Abstract
Objective
No drug is yet approved to treat the core symptoms of autism spectrum disorder (ASD). Low‐dose suramin was effective in the maternal immune activation and Fragile X mouse models of ASD. The Suramin Autism Treatment‐1 (SAT‐1) trial was a double‐blind, placebo‐controlled, translational pilot study to examine the safety and activity of low‐dose suramin in children with ASD.
Methods
Ten male subjects with ASD, ages 5–14 years, were matched by age, IQ, and autism severity into five pairs, then randomized to receive a single, intravenous infusion of suramin (20 mg/kg) or saline. The primary outcomes were ADOS‐2 comparison scores and Expressive One‐Word Picture Vocabulary Test (EOWPVT). Secondary outcomes were the aberrant behavior checklist, autism treatment evaluation checklist, repetitive behavior questionnaire, and clinical global impression questionnaire.
Results
Blood levels of suramin were 12 ± 1.5 μmol/L (mean ± SD) at 2 days and 1.5 ± 0.5 μmol/L after 6 weeks. The terminal half‐life was 14.7 ± 0.7 days. A self‐limited, asymptomatic rash was seen, but there were no serious adverse events. ADOS‐2 comparison scores improved by −1.6 ± 0.55 points (n = 5; 95% CI = −2.3 to −0.9; Cohen's d = 2.9; P = 0.0028) in the suramin group and did not change in the placebo group. EOWPVT scores did not change. Secondary outcomes also showed improvements in language, social interaction, and decreased restricted or repetitive behaviors.
Interpretation
The safety and activity of low‐dose suramin showed promise as a novel approach to treatment of ASD in this small study.
Introduction
Autism affects 1–2% of children in the United States.1, 2 Dozens of single genes and chromosomal copy number variants (CNVs)3 increase the relative risk of autism spectrum disorder (ASD) nearly 5–50 times over the current background risk. Yet no single gene or CNV causes ASD in 100% of children who carry the mutation,4 and no single DNA mutation accounts for more than 1–2% of all ASD.5 Specific environmental factors have also been shown to increase the risk of ASD.6, 7 However, no single child has all of the known genetic risk factors for ASD, or is exposed to all the same environmental risks. Although the noncore symptoms of ASD are highly heterogeneous from child to child, making each child unique, the same core features used for diagnosis – abnormalities in social communication, restricted interests, repetitive behaviors, adherence to routine, and/or atypical sensory behaviors – are by definition expressed in every child. One approach to addressing the challenge of many etiologies of ASD is to define a common pathophysiology that can contribute to the core diagnostic symptoms, regardless of the initiating genetic and environmental triggers. We hypothesized that there is a conserved cellular response to metabolic perturbation or danger that is shared by all children with ASD. This is called the cell danger hypothesis.8 Aspects of the cell danger response (CDR) are also referred to as the integrated stress response.9, 10, 11 Preclinical studies showed that the cell danger response in mice produced a treatable metabolic syndrome that was maintained by purinergic signaling. Antipurinergic therapy with suramin corrected both the behavioral and metabolic features of these genetic and environmental mouse models of ASD.12, 13, 14
The formulation of the cell danger hypothesis was based on the recognition that similar metabolic pathways were coordinately regulated as an adaptive response to cellular threat regardless of whether the perturbation was caused by a virus,15 a bacterium,16 genetic forms of mitochondrial disease,10 or neurodevelopmental disorders with complex gene–environment pathogenic mechanisms like autism.17 These metabolic pathways traced to mitochondria. Mitochondria are responsible for initiating and coordinating innate immunity18 and produce stereotyped changes in oxidative metabolism under stress19 that lead to the regulated release of purine and pyrimidine nucleotides like ATP and UTP through cell membrane channels.20 Inside the cell, ATP is an energy carrier. Outside the cell, extracellular ATP (eATP) is a multifunctional signaling molecule, a potent immune modulator,21 and a damage‐associated molecular pattern (DAMP) that can activate microglia, and trigger IL‐1β production and inflammasome assembly.22 Extracellular purines like ATP, ADP, and adenosine, and pyrimidines like UTP are ligands for 19 different purinergic (P2X, P2Y, and P1) receptors.23 The intracellular concentration of ATP (iATP) in mammalian cells is typically 1–5 mmol/L,24 but drops when ATP is released through membrane channels under stress. Typical concentrations of extracellular adenine nucleotides in the unstirred water layer at the cell surface where receptors and ligands meet are about 1–10 μmol/L, near the effective concentration for most purinergic receptors,25 but can increase when ATP is released during cell stress. Concentrations of eATP in the blood are another 500 times lower (10–20 nmol/L).26 Purinergic effectors like ATP are also coreleased with canonical neurotransmitters like glutamate, dopamine, and serotonin during depolarization at every synapse in which they have been studied23 and play key roles in activity‐dependent synaptic remodeling.27 These and other features28, 29, 30 led us to test the hypothesis that the CDR8 was maintained by purinergic signaling.12, 13, 14
Suramin has many actions. One of its best‐studied actions is as an inhibitor of purinergic signaling. It is the oldest member of a growing class of antipurinergic drugs (APDs) in development.31 Suramin was first synthesized in 1916,32 making it one of the oldest manmade drugs still in medical use. It is used to treat African sleeping sickness (trypanosomiasis), and remains on the World Health Organization list of essential medications. Concerns about the toxicity of high‐dose suramin arose when the cumulative antitrypanosomal dose was increased 5 times or more over several months to treat AIDS or kill cancer cells during chemotherapy. When blood levels were maintained over 150 μmol/L for 3–6 months at a time to treat cancer, a number of dose‐limiting side effects were described.32 These included adrenal insufficiency, anemia, and peripheral neuropathy. In contrast, mouse studies suggested that high‐dose suramin was not necessary to treat autism‐like symptoms. These studies showed that low‐dose suramin that produced blood levels of about 5–10 μM was effective in treating ASD‐like symptoms and did not produce toxicity even when used for at least 4 months.12, 14
Here, we report the findings of the Suramin Autism Treatment‐1 (SAT‐1) trial, the first direct test of suramin, the cell danger hypothesis, and the relevance of abnormal purinergic signaling in children with ASD. These data help form the foundation for future studies that will test the safety and efficacy of suramin, provide fresh directions for the development of new antipurinergic drugs, and add support to the hypothesis that a potentially treatable metabolic syndrome may contribute to the pathogenesis of autism.
