Abstract
Human and animal studies have shown that physical challenges and stressors during adolescence can have significant influences on behavioral and neurobiological development associated with internalizing disorders such as anxiety and depression. Given the prevalence of asthma during adolescence and increased rates of internalizing disorders in humans with asthma, we used a mouse model to test if and which symptoms of adolescent allergic asthma (airway inflammation or labored breathing) cause adult anxiety- and depression-related behavior and brain function. To mimic symptoms of allergic asthma in young BALB/cJ mice (postnatal days [P] 7–57; N=98), we induced lung inflammation with repeated intranasal administration of house dust mite extract (most common aeroallergen for humans) and bronchoconstriction with aerosolized methacholine (non-selective muscarinic receptor agonist). Three experimental groups, in addition to a control group, included: (1) “Airway inflammation only”, allergen exposure 3 times/week, (2) “Labored breathing only”, methacholine exposure once/week, and (3) “Airway inflammation + Labored breathing”, allergen and methacholine exposure. Compared to controls, mice that experienced methacholine-induced labored breathing during adolescence displayed a ~20% decrease in time on open arms of the elevated plus maze in early adulthood (P60), a ~30% decrease in brainstem serotonin transporter (SERT) mRNA expression and a ~50% increase in hippocampal serotonin receptor 1a (5Htr1a) and corticotropin releasing hormone receptor 1 (Crhr1) expression in adulthood (P75). This is the first evidence that experimentally-induced clinical symptoms of adolescent asthma alter adult anxiety-related behavior and brain function several weeks after completion of asthma manipulations.
Keywords: Adolescence, Anxiety, Asthma, House dust mite, Methacholine, Sex Differences
1. INTRODUCTION
Adolescence is a unique and critical period of behavioral and neurobiological development with rapid and substantial maturation of brain regions and neurotransmitter systems involved in emotion regulation [1–7]. Repeated exposure to health and environmental challenges during this stage of life alter behavioral and physiological development related to internalizing disorders like anxiety and depression [8–10]. Allergic asthma represents a common chronic health challenge, affecting 25.7 million people (9.5% <18y/o, 7.7% >18y/o) in the United States [11], and it is associated with internalizing disorder co-morbidity occurring as early as adolescence [12–17]. Asthma symptoms are significant chronic stressors, and asthma is associated with glucocorticoid (GC) dysregulation and exogenous corticosteroid administration as medication [18–21]. Altered GC exposure in asthma, particularly during adolescence, may be a mechanism that primes individuals for internalizing disorders [22–28].
GCs affect neurons at the functional and transcriptional levels [28–30]. Human studies indicate a strong relationship between peri-adolescent stress and adult GC regulation and mental health [10,24]. Adults that experienced peri-adolescent abuse have lower basal cortisol levels and dampened cortisol responses, and GC dysregulation is a risk factor for anxiety and depression [24,26,28,31–34]. Adolescents may be particularly vulnerable to GC influences on hippocampal gene regulation and down-stream GC dysregulation [22,24,35–38]. Rodent models confirm that pre- and peri-adolescent chronic stress can have long-term influences on GC regulation and anxiety- and depression-related behaviors [39,40]. For example, chronic restraint or social stress elicit GC receptor (GR) downregulation in the prefrontal cortex (PFC), a flattened GC circadian rhythm, and increased anxiety- and depression-like behavior [33,40–42].
Allergic asthma has two distinct components that may affect GC production and/or anxiety/depression development. First, allergic asthma involves increased airway inflammation, which is associated with increased cytokine production that can alter GC production, potentially increasing risk for internalizing disorders [20,43–45]. Allergic asthma is driven by a predominant T helper 2 (Th2) response, and Th2 cytokines, interleukin 4 (IL-4), interleukin 5 (IL-5), and interleukin 13 (IL-13), are critical for the development of this disease. In various animal models and in humans, antibodies that block these cytokines have had varying efficacy in treating the symptoms of asthma [46–48]. Second, asthma involves unpredictable respiratory distress with heavily labored breathing. This hypoxic state can lead to limited airflow, increased airway muscle activity, risk for respiratory failure, and airway remodeling [49,50]. Given these two distinct symptoms of asthma – lung inflammation and labored breathing – we independently manipulated these two features in a mouse model.
To understand mechanistic links between peri-adolescent asthma and adult internalizing disorders, we validated a rodent model by experimentally testing if lung inflammation and/or labored breathing during peri-adolescence led to significant adult symptoms associated with human internalizing disorders. We quantified anxiety- and depression-related symptoms in adulthood by measuring exploratory and hedonic behavior, basal GC regulation by measuring serum and fecal GC concentrations, GR and mineralocorticoid receptor (MR) gene expression in the hippocampus [24,26,28,34,42], and serotonergic and corticotropin-releasing hormone (CRH) receptor/transporter gene expression in brainstem, hippocampus, and PFC [51–57]. Because sex differences exist in susceptibility to asthma and internalizing disorders, we conducted the study with both male and female mice [10,58–63].
2. MATERIALS AND METHODS
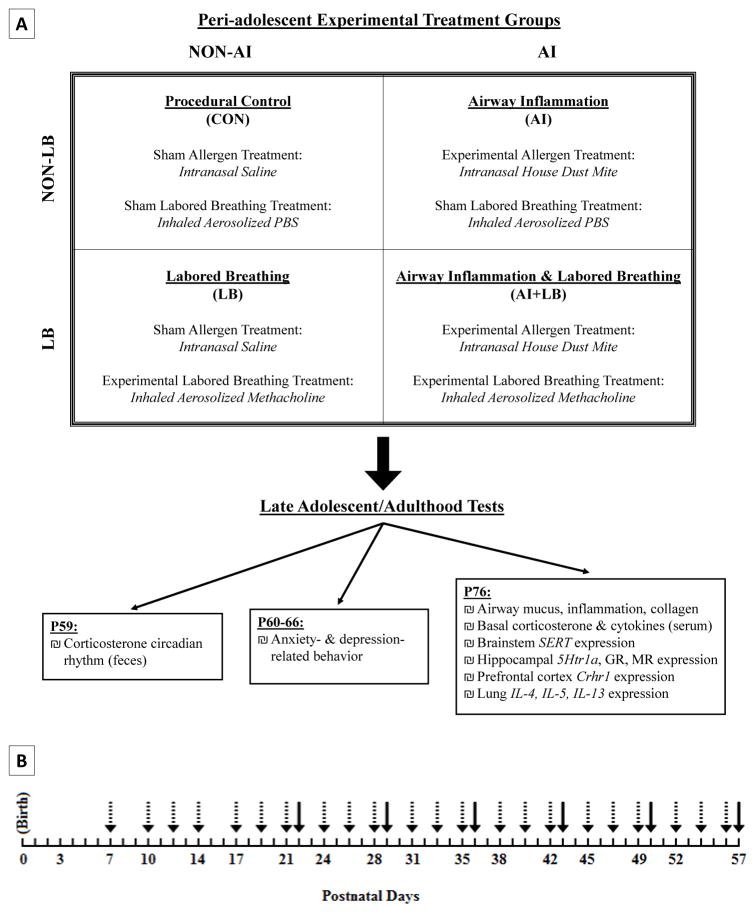
A longitudinal study was used to induce peri-adolescent allergic asthma symptoms and measure adult behavioral and neurobiological outcomes related to internalizing disorders. BALB/cJ mice – a strain susceptible to allergic airway inflammation and hyperresponsiveness [64,65] – were used. Four peri-adolescent conditions included: Airway Inflammation (AI), Labored Breathing (LB), Airway Inflammation + Labored Breathing (AI+LB), and Similarly-Handled Controls (CON; Figure 1A). Three consecutive cohorts were bred, providing 9+ offspring of each sex per condition (N=98 mice, 30–38 mice/cohort). Litter effects were controlled by evenly distributing same-sex siblings across all four conditions (3 experimental and 1 control). Each experimental group consisted of male and female pups selected from 14 litters (each experimental/control group had 12–14 males and 9–12 females). All procedures followed the National Institute of Health guide and were approved by the Pennsylvania State University IACUC committee.
Figure 1. Study design and timelines.
