549:489–500. doi: 10.1113/jphysiol.2002.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 321.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–78. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 323.Heinle H. Vasoconstriction of carotid artery induced by hydroperoxides. Arch Int Physiol Biochim. 1984;92:267–271. doi: 10.3109/13813458409071166. [DOI] [PubMed] [Google Scholar]
- 324.Reid MB, Khawli Fa, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. [accessed December 17, 2016];J Appl Physiol. 1993 75:1081–7. doi: 10.1152/jappl.1993.75.3.1081. http://www.ncbi.nlm.nih.gov/pubmed/8226515. [DOI] [PubMed] [Google Scholar]
- 325.Oba T, Koshita M, Yamaguchi M. H2O2 modulates twitch tension and increases Po of Ca2+ release channel in frog skeletal muscle. [accessed December 17, 2016];Am J Physiol. 1996 271:C810–C818. doi: 10.1152/ajpcell.1996.271.3.C810. http://www.ncbi.nlm.nih.gov/pubmed/8843710. [DOI] [PubMed] [Google Scholar]
- 326.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 327.Aghdasi B, Zhang JZ, Wut Y, Reid MB, Hamilton SL. Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. J Biol Chem. 1997;272:3739–3748. doi: 10.1074/jbc.272.6.3739. [DOI] [PubMed] [Google Scholar]
- 328.Nashawati E, Dimarco A, Supinski C. Effects Produced by Infusion of a Free Radical-Generating Solution into the Diaphragm. Am Rev Respir Dis. 1993;147:60–65. doi: 10.1164/ajrccm/147.1.60. [DOI] [PubMed] [Google Scholar]
- 329.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Brooks SV. Current Topics for Teaching Skeletal Muscle Physiology. Adv Physiol Educ. 2003;27:201–6. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 331.Wang X, Mccullough KD, Wang X, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J Biol Chem. 2001;276:28364–28371. doi: 10.1074/jbc.M102693200. [DOI] [PubMed] [Google Scholar]
- 332.Martín-Garrido A, Boyano-Adánez MC, Alique M, Calleros L, Serrano I, Griera M, Rodríguez-Puyol D, Griendling KK, Rodríguez-Puyol M. Hydrogen peroxide down-regulates inositol 1,4,5-trisphosphate receptor content through proteasome activation. Free Radic Biol Med. 2009;47:1362–1370. doi: 10.1016/j.freeradbiomed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 333.Wenceslau CF, Mccarthy CG, Szasz T, Webb RC. Lipoxin A4 mediates aortic contraction via rhoa/rho kinase, endothelial dysfunction and reactive oxygen species. J Vasc Res. 2014;51:407–417. doi: 10.1159/000371490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zerpa H, Berhane Y, Woodcock H, Elliott J, Bailey SR. Rho kinase activation and ROS production contributes to the cooling enhanced contraction in cutaneous equine digital veins. J Appl Physiol (Bethesda, Md 1985) 2010;109:11–18. doi: 10.1152/japplphysiol.01301.2009. [DOI] [PubMed] [Google Scholar]
- 335.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 336.Song YH, Cho H, Ryu SY, Yoon JY, Park SH, Il Noh C, Lee SH, Ho WK. L-type Ca2+ channel facilitation mediated by H2O2-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol. 2010;48:773–780. doi: 10.1016/j.yjmcc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 337.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 338.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 339.Wittköpper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsöld B, Kirchhof P, Maier LS, Hasenfuss G, Dobrev D, Eschenhagen T, El-Armouche A. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Stubbert D, Prysyazhna O, Rudyk O, Scotcher J, Burgoyne JR, Eaton P. Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension. 2014;64:1344–1351. doi: 10.1161/HYPERTENSIONAHA.114.04281. [DOI] [PubMed] [Google Scholar]
- 341.Raj U, Shimoda L. Oxygen-dependent signaling in pulmonary vascular smooth muscle. Am J Physiol Cell Mol Physiol. 2002;283:L671–L677. doi: 10.1152/ajplung.00177.2002. [DOI] [PubMed] [Google Scholar]
