Abstract
Restoration of functional independence in gait and vehicle transfer ability is a common goal of inpatient rehabilitation. Currently, ambulation changes tend to be subjectively assessed. To investigate more precise objective assessment of progress in inpatient rehabilitation, we quantitatively assessed gait and transfer performances over the course of rehabilitation with wearable inertial sensors for 20 patients receiving inpatient rehabilitation services. Secondarily, we asked physical therapists to provide feedback about the clinical utility of metrics derived from the sensors. Participant performance was recorded on a sequence of ambulatory tasks that closely resemble everyday activities. We developed a custom software system to process sensor signals and compute metrics that characterize ambulation performance. We quantify changes in gait and transfer ability by performing a repeated measures comparison of the metrics one week apart. Metrics showing the greatest improvement are walking speed, stride regularity, acceleration root mean square, walking smoothness, shank peak angular velocity, and shank range of motion. Furthermore, feedback from physical therapists suggests that wearable sensor-derived metrics can potentially provide rehabilitation therapists with additional valuable information to aid in treatment decisions.
Keywords: Accelerometry, activity monitor, ambulatory monitoring, gait analysis, inertial measurement units, physical therapy, rehabilitation, signal processing, wearable sensors
I. Introduction
The fundamental goals of inpatient rehabilitation are to restore function, mobility, and independence. Monitoring of motor recovery is typically accomplished by clinical observation using standard clinical rating scales, such as the Functional Independence Measure (FIM), to determine independence in activities of daily living at admission and discharge [1]. Between the admission and discharge FIM assessments, observations by therapists typically characterize progress and influence treatment decisions. Because this approach relies on intuition and subjective observations, it lacks detailed quantifiable information to characterize patient movement patterns. To gather more objective measurements of patients’ abilities, standardized clinical assessments, such as the Timed Up-and-Go (TUG) test [2], are administered by trained clinicians. The TUG test measures the time required to rise from a seated position in a chair, walk out 3 m, walk back to the chair, and sit down. Assessments like the TUG provide a high level overview of patient mobility, but are not sensitive enough to capture individual limb movements or changes in mobility and gait features [3]. More precise quantitative measurements of patient performance during rehabilitation can be collected via pervasive technology, such as wearable inertial measurement units (IMUs). Computations based on data collected from wearable IMU sensors can provide therapists with measures that are not open to the potential for inter-observer bias possible with subjective clinical judgments. These supplementary measurements can identify subtle performance changes during rehabilitation that are difficult to observe, such as changes in duration of single and double leg support.
Furthermore, IMUs are an ideal technology for tracking changes in movement because of their low cost, portability, reliability, and ease of attachment to the body. IMUs operate as a self-contained wireless network which can enable testing outside the lab and for any sequence of tasks. Also, IMUs do not interfere with the wearer’s movement. Several studies have used IMUs for analyzing gait and other movements as an inexpensive and unobtrusive substitute for other technologies [3]–[5]. In addition, we have shown in previous work that longitudinal changes in IMU metrics are predictive of patient FIM scores at discharge from inpatient rehabilitation [6].
In this paper, we report on a study that utilizes metrics and visualizations obtained from IMU data to characterize patient performance in an objective fashion. To produce clinically-meaningful metrics, we developed a standardized ambulation performance task, titled the ambulation circuit (AC), which involves a range of gait and transfer tasks. We fixed the interval of time over which repeated measurements of AC performance would be assessed (7 days) in order to quantify changes in movement parameters over one week of rehabilitation. Secondarily, we report feedback from physical therapists regarding the clinical utility of wearable sensors and IMU-derived measurements of gait and vehicle transfer ability.
II. Related Work
Wearable IMUs have been utilized extensively in healthcare applications [7], particularly for gait analysis [5], [8]. In addition, performance on common clinical assessments, such as the TUG test, have been characterized with IMUs [2], [8], [9]. Salarian and colleagues have a large body of research on iTUG, the instrumented TUG [8], [10]. To observe total body movement during the iTUG, seven inertial sensors were mounted on the body. The iTUG was segmented into four sections for computing movement metrics: sit-to-stand, steady-state gait, turning, and turn-to-sit. The research and success of iTUG led to a commercial sensing company, APDM. Existing commercial systems such as APDM offer IMU-based metrics; however, the testing protocols are specific to clinical laboratory-based assessments with a narrow range of ambulatory tasks.
While research in applications of IMUs for gait analysis and instrumenting clinical assessments is prevalent in the literature, to date only a few studies have focused on utilizing IMUs to quantify changes in mobility and gait parameters of impaired populations. These studies have investigated improvement in gait following surgery, such as hip arthroplasty surgery [11]; changes in gait after treatment for a specific injury or illness, such as Parkinson’s Disease [9], [12]; the relationship between changes in longitudinally collected gait parameters and changes in falls risk [13]; and changes in daily walking time over the course of rehabilitation for stroke inpatients [14]. Based on these findings, research quantifying fine-grained gait and transfer ability changes exhibited during rehabilitation with wearable inertial sensors represents a new direction to investigate. Consequently, the current study extends several areas of research, including IMU data processing, gait analysis, and rehabilitation research. More specifically, our work presents the following contributions:
Design and application of an ecological version of the TUG test (the ambulation circuit).
Computation of novel sensor-based metrics related to ecological gait and transfer ability (e.g. vehicle transfer and floor surface metrics).
A framework for measuring changes in IMU metrics for individual participants and participants as a group.
Insight into the recovery process for a multifarious population of inpatients (e.g. stroke, brain injury, etc.).
Feedback from therapists on the utility of wearable IMUs for rehabilitation.
In summary, we analyze changes in wearable sensor-derived metrics to gain insight into the recovery process and provide clinicians with more objective measurements of patient progress in clinical and natural environments.