(A) Study design with four experimental conditions to manipulate peri-adolescent asthma symptoms to determine adult behavioral and physiological outcomes. Each experimental/control condition received a control or experimental treatment from each category (airway inflammation and labored breathing). (B) Timeline for peri-adolescent experimental manipulations. Dashed arrows indicate house dust mite (HDM) treatments to stimulate lung inflammation. Solid arrows indicate methacholine (MCH) treatments to induce labored breathing. HDM began earlier than MCH because significant inflammation requires several weeks of HDM exposure [74].
2.1 MOUSE BREEDING AND HOUSING
BALB/cJ breeders were obtained from Jackson Laboratories (Bar Harbor, ME). Sister-pairs were mated with one male to produce ‘double-litters’ (14 double-litters, mean size: 9.7 pups, not culled). Pups were marked daily with non-toxic Sharpie ® marker for identification until given a unique ear notch (P9). To control for pup differences in pre-manipulation anxiety-prone phenotypes, we measured ultrasonic vocalizations (USV) on P3-5 (2 minutes/day) using the ‘Isolation’ method and recording at 65 Hz [66–69]. Consistently high- or low-calling pups within each litter were selected and equally-distributed among conditions. Pups were weaned at P22 into same-sex sibling groups in standard cages with corn-cob bedding (2–4 pups/cage, 28 cm x 17 cm x 12 cm). At P50, mice were individually housed in standard cages containing a red polypropylene tube for environmental enrichment and for low-stress transportation out of the home cage [70,71]. Colony and testing rooms were maintained at 21±1°C on a reverse 12:12 light:dark schedule (lights on 18:00 hours, lights off 06:00 hours) with ad libitum access to food and water. Labored breathing, fecal corticosterone metabolites, and anxiety-related behavior were measured from all offspring (n=98; 54 male, 44 female; 9–14 pups/sex/condition). Depression-related behavior was measured in half of these offspring (n=53; 30 male, 23 female, 5–9 pups/sex/condition); this smaller sample size was a result of procedural error for half of the mice. Lung mucus/inflammation/collagen, brain gene expression, and basal circulating corticosterone were measured in a subset of the 98 offspring (n=54; 28 male, 26 female; 6–8 pups/sex/condition); lung cytokine and circulating cytokine levels were measured in a third of the offspring (n=38; 19 male, 19 female; 4–5 pups/sex/condition).
2.2 INDUCTION OF PERI-ADOLESCENT ALLERGIC ASTHMA SYMPTOMS
2.2.1 Airway Inflammation
Young mice in the AI and AI+LB conditions were exposed to extract of Dermatophagoides pteronyssinus, commonly referred to as house dust mite (HDM; Greer Labs, NC) to induce chronic lung inflammation (3x/week; Figure 1B). Ovalbumin, often used to induce lung inflammation, was not used because tolerance occurs with repeat exposure [72,73]. During P7-15, mice received 10μg HDM (10μL of 1mg/ml protein weight solution in saline) per exposure. During P16-56, the dose increased to 15μg HDM (15μL) and was given under brief isoflurane anesthesia [65]. This method produces significant lung inflammation within 2 weeks of first dosage, which persists throughout treatment [74]. LB and CON mice were exposed to a control treatment of intranasal saline using the same exposure schedule, volumes, and procedures as those for HDM administration, including repeated exposure to isoflurane to administer the saline (Figure 1A).
2.2.2 Labored breathing (LB)
Young mice in the LB and AI+LB conditions were exposed to an inhaled muscarinic receptor agonist, methacholine (MCH; Sigma, St. Louis, MO), once per week during P22-57 (Figure 1B). To measure airway constriction/labored breathing, a whole-body plethysmograph was used (Data Sciences International, New Brighton, MN) for repeated non-invasive measures. Mice were placed in the plethysmograph chamber (diameter 7.5cm, height 7cm) and allowed to acclimate for 3 minutes, then exposed to five increasing doses of aerosolized MCH for 3 minutes per dose [65]. To induce similar levels of labored breathing in LB and AI+LB conditions, AI+LB mice received half-doses relative to LB mice (LB vs. AI+LB MCH doses: 0, 6.25, 12.5, 25, and 50ng/mL vs 0, 3.13, 6.25, 12.5, 25ng/mL in 100μL saline) based on preliminary data and prior studies [65]. AI and CON mice received the control treatment of aerosolized phosphate-buffered saline (PBS) in the plethysmograph chamber on the same time course used for MCH administration as explained above (Figure 1A). To verify MCH-induced bronchoconstriction for each dose, we recorded labored breathing behavior as present or absent, and we recorded and calculated mean enhanced pause (Penh) [75] with FinePointe software.
2.3 BEHAVIORAL TESTING
2.3.1 Anxiety-Like Behavior, Elevated Plus Maze (EPM)
At P60, anxiety-like behavior was measured on EPM, a common, pharmacologically-validated test for anxiety in mice [76–80]. Two open (30cm x 5cm) and two closed (30cm x 14.5cm x 5cm) flat perpendicular arms were elevated 42cm above the ground. Test orders were pseudo-randomized to balance across conditions. Mice were transported to the test room in their home cages approximately 1 hour before testing. Testing was conducted during the latter half of the dark phase (13:00–17:00 hours) under red-light illumination (<5 lux). Mice were placed in the center of the arena facing an open arm and video-recorded for 5 minutes. Entry into the maze arms was defined as 2-limbs passing the threshold boundary. EPM videos were scored for percent time on open arms, proportion of entries into open arms, and total number of entries into open and closed arms. Decreased time on or entries into open arms were used to quantify anxiety-like behaviour; arm entries were used to quantified locomotion [80].
2.3.2 Hedonic Behavior, Sucrose Preference Test (SPT)
On P66, we assessed anhedonic behavior by measuring free-choice sucrose consumption in SPT [81]. Forced swim test and tail suspension test were not used because performance in these tests (swimming and righting) are dependent on peak lung function, which was compromised in AI and AI+LB mice, regardless of affective state [82]. Mice were given 24 hours of free access to two water bottles, one with tap water and the other with 3% sucrose solution [83]. Bottle positions were switched after 12 hours to avoid side preference biases. Bottles were weighed before and after the 24-hr test to measure sucrose solution consumption relative to water. Decreased preference for sucrose solution is a sign of anhedonic behavior [3].
2.4 PHYSIOLOGICAL OUTCOMES
2.4.1 Corticosteroids in Feces and Serum
The immediate effects of peri-adolescent asthma symptoms on basal GC production were measured by collecting feces on P59 (late adolescence). Corticosteroids were extracted and measured from feces to provide a non-invasive measure of the circadian rhythm. These measures involved the following procedure and analysis for every experimental group (CON, AI, LB, and AI+LB). On P50, mice were moved from group-housing, solid-bottom cages to individual-housing, wire-bottom cages, which provided an 8-day acclimation period prior to fecal collection. Previous research has demonstrated that individual housing is a stressor for rodents [3,40,84], thus all experimental and control animals experienced the same individual housing. On P59, samples were collected every 4 hours, with samples at 08:00 and 12:00 hr (i.e. 2 and 6 hours after lights off) providing an estimate of daily peak production, and samples at 20:00 and 0:00 hr (i.e. 2 and 6 hours after lights on) providing an estimate of daily trough (accounting for lag time for circulating steroids to be metabolized and excreted in feces) [85–89]. All animals remained in individual housing in solid-bottom cages for the remainder of the study. To measure longer-term effects of peri-adolescent asthma on GC production, mice were sacrificed three weeks after asthma treatments (~P76; adulthood). Heart blood was collected on average 6 minutes from initial cage disruption and stored on ice until serum separated and stored at −80°C. Commercial [125I] radioimmunoassay kits (MP Biomedicals, Solon OH) were used to measure corticosteroid concentration in fecal extracts and serum using published methods [85–87]. Time to collect blood did not relate to serum corticosterone concentration (Pearson r=0.037, p=0.40, n=54), likely because mice were habituated to cage disturbance, which was a regular occurrence throughout the study, and mice were rapidly sacrificed and blood collected once their cage was disturbed. Samples were analyzed in duplicate; intra- and inter-assay coefficients of variation for low and high controls were 16.04 and 5.27, and 24.83 and 16.62 respectively.