- 342.Chiarugi P, Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal. 2007;19:672–682. doi: 10.1016/j.cellsig.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 343.Verbon EH, Post JA, Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene. 2012;511:1–6. doi: 10.1016/j.gene.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 344.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 345.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arter Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.ATV.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 346.Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 347.Arnold RS, Shi J, Murad E, Whalen aM, Sun CQ, Polavarapu R, Parthasarathy S, Petros Ja, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 349.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Lassègue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassègue B, Martín AS, Griendling KK. NADPH oxidase 4 mediates TGF-β-induced smooth muscle α-actin via p38MAPK and serum response factor. Free Radic Biol Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Takahashi Y, Ogra Y, Suzuki KT. Synchronized generation of reactive oxygen species with the cell cycle. Life Sci. 2004;75:301–311. doi: 10.1016/j.lfs.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 352.Hsieh C-Y, Chen C-L, Yang K-C, Ma C-T, Choi P-C, Lin C-F. Detection of Reactive Oxygen Species During the Cell Cycle Under Normal Culture Conditions Using a Modified Fixed-Sample Staining Method. J Immunoassay Immunochem. 2014;1819:37–41. doi: 10.1080/15321819.2014.910806. [DOI] [PubMed] [Google Scholar]
- 353.Goswami PC, Sheren J, Albee LD, Parsian A, Sim JE, Ridnour LA, Higashikubo R, Gius D, Hunt CR, Spitz DR. Cell cycle-coupled variation in topoisomerase IIalpha mRNA is regulated by the 3′-untranslated region. Possible role of redox-sensitive protein binding in mRNA accumulation. J Biol Chem. 2000;275:38384–38392. doi: 10.1074/jbc.M005298200. [DOI] [PubMed] [Google Scholar]
- 354.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–52. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 355.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/S0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 356.Blaker AL, Taylor JM, MacK CP. PKA-dependent phosphorylation of serum response factor inhibits smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol. 2009;29:2153–2160. doi: 10.1161/ATVBAHA.109.197285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Iyer D, Chang D, Marx J, Wei L, Olson EN, Parmacek MS, Balasubramanyam A, Schwartz RJ. Serum response factor MADS box serine-162 phosphorylation switches proliferation and myogenic gene programs. Proc Natl Acad Sci U S A. 2006;103:4516–21. doi: 10.1073/pnas.0505338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–44. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 359.Kanellos G, Frame MC. Cellular functions of the ADF/cofilin family at a glance. J Cell Sci. 2016;129:3211–8. doi: 10.1242/jcs.187849. [DOI] [PubMed] [Google Scholar]
- 360.Ramanathan SP, Helenius J, Stewart MP, Cattin CJ, Hyman Aa, Muller DJ. Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement. Nat Cell Biol. 2015;17:148–59. doi: 10.1038/ncb3098. [DOI] [PubMed] [Google Scholar]
- 361.Lancaster O, LeBerre M, Dimitracopoulos A, Bonazzi D, Zlotek-Zlotkiewicz E, Picone R, Duke T, Piel M, Baum B. Mitotic Rounding Alters Cell Geometry to Ensure Efficient Bipolar Spindle Formation. Dev Cell. 2013;25:270–283. doi: 10.1016/j.devcel.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 362.Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/S0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- 363.Yüce Ö, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Li J, Wang J, Jiao H, Liao J, Xu X. Cytokinesis and cancer: Polo loves ROCK ‘n’ Rho(A) J Genet Genomics. 2010;37:159–172. doi: 10.1016/S1673-8527(09)60034-5. [DOI] [PubMed] [Google Scholar]
- 365.Clement DL, De Buyzere ML, Duprez DA. Hypertension in peripheral arterial disease. Curr Pharm Des. 2004;10:3615–20. doi: 10.2174/1381612043382819. [DOI] [PubMed] [Google Scholar]
- 366.Madamanchi N, Hakim Z, Runge M. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3:254–267. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 367.Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med. 2010;14:70–78. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–71. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Madamanchi NR, Vendrov A, Runge MS. Oxidative Stress and Vascular Disease. Arterioscler Thromb Vasc Biol. 2004;25 doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 370.Li H, Witte K, August M, Brausch I, Gödtel-Armbrust U, Habermeier A, Closs EI, Oelze M, Münzel T, Förstermann U. Reversal of Endothelial Nitric Oxide Synthase Uncoupling and Up-Regulation of Endothelial Nitric Oxide Synthase Expression Lowers Blood Pressure in Hypertensive Rats. J Am Coll Cardiol. 2006;47:2536–2544. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 371.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 372.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.RES.80.1.45. [DOI] [PubMed] [Google Scholar]