III. Methods
The study followed a single-arm prospective cohort design with repeated measures of participant performance on standardized gait tasks on two different testing sessions separated by 7 days. The first test session (S1) occurred shortly after the participant became physically able to walk the distance required of the gait task (11.15 ± 4.75 days from admission). The second test session (S2) occurred within the final week of care (2.65 ± 2.25 days before discharge). During each test session, participant performance on the ambulation circuit was recorded two times, producing two separate trials at S1 and two separate trials at S2. In addition, physical measurements and information regarding participants’ rehabilitation impairment and other diagnoses were collected.
A. Participants
Participants were recruited from the inpatient rehabilitation population at a large inpatient rehabilitation facility. All participants met the following eligibility criteria: any rehabilitation diagnosis (e.g., stroke, spinal cord injury, debility), ≥ 18 years of age, no more than minimally cognitively impaired as signified by a score of one or greater on the Mini-Cog examination [15], able and willing to perform the walking task as signified by a score of four or better (minimal stand-by assistance) on the locomotion independence item of the FIM (using an ambulatory aid such as a cane or walker was acceptable). The study was approved by a regional hospital institutional review board and all participants gave written informed consent.
Twenty participants (Male = 14, Female = 6), between the ages of 52 and 88 years old (71.55 ± 10.62 years), participated in both testing sessions of the study (see Table I for participant characteristics). The majority (70%) of participants required a wheeled walker during both testing sessions. Three (15%) participants used a cane during both testing sessions. One participant transitioned from a walker to a cane between the sessions. Medical record review revealed rehabilitation diagnoses were varied, with fourteen (70%) participants undergoing post-stroke rehabilitation. Hemiparesis was present in 11 post-stroke participants.
TABLE I.
Participant Characteristics
| ID | Etiology | Sex | Age (years) |
Assistive Device |
Affected Side |
FIMA | FIMD |
|---|---|---|---|---|---|---|---|
| 001 | Stroke | Male | 73 | Walker | Left | 48 | 104 |
| 002 | Cardiac | Male | 84 | Walker | N/A | 59 | 92 |
| 003 | Debility | Male | 68 | Walker | N/A | 69 | 98 |
| 004 | Stroke | Male | 75 | N/A | Left | 39 | 78 |
| 005 | Stroke | Male | 63 | Cane | No paresis | 69 | 107 |
| 006 | Stroke | Female | 82 | Walker | Left | 46 | 86 |
| 007 | NTBI | Male | 52 | Walker | N/A | 64 | 93 |
| 009 | Stroke | Female | 85 | Walker | No paresis | 68 | 88 |
| 010 | TBI | Female | 67 | Walker | N/A | 41 | 68 |
| 011 | Stroke | Male | 74 | Walker/Cane | Left | 44 | 72 |
| 013 | NTSCI | Male | 76 | Walker | N/A | 51 | 88 |
| 014 | Stroke | Male | 55 | N/A | No paresis | 61 | 103 |
| 015 | Stroke | Male | 85 | Cane | Left | 87 | 113 |
| 016 | Stroke | Male | 54 | Walker | Left | 60 | 98 |
| 018 | Stroke | Male | 88 | Walker | Left | 53 | 92 |
| 019 | Stroke | Male | 65 | Walker | Right | 79 | 103 |
| 020 | Debility | Male | 74 | Walker | N/A | 52 | 91 |
| 021 | Stroke | Female | 74 | Walker | Right | 64 | 113 |
| 024 | Stroke | Female | 63 | Walker | Left | 67 | 104 |
| 025 | Stroke | Female | 74 | Cane | Right | 56 | 83 |
|
| |||||||
| Average | - | - | 71.55 | - | - | 58.65 | 93.70 |
| SD | - | - | 10.62 | - | - | 12.52 | 12.51 |
FIMA = admission total FIM, FIMD = discharge total FIM, NTBI = Non-traumatic brain injury, NTSCI = non-traumatic spinal cord injury, SD = standard deviation.
B. Standardized Gait Tasks: The Ambulation Circuit
We designed a standardized ambulation circuit to assess the mobility and physical ability of the participants during the test sessions. The AC is a continuous sequence of activities performed in a simulated community environment at the rehabilitation facility consisting of several indoor and outdoor modules. The ecological context provided by a simulated environment has been shown to produce a more representative assessment of an individual’s functionality than a controlled laboratory setting [16]. In ecological environments, patients adapt their movements to accomplish challenging tasks, such as transitioning from sitting to standing, navigating different flooring surfaces, and transferring into and out of motor vehicles.
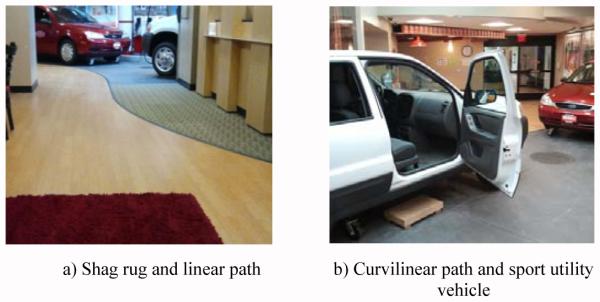
Fig. 1 illustrates the AC. The AC begins in a simulated hotel lobby area with the participant seated in a chair on a rectangular shag rug (see Fig. 2). The chair faces a linear path that leads to an outdoor area with several motor vehicles. On beginning the circuit, the participant rises from the sitting position, performing a sit-to-stand transition. Once standing, the participant walks across the remaining length of the shag rug. When the edge of the rug is reached, the participant performs a surface transition from the shag rug to smooth wood flooring. After walking for 3.66 meters (12 feet) on the smooth floor, a researcher asks the participant “When is your birthday?” during the first trial and “What is the date today?” during the second trial, to assess whether the participant stops walking or slows down. This is a variant of the Stops Walking When Talking Test, a simple method to determine risk for falling when simultaneously engaged in motor and cognitive tasks [17]. Participants are not informed that the question will be asked.