2.4.2 Lung Mucus, Inflammation, and Collagen
Lung sections were preserved in formalin and paraffin-embedded, sliced, and stained to visualize mucus (periodic acid-Schiff stain, PAS), inflammation (hematoxylin and eosin stain, H&E), and collagen (Masson’s trichrome stain, TRI). For each stain, 3 consecutive slices were mounted from posterior portions of the right inferior and left lobes. Mucus levels were quantified on a scale of 0–6 with increasing numbers indicating increasing mucus (Table 1). All 6 slices were quantified and an average mucus score calculated per mouse. Inflammation patches were counted if adjacent to blood vessels or airways, and thickness of the three largest patches were measured perpendicular to the airway/vessel membrane (20μm diameter or larger). In statistical analyses, we used total patch number and mean patch thickness calculated from the six largest patches (three from each lung)/mouse. If fewer than three patches were present, we quantified and averaged as many patches as possible. Collagen thickness was quantified around airways at least 150μm diameter, with five thickness measures for each of 3–5 airways on each of two slices per mouse. Mean collagen thicknesses across all airways was calculated for each mouse.
Table 1. Coding scheme for lung mucus.
A scale from 0-6 was used to code lung mucus on PAS-stained lung slides.
| Mucus Score | Description |
|---|---|
| 0 | No mucus or very small amounts of mucus |
| 1 | Partial thin outline of mucus on 1–2 inner airways |
| 2 | Full mucus outline around inner airway on 1–2 airway(s) |
| 3 | Full mucus outline on 3 airways |
| 4 | Mucus covering 1 entire airway OR mucus outline on 4+ airways |
| 5 | Mucus covering 1 entire airway AND mucus outline on 1+ airway(s) |
| 6 | Mucus covering 2+ airways AND mucus outline on 2+ airways |
2.4.3 Adult Lung Cytokine Gene Expression
At sacrifice, lungs were freshly dissected and stored in RNAlater (Ambion, Carlsbad, CA) for 24 hours prior to freezing at –80°C. Total cellular RNA was extracted from tissue using TRIzol reagent (Invitrogen; Carlsbad, CA) and Qiagen RNeasy columns (Qiagen; Germantown, MD). RNA quantity was measured with a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Wilmington, DE), and RNA quality checked with an Agilent 2100 BioAnalyzer™ (Agilent Technologies, Santa Clara, CA). RNA was reverse transcribed with High-Capacity cDNA Reverse Transcription kits (Applied Biosystems; Wilmington, DE). Resulting complementary DNA (cDNA) was examined for relative abundance of IL-4, IL-5, and IL-13 (Life Technologies, Mm00445259_m1, Mm00439646_m1, Mm00434204_m1) using quantitative real-time PCR (qRT-PCR). qRT-PCR reactions were prepared in triplicate in 96-well plates with validated TaqMan probes and the constitutively expressed beta actin gene (Actb, Life Technologies, Mm02619580_g1). The following cycle settings were used on the StepOnePlus Real-Time PCR System (Applied Biosystems, Wilmington, DE): 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C test for 60 seconds. Target gene expression was normalized to Actb expression by subtracting each sample’s gene of interest’s cycle threshold (CT) values from the corresponding Actb CT value, generating ΔCT values. Difference scores were standardized to the median CON mouse value, with all expression levels given relative to this median value. Relative abundance of each transcript per sample was determined using the 2 ΔΔCT method.
2.4.4 Adult Circulating Cytokine Levels
Serum was collected from whole blood obtained at sacrifice via mouse cardiac puncture (centrifugation at 12,000x g, 5 minutes, 4°C) and stored at −80°C. Cytokine concentrations (IL-4, IL-5, IL-6, IL-13, TNFα) were measured with MILLIPLEX® MAP Multiplex Immunoassay Kits (Mouse Cytokine/Chemokine Panel I, MCYTOMAG-70 K; Merck Millipore; Darmstadt, Germany). A Bio-Plex 200 System (Bio Rad; Hercules, CA) using a five-parameter logistic regression model created standards curves and calculated mean sample concentrations.
2.4.5 Adult Brain Serotonin- and HPA-Related Gene Expression
At sacrifice, brains were freshly-dissected to separate brainstem, hippocampus, and PFC. All sections were collected, processed, and analyzed as previously described for lung cytokine expression. Brain tissue TaqMan Gene Expression Assay primers and probes used included: SERT, 5Ht1ra, Crhr1, GR (Nr3c1), and MR (Nr3c2; Life Technologies, Mm00439391_m1, Mm00434106_s1, Mm00432670_m1, Mm00433832_m1, Mm01241596_m1).
2.5 STATISTICAL ANALYSES
Repeated measures ANOVAs were used to compare Penh across conditions. One ANOVA was conducted for each administration age (P22, 29, 36, 43, 50, 57) and the repeated measure at each age was mean Penh during each MCH dose. To compare behavioral and physiological outcome variables among conditions, all ANCOVAs were run with the following factors (each with 2-levels) – AI (intranasal saline vs. HDM exposure), LB (inhaled aerosolized saline vs. MCH exposure), sex (male vs. female), and USV category (high vs. low) – and with a covariate of mean cohort outcome value. The cohort covariate was used because a priori analyses (ANOVAs) indicated that several outcome variables were affected by cohort. All main and interaction effects with p<0.05 were reported. When ANCOVAs identified sex interactions, males and females analyses were conducted separately to determine specific sex effects. We compared results between each of the four experimental groups (CON, AI, LB, AI+LB), using ANCOVAs as described above with the AI and LB factors replaced with a 4-level Group factor. Outcomes were compared among the four groups using contrasts to compare the three experimental groups to the control group. Further, non-hypothesis-driven pairwise comparisons were conducted among the four groups using Sidak correction for multiple comparisons. Results of these post-hoc analyses are reported in the figure legends. For all tests, variable distribution was checked for normality; several variables (mucus, fecal corticosterone, serum corticosterone and cytokines, and gene expression) were log-transformed to achieve normal distribution for parametric statistics. No outliers were identified. As a result of technical issues, there was one missing data point in each of the following outcome variables: elevated plus maze time on open arms, circulating IL-4 concentration, and lung mucus, inflammation, and collagen measures. All other outcome variables included the full sample size indicated in Section 2.1. Figures depict untransformed estimated marginal means.
3. RESULTS
3.1 PERI-ADOLESCENT BRONCHOCONSTRICTION (P22-57)
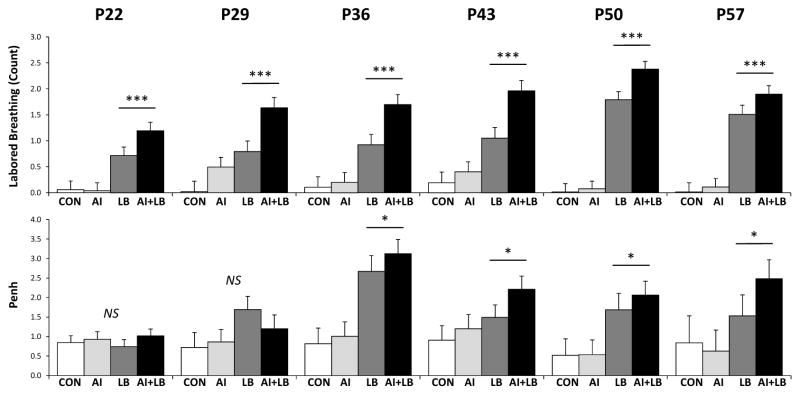
MCH led to increased labored breathing at every age of administration (Figure 2; Table 2). Penh values were not reliably higher in LB vs non-LB mice at P22 and 29, but were significantly higher at P36, 43, 50, and 57 (Figure 2; Table 2).
Figure 2. Peri-adolescent labored breathing and enhanced pause (Penh) values.
Top: The mean number of labored breathing events during weekly methacholine (MCH) or vehicle exposure presented for the four treatment groups: Control (CON), Airway Inflammation (AI), Labored Breathing (LB), and Airway Inflammation + Labored Breathing (AI+LB). Mice that experienced MCH (LB and AI+LB) had significantly higher scores than those that did not experience MCH (CON and AI), indicated by *** (p<0.001). Bottom: MCH treatments also caused significantly higher mean enhanced pause (Penh) values compared to saline on P36, 43, 50, and 57, indicated by * (p<0.05).
