Abstract
Background
Maternal supply of thyroid hormones during pregnancy serves a critical role in fetal development. Although animal and in vitro studies provide evidence for thyroid hormone disruption as a result of bisphenol A (BPA) exposure, there is still a lack of evidence in human studies, particularly in the context of pregnancy.
Objectives
We aimed to explore the associations between urinary BPA concentrations and plasma thyroid hormone parameters during gestation in pregnant women, and also investigated potential windows of vulnerability during gestation.
Methods
Our study population included 116 cases of preterm birth and 323 controls from a nested case-control study. We measured BPA in urine and thyroid hormone parameters in plasma samples collected at up to four study visits during pregnancy (median for each visit: 9.64, 17.9, 26.0, and 35.1 weeks gestation). We used linear mixed models for repeated measures analyses, and multivariate linear regression models stratified by study visit to explore potential windows of susceptibility.
Results
In our repeated measures analysis, BPA and thyroid stimulating hormone (TSH) were inversely associated. An interquartile range (IQR) increase in BPA was associated with an 8.21 % decrease in TSH (95% confidence interval [CI]: −14.2, −1.83), and a 4.79% increase in free T4 (95% CI: 0.82, 8.92). BPA and TSH were also inversely associated in our cross-sectional analyses at visits 3 and 4.
Conclusions
Our results suggest that TSH is inversely associated with urinary BPA in a consistent manner across pregnancy. Disruption of TSH levels during pregnancy can potentially impact child development and interfere with normal birth outcomes.
1. INTRODUCTION
Although regulations have increasingly restricted the production and use of bisphenol A (BPA) in several consumer products made of polycarbonate plastics (National Conference of State Legislature 2015), there is continuing evidence that widespread exposure to BPA still exists in the U.S. population (Andra et al. 2015). Furthermore, previous human exposure studies have shown detectable levels of urinary BPA in a majority of pregnant women in the U.S. (Braun et al. 2011; Woodruff et al. 2011). Human exposure to BPA has been hypothesized to disrupt critical hormones in the endocrine system (Vanderberg et al. 2012). There are several animal and in-vitro studies providing evidence that BPA exposure can affect biochemical processes that disrupt the production, secretion, or circulation of thyroid hormones (Boas et al. 2012; Richter et al. 2007; Tilley et al. 2015).
Within the hypothalamic-pituitary-thyroid (HPT) axis, thyrotropin (TSH) – a glycoprotein hormone originating from the pituitary gland – stimulates the thyroid gland to produce the thyroid hormones triiodothyronin (T3), and thyroxine (T4) (Tingi et al. 2016). T3 and T4 circulate throughout the body to affect target sites, in addition to negatively regulating TSH production at the pituitary gland (Tingi et al. 2016). During gestation, homeostatic regulation of the HPT axis is critical for the developing fetus, and maternal thyroid hormone levels have varying impacts on the fetus throughout periods of gestation (Tingi et al. 2016). During the first 10–12 weeks of gestation, the fetus’s entire supply of thyroid hormones comes from the mother, and coincides with fetal neurodevelopmental events such as brain stem and cerebral neurogenesis (Moog et al. 2017; Préau et al. 2015). After this period, the fetal thyroid gland begins to mature and produce thyroid hormones, but is not fully functional until 18 – 20 weeks gestation (Moog et al. 2017, Tingi et al. 2016). Maternal thyroid hormones also affect the maturation of various other tissues and organs in the fetus, in addition to stimulating the placental secretion of hormones that influence the supply of fetal glucose (Moog et al. 2017; Vissenberg et al. 2015).
Thyroid disease states such as hyperthyroidism and hypothyroidism are associated with adverse health outcomes during pregnancy (Institute of Medicine 2007; Krassas et al. 2010; Neale and Burrow 2004), and associated with stillbirth, miscarriage, preeclampsia, preterm delivery, cardiovascular disorders, and neurodevelopmental disorders in fetuses (Haddow et al. 1999; Luewan et al. 2011; Männistö et al. 2013). In addition to overt thyroid disease, subclinical perturbations of thyroid hormone levels are also suspected of adversely affecting child and maternal health during pregnancy (Negro and Mestman 2011). Recent review articles and meta-analyses suggest that subclinical hypothyroidism is associated with a higher risk for pre-eclampsia, pregnancy loss, placental abruption, premature rupture of membranes, preterm delivery, and neonatal mortality (Maraka et al. 2016; Tingi et al. 2016; van den Boogaard et al. 2011). These findings emphasize the need to continue investigating potential causes and consequences of changes in subclinical thyroid hormone levels during pregnancy.
Of particular relevance to child and maternal health are the interactions between BPA and thyroid hormones during pregnancy. Human health studies that examined BPA and thyroid hormones in adults (Geens et al. 2015; Meeker et al. 2009a; Meeker and Ferguson 2011; Sriphrapradang et al. 2013; Wang et al. 2012; Wang et al. 2013), and in pregnant women (Chevrier et al. 2013; Romano et al. 2015), have provided evidence suggesting that BPA may affect circulating levels of several thyroid hormone parameters. One of the studies of pregnant women indicated that maternal BPA levels were inversely associated with total T4 (Chevrier et al. 2013), whereas the other study did not show significant relationships between maternal BPA and any of the thyroid hormone parameters measured (Romano et al. 2015). Some of these studies were limited by collection of only a single spot urine and/or blood sample for measurement of BPA and thyroid hormones, having a relatively small sample size, or assessing associations with only a cross-sectional design. Our study is the first to examine this relationship in repeated measurements during pregnancy, which not only provides greater statistical power to assess a more robust exposure assessment, but also characterizes associations between BPA and thyroid hormones across time during gestation by controlling for individual variability. Specifically in our current study, we evaluated the associations between four repeated measures of urinary BPA and plasma thyroid hormone parameters – TSH, total T3, and free and total T4 – collected at up to four study visits in pregnancy. We also sought to identify potential windows of vulnerability during pregnancy by assessing the extent to which these associations varied by gestational age.