Fig. 1.
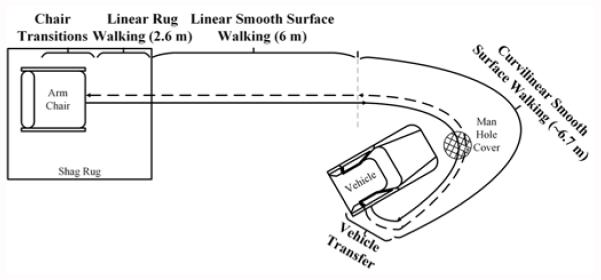
The ambulation circuit. The solid line represents the way out and the dashed line represents the mirrored return portion. Key circuit subtasks are labeled with distances in meters.
Fig. 2.
The simulated community environment at St. Luke’s Rehabilitation Institute.
Next, the participant approaches the front of a sport utility vehicle and begins a curvilinear path around the vehicle to approach an open passenger side door. The curvilinear path length is approximately 6.7 meters (22 feet). The curvilinear path contains a simulated sewer drain lid (manhole cover) over which the participant has to maneuver. As the participant approaches the vehicle passenger seat, the participant performs a transfer into and then out of the vehicle front passenger seat. After transferring out of the vehicle, the participant walks the AC route in reverse, returning to the chair in the simulated hotel lobby and sits down, ending the AC. The Stops Walking When Talking Test is not performed on the way back in order to provide 6.4 meters (21 feet) of uninterrupted smooth linear walking for gait analysis. Time taken to complete the AC officially stops once the participant’s back is fully rested against the back of the chair.
In summary, the AC is an extension of the common clinical assessment, the TUG, including a greater range of functional tasks (e.g., car transfers) and situational challenges (e.g., different flooring surfaces; a curvilinear pathway) than is found in more common assessments. This greater range of cognitive and motor challenges enhances the potential usefulness of the sensor data as a means to show change across time. The majority of the metrics we report can be computed from any assessment in any environment involving a chair transfer and walking (5 Times Sit-to-Stand, TUG, etc.).
C. Instrumentation
Using three Shimmer3 wireless IMUs, we recorded participant motion as they ambulated through the AC. The Shimmer3 platform contains a tri-axial accelerometer and a tri-axial gyroscope. The accelerometers and gyroscopes of all three sensor platforms were calibrated using the software provided by the manufacturer. One IMU was placed centrally on the lumbar spine at the level of the third vertebrae, near the individual’s center of mass (COM) [18]. Additionally, one sensor was placed on each shank, above the ankle and in line with the tibia. Positioning the sensor along the tibia reduced mounting error as the sensors were always positioned at approximately the same angle relative to the sagittal plane. The flatness of the tibia bone also prevented the sensor from moving during the activities. The sensor modules were securely attached to the body with elastic straps. Shank sensor mounting locations were measured at S1 and S2 for consistency. Fig. 3 illustrates the shank mounting locations and axes of the sensors. The accelerometer range was set to ± 2g for the COM sensor and ± 4g for the shanks. The gyroscope ranges for the shank and COM sensors were set at 500 °/s and 250 °/s, respectively. The data were collected at a sampling frequency of 51.2 Hz for all sensor platforms.
Fig. 3.
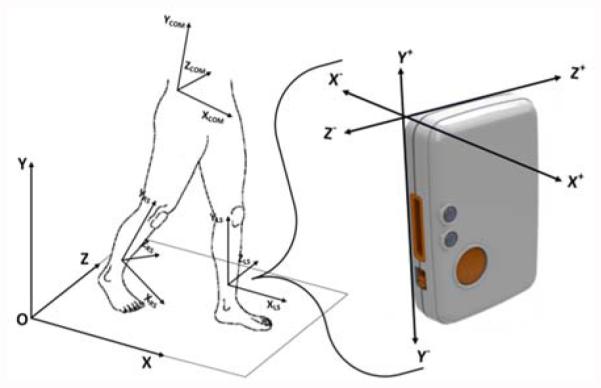
Sensor placement and axes orientation. Sensor units were mounted on the center of mass (COM), left shank (LS), and right shank (RS).
The inertial movement data and segment times are processed with a custom Python program designed for the AC data. First, the timestamps are aligned from the three different sensor platforms. Next, to correct for the orientation of the shank sensors along the tibia, the sensor local coordinate system is transformed to the body coordinate system [19]; a right handed system with the X-axis along the anterior-posterior body axis, the Y-axis along the vertical body axis, and the Z-axis along the medial-lateral body axis. Acceleration data are filtered with a 4th order zero-phase band pass Butterworth filter using cutoff frequencies of 0.1 Hz and 3 Hz for the COM accelerometer and 0.1 Hz and 10 Hz for the shanks. The gyroscope signals for all sensors are low passed filtered at 4 Hz.
From the processed data we compute metrics representing participants’ performance on the AC. AC task durations were recorded by a researcher using a stopwatch. The times are used to segment the data into the different tasks for computing metrics for each of the AC sections. Fig. 4 illustrates the tri-axial COM acceleration and left and right shank gyroscope data from a participant partitioned into the key sections of the AC.
Fig. 4.
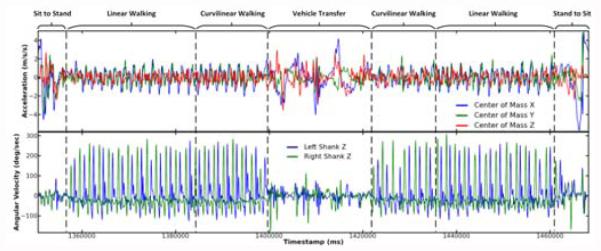
Sensor signals recorded during the AC. The center of mass (top plot: accelerometer) and shank (bottom plot: gyroscope) sensor signals were analyzed to quantify the rehabilitation process.