Table 2. Statistics on the effect of peri-adolescent MCH administration on labored breathing and Penh.
‘Labored Breathing’ column: ANOVA results for MCH main effect on labored breathing behavior at each age of administration. ‘Penh’ column: repeated measures ANOVA result for the effect of increasing MCH dose (time x LB interaction effect) on Penh values at each administration age. Significant results indicate a difference between LB vs. non-LB groups.
| Age (Postnatal Days) | Labored Breathing | Penh |
|---|---|---|
| P22 | F1,81 = 31.03, p<0.001 | F15,86 = 0.80, p=0.67 |
| P29 | F1,81 = 23.50, p<0.001 | F15,131 = 1.22, p=0.26 |
| P36 | F1,81 = 32.01, p<0.001 | F15,155 = 2.74, p<0.05 |
| P43 | F1,81 = 36.70, p<0.001 | F15,212 = 2.08, p<0.05 |
| P50 | F1,81 = 175.87, p<0.001 | F15,137 = 1.79, p<0.05 |
| P57 | F1,81 = 94.54, p<0.001 | F15,32 = 1.97, p=0.05 |
3.2 LATE ADOLESCENT FECAL CORTICOID RHYTHM (P59)
Fecal peak and trough corticoid concentrations were 6 times higher in females than males (Sex effect for peak and trough: F1,81=209.50, p<0.0001 and F1,81=268.63, p<0.0001). LB females trended towards elevated trough corticoid levels relative to non-LB females, whereas males showed an opposite trend (ANCOVA, Sex x LB interaction: F1,81=5.68, p<0.05; Figure 3). There were no effects of AI or USV, nor any interaction effects, on trough fecal corticoid production (Fs<2.44, ps>0.12). Peak fecal corticoid production was not affected by AI, LB, USV, or any interactions (Fs<3.60, ps>0.10).
Figure 3. Late adolescent fecal corticoid concentrations.
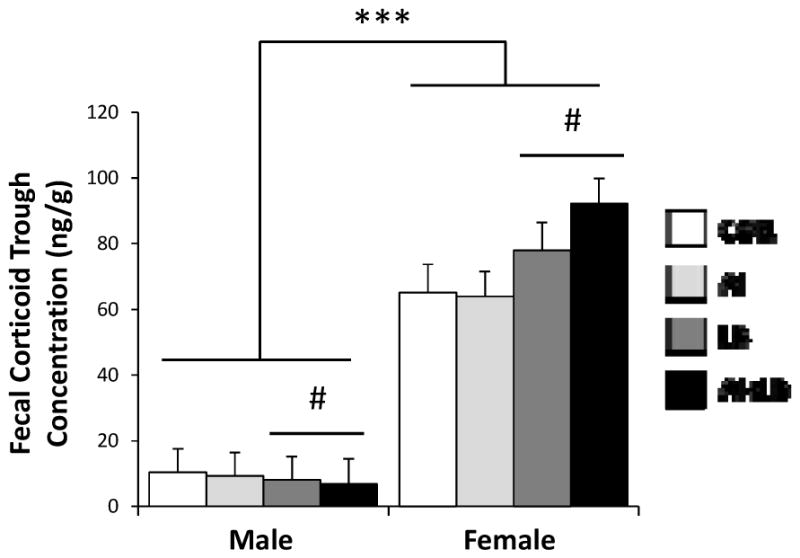
Mean fecal corticoid metabolite concentrations in late adolescent (P59) male and female mice in the four experimental groups: Control (CON), Airway Inflammation (AI), Labored Breathing (LB), and Airway Inflammation + Labored Breathing (AI+LB). Female mice had significantly greater fecal corticosteroid metabolite concentrations during the diurnal trough compared to males, indicated by *** (p<0.001). Left side: Male mice that experienced MCH (LB and AI+LB groups) had a trend toward decreased fecal corticoid concentrations compared to males that did not experience MCH (CON and AI groups), indicated by # (p<0.10). Right side: The reverse was true for female mice, with a trend toward increased fecal corticoid concentrations in MCH (LB and AI+LB) vs. non-MCH (CON and AI) mice, indicated by # (p<0.10). Bars indicate means with standard error. [Contrasts & pairwise group comparisons: For females, there was a trend for increased fecal corticoids in AI+LB vs. CON females (p<0.10) with no other group differences identified in pairwise comparisons. For males, there were no differences between individual groups.]
3.3 ADULT BEHAVIOR
3.3.1 Elevated Plus Maze (P60)
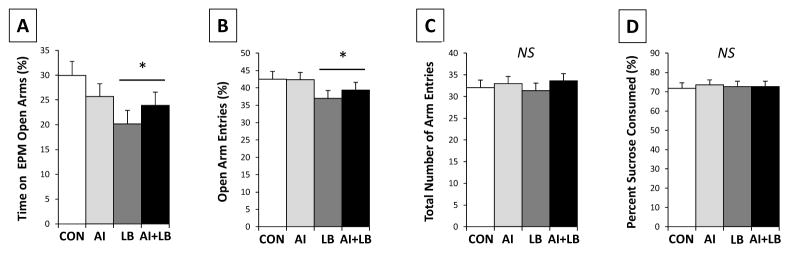
Peri-adolescent LB caused increased anxiety-related behavior in young adults. LB animals spent less time on the open arms (F1,80=5.230, p<0.05; Figure 4A) and had a lower proportion of open arm entries than non-LB mice (F1,81= 4.220, p<0.05; Figure 4B). There were no significant differences in locomotion (number of entries into open and closed arms) among LB groups (Fs<1.804, ps>0.183; Figure 4C). There were no main effects of sex, AI, or USV, and no significant interaction effects for any of the EPM behaviors (Fs<2.195, ps>0.142).
Figure 4. Adult behavior.
Behavioral measures for the following four treatment groups: Control (CON), Airway Inflammation (AI), Labored Breathing (LB), and Airway Inflammation + Labored Breathing (AI+LB). Adult mouse (P60) behavior in elevated plus maze (EPM) (A–C) indicates that adolescent MCH caused increased adult anxiety-live behavior but not locomotion: there was an LB main effect on time on open arms (A) and entries into open arms (B), but not on total entries into open and closed arms (C), main effect of LB indicated by * (p<0.05). No significant differences were observed in percent sucrose consumed by adult mice (P66) in sucrose preference test (SPT) (D). Bars indicate means with standard error. [Contrasts & pairwise group comparisons: LB and AI+LB groups spent less time on open arms of the EPM than the CON group (p<0.01 and 0.05); pairwise comparisons indicated no other differences among groups.]
3.3.2 Sucrose Preference Test (P66)
Neither AI nor LB predicted the relative amount of sucrose solution consumed (Fs<0.272, ps>0.605; Figure 4D). There was a significant interaction of Sex and USV on proportion of sucrose solution consumed over 24 hours; high-calling males consumed more sucrose compared to low-calling males, and the reverse was true for females (F1,36=4.815, p<0.05; interaction not depicted in figure). There were no other main effects or interactions (Fs<1.990, ps>0.167).
3.4 ADULT PHYSIOLOGY (P76)
3.4.1 Lung Mucus, Inflammation, and Collagen
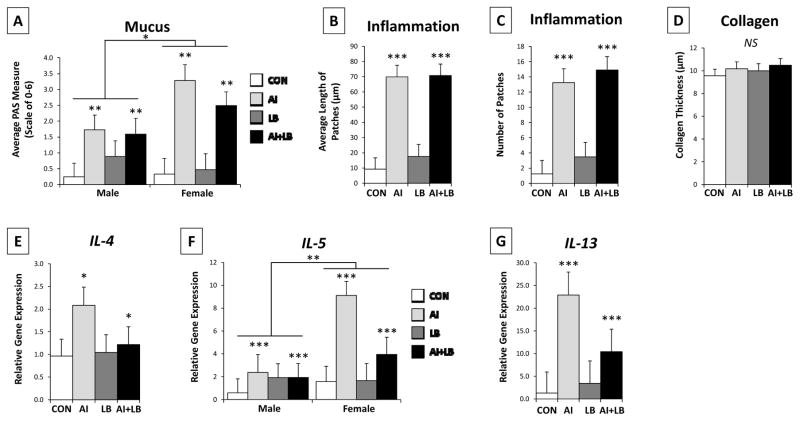
Three weeks after HDM and MCH treatments ended, AI mice had more lung mucus and inflammation than non-AI mice (mucus: F1,36=9.692, p<0.01, Figure 5A; inflammation patch size: F1,36=55.51, p<0.001, Figure 5B; number of inflammation patches: F1,36=41.97, p<0.001; Figure 5C). AI females had more mucus than AI males (AI x Sex interaction: F1,36=4.454, p<0.05). There was a trend for greater mucus production in high-USV mice (F1,36=3.587, p<0.10) and a significant interaction between AI and USV (F1,36=6.151, p<0.05). Within the AI group, high-calling mice produced more mucus than low-calling mice (F1,24=7.847, p<0.01). There were no group differences in amount of collagen (Fs<3.043, ps>0.090; Figure 5D).