- 373.Warnholtz a, Nickenig G, Schulz E, Macharzina R, Bräsen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Böhm M, Meinertz T, Münzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–33. doi: 10.1161/01.CIR.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 374.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis. 2011;216:321–6. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Judkins CP, Diep H, Broughton BRS, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 376.Lozhkin A, Vendrov AE, Pan H, Wickline SA, Madamanchi NR, Runge MS. NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis. J Mol Cell Cardiol. 2016;102:10–21. doi: 10.1016/j.yjmcc.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 378.Kim N, Hwangbo C, Lee S, Lee JH. Eupatolide inhibits PDGF-induced proliferation and migration of aortic smooth muscle cells through ROS-dependent heme oxygenase-1 induction. Phyther Res. 2013;27:1700–1707. doi: 10.1002/ptr.4924. [DOI] [PubMed] [Google Scholar]
- 379.Xi XP, Graf K, Goetze S, Fleck E, Hsueh WA, Law RE. Central role of the MAPK pathway in ang II-mediated DNA synthesis and migration in rat vascular smooth muscle cells. [accessed February 2, 2017];Arterioscler Thromb Vasc Biol. 1999 19:73–82. doi: 10.1161/01.atv.19.1.73. http://www.ncbi.nlm.nih.gov/pubmed/9888869. [DOI] [PubMed] [Google Scholar]
- 380.Zhang H, Facemire CS, Banes AJ, Faber JE. Different alpha-adrenoceptors mediate migration of vascular smooth muscle cells and adventitial fibroblasts in vitro. Am J Physiol Heart Circ Physiol. 2002;282:H2364–70. doi: 10.1152/ajpheart.00858.2001. [DOI] [PubMed] [Google Scholar]
- 381.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Intengan HD, Schiffrin EL. Vascular Remodeling in Hypertension. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 383.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling —Cardiovasc Res. Cardiovasc Res. 2008;78 doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 384.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 385.Flavahan NA, Bailey SR, Flavahan WA, Mitra S, Flavahan S. Imaging remodeling of the actin cytoskeleton in vascular smooth muscle cells after mechanosensitive arteriolar constriction. Am J Physiol Heart Circ Physiol. 2005;288:H660–H669. doi: 10.1152/ajpheart.00608.2004. [DOI] [PubMed] [Google Scholar]
- 386.Staiculescu MC, Galiñanes EL, Zhao G, Ulloa U, Jin M, Beig MI, Meininger GA, Martinez-Lemus LA. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res. 2013;98:428–436. doi: 10.1093/cvr/cvt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Nakamura M, Sunagawa M, Kosugi T, Sperelakis N. Actin filament disruption inhibits L-type Ca(2+) channel current in cultured vascular smooth muscle cells. [accessed October 13, 2016];Am J Physiol Cell Physiol. 2000 279:C480–7. doi: 10.1152/ajpcell.2000.279.2.C480. http://www.ncbi.nlm.nih.gov/pubmed/10913014. [DOI] [PubMed] [Google Scholar]
- 388.Samain E, Bouillier H, Perret C, Safar M, Dagher G. ANG II-induced Ca2+ increase in smooth muscle cells from SHR is regulated by actin and microtubule networks. Am J Physiol - Hear Circ Physiol. 1999;277:H834–841. doi: 10.1152/ajpheart.1999.277.2.H834. [DOI] [PubMed] [Google Scholar]
- 389.El-Yazbi AF, Abd-Elrahman KS, Moreno-Dominguez A. PKC-mediated cerebral vasoconstriction: Role of myosin light chain phosphorylation versus actin cytoskeleton reorganization. Biochem Pharmacol. 2015;95:263–278. doi: 10.1016/j.bcp.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 390.Itoh S, Umemoto S, Hiromoto M, Toma Y, Tomochika Y, Aoyagi S, Tanaka M, Fujii T, Matsuzaki M. Importance of NAD(P)H oxidase-mediated oxidative stress and contractile type smooth muscle myosin heavy chain SM2 at the early stage of atherosclerosis. Circulation. 2002;105:2288–2295. doi: 10.1161/01.CIR.0000015607.33345.1F. [DOI] [PubMed] [Google Scholar]