2. METHODS
2.1 Study Population
Participants of this current study comprised a subset of pregnant women drawn from a nested case-control study who were initially recruited for a prospective, longitudinal birth cohort at the Brigham and Women’s Hospital in Boston, MA (Ferguson et al 2014a; Ferguson et al 2015). In previous studies of this cohort, exposures to environmental phthalates and health outcomes such as preterm birth were investigated (Ferguson et al 2014b), in addition to markers of thyroid function (Johns et al 2016). The initial LifeCodes birth cohort enrolled 1600 women between 2006 and 2008, and of those, 1,181 pregnant women were recruited and followed to term, and delivered live, singleton infants. The nested case-control study included 130 women who delivered preterm (<37 full weeks of gestation) and 352 randomly selected controls of women who delivered a singleton infant after 37 full weeks of gestation. For the present study we excluded participants from the original nested case-controls study who had pre-existing thyroid conditions such as thyroid cancer, Grave’s disease, and hyper- or hypothyroidism (N=41), in addition to participants who lacked stored plasma samples from at least one study visit (N=2). Our final study population (N= 439) included 116 preterm birth cases and 323 controls.
Additional information regarding participant recruitment, eligibility criteria, and follow-up has been previously described (Ferguson et al. 2014a). Briefly, at each visit during follow-up, body metrics were recorded, and urine and blood were collected to measure biomarkers of chemical exposures and endogenous biological compounds. Biological samples were collected from the participants at four study visits across pregnancy: visit 1 (9.64 weeks of gestation [5.43 – 19.1 weeks]), visit 2 (17.9 weeks of gestation [14.9 – 32.1 weeks]), visit 3 (26.0 weeks of gestation [22.9 – 36.3 weeks]), and visit 4 (35.1 weeks of gestation [33.1 – 38.3]). During visit 1, a questionnaire was administered to collect demographic and other health information.
2.2 BPA Measurement
Urine samples collected from 439 participants (N=1,543 samples) were available for BPA measurements. Total BPA (free plus conjugated) was measured using isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) at NSF International (Ann Arbor, MI). The analytical methodology was modified from a method developed by the Centers for Disease Control and Prevention (CDC) and described in a previous study (Lewis et al. 2013). In summary, urine samples underwent enzymatic deconjugation, solid phase extraction, and were analyzed with a triple quadrupole mass spectrometer. For samples where BPA measurements were below the limit of detection (LOD), we assigned a value of the LOD divided by the square root of 2 because the percent of non-detectable samples was less than 50, and the distribution of BPA was not highly skewed (geometric standard deviation without samples below LOD = 2.03, and with samples below LOD = 2.82) (Hornung and Reed 1990).
To account for urinary dilution, in our preliminary analyses we corrected BPA concentrations using specific gravity (SG) as follows: BSG = B [(1.015 – 1)/(SG – 1)], where BSG is the specific gravity-adjusted BPA concentration (ug/L), B is the observed BPA concentration, the constant 1.015 is the specific gravity population median, and SG is the specific gravity of the individual urine sample (Meeker et al. 2009b). To account for this variability in urinary dilution between individuals in multivariate analyses, we applied a recent method developed by O’Brien et al. (2016). Briefly, we calculated the ratio of SG to predicted values of SG – which were extracted from a linear mixed model (LMM) of SG regressed on maternal age – and then standardized urinary BPA measurements by this ratio. In all subsequent LMMs and linear regression models, we used this covariate adjusted standardized BPA value as our predictor and further adjusted for SG as a covariate.
2.3 Thyroid Hormone Measurements
Plasma samples for 439 participants were available (N=1,443 samples) from at least one of the four study visits. Samples were assayed for TSH, total T3, and free and total T4 at the Clinical Ligand Assay Service Satellite (CLASS) lab at the University of Michigan (Ann Arbor, MI). TSH as well as total T3 and T4 were measured using an automated chemiluminescence immunoassay (Bayer ADVIA Centaur, Siemens Health Care Diagnostics, Inc.). Free T4 was assayed using direct equilibrium dialysis followed by radioimmunoassay (IVD Technologies). The LOD for TSH, total T3, total T4, and free T4 were 0.01 μIU/mL, 0.1 ng/mL, 0.3 μg/dL, and 0.1 ng/dL, respectively. Among the plasma samples, there was 100% detection for total T4 and T3. Meanwhile, 99.5% of TSH measurements were above the LOD, resulting in six samples below the LOD. Of those six samples, five had corresponding free T4 measurements at the ~75th percentile or greater within our study population, and we imputed TSH levels for those five samples by assigning them the TSH LOD value (0.01 μIU/mL). For free T4, we observed a detection rate of 98%, resulting in 27 samples below the LOD. Given that the LOD for free T4 is not biologically feasible, these samples were regarded as missing values in our statistical analyses.
2.4 Statistical Analyses
To enhance generalizability of results from our study population, inverse probability weighting was applied to all statistical analyses presented in this analysis. Specifically, we corrected for over-representation of preterm birth cases by applying study-specific weights related to the inverse probability of inclusion of cases of preterm birth, making the relative weights of cases and controls in our study population resemble observed proportions in a general population (Richardson et al. 2007).
Of the biomarkers we measured, the distributions of BPA, TSH, and free T4 were right-skewed; thus, we transformed each of those variables using the natural log in statistical analyses to comply with assumptions of normality for linear regression and LMMs. We confirmed the appropriateness of log-transformation of TSH and free T4 for repeated measures analyses by comparing marginal raw residuals and studentized conditional residuals of LMMs with and without transformation of these biomarkers. Models with log-transformed response variables had residuals that were more normally distributed and less skewed. Total T3 and T4 were normally distributed, and therefore remained untransformed in statistical analyses. Geometric means and standard deviations were calculated for BPA by study visit and demographic variables. We tested the difference in mean BPA levels across demographic variables using LMMs with a random intercept for each subject.