Figure 5. Adult lung state and cytokine expression.
Lung function measures for the four treatment groups: Control (CON), Airway Inflammation (AI), Labored Breathing (LB), and Airway Inflammation + Labored Breathing (AI+LB). Adolescent HDM treatments (AI and AI+LB groups) led to increased mucus (A), average inflammation patch length (B), number of inflammation patches (C), and gene expression (mRNA) for IL-4 (E), IL-5 (F), and IL-13 (G). Mucus and IL-5 expression were both greater in females compared to males. Collagen measures were not significantly different across groups (D). Main effects of AI and Sex indicated by * (p<0.05), ** (p<0.01), and *** (p<0.001). [Contrasts & pairwise group comparisons: AI and AI+LB groups had significantly more mucus (p<0.001) and more inflammation (length and number of patches, p<0.001) than both CON and LB groups. Compared to the CON group, AI had more gene expression of IL-4 (p<0.05), and AI and AI+LB groups had more expression of IL-5 (p<0.01 and 0.05), and IL-13 (p<0.001). Further pairwise comparisons indicated that the AI group had greater expression of IL-5 (p<0.01) and IL-13 (p<0.001) than the LB group, and the AI+LB group had greater IL-13 expression than the LB group (p<0.05).]
3.4.2 Lung Cytokine Gene Expression
Three weeks after final peri-adolescent asthma treatments, IL-4, IL-5, and IL-13 gene expression in lungs was increased in AI compared to non-AI mice (F1,22=4.56, p<0.05, F1,22=21.51, p<0.001, F1,22=38.79, p<0.001; Figure 5E-G). There was a significant sex difference in IL-5 expression, with females showing greater expression than males (F1,22=14.20, p<0.01). An AI x LB interaction in IL-5 and IL-13 expression indicated dampened expression in AI+LB mice compared to AI only mice (F1,22=4.98, p<0.05; F1,22=3.82, p<0.10). A main effect of USV emerged for IL-13, where high-calling mice had greater expression than low-calling mice (F1,22=5.60, p<0.05). No other main or interaction effects occurred (Fs<2.49, ps>0.128).
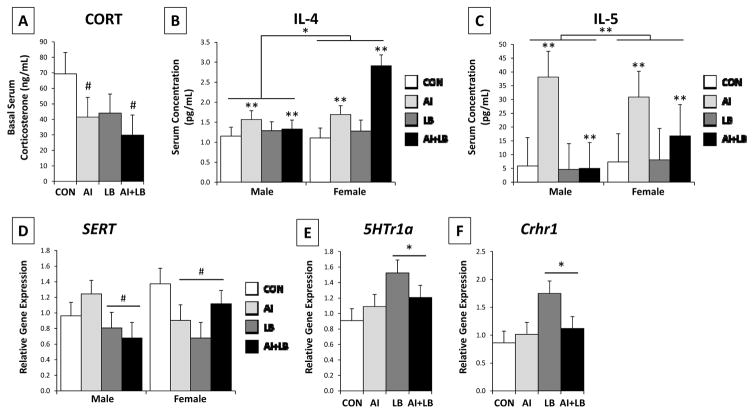
3.4.3 Circulating Basal Glucocorticoid Levels and Peripheral Circulating Cytokine Levels
Three weeks after final asthma treatments, there was a trend for AI mice to have decreased circulating corticosterone levels compared to non-AI mice (F1,37=2.81, p=0.10; Figure 6A). These results did not change when time to collect blood was included as a covariate (F1,37=2.60, p=.12). AI also increased circulating IL-4 and IL-5 concentrations relative to non-AI (F1,23=13.30, p<0.01; F1,22=8.29, p<0.01; Figure 6B-C), with females having higher concentrations than males (F1,23=5.58, p<0.05; F1,22=9.58, p<0.01). The IL-13 antibody did not perform well, so results are not reported here. As control measures, circulating IL-6 and TNF-α concentrations were quantified and indicated no effects of AI, LB, Sex, or USV (Fs<1.84, ps>0.18).
Figure 6. Adult basal corticosterone and cytokine concentrations in serum, and transporter/receptor gene expression in the brain.
The four experimental groups include: Control (CON), Airway Inflammation (AI), Labored Breathing (LB), and Airway Inflammation + Labored Breathing (AI+LB). Trending effect of AI indicated by # (p<0.10) for mean basal serum corticosterone concentrations (A). Main effect of AI or Sex indicated by * (p<0.05), and ** (p<0.01) for circulating IL-4 (B) and IL-5 levels (C). There was a main effect of LB on brainstem serotonin transporter (SERT) (D), hippocampal serotonin receptor 1a (5Htr1a) (E), and hippocampal corticotropin releasing hormone receptor 1 (Crhr1) (F), indicated by * (p<0.05) on E and F, and excluded on D in order to show sex-specific trends of LB and AI/LB on SERT expression, indicted with # (p<0.10). [Contrasts & pairwise group comparisons: Compared to CON mice, AI and AI+LB mice had significantly greater circulating IL-4, AI had greater IL-5 levels, and LB had significantly greater 5Htr1a and Crhr1 expression (p<0.05 and 0.01). Pairwise comparisons revealed no other group differences.]
3.4.5 Brain Serotonin and Anxiety/Stress-Related Gene Expression
Peri-adolescent LB altered adult gene expression related to serotonergic and anxiety/stress regulation. Adult LB mice had decreased brainstem SERT (F1,37=4.83, p<0.05, Figure 6D) and increased hippocampal 5Ht1ra and Crhr1 expression (F1,37=5.28, p<0.05, Figure 6E; F1,37=5.08, p<0.05, Figure 6F) compared to non-LB mice. There was a significant interaction of Sex, AI, and LB on SERT expression (F1,37=5.67, p<0.05); LB males showed a trend toward downregulated SERT expression (F1,19=3.54, p<0.10), whereas female expression was downregulated by both AI and LB (F1,17=4.23, p=0.055). There were no main effects of AI, LB, Sex or USV, nor interaction effects, on hippocampal MR or GR, nor for hippocampal Crhr1 levels. There was a trend for peri-adolescent LB to cause increased adult MR expression (F1,37=3.12, p<0.10). In PFC, a USV trend for Crhr1 expression in PFC indicated high-calling mice expressed more Crhr1 mRNA than low-calling mice (F1,37=3.83, p<0.10).
4. DISCUSSION
The current study confirmed that frequent peri-adolescent intranasal house dust mite extract (HDM) treatment produced airway inflammation in a mouse model, and that this state persisted several weeks beyond the treatment period [74]. In addition, although methacholine (MCH) is often used to verify airway hyperresponsiveness [50,74,90], here, we showed that weekly peri-adolescent MCH treatments effectively induced labored breathing bouts at multiple ages throughout mouse peri-adolescence. Previous studies have demonstrated that late adolescent/early-adult respiratory allergies and inflammation lead to increased anxiety [91]. In the current study, we found that labored breathing induced by weekly MCH exposure, and not lung inflammation induced by HDM treatments, led to the most significant increases in adult anxiety-related outcomes (specifically, behavior and gene expression associated with serotonergic and CRH function). Interestingly, peri-adolescent HDM and MCH treatment did not have a synergistic effect on adult symptoms associated with human internalizing disorders, although this might be attributed to the half MCH dose used in the HDM+MCH treated AI+LB group. These findings suggests that asthma-like repeated acute hypoxic labored breathing experiences, independent of airway inflammation, can affect physiological processes that contribute to later anxiety-related symptoms. Prior studies have established that hypoxia contributes to severity of asthma in an adult mouse model, that chronic postnatal hypoxia (P3–11) leads to increased adolescent anxiety-like behavior, and that hypoxia predicts panic disorder in asthmatic humans [50,92,93]. The current study extends these prior findings to suggest for the first time that brief, repeated asthma-like labored breathing events during mouse peri-adolescence cause changes in adult behavior and brain gene expression that are associated with an anxiety phenotype.