D. Computed Metrics
For a unique analysis of sensor-based gait information in a rehabilitation setting, we compute metrics from three main components of the AC. The first component consists of the chair sit-to-stand and stand-to-sit movements at the beginning and ending of the AC. The second component consists of the vehicle transfer and the third component consists of the ambulation occurring between the chair and the vehicle. This ambulation section includes the linear path on the smooth floor that is used to compute the majority of the gait cycle metrics.
For the ambulation section, an algorithm was developed to detect the gait cycle events of initial contact, terminal contact, and mid swing. Initial contact is the moment the heel strikes the ground and terminal contact is the moment the toes leave contact with the ground. The algorithm operates on the left and right shank medial-lateral (Z-axis) gyroscope data. The algorithm utilizes peak detection and thresholding techniques that were implemented with high accuracy by previous studies [10], [19]. By locating these key gait events, the gait cycle is defined (the time interval between two successive initial contacts of the same leg) and several metrics related to walking are computed. Table II presents the metrics we compute and groups the metrics into three categories:
Clinical assessments of progress (CAP). CAP metrics are commonly used approaches for assessing mobility in a clinical setting by recording the duration of a standardized activity, such as walking a fixed distance, rising from a chair, or the TUG assessment.
Whole body movement (WBM). WBM metrics are computed from data collected from the COM sensor. An example WBM metric is COM peak angular velocity.
Gait features (GF). GF are computed from data collected from the shank sensors. Examples of GF include cadence and shank range of motion, which are based on the aforementioned gait cycle event detection algorithm.
TABLE II.
Metric Descriptions
| Category | Metric | Units | Qualitative Description | Refer -ence |
|---|---|---|---|---|
| CAP | Duration | S | Total time to complete the ambulation circuit or a subtask of the ambulation circuit. | |
| Floor surface speed ratio | Measures the effect of walking velocity on two different floor surfaces. | |||
| Walking speed | m/s | The walking velocity as determined by distance divided by time. | ||
| WBM | COM peak angular velocity |
°/s | Maximum rotational velocity of the COM around the Z-axis while rising from a seated position in the chair to a standing position. |
|
| Root mean square (RMS) | m/s2 /s | Square root of the mean of the squares of each axes of the acceleration signal on the COM. Represents the magnitude of the signal (normalized by time). |
[3] | |
| Smoothness index | Ratio of even to odd harmonics of the vertical Y-axis COM acceleration signal. A higher harmonic ratio represents a smoother walking pattern. |
[4] | ||
| Smoothness of RMS | m/s3 /s | Root mean square of the derivatives of each X, Y, and Z signal. Synonymous with RMS of jerk (normalized by time). |
[3] | |
| GF | Cadence | steps/min | Step rate as expressed by the number of steps per minute. | |
| Double support percent | % | Percentage of the gait cycle that both feet are on the ground. Computed as the sum of the initial double support time and the terminal double support time. |
[8], [10] |
|
| Gait cycle time | S | Duration to complete one stride (time between two consecutive initial contacts of the same foot). |
[8], [10] |
|
| Number of gait cycles | The number of complete gait cycles (strides) that occurred. | |||
| Shank peak angular velocity |
°/s | Maximum rotational velocity of the shank around the Z-axis during the gait cycle. This occurs during the swing phase. |
||
| Shank range of motion | ° | Integrated angular velocity for each gait cycle. Provides an estimate of the degrees of shank movement. |
[8], [10] |
|
| Step length | m | Distance between initial contacts of opposite feet. | [18] | |
| Step regularity | % | Expression of the regularity of the acceleration of sequential steps. Computed using the autocorrelation of the vertical Y-axis of the COM acceleration. |
[18] | |
| Stride regularity | % | Expression of the regularity of the acceleration of sequential strides (see step regularity). | [18] | |
| Step symmetry | % | Ratio of step regularity to stride regularity. | [18] |
CAP = clinical assessments of progress, COM = center of mass, GF = gait features, m = meters, S = seconds, WBM = whole body movement, ° = degrees.
E. Data Analysis
Sensor-based metrics are statistically analyzed to identify clinically significant changes in the repeated measures data. Detected changes in patient performance offer additional insights to clinicians, as well as demonstrate the benefit of sensor-based analysis of rehabilitation. The statistical analyses we apply to the wearable sensor data at the group and individual levels are summarized below.
1) Quantifying Group Changes
An effect size (ES) based on Cohen’s d for repeated measures (RM) data is used to quantify the strength of changes in each of the computed metrics [20]:
| (1) |
Where is the mean group score from data collected at S1, is the mean group score from data collected at S2, and SD represents the standard error of difference between S1 and S2 scores. In cases where the S1 and S2 scores have equal variance, SD is calculated using the formula [20]:
| (2) |
In Equation 2, sS1 is the standard deviation for the S1 participant pool and r is the test-retest reliability measured between trial 3 and 4 at S2 testing. An unbiased estimation of population test-retest reliability is derived using r [20].
When S1 and S2 variances are not equal, as determined by the Levene’s test of equal variances, an adjustment is applied to the estimate of SD [20]:
| (3) |
The resulting effect sizes, dRM (Equation 1), are used to evaluate group changes in gait parameters over the course of one week of inpatient rehabilitation. Additionally, the confidence intervals for each ES are computed using a small sample size approximation with alpha set at 95% [21].