Materials and Methods
Study design and participants
The SAT‐1 trial was an investigator‐initiated, phase I/II, double‐blind, placebo‐controlled, randomized clinical trial to examine the safety and activity of single‐dose suramin or placebo in 10 children with autism spectrum disorders (ASD). All children met DSM‐5 diagnostic criteria for autism spectrum disorder, and received confirmatory testing by Autism Diagnostic Observation Schedule, 2nd edition (ADOS‐2) examination. Inclusion criteria were male subjects, ages 4–17 years, living in the San Diego, California region, with a confirmed diagnosis of ASD. Exclusion criteria included children who weighed less than the 5th percentile for age, took prescription medications, or had laboratory evidence of liver, kidney, heart, or adrenal abnormalities. Children living more than a 90‐min drive from the testing sites in La Jolla, CA were excluded to eliminate the possibility of aberrant behaviors resulting from extended car travel. Children with known syndromic forms of ASD caused by DNA mutation or chromosomal copy number variation (CNV) were excluded in this first study. Families were asked not to change their children's therapy (e.g., supplements, speech, and behavioral therapies) or diet throughout the study period. The study was conducted between 27 May 2015 (date of the first child to be enrolled) and 3 March 2016 (date of the last child to complete the study).
Regulatory approvals, registration, CONSORT, and informed consent
The research plan, clinical trial protocol, informed consents, advertising, and amendments were approved by the University of California, San Diego (UCSD) Institutional Review Board (IRB Project #150134) before implementation. The study was authorized by the U.S. Food and Drug Administration (IND#118212), and conformed to the World Medical Association Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects,33 and the International Council for Harmonization (ICH) E6 Good Clinical Practice (GCP) guidelines. The trial was registered with clinical trials.gov (https://clinicaltrials.gov/ct2/show/NCT02508259). Reporting of the SAT‐1 trial conformed to CONSORT 2010 guidelines.34 Signed informed consent, with additional consent for video and still image photography, were obtained from the parents of all participants before enrollment and randomization. Storyboards and social stories were created to review with parents, help children visualize and prepare for the study, and create the opportunity to ask questions (Figure S1, Data S2).
Randomization and masking
Twenty male subjects with ASD were screened. Sixteen met entry criteria. Ten participants could be matched by age, nonverbal IQ, and ADOS scores into five pairs. The randomization sequence was generated electronically by the biostatistical team. Subjects within each pair were allocated to receive suramin or saline according to the prospectively determined randomization sequence. The randomization sequence was concealed from the clinical team and implemented by the UCSD investigational pharmacy, which prepared drug and placebo for infusion. The design was double blind. The mask was not broken until all subjects had completed the study and all clinical data had been collected.
Diagnostic and outcome procedures
The diagnosis of each of the enrolled participants was confirmed by ADOS‐235 comparison scores of ≥7. Nonverbal IQ was tested by Leiter‐3 examination.36 The primary behavioral outcomes were ADOS scores and language assessed by standardized vocabulary testing. Expressive vocabulary was assessed by Expressive One‐Word Picture Vocabulary Test (EOWPVT).37 Primary outcomes were measured at baseline, and 2 days and 6 weeks after infusion. Secondary outcomes were the Aberrant Behavior Checklist (ABC),38 Autism Treatment Evaluation Checklist (ATEC),39, 40 Clinical Global Impression of Improvement (CGI)41 (Data S1), and Repetitive Behavior Questionnaire (RBQ).42 Secondary outcomes were measured at baseline, and 7 days and 6 weeks after infusion.
Protocol deviations
The original protocol was designed to collect electroencephalography (EEG), heart rate variability (HRV), balance, gait, fine motor, and sensory motor data as secondary outcomes. However, the wide range in ages and abilities, small subject numbers, and task compliance difficulties made collection of these data incomplete and insufficiently powered to draw any conclusions. In addition, we found that major language advances were in the form of new speech fluency and new interest in speech and social communication, and not in new vocabulary. Peabody Picture Vocabulary Testing (PPVT) did not capture this new interest in communication. These data were incomplete and insufficiently powered for analysis.
Drug and placebo administration
Suramin was provided as the hexasodium salt (MW 1429.2 g/mol) in 1 g lyophilized vials by Bayer Pharma AG (Leverkusen, Germany), under Dr. Naviaux's IND #118212. Lot #BXNOGW1, expiration date of 3 September 2018, was used in these studies. A 1 g vial was reconstituted in 10 mL of sterile water for infusion to prepare a 10% (100 mg/mL) solution. All infusions were conducted at the University of California, San Diego School of Medicine Clinical and Translational Research Institute (CTRI) in La Jolla, CA. Height and weight were recorded, vital signs and capillary oxygen saturation (pulse oximetry) measured, physical and neurological examinations were conducted, and urine and blood for safety monitoring, pharmacology, and metabolomics were collected before the infusion. Each child then received a 50 mg test dose (0.5 mL of a freshly reconstituted 10% solution) of suramin in 5 mL of saline, or 5 mL of saline only given by slow intravenous (IV) push over 3 min, followed by a 10‐mL flush of saline. One hour after the test dose, vital signs were repeated and a single infusion of either suramin (20 mg/kg, minus the 50 mg test dose, in 50 mL, up to a maximum of 1 g) or saline (50 mL IV) was given over 30 min, followed by a 10‐mL flush of saline. One hour after completion of the infusion, vital signs and the physical and neurological examinations were repeated, blood was collected for safety monitoring and pharmacology, and the family discharged to home. A typical infusion visit to the Clinical Translational Research Institute (CTRI) lasted about 4 h from start to finish.
Safety and adverse event monitoring
Blood and urine samples were collected for safety and toxicity monitoring at 5 times throughout the study: at baseline (32 ± 6 days before the infusion; mean ± SEM), immediately before the infusion, 1 h after the infusion, 2 days after, and 45 days after the infusion. Unexpected and adverse events were recorded as they occurred and graded in severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 (CTCAE) scale. Additional pharmacovigilence monitoring included daily scripted phone calls in the first week, then 4 weekly calls until the exit examinations at 6 weeks. Each child received a formal neurological examination by a board‐certified pediatric neurologist at baseline and at the end of the study. An independent data safety monitoring board (DSMB) reviewed the data and IRB communications for the study.
Pharmacokinetics
Plasma samples were collected for suramin pharmacokinetics (PK) before the infusion, at 1 h, 2 days, and 45 days postinfusion. Suramin concentrations were measured by high‐performance liquid chromatography and tandem mass spectrometry (LC‐MS/MS) as described previously.13 See Supplemental Methods for details. The small number of PK samples per subject prevented a standard, noncompartmental analysis in individual subjects. The suramin drug concentrations were analyzed using a population PK approach with post hoc empiric Bayesian estimate of PK parameters in individual subjects. The PK data were fit to a two‐compartment model using the computer program NONMEM (ICON, Dublin, Ireland).43 PK parameters were scaled allometrically with volume terms scaled to linear body weight (kg1.0) and clearance terms scaled to weight (kg0.75). Scaled adult suramin parameters of compartmental volumes of distribution and clearance were used as initial parameter estimates and between subject variability only estimated for clearance (CL) and the peripheral volume of distribution (V d).