The current study suggests that chronic peri-adolescent asthma symptoms, which represent a real-world, common chronic stressor for many adolescents, can lead to subtle alterations in adult glucocorticoid (GC) regulation that includes young-adult basal GC upregulation with later adult downregulation. Persistent peri-adolescent lung inflammation led to dampened adult basal serum GC levels, but it did not contribute to late adolescent GC production or adult anxiety-related symptoms. In humans, experiencing asthma serves as a chronic stressor throughout development, and chronic stress is notorious for inducing GC dysregulation [20–22,28]. The current study suggests that labored breathing aspects of asthma may cause short-term alterations in GC regulation, whereas lung inflammation may lead to longer-term alterations. Further, our results indicate that peri-adolescent labored breathing causes an acute increase in basal GC production during the circadian trough in females but not in males. This is concordant with previous research demonstrating that females have higher endocrine responses than males following stress [59,84,94,95]. In humans, females are more vulnerable to developmental perturbations, and this has been associated with increased anxiety and depression rates in females compared to males [10,60]. Based on results from the current study, further investigation is needed on differential male and female late-adolescent physiological responses to acute labored breathing attacks. Sex differences in acute responses to this adolescent clinical experience may help to identify mechanisms that underlie female susceptibility to dysregulation and/or identify additional symptoms of asthma-associated internalizing disorders. The current study did not use different doses of HDM or MCH to accommodate sex differences in body weight that emerge during adolescence. Thus, future dose-response studies are required to determine if the effects observed here persist if doses are equalized for body weight.
Asthma and internalizing disorders occur at different rates in females and males. Female adolescents exhibit higher rates of asthma than male adolescents, and female adolescents and adults are hospitalized for asthma-related symptom flares more frequently than males of the same age [61–63,96,97]. Additionally, females are more sensitive to the consequences of childhood and adolescent stressors compared to males, which may contribute to higher rates of anxiety and depression in adolescent and young adult females; these female-biased effects persist into adulthood [10,58–60]. The current study, which suggests that peri-adolescent asthma symptoms lead to adult anxiety-related symptoms, sets the stage for follow-up investigations on asthma-induced anxiety-related neural substrates and sex differences in mechanisms that underlie the co-morbidity of asthma and internalizing disorders.
Serotonin function plays an important role in internalizing disorders, with decreased amounts of serotonin and reuptake ability being characteristic of depressive and anxious phenotypes [52]. Peripheral serotonin has also been demonstrated to play an important role in Th2 immune response, and recent evidence indicates that serotonin receptor agonists prevent symptoms of airway inflammation in a mouse model [98,99]. Previous human research has indicated that sex differences exist in peripheral serotonin receptor and transporter binding at baseline and in suicide victims [100,101]. In the current study, we found that adult female SERT expression in the brainstem was affected by both peri-adolescent HDM and MCH treatments, whereas in males this was driven by MCH treatments only. Additionally, females had a greater HDM-induced increase in Th2 cytokines in lung (IL-5) and serum (IL-5 and IL-4) compared to males. Considering that serotonin is involved in this response and that sex differences exist in serotonin-related substrates, it is evident that our asthma treatments drove a greater female than male Th2 response in lungs and serum, and that this sex difference was not present in untreated (CON) mice. Based on results of the current and prior studies, an accentuated Th2 response in allergic responses may be one process that modulates serotonergic function, or vice versa, and that these processes may be more pronounced in females compared to males.
As previously stated, ultrasonic vocalizations (USV) rates during early postnatal life (P3-5) predict later-life temperament [66–69]. To control for different pup USV rates, indicative of an anxiety-prone phenotype, we evenly distributed high- and low-calling pups among treatment groups, and used USV call rate in statistical analyses to determine if developmental asthma affects fear-prone individuals more than others. However, we found that USV classification had no significant main or interaction effects on results in the current study. Some results indicated that experimental asthma treatments were differentially effective in high-calling relative to low-calling pups; for example, when verifying effects of HDM in the AI treatment group, high-calling (anxiety-prone) mice produced more mucus than low-calling mice. These findings suggest that in the mouse model, as with humans, early-life temperament may influence the severity of asthma symptoms later in life [102,103]. However, we found no evidence that early-life temperament exacerbated an individual’s susceptibility to asthma-induced adult anxiety symptoms.
Individual housing from P50-76 is a potential limitation to the current study. Individual housing was necessary to measure individual basal corticosteroid diurnal rhythm at the end of the asthma treatments, and this housing could have affected the study results. Individual-housing is a documented stressor for rodents and has been shown to cause anxiety-like behavior [3,40,84]. The present study implemented individual housing across all experimental conditions, so any anxiety-related symptoms that resulted from this housing affected all groups. However, susceptibility to single-housing-induced anxiety may have differed among the peri-adolescent treatment groups. For example, the labored breathing mice may have had a greater anxiety-like response to single-housing than other groups. On the other hand, if single-housing increased anxiety-like behavior across all groups, the effects of our asthma treatments on adult anxiety-like symptoms may have been muted. Thus, the results presented in the current study should be interpreted with the understanding that late adolescent individual-housing may have either accentuated, muted, or otherwise altered influences of peri-adolescent labored breathing and airway inflammation on adult behavior and physiology.
4.1 CONCLUSIONS
A validated mouse model is an essential first step for experimental studies on the independent effects of labored breathing and chronic lung inflammation on behavioral and neurobiological development. In addition, a mouse model further allows us to study bidirectional interactions of internalizing behavior and asthma. Here we documented that repeated peri-adolescent allergen and muscarinic receptor agonist exposure caused airway inflammation and labored breathing, respectively, in a mouse model. These results indicate that this model can be used to determine the independent influences of different asthma symptoms on adolescent development. More importantly, these manipulations led to changes in adult behavioral and physiological processes that have been associated with an anxiety-like phenotype. This study is the first of its kind, and while our goal was to develop a sound model for human internalizing disorders in response to peri-adolescent asthma, further investigation and improvement of this model will allow us to more confidently associate our observed results with behavior and physiology indicative of those observed in human patients. Specifically, labored breathing alone may drive increased anxiety-like symptoms in asthma patients, and chronic peri-adolescent lung inflammation may have a long-term impact on hypothalamic-pituitary-adrenal axis regulation, a risk factor for internalizing disorders. While the present study documented significant acute behavioral and neurobiological effects of peri-adolescent lung inflammation and labored breathing, further studies are necessary to: (1) address the relative permanence of these effects, (2) determine other processes affected by peri-adolescent asthma, and (3) test the influence of environmental and pharmacological interventions on minimizing adult psychiatric symptoms. Additionally, future work will require dose-response studies to elucidate the relative influence of labored breathing and airway inflammation. Based on results from the current study, the mouse model presented here provides a good foundation for follow-up studies to inform intervention, therapy, and medication regimens in young patients with asthma, with the ultimate goal of minimizing internalizing disorders and related symptoms in this population.
HIGHLIGHTS.
Experimental conditions induced asthma-like symptoms in adolescent mice.
Chronic adolescent asthma symptoms caused adult anxiety-related symptoms.
Induced labored breathing raised adult anxiety behavior and brain gene expression.
Induced airway inflammation led to decreased adult basal corticosterone production.
Acknowledgments
FUNDING: This work was supported by the National Institutes of Health (5R21MH092667) and Pennsylvania State Institute for Neuroscience.