2) Quantifying Individual Changes
At the individual level, changes in gait metrics one week apart are characterized with the reliable change index (RCI) [22]:
| (4) |
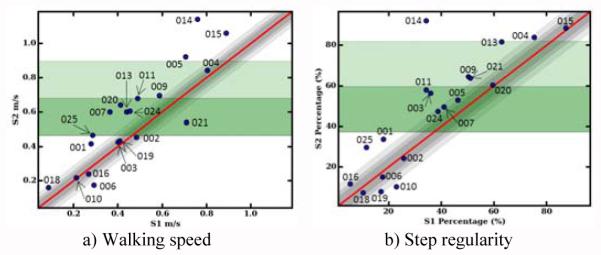
Where xS1 is an individual participant’s score from data collected at S1 and xS2 is the same participant’s score from data collected at S2. In addition to numeric RCI statistics, comparison of individuals to the group for change between S1 and S2 are accomplished graphically with RCI plots. Fig. 5 shows an example RCI plot of the walking smoothness index metric (see Fig. 6 for additional RCI plots). The values measured for the smoothness index at S1 (X-axis) are plotted against S2 (Y-axis). The red diagonal line intersecting the plot represents an absence of change from S1 to S2. The shaded gray diagonal areas represent confidence intervals based on standard error of measurement and criteria suggested by Wise [23]. The green bands represent the mean value for S1, plus one and two standard deviations respectively. As Fig. 5 shows, the majority of participants showed improvement between S1 and S2 for walking smoothness. Four smoothness indices fell below the diagonal line (indicating lack of response) and ten smoothness indices appeared above the 95% confidence interval, indicating substantial response.
Fig. 5.
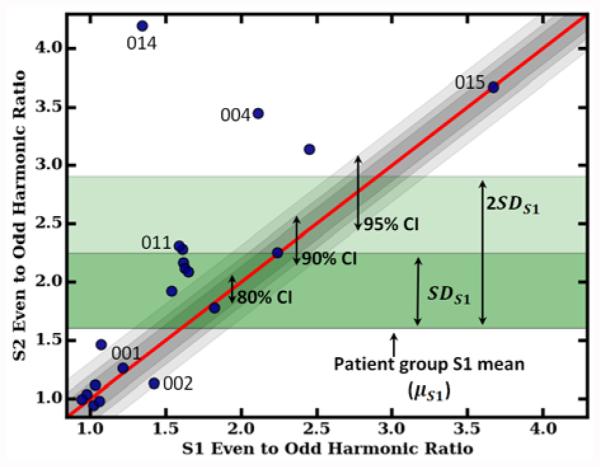
The smoothness index metric as an example reliable change index plot. Participant session 1 (S1) scores are plotted against session 2 (S2) scores. Also plotted are confidence intervals (CI) and S1 standard deviations (SD). Select individuals are labeled with their participant identification number.
Fig. 6.
Walking speed (a) and step regularity (b) reliable change index plots. Individuals are labeled with their participant identification number.
IV. Results
Tables III-V contain results for CAP, WBM, and GF metrics, respectively. Reported statistics for each metric include the mean and standard deviation for S1 and S2 (μS1, SDS1, μS2, and SDS2) and the standardized mean difference effect size. To facilitate analysis and insights at the individual patient level, smoothness index (see Fig. 5), walking speed (see Fig. 6a), and step regularity (see Fig. 6b) are displayed as RCI plots.
TABLE III.
Clinical Assessments of Progress (CAP) Metric Results
| Metric | μ S1 | SDS1 | μ S2 | SDS2 | Effect Size |
|---|---|---|---|---|---|
| Curvilinear walking duration |
24.43 | 18.14 | 19.48 | 12.23 | −1.03* |
| Duration | 177.85 | 129.53 | 137.36 | 88.56 | −1.08* |
| Floor surface speed ratio |
0.75 | 0.13 | 0.80 | 0.14 | 0.48* |
| Sit-to-stand duration | 6.84 | 6.10 | 4.79 | 3.03 | −0.49* |
| Stand-to-sit duration | 12.94 | 12.05 | 11.13 | 7.13 | −0.34* |
| Vehicle challenge duration |
47.81 | 36.36 | 34.75 | 28.10 | −0.55* |
| Walking speed | 0.47 | 0.22 | 0.57 | 0.28 | 1.58* |
S1 = session 1, S2 = session 2, SD = standard deviation, μ = mean,
= significant at the 95% confidence level.
TABLE V.
Gait Features (GF) Metric Results
| Metric | μ S 1 | SDS1 | μ S2 | SDS2 | Effect Size |
|---|---|---|---|---|---|
| Cadence | 64.88 | 17.67 | 70.54 | 20.32 | 1.38* |
| Double support percent | 33.79 | 11.97 | 32.19 | 13.72 | −0.49* |
| Gait cycle time | 1.96 | 0.66 | 1.87 | 0.68 | −0.64* |
| Affected side peak angular velocity |
190.09 | 67.88 | 208.79 | 72.50 | 1.52* |
| Affected side shank range of motion |
47.34 | 13.22 | 50.02 | 11.44 | 1.20* |
| Left side peak angular velocity | 195.98 | 61.01 | 213.09 | 68.84 | 1.30* |
| Left side shank range of motion |
47.24 | 12.66 | 50.93 | 12.59 | 1.73* |
| Number of gait cycles | 18.95 | 9.32 | 16.38 | 5.59 | −0.90* |
| Right side peak angular velocity |
217.65 | 43.80 | 244.53 | 51.02 | 2.02* |
| Right side shank range of motion |
50.41 | 10.91 | 54.70 | 9.43 | 1.45* |
| Step length | 0.21 | 0.07 | 0.23 | 0.06 | 0.64* |
| Step regularity | 37.29 | 22.36 | 46.91 | 28.11 | 1.31* |
| Stride regularity | 40.88 | 22.73 | 51.53 | 24.45 | 0.55* |
| Step symmetry | 63.57 | 27.50 | 70.80 | 26.31 | 0.35 |
| Unaffected side peak angular velocity |
231.38 | 39.33 | 255.20 | 40.62 | 1.91* |
| Unaffected side shank range of motion |
51.91 | 11.96 | 55.69 | 8.40 | 1.56* |
S1 = session 1, S2 = session 2, SD = standard deviation, μ = mean,
= significant at the 95% confidence level.