Pharmacometabolomics
Targeted, broad‐spectrum, plasma metabolomic analysis, covering 63 biochemical pathways, was performed by LC‐MS/MS as described previously44 with minor modifications. In all, 431 of 610 targeted metabolites were measureable in plasma. See Supplemental Methods for details.
Sample size calculation and statistical analysis
This was a pilot study designed to obtain activity data and effect size estimates upon which future sample size calculations could be based. No data on suramin in autism were available for sample size calculations prior to this study. Each child was used as his own control to examine before and after treatment effects in a paired t‐test design for the analysis of the ADOS, EOWPVT, ABC, ATEC, RBQ, and blood and urine safety data. Paired, nonparametric analysis was done by Wilcoxon signed‐rank sum test. Categorical data, such as the presence or absence of adverse events or historical symptoms, was analyzed by Fisher's exact test. Two‐way ANOVA (treatment × time), with Sidak post hoc correction, was used to analyze the 6‐week summaries captured by the ADOS, CGI, and blood and urine safety analysis. Cohen's d – calculated as the mean difference of the paired, within‐subject scores before and after treatment, divided by the standard deviation of the differences – was used as an estimate of effect size. Metabolomic data were log‐transformed, scaled by control standard deviations, and analyzed by multivariate partial least squares discriminant analysis (PLSDA), with pairwise comparisons and post hoc correction for multiple hypothesis testing using Fisher's least significant difference method in MetaboAnalyst,45 or the false discovery rate (FDR) method of Benjamini and Hochberg. Metabolites with variable importance in projection (VIP) scores determined by PLSDA that were greater than 1.5 were considered significant. Methods were implemented in Stata (Stata/SE12.1, StataCorp, College Station, TX), Prism (Prism 6, GraphPad Software, La Jolla, CA), or R. Significant metabolites were grouped into pathways and their VIP scores summed to determine the rank‐ordered significance of each biochemical pathway.
Results
Participant disposition and demographics
Figure 1 illustrates the CONSORT flow diagram for patient recruitment, allocation, and analysis in the SAT‐1 study. The two treatment groups were well matched (Table 1). The mean age was 9.1 years (range = 5–14). The mean nonverbal Leiter IQ was 80 (range = 66–92). The mean ADOS‐2 comparison score was 9.0 (range = 7–10).
Figure 1.
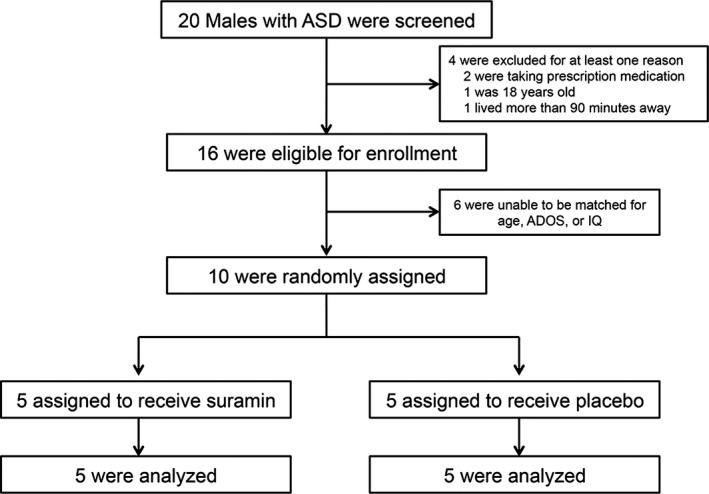
Trial profile.
Table 1.
Group characteristics
| Parameter | Suramin group Mean ± SD (range) or Number | Placebo group Mean ± SD (range) or Number | P valueb |
|---|---|---|---|
| Number (male subjects) | 5 | 5 | N/A |
| Age (years) | 8.9 ± 3.3 (5.7–13.6) | 9.2 ± 3.8 (6.2–14.7) | 0.88 |
| Leiter IQ | 82 ± 7.8 (75–92) | 79 ± 8.8 (66–87) | 0.69 |
| ADOS Score | 8.6 ± 0.9 (8–10) | 9.4 ± 1.3 (7–10) | 0.30 |
| Weight (kg) | 32 ± 14 (23–55) | 40 ± 23 (24–80) | 0.53 |
| Weight percentile | 64 ± 16 (42–84) | 78 ± 30 (25–98) | 0.40 |
| Height (cm) | 136 ± 23 (118–174) | 137 ± 28 (113–180) | 0.92 |
| BSAa (m2) | 1.09 ± 0.32 (0.87–1.63) | 1.21 ± 0.46 (0.87–1.99) | 0.64 |
| Body mass index (kg/m2) | 16.8 ± 1.1 (15.5–18.1) | 19.9 ± 3.1 (16.2–24.7) | 0.07 |
| Head circumference (cm) | 54.3 ± 2.8 (51.5–57.5) | 54.5 ± 2.3 (51.5–57) | 0.90 |
| HC percentile | 75 ± 30 (35–99) | 75 ± 27 (42–97) | 0.97 |
| Age at ASD diagnosis (yrs) | 3.2 ± 0.5 (2.5–3.75) | 2.7 ± 0.3 (2.5–3.0) | 0.10 |
| Paternal age at birth (yrs) | 37 ± 3.2 (35–41) | 43 ± 12 (33–64) | 0.62 |
| Maternal age at birth (yrs) | 35 ± 2.8 (32–38) | 41 ± 6 (33–47) | 0.053 |
| Sibling with ASD | 0 | 1 | 0.99 |
| History of GI issues – current | 0 | 1 | 0.99 |
| Maintains a gluten‐free diet | 0 | 1 | 0.99 |
| IVF conception | 1 | 0 | 0.99 |
| C‐section delivery | 1 | 1 | 0.99 |
| History of premature birth | 0 | 1 | 0.99 |
| History of epilepsyc – current | 0 | 0 | 0.99 |
| History of developmental regression(s) | 3 | 2 | 0.99 |
| History of asthma – current | 0 | 0 | 0.99 |
| ASD symptom improvement with fever | 2 | 1 | 0.99 |
BSA, body surface area; HC, head circumference; GI, gastrointestinal; IVF, in vitro fertilization; ASD, autism spectrum disorder.
Mosteller method.
Student's t‐test for continuous data; Fisher's exact test for categorical data.
Patients taking prescription drugs were excluded from the study. This included anticonvulsant medications.