For our repeated measures analyses, BPA was regressed on each thyroid hormone in separate LMMs, which included subject-specific random intercepts to account for intra-individual correlation of repeated measures over time. When constructing the LMMs, variables were considered as potential covariates if they were significantly or marginally associated with both thyroid hormone parameters and BPA in bivariate analyses, and included: maternal age, race/ethnicity, education, BMI at initial study visit, tobacco use, alcohol use, fetal sex and health insurance provider (an indicator of socioeconomic status). In addition to urinary BPA, crude LMMs consisted of fixed effects terms for gestational age at sample collection and urinary SG. Of the potential covariates, maternal age, race/ethnicity, education, health insurance provider and BMI at initial study visit were each associated with thyroid hormone parameters and BPA. These variables were then selected for the final model in a stepwise approach if they changed the beta coefficient of BPA by 10 percent or more. We also considered inclusion of covariates by how each impacted model fit, based on Aikaike information criterion (AIC). The covariates in the final LMMs included urinary specific gravity, gestational age at time of sample collection, maternal age at enrollment, BMI at time of sample collection, and health insurance provider. Of these variables, maternal age was the only variable not significantly associated with thyroid hormones in bivariate analyses, but was kept in the model due to a priori knowledge that maternal age is a likely confounder in the association between BPA exposure and thyroid hormones (Chevrier et al. 2013; Romano et al. 2015). To determine potential non-linear relationships, we further assessed the appropriateness of fitting maternal age and gestational age using quadratic and cubic terms. Maternal age appeared to have a linear relationship with the outcome variables, however, gestational age was more appropriately fit in our LMMs as a quadratic term. For LMMs, we considered the following options for covariance matrices: variance components, compound symmetry, heterogeneous compound symmetry, first order autoregressive, heterogeneous first order autoregressive, and unstructured. Among these covariance matrices, AIC indicated that variance components were the most appropriate for our analyses.
To investigate potential windows of vulnerability during pregnancy, we stratified linear regression models by study visit of sample collection. Our regression models included individual thyroid hormones as the continuous outcome variable, natural log transformed BPA as the continuous predictor variable, and the same covariates as those used in LMMs. To enhance the interpretability of effect estimates from models with log-transformed outcome and/or exposure variables in both our cross-sectional and repeated measures analyses, beta estimates and their respective 95% confidence intervals (CI) were converted to the percent change in individual thyroid hormone levels corresponding to an interquartile rage (IQR) difference in urinary BPA concentrations.
To examine potential non-linear associations between BPA and thyroid hormone parameters, we conducted additional cross-sectional analyses where we regressed individual hormone parameters by quartiles of SG-corrected BPA while controlling for the same covariates used in LMM. We treated quartiles of BPA as an ordinal variable to test for trends across increasing quartiles. We also assessed potential non-linear relationships in LMMs by modeling BPA as a quadratic term. Based on AIC values and residual distribution, BPA was more appropriately fit as a linear term. The threshold for statistical significance and marginal significance corresponded to p-values of 0.05 and 0.10 respectively. All of the aforementioned statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
2.5 Sensitivity Analyses
In order to evaluate the robustness of our analyses, we conducted additional sensitivity analyses. Firstly, we conducted additional stratified analyses with four non-overlapping ranges of gestational age as opposed to study visit. Secondly, to explore potential confounding by case-control status, we conducted a sensitivity analysis wherein we conducted our repeated measures analyses among only women who delivered at term.
Given that previous analyses of this cohort have investigated the relationship between phthalates and thyroid hormone parameters (Johns et al. 2016), we explored the potential influence of phthalates on the relationship between BPA and thyroid hormone parameters. We selected phthalates that were previously shown to be associated with thyroid hormones, and adjusted for individual phthalate metabolites (mono-2-ethylhexyl phthalate [MEHP], mono-iso-butyl phthalate [MiBP], and mono-3-carboxypropyl phthalate [MCPP]) as a covariate in our repeated measures analyses. Finally, due to the close feedback relationship between free T4 and TSH, we assessed the potential influence of free T4 on the relationship between BPA and TSH by modeling for free T4 as a covariate.
3. RESULTS
Previous studies of this case-control study population have characterized distributions of BPA and thyroid hormone levels by study visit of sample collection (Cantonwine et al. 2015; Johns et al. 2016). The overall detection rate of BPA in our study sample was 81.1%. Geometric means and standard deviations for all thyroid hormones by study visit were calculated and presented in a previous study (Johns et al. 2016). In Table 1., we present selected percentiles of BPA concentrations by demographic characteristics. BPA was statistically significantly lower among women above 24 years of age in comparison to 18–24 year-olds, among women with college level education compared to high school graduates, and in women who did not smoke during pregnancy compared to women who reported smoking. BPA concentrations were significantly higher in non-white women than in white women, women with public health insurance compared to those with private insurance, and in obese women (greater than 30 kg/m2) in comparison to women with a BMI of less than 25 kg/m2. In comparison to the original study population, our nested case-control population had a similar proportion of White women, but slightly higher proportion of African-American women (17% in the present study population compared to 15.8% in the original population). Maternal age in our nested study (Mean [standard deviation (SD)] = 31.8 [5.3]) was similar to the parent cohort (Mean [S.D.] = 32 [5.7]). BMI at initial study visit was also similar in the nested study (Mean [S.D.] = 26.5 [6.14]) compared to the parent cohort (Mean [S.D.] = 27 [6.5]).
Table 1.
SG-corrected BPA measurements (weighted median [25th, 75th percentiles]) by demographic characteristics in all samples measured (N=439 participants, 1,443 plasma samples).