We would like to acknowledge the intense assistance of many students in the Behavioral Neuroendocrinology Lab: AG Agasar, SJ Allen, AD Bao, AK Bossert, AP Bruscke, DC Cardell, JT Carp, HS Chaudhry, SL Cooperstein, TL Coppage, K Craig, M DeNicola, CG Firely, JL Fox, OM Francois, S Gnanarajah, II Guo, IM Kaplan, A Kech, SZ Kidder, HO Knisley, SM Koo, EL Mercier, AC Motchenbacher, CM Ragan, AJ Rodriguez, JD Senville, MH Woehling, and JA Wisniewski.
We dedicate this paper to Robert H. Bonneau, esteemed colleague and supportive mentor, who passed away before manuscript completion. This work would not have been possible without him. He is greatly missed.
ABBREVIATIONS
- Y/O
Years old
- CON
Control (similarly handled control group)
- AI
Airway Inflammation (experimental group)
- LB
Labored Breathing (experimental group)
- AI+LB
Airway Inflammation + Labored Breathing (experimental group)
- HDM
House dust mite
- MCH
Methacholine
- PBS
Phosphate-buffered saline
- Penh
Enhanced pause
- USV
Ultrasonic vocalization
- GC
Glucocorticoids
- P
Postnatal Day
- Th2
T helper 2
- PFC
Prefrontal cortex
- CT
Cycle threshold
- qRT-PCR
Quantitative real time polymerase chain reaction
- CRH
Corticotropin releasing hormone
- GR
Glucocorticoid receptor
- MR
Mineralocorticoid receptor
- SERT
Serotonin transporter
- 5Htr1a
Serotonin receptor 1a
- Crhr1
Corticotropin releasing hormone receptor 1
- EPM
Elevated plus maze
- SPT
Sucrose preference test
- PAS
Periodic acid-Schiff stain
- H&E
Hematoxylin and eosin stain
- TRI
Masson’s trichrome stain
- IL-4
Interleukin 4
- IL-5
Interleukin 5
- IL-6
Interleukin 6
- IL-13
Interleukin 13
- TNFα
Tumor necrosis factor alpha
- SPSS
Statistical Package for the Social Sciences
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- 2.Romeo RD. Perspectives on stress resilience and adolescent neurobehavioral function. Neurobiol Stress. 2015;1:128–133. doi: 10.1016/j.ynstr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: Investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- 4.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. http://dx.doi.org/10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 5.Sachser N, Hennessy MB, Kaiser S. Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci Biobehav Rev. 2011;35:1518–1533. doi: 10.1016/j.neubiorev.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annuals.1308.001. [DOI] [PubMed] [Google Scholar]
- 7.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/S0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JG, Cohen P, Pine DS, Klein DF, Kason S, Brook S, Judith Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA. 2000;284:2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- 9.Moretti MM, Craig SG. Maternal versus paternal physical and emotional abuse, affect regulation and risk for depression from adolescence to early adulthood. Child Abus Negl. 2013;37:4–13. doi: 10.1016/j.chiabu.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the national comorbidity survey. Am J Public Health. 2001;91:753–760. doi: 10.2105/AJPH.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in Asthm a Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. NCHS Data Brief. 2012 [PubMed] [Google Scholar]
- 12.Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Arch Gen Psychiatry. 2003;60:1125–1130. doi: 10.1001/archpsyc.60.11.1125. [DOI] [PubMed] [Google Scholar]
- 13.Ross CJM, Davis TMA. Screening and assessing adolescent asthmatics for anxiety disorders. Clin Nurs Res. 2007;16:5–24. doi: 10.1177/1054773806295235. [DOI] [PubMed] [Google Scholar]
- 14.Peters TE, Fritz GK. Psychological considerations of the child with asthma. Pediatr Clin N Am. 2011;58:921–935. doi: 10.1016/j.pcl.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Katon W, Lozano P, Russo J, McCauley E, Richardson L, Bush T. The Prevalence of DSM-IV anxiety and depressive disorders in youth with asthma compared with controls. J Adolesc Heal. 2007;41:455–463. doi: 10.1016/j.jadohealth.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afari N, Schmaling K, Barnard S, Buchwald D. Psychiatric comorbidity and functional status in adult patients with asthma. J Clin Psychol Med Settings. 2001;8:245–252. doi: 10.1023/A:1011912712262. [DOI] [Google Scholar]
- 17.Nascimento I, Nardi AE, Valença AM, Lopes FL, Mezzasalma MA, Nascentes R, Zin WA. Psychiatric disorders in asthmatic outpatients. Psychiatry Res. 2002;110:73–80. doi: 10.1016/S0165-1781(02)00029-X. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 19.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 20.Priftis KN, Papadimitriou A, Nicolaidou P, Chrousos GP. The hypothalamic-pituitary-adrenal axis in asthmatic children. Trends Endocrinol Metab. 2008;19:32–38. doi: 10.1016/j.tem.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Landstra AM, Postma DS, Boezen HM, Van Aalderen WMC. Role of serum cortisol levels in children with asthma. Am J Respir Crit Care Med. 2002;165:708–712. doi: 10.1164/rccm.2102115. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 23.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 24.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- 25.Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Feder A, Copland JD, Goetz RR, Mathew SJ, Pine DS, Dahl RE, Ryan ND, Greenwald S, Weissman MM. Twenty-four-hour cortisol secretion patterns in prepubertal children with anxiety or depressive disorder. Biol Psychiatry. 2004;56:198–204. doi: 10.1016/j.biopsych.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 28.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 29.Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joëls M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 30.Morsink MC, Joëls M, Sarabdjitsingh RA, Meijer OC, De Kloet ER, Datson NA. The dynamic pattern of glucocorticoid receptor-mediated transcriptional responses in neuronal PC12 cells. J Neurochem. 2006;99:1282–1298. doi: 10.1111/j.1471-4159.2006.04187.x. [DOI] [PubMed] [Google Scholar]
- 31.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/S0954579401003066. [DOI] [PubMed] [Google Scholar]
- 32.Van den Bergh BRH, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children’s Depression Inventory. Psychoneuroendocrinology. 2009;34:791–794. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro-Psychopharmacology Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Horm Behav. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of Childhood Emotional Abuse and Age on Cortisol Responsivity in Adulthood. Biol Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee PR, Brady D, Koenig JI. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Brain Res Mol Brain Res. 2003;115:55–62. doi: 10.1016/s0169-328x(03)00180-3. S0169328X03001803 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Holder MK, Blaustein JD. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol. 2014;35:89–110. doi: 10.1016/j.yfrne.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan R, Wilson DA, Feldon J, Yee BK, Meyer U, Richter-Levin G, Avi A, Michael T, Gruss M, Bock J, Helmeke C, Braun K. Impact of early life experiences on brain and behavioral development. Dev Psychobiol. 2006;48:583–602. doi: 10.1002/dev.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Müller MB, Schmidt MV. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Horm Behav. 2008;53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Jäättelä M, Ilvesmäki V, Voutilainen R, Stenman U, Saksela E. Tumor necrosis factor as a potent inhibitor of adrenocorticotropin-induced cortisol production and steroidogenic P450 enzyme gene expression in cultured human fetal adrenal cells. Endocrinology. 1991;128:623–629. doi: 10.1210/endo-128-1-623. [DOI] [PubMed] [Google Scholar]
- 44.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 45.Adcock IM, Ford PA, Bhavsar P, Ahmad T, Chung KF. Steroid resistance in asthma: Mechanisms and treatment options. Curr Allergy Asthma Rep. 2008;8:171–178. doi: 10.1007/s11882-008-0028-4. [DOI] [PubMed] [Google Scholar]
- 46.Corren J. Anti-interleukin-5 antibody therapy in asthma and allergies. Curr Opin Allergy Clin Immunol. 2011;11:565–70. doi: 10.1097/ACI.0b013e32834c3d30. [DOI] [PubMed] [Google Scholar]
- 47.Buzney CD, Gottlieb AB, Rosmarin D. Asthma and atopic dermatitis: a review of targeted inhibition of Interleukin-4 and Interleukin-13 as therapy for atopic disease. J Drugs Dermatology. 2016;15:165–71. [PubMed] [Google Scholar]
- 48.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75:68–78. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith TF, Hudgel DW. Decreased ventilation response to hypoxia in children with asthma. J Pediatr. 1980;97:736–741. doi: 10.1016/S0022-3476(80)80255-1. [DOI] [PubMed] [Google Scholar]
- 50.Ahmad T, Kumar M, Mabalirajan U, Pattnaik B, Aggarwal S, Singh R, Singh S, Mukerji M, Ghosh B, Agrawal A. Hypoxia response in asthma: Differential modulation on inflammation and epithelial injury. Am J Respir Cell Mol Biol. 2012;47:1–10. doi: 10.1165/rcmb.2011-0203OC. [DOI] [PubMed] [Google Scholar]