Safety monitoring and adverse events
Extensive monitoring revealed no serious toxicities (CTCAE grades 3–5). Neurologic examinations showed there was no peripheral neuropathy (Table 2). Analysis of free cortisol, hemoglobin, white blood cell count (WBC), platelets, liver transaminases, creatinine, and urine protein showed no differences in children who received suramin and placebo (Fig. 2). Five children who received suramin developed a self‐limited, evanescent, asymptomatic, fine macular, patchy, morbilliform rash over 1–20% of their body (Fig. 2HI). This peaked 1 day after the infusion and disappeared spontaneously in 2–4 days. The mean number of AEs per participant was 1.9 (1.2 in the placebo group and 2.6 in the suramin group; 1.6 in the suramin group for a nonrash AE; RR = 1.3; 95% CI = 0.5–3.4; P = 0.77; Table 2). No serious adverse events (SAEs) occurred in this study. An independent data and safety monitoring board (DSMB) reviewed this information, as well as the clinical safety and toxicity data and IRB communications from the study, and found no safety concerns.
Table 2.
Summary of adverse or unanticipated events
| No. | Events | Suramin (N = 5) | CTCAEa grade | Placebo (N = 5) | CTCAEa grade | P valueb |
|---|---|---|---|---|---|---|
| 1 | Asymptomatic rash | 5 | 1 | 0 | – | 0.0079 |
| 2 | Uncomplicated URIc | 2 | 1 | 2 | 1 | 0.99 |
| 3 | Headache | 1 | 1 | 0 | – | 0.99 |
| 4 | Emesis × 1 | 1d | 1 | 1e | 1 | 0.99 |
| 5 | Hyperactivity | 2f | 1 | 1 | 1 | 0.99 |
| 6 | Hypoglycemiaf | 1 | 2 | 1 | 2 | 0.99 |
| 7 | Leukocytosis | 0 | – | 1g | 1 | 0.99 |
| 8 | Enuresis | 1h | 1 | 0 | – | 0.99 |
| 9 | Peripheral neuropathy | 0 | – | 0 | – | 0.99 |
| Total: | 13 | – | 6 | – | 0.12 | |
| Nonrash AEs: | 8 | – | 6 | – | 0.77 |
CTCAE, common terminology criteria for adverse events v4.03. Mild to moderate = Grades 1–2; Serious = Grades 3–5.
Fisher's exact test.
URI, upper respiratory tract infection, common cold. Infusions occurred October–February.
In 7‐year‐old after pizza and slushee consumption after playing youth league basketball.
In a 6‐year‐old after a car ride.
In a 5‐ and 14‐year‐old intermixed with periods of calm focus in first week (the 14‐year‐old) or first 3 weeks (the 5‐year‐old).
Six weeks after the infusion, after several days of a URI and fasting before lunch. Hypoglycemia was asymptomatic and corrected after a normal lunch.
Leukocytosis (12.2k WBC) occurred on the day of the saline infusion and preceded a URI.
In a 7‐year‐old briefly for a few days while sick with a cold. None of the events required medical intervention. No serious adverse events (SAEs) occurred in this study.
Figure 2.
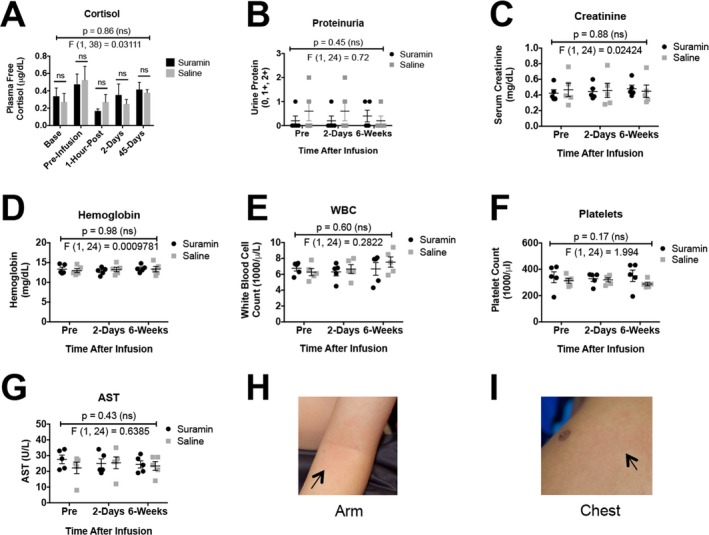
Safety monitoring. (A) Free cortisol, (B) proteinuria, (C) creatinine, (D) hemoglobin, (E) white blood cells (WBC), (F) platelets, (G) aspartate aminotransferase (AST), (H) rash – antecubital fossa, (I) chest. Data were analyzed by two‐way ANOVA to test for treatment, time, and treatment × time interaction effects. P and F values reflect the treatment effect. Only the rash was significantly different between suramin and placebo groups.
Pharmacokinetics
Pharmacokinetic analysis showed that at 1 h after intravenous infusion of 20 mg/kg (558 ± 41 mg/m2; mean ± SD; Table S1), the suramin concentration was 104 ± 11.6 μmol/L (Fig. 3A). The distribution phase half‐life was 7.4 ± 0.55 h. The suramin levels rapidly fell below 100 μmol/L and into the target range before day 2 in all subjects, with an average plasma level of suramin of 12.0 ± 1.5 μmol/L on day 2 (Fig. 3B, Table S1). Target concentrations of 1.5–15 μmol/L were maintained between 2 days and 6 weeks following the dose (Fig. 3). The steady‐state volume of distribution was 0.83 ± 0.014 L/kg (22.7 ± 2.6 L/m2). The clearance was 1.95 ± 0.21 mL/h/kg (0.056 ± 0.011 L/h/m2). The terminal elimination phase half‐life (t1/2) was 14.7 ± 1.4 days (Fig. 3B,D). A two‐compartment PK model showed excellent fit between measured and predicted plasma levels (r 2 = 0.998; Fig. 3C). These data are the first in the published literature on the pharmacokinetics of suramin in a pediatric population.
Figure 3.
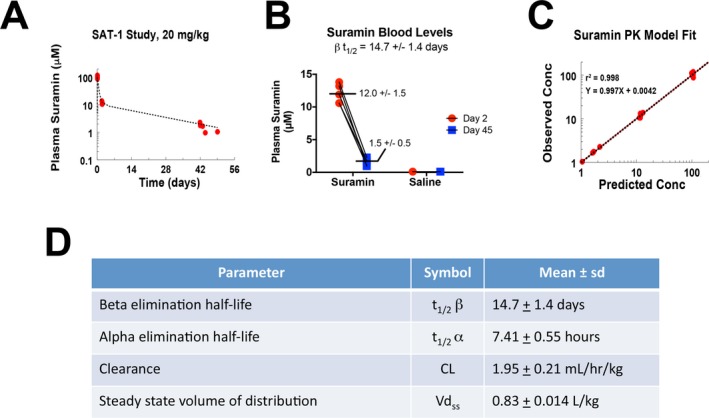
Pharmacokinetics of single‐dose suramin in children with autism spectrum disorders. (A) Two‐compartment model of suramin blood concentrations. The first 48 h were dominated by the distribution phase. Over 90% of the model is described by the elimination phase. (B) Plasma suramin concentrations. (C) A two‐compartment model correlated well with measured values. (D) Pediatric PK parameters of suramin.