| Population Characteristics | Count (Percent) of Total Populationa | BPA (ug/L) | |
|---|---|---|---|
| Age | 18 – 24 years old [ref] | 54 (12%) | 2.1 (1.19, 3.28) |
| 25 – 29 years old | 92 (21%) | 1.26 (0.81, 1.99)* | |
| 30 – 34 years old | 176 (40%) | 1.15 (0.72, 1.93)* | |
| 35 + years old | 117 (27%) | 1.25 (0.77, 1.99)* | |
| Race/ethnicity | White [ref] | 247 (56%) | 1.13 (0.7, 1.86) |
| African – American | 75 (17%) | 1.66 (1.05, 2.65)* | |
| Other | 117 (27%) | 1.37 (0.85, 2.26)* | |
| Education | High School Degree (13 years) [ref] | 67 (16%) | 1.69 (1.09, 3.03) |
| Technical School (> 13 years) | 76 (18%) | 1.47 (0.94, 2.58) | |
| Junior College or Some College (> 13years) | 127 (29%) | 1.22 (0.73, 1.98)* | |
| College Graduate (16 + years) | 159 (37%) | 1.09 (0.70, 1.78)* | |
| Health Insurance Provider | Private/HMO/Self-pay [ref] | 344 (81%) | 1.19 (0.72, 1.98) |
| Medicaid/SSI/MassHealth | 83 (19%) | 1.76 (1.13, 3.01)* | |
| BMI at Initial Visit | <25 kg/m2 [ref] | 227 (52%) | 1.15 (0.72, 1.98) |
| 25 – 29.9 kg/m2 | 113 (26%) | 1.27 (0.77, 2.09) | |
| ≥30 kg/m2 | 99 (22%) | 1.59 (1.1, 2.54)* | |
| Tobacco Use | Smoked During Pregnancy [ref] | 31 (7%) | 1.69 (1.13, 3.01) |
| No Smoking During Pregnancy | 402 (93%) | 1.25 (0.81, 2.09)* | |
| Alcohol Use | Alcohol Use During Pregnancy [ref] | 18 (4%) | 1.05 (0.61, 1.67) |
| No Alcohol use During Pregnancy | 412 (96%) | 1.3 (0.83, 2.12) | |
| Fetal Sex | Male [ref] | 198 (45) | 1.22 (0.79, 2.07) |
| Female | 241 (55%) | 1.31 (0.84, 2.1) |
Abbreviations: BMI, Body Mass Index; HMO, Health Maintenance Organization; SSI, Supplemental Security Income.
Weighted by case-control sampling probabilities to represent the general sampling population
Significant difference (p<0.01) in BPA concentration in the category compared to reference (first category listed) using linear mixed models adjusted for specific gravity and with a random intercept for each subject.
Table 2 shows that the geometric mean concentrations of BPA were significantly lower during study visits 2 and 4 in comparison to the initial visit.
Table 2.
Weighted distributions of SG-corrected urinary BPA by study visit of sample collection in pregnancy (N=439 subjects)
| Visit | # Samples | % <LOD | Geometric Mean (Geometric SD) | Percentiles
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 90 | 95 | Max | ||||
| Total | 1543 | 18.9 | 1.18 (2.82) | 0.83 | 1.28 | 2.10 | 3.68 | 5.59 | 95.7 |
| 1 | 438 | 15.8 | 1.33 (2.84) | 0.81 | 1.31 | 2.09 | 3.57 | 5.59 | 53.3 |
| 2 | 382 | 24.9 | 1.04 (2.85)* | 0.77 | 1.25 | 2.11 | 3.28 | 4.76 | 63.8 |
| 3 | 375 | 17.6 | 1.22 (2.84) | 0.85 | 1.30 | 2.08 | 4.13 | 5.62 | 78.3 |
| 4 | 348 | 17.5 | 1.12 (2.70)* | 0.80 | 1.28 | 2.12 | 3.68 | 5.63 | 95.7 |
Significant difference (p<0.01) in BPA concentration in study visit compared to visit 1 using linear mixed models with a random intercept for each subject
The percent changes in thyroid hormones associated with an IQR increase in BPA concentrations across study visits are presented in Table 3. Our repeated measures analyses showed that an IQR increase in BPA in our final model was significantly associated with an 8.21% decrease in TSH (95% confidence interval [CI]: −14.2, −1.83). Similar relationships were observed in both full and crude models of TSH. In the final LMM of free T4, we observed a significant positive relationship with BPA such that an IQR increase in BPA was associated with a 4.79% increase in free T4 (95% CI: 0.82, 8.92). This significant positive relationship between BPA and free T4 was also observed in the full model but not the crude model. The relationship between BPA and total T3 was nearly null, meanwhile BPA and total T4 were inversely related, but that relationship was not significant.
Table 3.
Repeated measures analysis: percent change (95% CIs) in thyroid hormone concentrations in relation to interquartile range increase in BPA concentrations
| Thyroid Hormone Parameter | Model 1a | Model 2b | Model 3c | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | %Δ (95%CI) | p-value | n | %Δ (95%CI) | p-value | n | %Δ (95%CI) | p-value | |
| Ln-TSH | 1107 | −7.07 (−13.1, −0.57)* | 0.03 | 1152 | −8.89 (−14.7, −2.64)* | 0.006 | 1118 | −8.21 (−14.2, −1.83)* | 0.01 |