- 391.de la Cuesta F, Zubiri I, Maroto AS, Posada M, Padial LR, Vivanco F, Alvarez-Llamas G, Barderas MG. Deregulation of smooth muscle cell cytoskeleton within the human atherosclerotic coronary media layer. J Proteomics. 2013;82:155–165. doi: 10.1016/j.jprot.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 392.Touré F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, Schmidt AM. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110:1279–1293. doi: 10.1161/CIRCRESAHA.111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wang J-N, Shi N, Chen S-Y. Manganese superoxide dismutase inhibits neointima formation through attenuation of migration and proliferation of vascular smooth muscle cells. Free Radic Biol Med. 2012;52:173–81. doi: 10.1016/j.freeradbiomed.2011.10.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Colangelo S, Langille BL, Steiner G, Gotlieb AI. Alterations in endothelial F-actin microfilaments in rabbit aorta in hypercholesterolemia. [accessed October 13, 2016];ArteriosclerThrombVascBiol. 1998 18:52. doi: 10.1161/01.atv.18.1.52. http://www.ncbi.nlm.nih.gov/pubmed/9445256. [DOI] [PubMed] [Google Scholar]
- 395.Ai L, Rouhanizadeh M, Wu JC, Takabe W, Yu H, Alavi M, Li R, Chu Y, Miller J, Heistad DD, Hsiai TK. Shear stress influences spatial variations in vascular Mn-SOD expression: implication for LDL nitration. Am J Physiol Cell Physiol. 2008;294:C1576–85. doi: 10.1152/ajpcell.00518.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med Biol Eng Comput. 2008;46:469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Weber DS, Webb RC. Enhanced relaxation to the Rho-kinase inhibitor Y-27632 in mesenteric arteries from mineralocorticoid hypertensive rats. Pharmacology. 2001;63:129–133. doi: 10.1159/000056123. [DOI] [PubMed] [Google Scholar]
- 398.Asano M, Nomura Y. Comparison of inhibitory effects of Y-27632, a Rho kinase inhibitor, in strips of small and large mesenteric arteries from spontaneously hypertensive and normotensive Wistar-Kyoto rats. [accessed November 15, 2016];Hypertens Res. 2003 26:97–106. doi: 10.1291/hypres.26.97. http://www.ncbi.nlm.nih.gov/pubmed/12661918. [DOI] [PubMed] [Google Scholar]
- 399.Masumoto a, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita a. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 400.Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension: comparison with protein kinase C. Circ Res. 2001;88:774–9. doi: 10.1161/hh0801.090441. [DOI] [PubMed] [Google Scholar]
- 401.Chitaley K, Weber DS, Webb RC. RhoA/rho-kinase, vascular changes, and hypertension. Curr Hypertens Rep. 2001;3:139–144. doi: 10.1007/s11906-001-0028-4. [DOI] [PubMed] [Google Scholar]
- 402.Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 403.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 404.Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, Kaibuchi K, Takeshita a. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 2001;15:1062–4. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 405.Wang H-W, Liu P-Y, Oyama N, Rikitake Y, Kitamoto S, Gitlin J, Liao JK, Boisvert Wa. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. FASEB J. 2008;22:3561–3570. doi: 10.1096/fj.08-108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Shimokawa H, Morishige K, Miyata K, Kandabashi T, Eto Y, Ikegaki I, Asano T, Kaibuchi K, Takeshita A. Long-term inhibition of Rho-kinase induces a regression of arteriosclerotic coronary lesions in a porcine model in vivo. Cardiovasc Res. 2001;51:169–177. doi: 10.1016/S0008-6363(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 407.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-Associated Protein Kinase Contributes to Early Atherosclerotic Lesion Formation in Mice. Circ Res. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 408.Eto Y, Shimokawa H, Hiroki J, Morishige K, Kandabashi T, Matsumoto Y, Amano M, Hoshijima M, Kaibuchi K, Takeshita a. Gene transfer of dominant negative Rho kinase suppresses neointimal formation after balloon injury in pigs. [accessed October 13, 2016];Am J Physiol Heart Circ Physiol. 2000 278:H1744–50. doi: 10.1152/ajpheart.2000.278.6.H1744. http://www.ncbi.nlm.nih.gov/pubmed/10843868. [DOI] [PubMed] [Google Scholar]