Pharmacometabolomics
Targeted plasma metabolomics was performed immediately before infusion, at 2 days, and 6 weeks after the infusion. The rank order of the top 35 of 48 significant metabolites 6 weeks after suramin treatment is illustrated in Figure 4. The rank order after 2 days is illustrated in Figure S2. Consistent with our previously published work using mouse models, the metabolic effects of suramin resulted in a decrease of the cell danger response8 and restored more normal metabolism.12, 13 Purine metabolism was the single most changed pathway (Table 3, Table S2). Suramin increased healthy purines such as AICAR, which is an activator of the master metabolic regulator AMP‐dependent protein kinase (AMPK). 1‐Methyl‐adenine (1‐MA) was also increased. 1‐MA is derived from 1‐methyl‐adenosine, a recently recognized marker of new protein synthesis and cell growth. Suramin decreased other purines in the plasma such as cAMP and dGDP (Fig. 4, Tables S3 and S4). Improvements in 1‐carbon, folate, methionine, and cysteine metabolism were also found (Table 3, and Figure S3). Figure 5 illustrates the similarities found in the pharmacometabolomic response to suramin in MIA13 and Fragile X mouse models12 and in children with ASD in this study. Twenty‐one of the 28 (75%) pathways changed in ASD were also changed by suramin treatment in the mouse models of ASD (Fig. 5).
Figure 4.
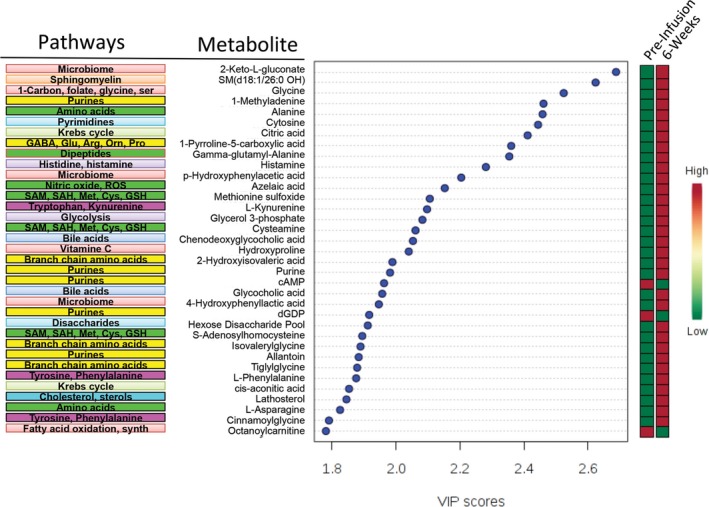
Suramin pharmacometabolomics. Rank order of metabolites and pathways that were changed by suramin at 6 weeks after treatment.
Table 3.
Suramin pharmacometabolomics: biochemical pathways changed at 6‐weeks
| No. | Pathway name | Measured metabolites in the pathway (N) | Expected pathway proportion (P = N/429) | Expected hits in sample of 48 (P × 48) | Observed hits in the top 48 metabolites | Fold enrichment (obs/exp) | Impact (sum VIP score) | Fraction of impact (VIP score) explained (% of 94.6) | Increased | Decreased |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Purine metabolism | 26 | 0.061 | 2.9 | 5 | 1.7 | 10.2 | 11% | 3 | 2 |
| 2 | SAM, SAH, methionine, cysteine, glutathione | 15 | 0.035 | 1.7 | 5 | 3.0 | 9.5 | 10% | 5 | 0 |
| 3 | Microbiome metabolism | 18 | 0.042 | 2.0 | 4 | 2.0 | 8.4 | 9% | 4 | 0 |
| 4 | Branch chain amino acid metabolism | 12 | 0.028 | 1.3 | 4 | 3.0 | 7.4 | 8% | 4 | 0 |
| 5 | Bile acid metabolism | 6 | 0.014 | 0.7 | 3 | 4.5 | 5.7 | 6% | 3 | 0 |
| 6 | Fatty acid oxidation and synthesis | 37 | 0.086 | 4.1 | 3 | 0.7 | 5.0 | 5% | 0 | 3 |
| 7 | Amino acid metabolism (alanine) | 4 | 0.009 | 0.4 | 2 | 4.5 | 4.3 | 5% | 2 | 0 |
| 8 | Krebs cycle | 9 | 0.021 | 1.0 | 2 | 2.0 | 4.3 | 5% | 2 | 0 |
| 9 | Pyrimidine metabolism | 9 | 0.021 | 1.0 | 2 | 2.0 | 4.2 | 4% | 2 | 0 |
| 10 | Sphingomyelin metabolism | 36 | 0.084 | 4.0 | 2 | 0.5 | 4.1 | 4% | 2 | 0 |
| 11 | 1‐Carbon, folate, formate, glycine, serine | 5 | 0.012 | 0.6 | 2 | 3.6 | 4.0 | 4% | 2 | 0 |
| 12 | GABA, glutamate, arginine, ornithine, proline | 6 | 0.014 | 0.7 | 2 | 3.0 | 3.9 | 4% | 2 | 0 |
| 13 | Tyrosine and phenylalanine metabolism | 3 | 0.007 | 0.3 | 2 | 6.0 | 3.7 | 4% | 2 | 0 |
| 14 | Cholesterol, cortisol, nongonadal steroid | 16 | 0.037 | 1.8 | 2 | 1.1 | 3.5 | 4% | 2 | 0 |
| 15 | Gamma‐glutamyl and other dipeptides | 2 | 0.005 | 0.2 | 1 | 4.5 | 2.4 | 2% | 1 | 0 |
| 16 | Histidine, histamine, carnosine metabolism | 4 | 0.009 | 0.4 | 1 | 2.2 | 2.3 | 2% | 1 | 0 |
| 17 | Nitric oxide, superoxide, peroxide metabolism | 2 | 0.005 | 0.2 | 1 | 4.5 | 2.2 | 2% | 1 | 0 |
| 18 | Tryptophan, kynurenine, serotonin, melatonin | 6 | 0.014 | 0.7 | 1 | 1.5 | 2.1 | 2% | 1 | 0 |
| 19 | Glycolysis and gluconeogenesis metabolism | 7 | 0.016 | 0.8 | 1 | 1.3 | 2.1 | 2% | 1 | 0 |
| 20 | Vitamin C (ascorbate) metabolism | 2 | 0.005 | 0.2 | 1 | 4.5 | 2.0 | 2% | 1 | 0 |
| 21 | Amino‐sugar, hexose metabolism | 5 | 0.012 | 0.6 | 1 | 1.8 | 1.9 | 2% | 1 | 0 |
| 22 | Phospholipid metabolism | 73 | 0.170 | 8.2 | 1 | 0.1 | 1.6 | 2% | 0 | 1 |
| Subtotal: | 42 | 6 | ||||||||
| Total: | 48 | |||||||||
Figure 5.