| Ln-Free T4 | 1288 | 4.48 (0.48, 8.65)* | 0.03 | 1343 | 3.64 (−0.21, 7.64) | 0.06 | 1301 | 4.79 (0.82, 8.92)* | 0.02 |
| Total T3 | 1030 | −0.02 (−0.04, 0.001) | 0.07 | 1075 | −0.01 (−0.03, 0.01) | 0.56 | 1040 | 0.01 (−0.03, 0.004) | 0.12 |
| Total T4 | 1275 | −0.85 (−2.24, 0.55) | 0.23 | 1328 | −0.20 (−1.56, 1.17) | 0.78 | 1287 | −0.61 (−2.00, 0.77) | 0.38 |
Full model: adjusted for SG, gestational age + gestational age2, maternal age, race/ethnicity, education level, health insurance provider, and BMI at first study visit
Crude model: adjusted for SG, gestational age + gestational age2
Final model: adjusted for SG, gestational age + gestational age2, maternal age, health insurance provider, and BMI at first study visit
p < 0.05
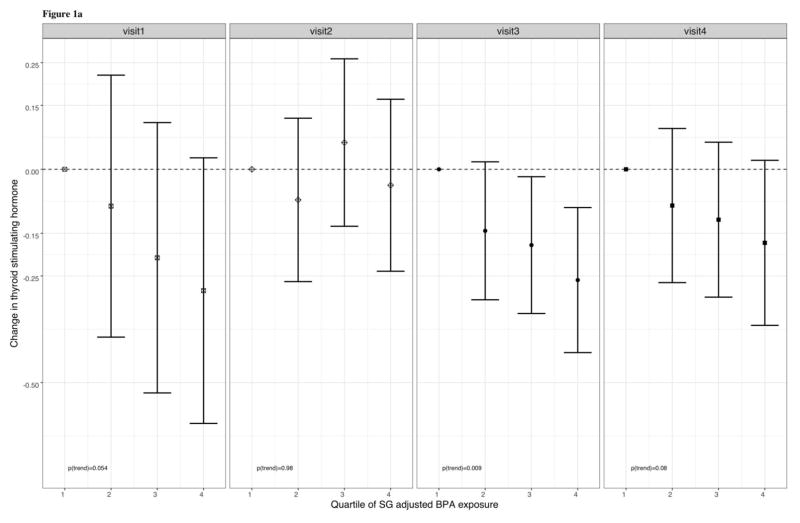
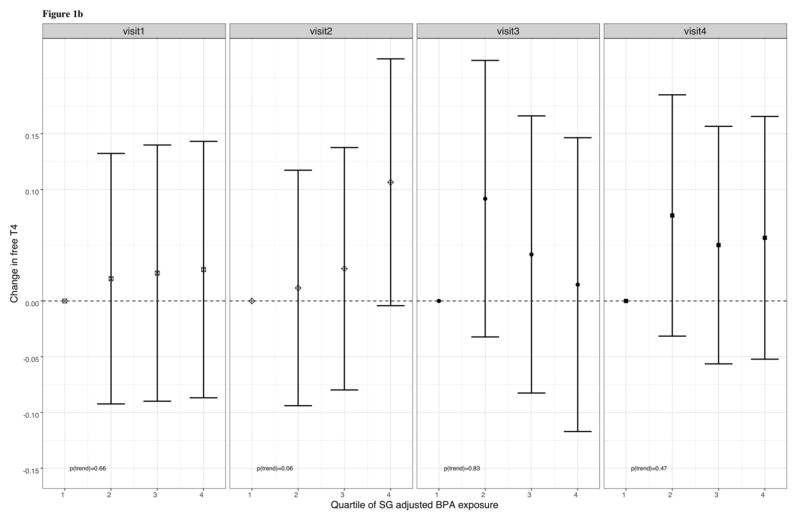
In Table 4, percent changes in thyroid hormones are presented in relation to an IQR increase in BPA at individual study visits and by four non-overlapping windows of gestation. These cross-sectional analyses showed that an IQR increase in BPA was associated with a statistically significant 13.5% decrease in TSH at visit 3 (95% CI: −21.9, −4.23) and a 17.3% decrease in TSH at visit 4 (95% CI: −27.9, −5.10), in addition to a marginally significant 16.4% decrease in TSH during visit 1 (95% CI: −31.2, 1.53). BPA was not significantly associated with total T3 or free T4 at any of the study visits. At visit 4, we observed that an IQR increase in total BPA was associated with a suggestive 3.68% increase in total T4 (95% CI: −0.59, 7.95). When stratified by non-overlapping windows of gestation, the direction and significance of relationships were similar to that of the stratified analyses by study visit. Similar to our cross-sectional analyses of BPA as a continuous predictor, we observed marginally significant inverse relationships between quartiles of SG-corrected BPA and TSH at visit 1 (p for trend = 0.054) and visit 4 (p for trend = 0.08), and a significant inverse relationship at visit 3 (p for trend = 0.009), and a marginally significant inverse relationship at visit 4 (p for trend = 0.08) (figure 1a.). Additionally, we found that BPA and free T4 were positively associated at visit 2 (p for trend = 0.06), but there were no significant trends between free T4 and quartiles of SG-corrected BPA at visits 1, 3, and 4(figure 1b.).
Table 4.
Cross-sectional analysis: percent change (95% CIs) in thyroid hormone concentrations in relation to interquartile range increase in urinary BPA concentrations by study visit during gestation and four non-overlapping windows of gestation
| Ln-TSH | Ln-Free T4 | Total T3 | Total T4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Visit | n | %Δ (95%CI) | p-value | n | %Δ (95%CI) | p-value | n | %Δ (95%CI) | p-value | n | %Δ (95%CI) | p-value |
| 1 | 303 | -16.4 (−31.2, 1.53) | 0.07 | 356 | 2.66 (−4.49, 10.3) | 0.47 | 290 | 0.03 (−0.04, 0.07) | 0.65 | 349 | −0.11 (−3.71, 3.49) | 0.95 |
| 2 | 297 | 4.14 (−14.7, 27.1) | 0.69 | 339 | 6.73 (−1.54, 15.7) | 0.11 | 282 | −0.02 (−0.09, 0.05) | 0.62 | 333 | 0.07 (−4.79, 4.93) | 0.98 |
| 3 | 276 | −13.5 (−21.9, −4.23)* | <0.01 | 311 | −1.09 (−9.26, 7.81) | 0.80 | 257 | −0.02 (−0.07, 0.02) | 0.32 | 304 | −1.51 (−5.15, 2.13) | 0.41 |
| 4 | 241 | −17.3 (−27.9, −5.10)* | <0.01 | 295 | 3.79 (−3.78, 12.0) | 0.33 | 211 | −0.05 (−0.11, 0.02) | 0.18 | 301 | 3.68 (−0.59, 7.95) | 0.09 |
|
| ||||||||||||
| Gestational Weeks | ||||||||||||
|
| ||||||||||||
| 5.43 – 13.9 | 290 | −10.7 (−26.7, 8.76) | 0.26 | 342 | 2.45 (−4.90, 10.4) | 0.52 | 279 | 0.01 (−0.04, 0.06) | 0.72 | 334 | −0.32 (−3.71, 3.49) | 0.86 |
| 14.0 – 19.7 | 292 | −9.83 (−26.9, 11.1) | 0.33 | 333 | 6.77 (−1.2, 15.4) | 0.097 | 279 | −0.01 (−0.09, 0.06) | 0.69 | 328 | 1.00 (−4.01, 6.02) | 0.69 |
| 19.9 – 28.0 | 283 | −13.1 (−21.5, −3.86)* | <0.01 | 320 | −1.05 (−9.18, 7.81) | 0.81 | 263 | 0.02 (−0.07, 0.02) | 0.26 | 312 | −2.06 (−5.57, 1.46) | 0.25 |
| 28.3 – 38.3 | 252 | −16.0 (−26.7, −3.89)* | 0.011 | 306 | 3.85 (−3.62, 11.8) | 0.32 | 219 | −0.05 (−0.11, 0.02) | 0.17 | 313 | 4.09 (−0.16, 8.34) | 0.06 |
Linear regression models adjusted for urinary specific gravity, gestational age + gestational age2 at time of sample collection, maternal age at enrollment, BMI at the first study visit, and health insurance provider.
p < 0.05
Figure 1.