V. Discussion
In this paper we investigate the insights that sensor-based quantifiable measures can supply in addition to observations by clinicians. While analyzing changes at the group level provides insight about the effects of therapy from a research perspective, the effects of rehabilitation on an individual basis can be established with wearable sensors and applied directly to patient care.
A. Group Responsiveness to Therapy
1) Clinical Assessments of Progress
As a group, AC metrics related to CAP demonstrate moderate improvements based on the magnitude of effect sizes (see Table III). For total AC duration, there is a 22.77% decrease in the total time required to complete the AC. Improved functionality is confirmed by increased ambulatory capabilities, completing the curvilinear section of the AC 20.26% faster. Responsiveness on the vehicle load/unload challenge is moderate with 27.32% decrease in duration observed on average. Walking speed is a typical metric for comparison among populations and for indication of ambulatory improvements. Group average performance during both sessions (walking speed S1 0.47 ± 0.22 m/s; walking speed S2 0.57 ± 0.28 m/s) as well as the large responsiveness across time (see Fig. 6a for an RCI plot) are in agreement with previous published studies analyzing walking speed in post-stroke populations [24].
2) Whole Body Movement Metrics
Large levels of responsiveness are observed for RMS during linear path gait (see Table IV). While there is a relationship between COM RMS and walking speed [4], the large responsiveness across time is due in part to the participant-dictated speed of movement. During the vehicle transfer, the ES for COM RMS during the load and unload tasks suggest substantial progress from one week of rehabilitation therapy. Similar changes in COM RMS are also present on the chair task.
TABLE IV.
Whole Body Movement (WBM) Metric Results
| Metric | μ S 1 | SDS1 | μ S 2 | SDS2 | Effect Size |
|---|---|---|---|---|---|
| COM RMS | 0.08 | 0.06 | 0.11 | 0.10 | 1.87* |
| COM Smoothness of RMS |
0.75 | 0.59 | 1.10 | 1.04 | 1.90* |
| Sit-to-stand RMS | 0.65 | 0.64 | 0.85 | 0.67 | 0.71* |
| Sit-to-stand peak angular velocity |
84.02 | 37.96 | 72.46 | 44.47 | −0.37 |
| Smoothness index | 1.60 | 0.65 | 2.01 | 0.97 | 1.82* |
| Stand-to-sit RMS | 0.38 | 0.45 | 0.36 | 0.30 | −0.07 |
| Stand-to-sit peak angular velocity |
126.87 | 36.75 | 118.45 | 42.08 | −0.26 |
| Vehicle load RMS | 0.10 | 0.11 | 0.16 | 0.16 | 1.37* |
| Vehicle load peak angular velocity |
81.94 | 36.62 | 78.27 | 26.32 | −0.10 |
| Vehicle unload RMS | 0.16 | 0.12 | 0.29 | 0.37 | 2.71* |
| Vehicle unload peak angular velocity |
74.45 | 47.85 | 68.45 | 35.89 | −0.15 |
COM = center of mass, RMS = root mean square, S1 = session 1, S2 = session 2, SD = standard deviation, μ = mean,
= significant at the 95% confidence level.
Another WBM metric, the smoothness index of walking (see Fig. 5), characterizes gait harmonics to quantify cyclical movements independent of speed [4]. The calculated ES for change in smoothness index emphasizes the influence rehabilitation has on developing a more stable pattern of locomotion. As a group, the participants demonstrate a 25.63% improvement in the smoothness index.
3) Gait Features
Changes in metrics describing gait quality in terms of symmetry, regularity, and consistency are observed during the straight path portion of the AC (see Table V). During one week of rehabilitation, the increased walking speed is accompanied by an average increase of 8.72% in cadence. Another important outcome is the 4.74% decrease in the amount of double limb support in the gait cycle. In addition, improvement is observed in gait consistency, measured with stride and step regularity (see Fig. 6b for an RCI plot). These metrics indicate that patients are beginning to produce more consistent walking patterns over one week, increasing the load carried by the affected limb.
Changes are also observed in individual leg movements. Large levels of responsiveness are detected in peak angular velocity, measured at each shank. Along with faster leg movements during the swing phase, there is a strong indication of increased limb range of motion during gait. To perform sub-group analyses of stroke patients with hemiparesis, each limb is re-classified as affected (paretic) or unaffected (non-paretic), instead of left or right. The recategorization produces a slightly different ES for shank peak angular velocity and range of motion. Tracking changes in the affected side of the body offers additional insight for clinicians treating stroke patients and injuries affecting one side of the body more than the other side.
B. Individual Responsiveness to Therapy
RCI analyses suggest that recovery is not consistent for all patients over one week of inpatient rehabilitation (see Fig. 5 and 6). For example, participant #014 experienced a substantial amount of recovery compared to the rest of the participants as assessed through RCI plots. This finding is corroborated by the conventional method of using the FIM to characterize functioning at admission (see Table I). By contrast, participant #015 did not demonstrate significant change in smoothness of walking or step regularity. At admission to the inpatient facility the functional capabilities for this participant were close to independent, rendering a small window for improvement across time.
The RCI visualization of performance at the individual level can track progress by assessing performance on multiple metrics. For example, a few participants with moderate responsiveness for walking speed (#007, #015, and #020) did not show change in the smoothness index metric and vice versa (#004). Therefore, analysis of multiple metrics, such as smoothness index along with walking speed, highlights the differences in individual recovery.