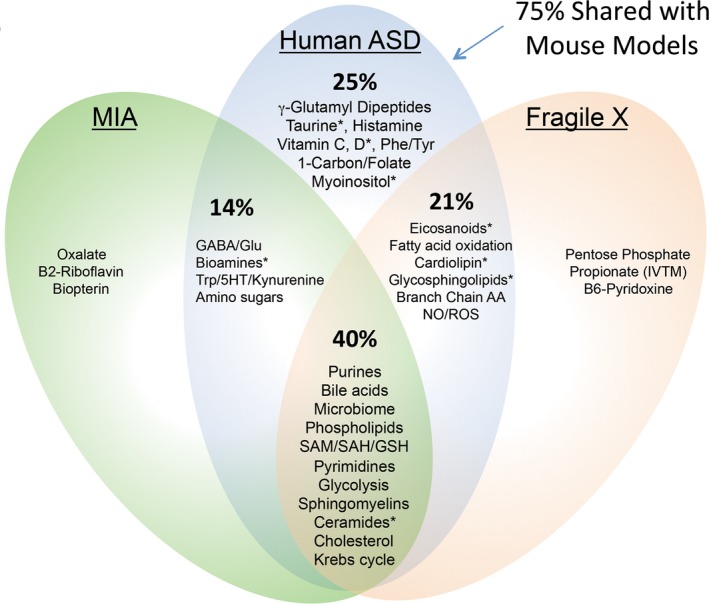
Shared biochemical pathways. 75% of the pathways that were altered by suramin in children with ASD were also altered in the mouse models. Asterisks (*) indicate pathways that were changed at 2 days, but not at 6 weeks after treatment.
Outcomes
The primary outcome measures were ADOS‐2 and Expressive One‐Word Picture Vocabulary (EOWPVT) scores (Table 4). Parents reported that after suramin treatment, the rate of language, social, behavioral, and developmental improvements continued to increase for 3 weeks, then gradually decreased toward baseline over the next 3 weeks. The blood levels of suramin at 3 weeks were estimated to be 4.2 ± 0.5 μmol/L using our PK model. ADOS‐2 comparison scores at 6 weeks improved by an average of −1.6 ± 0.55 points (mean ± SD; n = 5; 95% CI = −2.3 to −0.9; Cohen's d = 2.9; P = 0.0028) in the suramin treatment group and did not change in the saline group. We calculated P values by both parametric and nonparametric methods (Table 4). The mean ADOS comparison score in the suramin‐treated group was 8.6 ± 0.4 at baseline and 7.0 ± 0.3 at 6 weeks. Two‐way ANOVA of ADOS scores of suramin and placebo groups measured at baseline and at 6 weeks were also significant (treatment × time interaction F(1, 8) = 12.0; P = 0.0085; Figure S4A). ADOS scores were not changed in the saline‐treated group (Table 4). EOWPVT scores did not change (Table 4). Several secondary outcome measures also showed improvements. These included improvements in ABC, ATEC, and CGI scores (Table 4). The Repetitive Behavior Questionnaire (RBQ) scores did not capture a change.
Table 4.
Outcomes
| Outcome | Suramin | Placebo | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | Factor or behavior | Time after treatment (days) | Difference from baseline (mean ± SD) | 95% CI | d a | N | P b | P c | Difference from baseline (mean ± SD) | 95% CI | d a | N | P b | P c |
| Primary outcomes | ||||||||||||||
| ADOS‐2 | Comparison | 45 | −1.6 ± 0.55 | −2.3 to −0.9 | 2.9 | 5 | 0.0028 | 0.038 | −0.4 ± 0.55 | −1.1 to +0.28 | 0.7 | 5 | 0.18 | 0.16 |
| Raw | 45 | −4.6 ± 1.9 | −7.0 to −2.2 | 2.4 | 5 | 0.0062 | 0.039 | −0.4 ± 1.8 | −2.7 to +1.9 | 0.22 | 5 | 0.65 | 0.58 | |
| Social | 45 | −3.2 ± 1.9 | −5.6 to −0.8 | 1.7 | 5 | 0.020 | 0.043 | 0.0 ± 1.7 | −2.2 to +2.2 | 0 | 5 | 0.99 | 0.71 | |
| Restr/Rep | 45 | −1.4 ± 0.89 | −2.5 to −0.29 | 1.6 | 5 | 0.025 | 0.059 | −0.4 ± 2.1 | −3.0 to +2.2 | 0.19 | 5 | 0.69 | 0.58 | |
| EOWPVT | Vocabulary | 45 | −4.2 ± 8.3 | −14.5 to +6.1 | −0.51 | 5 | 0.32 | 0.50 | +2.0 ± 4.6 | −3.8 to +7.8 | 0.43 | 5 | 0.39 | 0.50 |
| Secondary outcomes | ||||||||||||||
| ABC | Stereotypy | 7 | −3.6 ± 2.1 | −6.2 to −1.0 | 1.7 | 5 | 0.018 | 0.043 | +0.4 ± 1.9 | −2.0 to +2.8 | −0.21 | 5 | 0.67 | 0.68 |
| Stereotypy | 45 | −4.0 ± 2.3 | −6.9 to −1.1 | 1.7 | 5 | 0.019 | 0.042 | +1.0 ± 4.3 | −4.3 to +6.3 | −0.23 | 5 | 0.63 | 0.69 | |
| ATEC | Total | 7 | −10 ± 7.7 | −20 to −0.46 | 1.3 | 5 | 0.044 | 0.043 | +7.2 ± 14 | −10 to +25 | −0.51 | 5 | 0.32 | 0.35 |
| Language | 7 | −2.2 ± 1.5 | −4.0 to −0.36 | 1.4 | 5 | 0.021 | 0.059 | 0.0 ± 4.1 | −5.0 to +5.0 | 0 | 5 | 0.99 | 0.89 | |
| Sociability | 7 | −3.6 ± 2.6 | −6.8 to −0.36 | 1.4 | 5 | 0.025 | 0.063 | −0.8 ± 2.8 | −4.3 to +2.6 | 0.29 | 5 | 0.55 | 0.58 | |
| Language | 45 | −2.0 ± 1.4 | −2.7 to −0.49 | 1.4 | 5 | 0.034 | 0.059 | −0.2 ± 2.9 | −3.8 to +3.4 | 0.07 | 5 | 0.88 | 0.79 | |
| CGI | Overall ASD | 45 | −1.8 ± 1.04 | −3.4 to −0.15 | 1.7 | 5 | 0.05 | n/a | 0.0 ± 0.34 | −0.55 to +0.55 | 0 | 5 | 0.99 | n/a |
| E. Language | 45 | −2.0 ± 1.04 | −3.6 to −0.35 | 1.9 | 5 | 0.01 | n/a | 0.0 ± 0.34 | −0.55 to +0.55 | 0 | 5 | 0.99 | n/a | |
| Social Inter. | 45 | −2.0 ± 1.04 | −3.6 to −0.35 | 1.9 | 5 | 0.01 | n/a | 0.0 ± 0.34 | −0.55 to +0.55 | 0 | 5 | 0.99 | n/a | |
| RBQ | Total | 45 | −3.2 ± 5.8 | −10.4 to +4.0 | 0.55 | 5 | 0.28 | 0.22 | −0.8 ± 3.3 | −4.9 to 3.3 | 0.24 | 5 | 0.62 | 0.47 |
ADOS‐2, autism diagnostic observation schedule, 2nd edition; EOWPVT, Expressive One‐Word Picture Vocabulary Test; ABC, aberrant behavior checklist; ATEC, autism treatment evaluation checklist; CGI, clinical global impression survey; RBQ, repetitive behavior questionnaire; Restr/Rep, restricted or repetitive behaviors; Overall ASD Sx, overall ASD symptoms; E. Language, expressive language; Social Inter., social interaction. Analysis . ADOS, EOWPVT, ABC, ATEC, and RBQ scores were analyzed by paired analysis before and after treatment using each subject as their own control. CGI was analyzed by two‐way ANOVA (symptom × time before and after treatment) with post hoc correction. Nonparametric P values were not calculated (n/a). Interpretation . ADOS, ABC, ATEC, CGI, and RBQ are severity scores; negative differences from baseline reflect decreased severity, that is, improvement. EOWPVT is a performance score; negative differences reflect a decrease.