Figure 1a. Adjusted regression coefficients for change in TSH in relation quartiles of urinary BPA
Figure 1.a. Adjusted regression coefficients for change in plasma TSH in relation to SG-adjusted BPA quartiles. Sampling weights were used in addition to adjustment for the covariates: gestational age + gestational age2 at time of sample collection, maternal age at enrollment, body mass index (BMI) at the first study visit, and health insurance provider.
Figure 1b. Adjusted regression coefficients for change in FT4 in relation to quartiles of urinary BPA
Figure 1.b. Adjusted regression coefficients for change in plasma free T4 in relation to SG-adjusted BPA quartiles. Sampling weights were used in addition to adjustment for the covariates: gestational age + gestational age2 at time of sample collection, maternal age at enrollment, body mass index (BMI) at the first study visit, and health insurance provider.
Results from our sensitivity analysis among only control women were similar to those reported in the weighted case-control study population (Supplemental Tables 1 and 2). The addition of individual phthalate metabolites and free T4 as a covariate did not substantially change the beta coefficient of BPA and the significance level remained unchanged (Supplemental Tables 1 and 2).
4. DISCUSSION
To our knowledge, this is the first epidemiologic study to assess the associations between repeated measures of BPA and maternal thyroid hormone parameters in pregnancy. Using repeated measures, our analysis demonstrated that urinary BPA concentrations were inversely associated with levels of TSH in pregnant women and positively associated with free T4. In our stratified analyses, BPA was significantly inversely associated with TSH at visits 3 and 4. BPA was also marginally associated with decreased TSH at visit 1 (p = 0.07). With the exception of visit 2, the magnitude of these effect estimates did not appreciably differ between study visits, therefore there is not enough evidence in the present study implicating windows of vulnerability. In regards to free T4, cross-sectional analyses suggest that the relationship between BPA and free T4 were not significant by study visit or non-overlapping windows of gestation. At visit 4, we also observed a positive association between BPA and total T4, but this was not statistically significant (p = 0.09).
Two previous epidemiological studies have investigated the relationship between BPA and thyroid hormones in pregnant women (Chevrier et al. 2013; Romano et al. 2015). In a sample of 335 pregnant women, Chevrier et al. (2013) measured urinary BPA in the first half (12.4 ± 3.8 weeks) and second half (26.2 ± 2.2 weeks) of gestation, and thyroid hormone parameters in the second trimester (~26 weeks). Similar to what we observed at visit 3 (median 26 weeks of gestation), Chevrier et al. (2013) observed null associations between free T4 and urinary BPA at the second half of gestation. In contrast to our study, Chevrier et al. (2013) reported a significant inverse relationship between total T4 and urinary BPA during the second half of gestation. An inverse association between TSH and urinary BPA was observed at the second half of gestation, but unlike our study, those results were not statistically significant (Chevrier et al. 2013). In another study, Romano et al. (2015) measured maternal urinary BPA twice – at 16 and 26 weeks gestation – and thyroid hormone parameters at 16 weeks gestation in a sample of 181 pregnant women. Similar to our findings at visit 2 (median 18 weeks of gestation), the authors found that maternal urinary BPA was not significantly associated with TSH, total and free T4, or free T3 at 16 weeks gestation (Romano et al. 2015).
Although the results of these two studies disagree with some of our findings, it should be noted that the discrepancies might be due to differences in populations, study design, or related to the inaccuracy of single spot urine measures of BPA. Chevrier et al. (2013) conducted their analyses from a cohort through the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), which compared to our study, had a higher proportion of participants under 25 years of age that were from an agricultural region and predominantly Latin American, underlining potential differences in exposure levels due to socioeconomic status, cultural practices, and geographical location. Another possible reason for observed differences could have been due to the fact that Chevrier et al. (2013) accounted for urinary dilution using urinary creatinine, whereas we utilized specific gravity. Romano et al. (2015) presented results from the Health Outcomes and Measures of the Environment (HOME) Study, and their study population had a higher proportion of women under 25 and Black women. Furthermore, differences may have been observed due to the fact that Romano et al. (2015) chose to adjust for covariates that were absent in our data, such as measurements of iodine intake, polychlorinated biphenyls, and hexachlorobenzene. In regards to the limitation of single spot measurements, non-persistent organic chemicals such as BPA are metabolized quickly, and concentrations within humans are highly variable (Barr et al. 2005b). Single spot urine measurements estimate exposures that recently took place and lack the ability to characterize long-term continuous exposures, which can be better addressed using repeated measurements (Barr et al. 2005b).
Several epidemiological studies have investigated the relationship between BPA and thyroid hormones, specifically TSH in adult men and non-pregnant women (Geens et al. 2015; Meeker et al. 2009a; Meeker and Ferguson 2011; Wang et al. 2013). Consistent with our results, two of these studies found suggestive inverse relationship between urinary BPA and serum TSH in adults and children (N= 1,675) (Meeker and Ferguson 2011) and adult men alone (N=75) (Meeker et al. 2009a). From a larger study of adults in China (N=3,394), Wang et al. (2013) observed a significant inverse relationship between these biomarkers as well. Conversely, another study reported a significant positive association between urinary BPA and serum TSH in 43 lean adults but not in 151 obese adults (Geens et al. 2015). Since these studies represent non-pregnant women or men, they cannot be directly compared to our findings, but they do provide evidence that BPA exposure may be related to thyroid hormone disruption in humans. Other factors besides sex and pregnancy status that may lead to contrasting findings include age ranges of participants, and physiological states such as obesity.