C. Therapist Feedback
To further investigate the clinical utility of wearable sensor-derived metrics, we presented AC data to physical therapists (N = 5) and physical therapy assistants (N = 2) to collect feedback. The group had a mean age of 40.14 ± 9.49 years (M = 1, F = 6) and had been working in rehabilitation for 11.86 ± 12.56 years. Quantitative responses of questions asked were scored by the therapists on a scale from 1 (strongly disagree/not useful) to 5 (strongly agree/very useful). The interviews reveal that therapists are quite comfortable with technology (4.00 ± 0.82), willing to learn new technology (4.29 ± 0.76), and interested in using wearable technology for their patients (4.43 ± 0.53). To evaluate patient gait and transfer ability, therapists primarily use observation and an estimate of the amount of physical assistance the patient requires performing certain tasks. To evaluate change in patient gait and transfer ability, therapists use their memory to compare previous observations to current ones.
Additionally, wearable sensor-derived metrics were presented and explained to the therapists. The therapists then rated each metric for its usefulness in providing therapy services for patients. The results indicate therapists believe sit-to-stand duration is the most useful metric (4.14 ± 1.46). Walking speed (4.00 ± 1.41) and cadence (4.00 ± 1.00) are also highly rated metrics. The metrics with the lowest rated usefulness include COM peak angular velocity (2.86 ± 1.07) and vehicle load/unload duration (3.14 ± 1.07). For the vehicle metrics, two therapists stated a patient’s ability to complete these tasks is more important than the amount of time the patient requires.
The feedback received from physical therapists emphasizes that the aim of quantifying progress with wearable sensors is useful for rehabilitation settings; however, wearable sensors should not be regarded as a replacement for experienced clinical judgment. The overarching goal of technology platforms for rehabilitation should strive to be assistive: providing insightful information, adapting to ecological conditions, and incorporating the context of the patient’s progress. Feedback should also be provided to the patient, as patient motivation and engagement is associated with positive rehabilitation outcomes [25].
A limitation of this study is the metric computations have not been laboratory validated; however, all of the algorithms are derived from previously-published and validated sources. Other limitations include:
Non-uniform days between admission and S1 AC testing. Participants were tested when their physical therapists determined they were able to safely perform the AC.
-
The use of human-operated stopwatch times to segment the AC into its subtasks. The times recorded by the researchers could impose non-systematic error.
A direction for future work includes recruiting healthy individuals to perform the ambulation circuit to provide reference data for comparison to patient data.
VI. Conclusion
Inpatient rehabilitation contains a wide spectrum of challenges that are tackled uniquely by different patients, depending on their pre-morbid state, injury, drive to improve, and compensatory strategies. Changes measured in movement profiles over the course of one week of therapy and feedback from therapists indicate wearable IMUs provide a viable platform for gaining insight into these complex recovery processes. The ambulation circuit presented in this study allowed data collection to capture performance of real-world challenges in ecological environments. Several gait and transfer features exhibit statistically significant differences in value from session one to session two, which indicates wearable sensor-derived metrics may be practical for clinicians to use in addition to observation to quantify gait and vehicle transfer improvement. Specifically, metrics showing the greatest change are walking speed, stride regularity, acceleration root mean square, walking smoothness, shank peak angular velocity, and shank range of motion. Of these metrics, only walking speed does not make use of wearable inertial sensor data, indicating that wearable sensors can capture details about changes in movement patterns that cannot be acquired from standardized subjective clinical assessments.
Acknowledgment
We wish to thank our therapist collaborators at St. Luke’s Rehabilitation Institute. This work is funded in part by National Science Foundation grant 0900781.
This work was partially supported by National Science Foundation grant 0900781.
Biographies

Vladimir Borisov received a B.S. degree in bioengineering from Washington State University, Pullman, WA, in 2009. He is pursuing his Ph.D. in bioengineering at Washington State University. His research interests include wearable monitoring systems, using simulations for studying complex biomechanical systems, and practical commercialization of technologies with healthcare applications.

Gina Sprint received a B.S. degree in computer science from Eastern Washington University, Cheney, WA, in 2012. She is currently completing a Ph.D. degree in computer science at Washington State University. Her research interests include wearable computing, machine learning, technology applications for healthcare, and computer science education.

Diane J. Cook received a B.A. from Wheaton College, a M.S. from the University of Illinois, and a Ph.D. from the University of Illinois. She is a Huie-Rogers Chair Professor at Washington State University. Her research interests include machine learning, smart environments, and automated health assessment and intervention.

Douglas L. Weeks received a B.S. degree in biological sciences and kinesiology and a M.S. degree in human motor control from Texas A&M University. He obtained a Ph.D. from the University of Colorado, and completed post-doctoral research at the Rehabilitation Institute of Montreal. He currently directs the research program at St. Luke’s Rehabilitation Institute in Spokane, WA.
Contributor Information
Vladimir Borisov, Voiland School of Chemical and Bioengineering, Washington State University, Pullman, WA 99164 USA (vladimir.borisov@wsu.edu).
Gina Sprint, School of Electrical Engineering and Computer Science, Washington State University, Pullman, WA 99164 USA (gsprint@eecs.wsu.edu).
Diane J. Cook, School of Electrical Engineering and Computer Science, Washington State University, Pullman, WA 99164 USA (cook@eecs.wsu.edu).