A positive Cohen's d reflects improvement, and a negative d reflects a decrease by convention. Cohen's d is likely an overestimate of the actual treatment effect based on the large mean differences and small standard deviations found before and after treatment in this small study.
P value from parametric paired t‐test analysis.
P value from nonparametric paired Wilcoxon signed‐rank sum analysis.
Discussion
The aim of the SAT‐1 trial was to test the safety, pharmacokinetics, and pharmacodynamics of low‐dose suramin in children with ASD. A self‐limited rash was seen, but no serious adverse events occurred. Pharmacometabolomic analysis showed that the pathways changed by suramin treatment in ASD were previously known mediators of the cell danger response (CDR)8 and that purine metabolism was changed most. Seventy‐five percent of the pathways changed by suramin in children with ASD were also changed by suramin in mouse models.12, 13, 14
Safety
Suramin has been used safely for nearly a century to treat both children and adults with African sleeping sickness. Although side effects occurred occasionally, these could be minimized by attention to patient nutritional status, proper dose, administration procedures, and measured blood levels of suramin.46 The low dose of suramin used in this study produced blood levels of 1.5–15 μmol/L for 6 weeks. Previous studies have never examined the side‐effect profile of suramin in this low‐dose range. The side‐effect profile of high‐dose suramin (150–270 μmol/L) is known from cancer chemotherapy studies.32 The side‐effect profile from medium‐dose suramin (50–100 μmol/L) is known from African sleeping sickness studies.46 However, the side‐effect profile of low‐dose suramin (5–15 μmol/L) used for antipurinergic therapy (APT) in autism is unknown. Low‐dose suramin was found to be safe in five children with ASD, ages 5–14 years, in this study.
Study limitations
Limitations of the SAT‐1 study included its small size and the suboptimal timing of the outcome measurements. Parents reported that the rate of new behavioral and developmental improvements continued to increase for the first 3 weeks after the single dose of suramin, as blood levels of suramin fell from 12 to 4 μmol/L, then gradually decreased toward baseline over the next 3 weeks, as blood levels fell further from 4 to 1.5 μmol/L. This pattern of response suggested a threshold effect at about 4 μmol/L that could not have been predicted on the basis of what was known about suramin before this study, and outcomes were not measured at 3 weeks.
Another potential limitation of the trial was the self‐limited rash. The rash was asymptomatic and resolved spontaneously in a few days. In theory, the rash may have biased parents in a way that caused them to either improve their scores on the ABC, ATEC, RBQ, and CGI, or to report more side‐effects or adverse behaviors at both the 7‐day and 6‐week reports. Examiner‐based ADOS scoring was more resistant to this potential bias, since the rash was not visible on exposed skin to the outcome examiners at any time. However, a design limitation of the study was that one of the two ADOS examiners was also assigned to conduct scripted phone interviews with the families, and might have been susceptible to unconscious bias even though the study remained blinded and the rash preceded any significant examiner‐based outcomes by one and a half months.
Another potential weakness of this study was that ADOS scoring was not designed to be, and is not typically used as, a repeated measure of outcomes in autism treatment studies. This has occurred historically for two counterbalancing reasons: (1) because it is generally believed that ADOS scores are diagnostic and are not sensitive to change once the diagnosis is established, and (2) because training effects have the potential to produce improvements that are artifactual. With regard to the first point, under the right circumstances ADOS scores can be sensitive to change and have recently been used successfully as an outcome measure in a large autism treatment study.47 With regard to the second point, if training effects occurred, they were asymmetric, since improvements were only observed in the suramin treatment group and were not observed in the placebo group (Table 4).
Psychopharmacology
Suramin has objective central nervous system (CNS) effects in animal models12, 13, 14 and children with autism despite being unable to penetrate the blood–brain barrier.48 Suramin also has a number of peripheral effects on innate immunity, metabolism, pain, gut, autonomic, inflammatory, and other pathways regulated by purinergic signaling that may contribute to the beneficial effects observed.8, 23 Previous studies have shown that suramin is taken up into the CNS at the level of the brainstem, although not appreciably into the cerebrum or cerebellum.13 There are eight circumventricular organs (CVOs) in the brain that contain neurons that lack a blood–brain barrier.49 The area postrema in the brainstem is one of these CVOs that monitors the chemistry of the blood and transduces this information to higher centers in the brain for neuroendocrine, affective, cognitive, and behavioral integration. Rather than being a disadvantage, the peripheral actions and indirect CNS effects of suramin may have certain advantages by minimizing the risk of CNS toxicity. While new antipurinergic drugs (APDs) may soon be developed that can pass the blood–brain barrier, this appears not to be required to produce the behavioral effects of suramin in ASD.
Conclusions
The SAT‐1 trial examined the effects of low‐dose suramin or placebo in 10 children with autism spectrum disorder. No safety concerns were found. A two‐compartment pharmacokinetic model permitted accurate forecasting of plasma drug levels from 1 h to 6 weeks after the infusion. Metabolomic studies confirmed the importance of the cell danger response (CDR)8 and purinergic signaling.12, 13, 14 A single intravenous dose of suramin was associated with improved scores for language, social interaction, and decreased restricted or repetitive behaviors measured by ADOS, ABC, ATEC, and CGI scores. None of these improvements occurred in the five children who received placebo. The generalizability of these findings is unknown. Future studies will be needed to confirm these findings in larger numbers of children with ASD, and to evaluate whether a few doses of suramin given over a few months are safe and might facilitate continued improvements.