Previous studies within this cohort have examined maternal urinary BPA levels and thyroid hormones separately in relation to birth outcomes (Catonwine et al. 2015; Johns et al. 2017). Johns et al. (2017) reported that higher levels of total T3 were significantly associated with higher odds of overall preterm birth at study visits 1 and 3, whereas free T4 was protective at visit 3, and significantly associated with lower odds of preterm birth. Cantonwine et al. (2015) did not observe significant associations between overall pre-term birth and BPA. These results suggest that thyroid hormones may not mediate the effect of BPA on preterm birth; however, BPA may still influence other critical physiological processes during pregnancy through disruption of thyroid hormone levels as indicated in the present study.
Although the mechanisms of action have not been confirmed, some authors have hypothesized that one manner in which BPA disrupts thyroid function is by binding to the thyroid hormone receptor, acting as an antagonist to free T3, and impacting transcriptional regulation of T3 (Boas et al. 2009; Moriyama et al. 2002). Our results support another potential pathway, where BPA could disrupt normal thyroid functions by altering circulating TSH levels. Cells of the pituitary gland express estrogen receptors, making them susceptible to interactions with estrogen mimicking toxicants such as BPA (Dang et al. 2009). Some animal studies have demonstrated that BPA exposure can impact synthesis of hormones in the pituitary gland and increase the weight of the pituitary gland in rats (Dang et al. 2009; Goloubkova et al. 2000). It is possible that BPA exposure could affect TSH production at the pituitary gland through estrogen receptor signaling.
Another possible explanation as to why we observed an inverse relationship between BPA and TSH could be due to the fact that circulating T3 and T4 hormones also inhibit TSH synthesis via receptor binding at the pituitary gland (Ahmed et al. 2008). If BPA interacted with the thyroid gland to temporarily cause subclinical perturbations to T3 or T4 production, an increase above certain thresholds could lead to a feedback mechanism where the pituitary gland is signaled to decrease the production of TSH. This hypothesis is supported by the fact that BPA was positively associated with free T4 and inversely associated with TSH in repeated measures analyses. However, although we observed an overall positive relationship between BPA and free T4 in our repeated measures analyses, the lack of consistent positive associations in cross-sectional analyses suggest that perhaps TSH is a more sensitive biomarker compared to the other thyroid hormones and discernible to the thyroid disrupting potential of BPA. If fluctuations in TSH levels are mechanistically influenced by BPA exposure, it may have implications for the health of developing fetuses during pregnancy. Moreover, since TSH is closely involved with negative feedback mechanisms of T3 and T4 production, the marginally positive relationship between BPA and total T4 at visit 4 may have been a result of sustained disruption of TSH via BPA exposure. Alternatively, fluctuations in free T4 and TSH are also naturally variable throughout windows of pregnancy, which may be contributing to some of the trends that we see in our results (de Escobar et al. 2004).
A general limitation of our study is that despite the use of sampling weights in our analysis, there may still be constraints for the generalizability of our results to the broader U.S. population due to our sampling approach. Another limitation in our study is that we did not assess thyroid autoimmunity via measurement of thyroperoxidase antibodies, which may influence thyroid function in pregnancy (van den Boogaard et al. 2011). We also lacked the measurement of human chorionic gonadotropin (hCG), which is a molecule with similar homology to TSH and can stimulate the TSH receptor to influence maternal thyroid function during the first trimester in pregnancy (Tingi et al. 2016). What distinguishes the methodological approach of our current study is the advantage related to having up to four repeated measurements from a large sample of pregnant women. Having repeated measures enables us to control for intra-individual variability of our sample population in regression analyses and increases statistical power to assess associations between BPA and thyroid hormone parameters. Additionally, our measurement of free T4 also provides an advantage for better characterizing the HPT axis.
5. CONCLUSIONS
In summary, the present study provides further evidence that BPA exposure has the potential to disrupt thyroid hormones in humans. In order to elucidate the mechanisms that may explain these associations, further animal and human studies must be conducted. Given that pregnancy is an especially vulnerable period of time for child and maternal health, it is important to continue advancing our knowledge of the health effects of environmental exposures during pregnancy. Future studies that aim to further characterize the health effects of environmental chemicals during pregnancy should pay close attention to the role of the HPT axis and windows of vulnerability.
Supplementary Material
Widespread exposure to BPA still exists in the U.S.
We measured BPA and thyroid hormone parameters during pregnancy
Maternal BPA was inversely associated with TSH across pregnancy
Thyroid hormone disruption during pregnancy could influence fetal development
Acknowledgments
We thank K. Kneen, S. Clipper, G. Pace, D. Weller, and J. Bell of NSF International in Ann Arbor, Michigan, for urine BPA analysis; D. McConnell of the CLASS Lab at University of Michigan for assistance in hormone analysis.
Funding: Subject recruitment and sample collection was originally funded by Abbott Diagnostics. This work was also supported by the National Institute of Environmental Health Sciences, National Institutes of Health (grants R01ES018872, P42ES017198, P01ES022844, P30ES017885, P50ES026049, and T32ES007062). Support for Max Aung was provided in part by a grant from the Robert Wood Johnson Foundation Health Policy Research Scholars program.