Douglas L. Weeks, St. Luke’s Rehabilitation Institute, Spokane, WA 99202 USA (weeksdl@inhs.org)
References
- [1].Hamilton BB, Granger CV, Sherwin FS, Zielezny M, Tashman JS. Rehabilitation Outcomes: Analysis and Measurement. Paul H. Brookes Publishing Company; Baltimore, MD: 1987. A Uniform National Data System for Medical Rehabilitation; pp. 137–150. [Google Scholar]
- [2].Sprint G, Cook D, Weeks D. Towards Automating Clinical Assessments: A Survey of the Timed Up and Go (TUG) Biomed. Eng. IEEE Rev. 2015;PP(99):1–1. doi: 10.1109/RBME.2015.2390646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang M, Lange B, Chang C-Y, Sawchuk AA, Rizzo AA. Beyond the standard clinical rating scales: fine-grained assessment of post-stroke motor functionality using wearable inertial sensors. Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE. 2012:6111–6115. doi: 10.1109/EMBC.2012.6347388. [DOI] [PubMed] [Google Scholar]
- [4].Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture. 2003;18(1):35–46. doi: 10.1016/s0966-6362(02)00159-5. [DOI] [PubMed] [Google Scholar]
- [5].Tao W, Liu T, Zheng R, Feng H. Gait Analysis Using Wearable Sensors. Sensors. 2012 Feb;12(12):2255–2283. doi: 10.3390/s120202255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sprint G, Cook DJ, Weeks DL, Borisov V. Predicting Functional Independence Measure Scores During Rehabilitation With Wearable Inertial Sensors. IEEE Access. 2015;3:1350–1366. doi: 10.1109/ACCESS.2015.2468213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rodgers MM, Pai VM, Conroy RS. Recent Advances in Wearable Sensors for Health Monitoring. IEEE Sens. J. 2015 Jun;15(6):3119–3126. [Google Scholar]
- [8].Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010;18(3):303–310. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Galli M, Kleiner AFR, Gaglione M, Sale P, Albertini G, Stocchi F, De Pandis MF. Timed Up and Go test and wearable inertial sensor: a new combining tool to assess change in subject with Parkinson’s disease after automated mechanical peripheral stimulation treatment. Int. J. Eng. Innov. Technol. 2015;4:155–163. [Google Scholar]
- [10].Salarian A, Russmann H, Vingerhoets FJG, Dehollain C, Blanc Y, Burkhard PR, Aminian K. Gait Assessment in Parkinson’s Disease: Toward an Ambulatory System for Long-Term Monitoring. IEEE Trans. Biomed. Eng. 2004 Aug;51(8):1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- [11].Rapp W, Brauner T, Weber L, Grau S, Mündermann A, Horstmann T. Improvement of walking speed and gait symmetry in older patients after hip arthroplasty: a prospective cohort study. BMC Musculoskelet. Disord. 2015 Oct;16(1):291. doi: 10.1186/s12891-015-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kleiner A, Galli M, Gaglione M, Hildebrand D, Sale P, Albertini G, Stocchi F, De Pandis MF. The Parkinsonian Gait Spatiotemporal Parameters Quantified by a Single Inertial Sensor before and after Automated Mechanical Peripheral Stimulation Treatment. Park. Dis. 2015;2015 doi: 10.1155/2015/390512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Simila H, Immonen M, Merilahti J, Petakoski-Hult T. Gait analysis and estimation of changes in fall risk factors; 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2015. 2015. pp. 6939–6942. [DOI] [PubMed] [Google Scholar]
- [14].Dorsch AK, Thomas S, Xu X, Kaiser W, Dobkin BH, on behalf of the SIRRACT investigators. Emara T, Edwards D, Fonzetti P, Maasch J, Lee S-G, Owolabi MO, Hamzat TK, LeBlanc CJ, Morse R, Swaminathan N, Karatas GK, Boza R, Brown AW, Miyai I, Kawano T, Chen S-Y, Hanger HC, Zucconi C, Mammi S, Ghislanzoni C, Juan F, Lang CE. SIRRACT: An International Randomized Clinical Trial of Activity Feedback During Inpatient Stroke Rehabilitation Enabled by Wireless Sensing. Neurorehabil. Neural Repair. 2014 Sep; doi: 10.1177/1545968314550369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int. J. Geriatr. Psychiatry. 2000 Nov;15(11):1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [16].Mcmahon B. Work Worth Doing: Advances in Brain Injury Rehabilitation. 1 CRC Press; Orlando, FL: 1991. [Google Scholar]
- [17].Lundin-Olsson L, Nyberg L, Gustafson Y. ‘Stops walking when talking’ as a predictor of falls in elderly people. The Lancet. 1997;349(9052):617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- [18].Moe-Nilssen R, Helbostad JL. Estimation of gait cycle characteristics by trunk accelerometry. J. Biomech. 2004;37(1):121–126. doi: 10.1016/s0021-9290(03)00233-1. [DOI] [PubMed] [Google Scholar]
- [19].Chen S. Gait Feature Extraction from Inertial Body Sensor Networks for Medical Applications. University of Virginia; 2013. [Google Scholar]
- [20].Wolff Smith L, Beretvas SN. Estimation of the Standardized Mean Difference for Repeated Measures Designs. J. Mod. Appl. Stat. Methods. 2009;8(2):600–609. [Google Scholar]
- [21].Viechtbauer W. Approximate Confidence Intervals for Standardized Effect Sizes in the Two-Independent and Two-Dependent Samples Design. J. Educ. Behav. Stat. 2007 Mar;32(1):39–60. [Google Scholar]
- [22].Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- [23].Wise EA. Methods for Analyzing Psychotherapy Outcomes: A Review of Clinical Significance, Reliable Change, and Recommendations for Future Directions. J. Pers. Assess. 2004;82(1):50–59. doi: 10.1207/s15327752jpa8201_10. [DOI] [PubMed] [Google Scholar]
- [24].Mulroy SJ, Klassen T, Gronley JK, Eberly VJ, Brown DA, Sullivan KJ. Gait Parameters Associated With Responsiveness to Treadmill Training With Body-Weight Support After Stroke: An Exploratory Study. Phys. Ther. 2010 Feb;90(2):209–223. doi: 10.2522/ptj.20090141. [DOI] [PubMed] [Google Scholar]
- [25].Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011 May;377(9778):1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]