- 51.Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8\n4/2/109. [pii] [DOI] [PubMed] [Google Scholar]
- 52.Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- 53.Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- 54.Kalueff AV, Olivier JDA, Nonkes LJP, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Jørgensen HS. Studies on the neuroendocrine role of serotonin. Dan Med Bull. 2007;54:266–88. DMB3960 [pii] [PubMed] [Google Scholar]
- 56.Veenit V, Riccio O, Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J Psychiatr Res. 2014;53:1–7. doi: 10.1016/j.jpsychires.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Backström T, Winberg S. Central corticotropin releasing factor and social stress. Front Neurosci. 2013;7:1–10. doi: 10.3389/fnins.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rat’s anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- 59.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roza SJ, Hofstra MB, van der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. Am J Psychiatry. 2003;160:2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- 61.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992;268:3437–3440. doi: 10.1001/jama.1992.03490240045034. [DOI] [PubMed] [Google Scholar]
- 62.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47:537–542. doi: 10.1136/thx.47.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vink NM, Postma DS, Schouten JP, Rosmalen JGM, Boezen HM. Gender differences in asthma development and remission during transition through puberty: The TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126:498–504.e6. doi: 10.1016/j.jaci.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Finkelman FD, Wills-Karp M. Usefulness adn optimization of mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121 doi: 10.1038/nmeth.2250.Digestion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahu N, Morales JL, Fowell D, August A. Modeling susceptibility versus resistance in allergic airway disease reveals regulation by Tec kinase Itk. PLoS One. 2010;5:e11348. doi: 10.1371/journal.pone.0011348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: A tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/S0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- 67.Dichter GS, Brunelli SA, Hofer MA. Elevated plus-maze behavior in adult offspring of selectively bred rats. Physiol Behav. 1996;60:299–304. doi: 10.1016/0031-9384(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 68.Brunelli SA, Vinocur DD, Soo-Hoo D, Hofer MA. Five generations of selective breeding for ultrasonic vocalization (USV) responses in N:NIH strain rats. Dev Psychobiol. 1997;31:255–65. doi: 10.1002/(SICI)1098-2302(199712)31:4<255::AID-DEV3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 69.Hahn ME, Lavooy MJ. A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents. Behav Genet. 2005;35:31–52. doi: 10.1007/s10519-004-0854-7. [DOI] [PubMed] [Google Scholar]
- 70.Roy V, Belzung C, Delarue C, Chapillon P. Environmental enrichment in BALB/c mice: effects in classical tests of anxiety and exposure to a predatory odor. Physiol Behav. 2001;74:313–320. doi: 10.1016/S0031-9384(01)00561-3. [DOI] [PubMed] [Google Scholar]
- 71.Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Methods. 2010;7:825–826. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- 72.Tsitoura DC, Blumenthal RL, Berry G, DeKruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: Role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–246. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 73.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–85. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 74.Saglani S, Mathie SA, Gregory LG, Bell MJ, Bush A, Lloyd CM. Pathophysiological features of asthma develop in parallel in house dust mite-exposed neonatal mice. Am J Respir Cell Mol Biol. 2009;41:281–289. doi: 10.1165/rcmb.2008-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometic plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. http://dx.doi.org/10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 76.Pellow S, Chopin P, File SE, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 77.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 78.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 79.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 80.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/S0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 81.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 82.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 83.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 85.Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol. 2005;184:153–163. doi: 10.1677/joe.1.05935. [DOI] [PubMed] [Google Scholar]
- 86.Cavigelli SA, Guhad FA, Ceballos RM, Whetzel CA, Nevalainen T, Lang CM, Klein LC. Fecal corticoid metabolites in aged male and female rats after husbandry-related disturbances in the colony room. J Am Assoc Lab Anim Sci. 2006;45:17–21. [PubMed] [Google Scholar]
- 87.Thanos PK, Cavigelli SA, Michaelides M, Olvet DM, Patel U, Diep MN, Volkow ND. A non-invasive method for detecting the metabolic stress response in rodents: characterization and disruption of the circadian corticosterone rhythm. Physiol Res. 2009;58:219–228. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Touma C, Sachser N, Möstl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol. 2003;130:267–278. doi: 10.1016/S0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- 89.Harper JM, Austad SN. Fecal glucocorticoids : a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool Ecol Evol Approaches. 2000;73:12–22. doi: 10.1086/316721. [DOI] [PubMed] [Google Scholar]
- 90.Ohki Y, Tokuyama K, Mayuzumi H, Sato A, Koyama H, Takizawa T, Arakawa H, Mochizuki H, Morikawa A. Characteristic features of allergic airway inflammation in a murine model of infantile asthma. Int Arch Allergy Immunol. 2005;138:51–58. doi: 10.1159/000087357. http://dx.doi.org/10.1016/j.jaci.2004.12.214. [DOI] [PubMed] [Google Scholar]
- 91.Tonelli LH, Katz M, Kovacsics CE, Gould TD, Joppy B, Hoshino A, Hoffman G, Komarow H, Postolache TT. Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents. Brain Behav Immun. 2009;23:784–793. doi: 10.1016/j.bbi.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salmaso N, Silbereis J, Komitova M, Mitchell P, Chapman K, Ment LR, Schwartz ML, Vaccarino FM. Environmental enrichment increases the GFAP+ stem cell pool and reverses hypoxia-induced cognitive deficits in juvenile mice. J Neurosci. 2012;32:8930–8939. doi: 10.1523/JNEUROSCI.1398-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carr RE. Panic disorder and asthma: causes, effects, and research implications. J Psychosom Res. 1998;44:43–52. doi: 10.1016/s0022-3999(97)00137-2. http://dx.doi.org/10.1016/S0022-3999(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 94.Handa RJ, Burgess LH, Kerr JE, OJA Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 95.Heinsbroek RPW, Van Haaren F, Feenstra MGP, Endert E, Van de Poll NE. Sex- and time-dependent changes in neurochemical and hormonal variables induced by predictable and unpredictable footshock. Physiol Behav. 1991;49:1251–1256. doi: 10.1016/0031-9384(91)90359-v. http://dx.doi.org/10.1016/0031-9384(91)90359-V. [DOI] [PubMed] [Google Scholar]
- 96.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61:722–728. doi: 10.1136/thx.2005.045161. 61/8/722 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCallister JW, Mastronarde JG. Sex differences in asthma. J Asthma. 2008;45:853–861. doi: 10.1080/02770900802444187. [DOI] [PubMed] [Google Scholar]
- 98.Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015;213:561–574. doi: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- 99.Nau F, Miller J, Saravia J, Ahlert T, Yu B, Happel KI, Cormier SA, Nichols CD. Serotonin 5-HT 2 receptor activation prevents allergic asthma in a mouse model. Am J Physiol - Lung Cell Mol Physiol. 2015;308:L191–L198. doi: 10.1152/ajplung.00138.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jovanovic H, Lundberg J, Karlsson P, Cerin Å, Saijo T, Varrone A, Halldin C, Nordström AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 101.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-S. [DOI] [PubMed] [Google Scholar]
- 102.Kim SP, Ferrara A, Chess S. Temperament of asthmatic children. A prelliminary study. J Pediatr. 1980;97:483–486. doi: 10.1016/s0022-3476(80)80214-9. [DOI] [PubMed] [Google Scholar]
- 103.Meldrum SJ, D’Vaz N, Dunstan JA, Mori TA, Hird K, Simmer K, Prescott SL. Allergic disease in the first year of life is associated with differences in subsequent neurodevelopment and behaviour. Early Hum Dev. 2012;88:567–573. doi: 10.1016/j.earlhumdev.2011.12.032. [DOI] [PubMed] [Google Scholar]