Support for Kelly Ferguson was provided in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed OM, El-Gareib AW, El-Bakry AM, El-Tawab SA, Ahmed RG. Thyroid hormones states and brain development interactions. International Journal of Developmental Neuroscience. 2008;26(2):147–209. doi: 10.1016/j.ijdevneu.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Andra SS, Charisiadis P, Arora M, van Vliet-Ostaptchouk JV, Makris KC. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environment international. 2015;85:352–379. doi: 10.1016/j.envint.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wang RY, Needham LL. Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children's Study. Environmental Health Perspectives. 2005b:1083–1091. doi: 10.1289/ehp.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Main KM, Feldt-Rasmussen U. Environmental chemicals and thyroid function: an update. Curr Opin Endocrinol Diabetes Obes. 2009;16:385–391. doi: 10.1097/MED.0b013e3283305af7. [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Molecular and cellular endocrinology. 2012;355(2):240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environmental health perspectives. 2011;119(1):131. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD. Urinary bisphenol A levels during pregnancy and risk of preterm birth. Environmental health perspectives. 2015;123(9):895. doi: 10.1289/ehp.1408126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, Harley KG. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environmental Health Perspectives (Online) 2013;121(1):138. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VH, Choi KC, Jeung EB. Estrogen receptors are involved in xenoestrogen induction of growth hormone in the rat pituitary gland. Journal of Reproduction and Development. 2009;55(2):206–213. doi: 10.1262/jrd.20147. [DOI] [PubMed] [Google Scholar]
- de Escobar GM, Obregón MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Practice & Research Clinical Endocrinology & Metabolism. 2004;18(2):225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014a;70:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014b;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect. 2015;123:210–216. doi: 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T, Dirtu AC, Dirinck E, Malarvannan G, Van Gaal L, Jorens PG, Covaci A. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environment international. 2015;76:98–105. doi: 10.1016/j.envint.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Goloubkova T, Ribeiro MFM, Rodrigues LP, Cecconello AL, Spritzer PM. Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Archives of toxicology. 2000;74(2):92–98. doi: 10.1007/s002040050658. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New England Journal of Medicine. 1999;341(8):549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hornung RWR, LD Estimation of average concentration in the presence of nondetectable values Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Institute of Medicine. The National Academies Collection: Reports funded by National Institutes of Health. In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US); 2007. [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environ Health Perspect. 2016 doi: 10.1289/EHP170. http://dx.doi.org/10.1289/EHP170. [DOI] [PMC free article] [PubMed]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Seely EW, Meeker JD. Longitudinal Profiles of Thyroid Hormone Parameters in Pregnancy and Associations with Preterm Birth. PloS one. 2017;12(1):e0169542. doi: 10.1371/journal.pone.0169542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, et al. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. 2013;93:2390–2398. doi: 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luewan S, Chakkabut P, Tongsong T. Outcomes of pregnancy complicated with hyperthyroidism: a cohort study. Archives of gynecology and obstetrics. 2011;283(2):243–247. doi: 10.1007/s00404-010-1362-z. [DOI] [PubMed] [Google Scholar]
- Männistö T, Mendola P, Grewal J, Xie Y, Chen Z, Laughon SK. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. The Journal of Clinical Endocrinology & Metabolism. 2013;98(7):2725–2733. doi: 10.1210/jc.2012-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraka S, Ospina NMS, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, Coddington CC, III, Stan MN, Murad MH, Montori VM. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26(4):580–590. doi: 10.1089/thy.2015.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environmental science & technology. 2009a;44(4):1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Téllez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environmental health perspectives. 2009b;117(10):1587. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in US adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environmental health perspectives. 2011;119(10):1396. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. doi: 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. The Journal of Clinical Endocrinology & Metabolism. 2002;87(11):5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- National Conference of State Legislature. [accessed 7.1.2016];NCLS policy update: state restrictions on bisphenol a (BPA) in consumer products. 2015 http://www.ncsl.org/research/environment-and-natural-resources/policy-update-on-state-restrictions-on-bisphenol-a.aspx.
- Neale D, Burrow G. Thyroid disease in pregnancy. Obstetrics and gynecology clinics of North America. 2004;31(4):893–905. doi: 10.1016/j.ogc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Negro R, Mestman JH. Thyroid disease in pregnancy. Best Practice & Research Clinical Endocrinology & Metabolism. 2011;25(6):927–943. doi: 10.1016/j.beem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Richardson DB, Rzehak P, Klenk J, Weiland SK. Analyses of case-control data for additional outcomes. Epidemiology. 2007;18:441–445. doi: 10.1097/EDE.0b013e318060d25c. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124:220–227. doi: 10.1289/ehp.1509693. http://dx.doi.org/10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Préau L, Fini JB, Morvan-Dubois G, Demeneix B. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2015;1849(2):112–121. doi: 10.1016/j.bbagrm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive toxicology. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Webster GM, Vuong AM, Zoeller RT, Chen A, Hoofnagle AN, Calafat AM, Karagas MR, Yolton K, Lanphear BP, Braun JM. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environmental research. 2015;138:453–460. doi: 10.1016/j.envres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriphrapradang C, Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. Association between bisphenol A and abnormal free thyroxine level in men. Endocrine. 2013;44(2):441–447. doi: 10.1007/s12020-013-9889-y. [DOI] [PubMed] [Google Scholar]
- Tilley SK, Fry RC. Hormone Response Pathways as Responders to Environmental Contaminants and Their Roles in Disease. Systems Biology in Toxicology and Environmental Health. 2015:225. [Google Scholar]
- Tingi E, Syed AA, Kyriacou A, Mastorakos G, Kyriacou A. Benign thyroid disease in pregnancy: A state of the art review. Journal of Clinical & Translational Endocrinology. 2016;6:37–49. doi: 10.1016/j.jcte.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine reviews. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. Significance of (sub) clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Human reproduction update. 2011;17(5):605–619. doi: 10.1093/humupd/dmr024. [DOI] [PubMed] [Google Scholar]
- Vissenberg R, Manders VD, Mastenbroek S, Fliers E, Afink GB, Ris-Stalpers C, Goddijn M, Bisschop PH. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Human reproduction update. 2015;21(3):378–387. doi: 10.1093/humupd/dmv004. [DOI] [PubMed] [Google Scholar]
- Wang F, Hua J, Chen M, Xia Y, Zhang Q, Zhao R, Zhou W, Zhang Z, Wang B. High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occupational and environmental medicine. 2012:oemed–2011. doi: 10.1136/oemed-2011-100529. [DOI] [PubMed] [Google Scholar]
- Wang T, Lu J, Xu M, Xu Y, Li M, Liu Y, Tian X, Chen Y, Dai M, Wang W, Lai S. Urinary bisphenol a concentration and thyroid function in Chinese adults. Epidemiology. 2013;24(2):295–302. doi: 10.1097/EDE.0b013e318280e02f. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environmental health perspectives. 2011;119(6):87. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.