- 409.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 410.Stocker R, Keaney J. Role of oxidative stress modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 411.Shafique E, Choy WC, Liu Y, Feng J, Arthur Lyra BC, Arafah M, Yassin-Kassab A, Zanetti AVD, Clements RT, Bianchi C, Benjamin LE, Sellke FW, Abid MR. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging (Albany NY) 2013;5:515–530. doi: 10.1038/nmeth866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Klomsiri C, Nelson KJ, Bechtold E, Soito L, Johnson LC, Lowther WT, Ryu S-E, King SB, Furdui CM, Poole LB. Use of Dimedone-Based Chemical Probes for Sulfenic Acid Detection. Thiol Redox Transitions Cell Signaling, Part A. 2010;473:77–94. doi: 10.1016/S0076-6879(10)73003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Kris-Etherton PM. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 414.Berndt C, Poschmann G, Stühler K, Holmgren A, Bräutigam L. Zebrafish heart development is regulated via glutaredoxin 2 dependent migration and survival of neural crest cells. Redox Biol. 2014;2:673–678. doi: 10.1016/j.redox.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Fedorova M, Kuleva N, Hoffmann R. Identification of cysteine, methionine and tryptophan residues of actin oxidized in vivo during oxidative stress. J Proteome Res. 2010;9:1598–1609. doi: 10.1021/pr901099e. [DOI] [PubMed] [Google Scholar]
- 416.Hamnell-Pamment Y, Lind C, Palmberg C, Bergman T, Cotgreave Ia. Determination of site-specificity of S-glutathionylated cellular proteins. Biochem Biophys Res Commun. 2005;332:362–369. doi: 10.1016/j.bbrc.2005.04.130. [DOI] [PubMed] [Google Scholar]
- 417.Su D, Shukla AK, Chen B, Kim JS, Nakayasu E, Qu Y, Aryal U, Weitz K, Clauss TRW, Monroe ME, Camp DG, Bigelow DJ, Smith RD, Kulkarni RN, Qian WJ. Quantitative site-specific reactivity profiling of S-nitrosylation in mouse skeletal muscle using cysteinyl peptide enrichment coupled with mass spectrometry. Free Radic Biol Med. 2013;57:68–78. doi: 10.1016/j.freeradbiomed.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Chen SC, Huang B, Liu YC, Shyu KG, Lin PY, Wang DL. Acute hypoxia enhances proteins’ S-nitrosylation in endothelial cells. Biochem Biophys Res Commun. 2008;377:1274–1278. doi: 10.1016/j.bbrc.2008.10.144. [DOI] [PubMed] [Google Scholar]
- 419.Shartava A, Monteiro Ca, Aladar Bencsath F, Schneider K, Chait BT, Gussio R, Casoria-Scott La, Shah AK, Heuerman Ca, Goodman SR. A posttranslational modification of β-actin contributes to the slow dissociation of the spectrin-protein 4.1-actin complex of irreversibly sickled cells. J Cell Biol. 1995;128:805–818. doi: 10.1083/jcb.128.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Dalle-Donne I, Giustarini D, Rossi R, Colombo R, Milzani a. Reversible S-glutathionylation of Cys374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic Biol Med. 2003;34:23–32. doi: 10.1016/S0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- 421.Pizarro GO, Ogut O. Impact of actin glutathionylation on the actomyosin-S1 ATPase. Biochemistry. 2009;48:7533–7538. doi: 10.1021/bi900669m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Dalle-Donne I, Milzani A, Giustarini D, Di Simplicio P, Colombo R, Rossi R. S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. J Muscle Res Cell Motil. 2000;21:171–181. doi: 10.1023/A:1005671319604. [DOI] [PubMed] [Google Scholar]
- 423.Tsapara a, Kardassis D, Moustakas a, Gravanis a, Stournaras C. Expression and characterization of Cys374 mutated human β-actin in two different mammalian cell lines: Impaired microfilament organization and stability. FEBS Lett. 1999;455:117–122. doi: 10.1016/S0014-5793(99)00848-0. [DOI] [PubMed] [Google Scholar]
- 424.Dalle-Donne I, Rossi R, Giustarini D, Gagliano N, Di Simplicio P, Colombo R, Milzani a. Methionine oxidation as a major cause of the functional impairment of oxidized actin. Free Radic Biol Med. 2002;32:927–937. doi: 10.1016/S0891-5849(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 425.Lusty CJ, Fasold H. Characterization of sulfhydryl groups of actin. Biochemistry. 1969;8:2933–2939. doi: 10.1021/bi00835a036. [DOI] [PubMed] [Google Scholar]