Special note from the authors
Suramin is not approved for the treatment of autism. Like many intravenous drugs, when administered improperly by untrained personnel, at the wrong dose and schedule, without careful measurement of drug levels and monitoring for toxicity, suramin can cause harm. Careful clinical trials will be needed over several years at several sites to learn how to use low‐dose suramin safely in autism, and to identify drug–drug interactions and rare side effects that cannot currently be predicted. We strongly caution against the unauthorized use of suramin.
Author Contributions
Dr. Robert Naviaux raised the funding, obtained the regulatory approvals, conceived, designed, and directed the trial, analyzed the data, prepared the figures, and wrote the manuscript. Dr. Curtis, Dr. Westerfield, and Ms. Mash performed the neurodevelopmental testing, provided clinical coordination, and edited the manuscript. Dr. Reiner helped design the study, coordinated patient infusions and clinical care, and edited the manuscript. Dr. Li, Dr. Jane Naviaux, and Dr. Wang performed the metabolomic and pharmacokinetic analysis, analyzed the data, prepared the figures, and wrote parts of the manuscript. Dr. Jain and Ms. He helped design the study, prepared the randomization key, performed biostatistical analyses, and edited the manuscript. Dr. Bright directed the data compilation, integrity, and completeness analysis, provided independent biostatistical analysis, and edited the manuscript. Dr. Goh helped design the study, performed neurologic examinations, and edited the manuscript. Dr. Alaynick helped design the study and edited the manuscript. Dr. Capparelli analyzed the pharmacokinetic data, prepared the figures, and wrote parts of the manuscript. Dr. Sun and Ms. Adams provided investigational pharmacy support, implemented the clinical mask, and edited the manuscript. Ms. Arellano provided clinical coordination and edited the manuscript. Dr. Chukoskie helped design the study, analyzed the data, critically reviewed and edited the manuscript. Dr. Lincoln and Dr. Townsend helped design the study, directed the neurodevelopmental studies, wrote and edited the manuscript.
Conflict of Interest
RKN has filed a provisional patent application related to antipurinergic therapy of autism and related disorders and is a scientific advisory board member for the Autism Research Institute and the Open Medicine Foundation. EVC is a DSMB member for Cempra Pharmaceuticals and The Medicines Company, and a consultant for Alexion. SG is co‐owner of MitoMedical. The other authors declare no conflicts of interest.
Supporting information
Table S1. Single‐dose suramin pharmacokinetics.
Table S2. Suramin pharmacometabolomics. Pathways changed at 2 days.
Table S3. Suramin pharmacometabolomics. Metabolites changed at 2 days.
Table S4. Suramin pharmacometabolomics. Metabolites changed at 6 weeks.
Figure S1. Storyboard illustration of each step of the infusion day visit.
Figure S2. Suramin pharmacometabolomics. Rank order of metabolites and pathways that were changed by suramin at 2 days after treatment.
Figure S3. Suramin pharmacometabolomics pathway visualization. (A) After 2 days. (B) After 6 weeks. Metabolites indicated in red were increased, and those in green were decreased compared to controls (see z‐score scale in upper right).
Figure S4. Outcomes. (A) 6 Weeks ADOS comparison scores by two‐way ANOVA. (B) 6 Weeks ADOS comparison score improvement after suramin. (C) 6 Weeks ADOS social affect score improvement after suramin. (D) 6 Weeks ADOS restricted and repetitive behavior score improvement after suramin. (E) 2 days ADOS comparison scores were not changed. (F) no change in 6 weeks ADOS scores in subjects receiving saline placebo. (G) no change in 6 weeks ADOS social affect scores in subjects receiving placebo. (H) no change in 6 weeks ADOS restricted and repetitive behavior scores in subjects receiving placebo. (I) no change in 6 weeks Expressive One‐Word Picture Vocabulary scores. (J) 7‐day improvement in ABC stereotypy scores after suramin. (K) 6‐week Improvement in ABC stereotypy scores after suramin. (L) 7‐day Improvement in ATEC total scores after suramin. (M) no change in 6 weeks EOWPVT scores after saline. (N) no change in 7 days ABC stereotypy scores after saline. (O) no change in 6 weeks ABC stereotypy scores after saline. (P) no change in 7 days ATEC total scores after saline. (Q) improved ATEC speech, language, and communication scores 7 days after suramin. (R) improved ATEC sociability scores 7 days after suramin. (S) improved ATEC speech, language, and communication scores 6 weeks after suramin. (T) improved ADOS comparison scores after dropping a subject who missed the 6‐week visit (N = 4). (U) no change in 7 days ATEC speech, language, and communication after saline. (V) no change in 7 days ATEC sociability after saline. (W) no change in 6 weeks ATEC speech, language, and communication scores 6 weeks after saline (X) no change in EOWPVT scores after dropping subject who missed the 6‐week visit (N = 4). (Y) no change in 2 days ADOS scores after suramin. (Z) no change in 6 weeks RBQ total scores after suramin. (aa) improved core symptoms of ASD and other behaviors by CGI at 6 weeks after suramin. P values: *0.05, **0.01, ***0.001. (bb) Top 3, most changed symptoms named by parents in the 6‐week CGI. (cc) no change in 2 days ADOS scores after saline. (dd) no change in 6 weeks RBQ total scores after saline.
Data S1. Clinical Global Impression (CGI) questionnaire.
Data S2. Social Stories to Accompany the Storyboard Panels Describing Each Step of the Infusion Day Visit.
Acknowledgments
RKN thanks the patients and families who gave their time and effort in helping to make this study possible. We thank Dr. Richard Haas, Dr. Doris Trauner, and Dr. Stephen Edelson for their advice in planning the study. We thank Dr. Judy S. Reilly for critical reading of the manuscript and suggestions for improvements. RKN also thanks Jonathan Monk for assistance with the Cytoscape visualizations, Marlene Samano and Nicole Suarez, and Maeve Taaffe, Lee Vowinkel, Dennis Perpetua, Jessica Nasca, Peewee Buquing, and Patricia Moraes for their expert clinical assistance at the UCSD Clinical Translational Research Institute, and Thaine Ross and Melinda Stafford for their expert assistance in the Investigational Pharmacy. RKN extends a special thanks to graphic artists Suzanne Parlett and Qamdyn Hale for help in creating the storyboards used in the study.
References
- 1. Zablotsky B, Black LI, Maenner MJ, et al. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health interview survey. Natl Health Stat Report 2015;13:1–20. [PubMed] [Google Scholar]
- 2. Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years–Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ 2016;65:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinto D, Pagnamenta AT, Klei L, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010;466:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richards C, Jones C, Groves L, et al. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta‐analysis. Lancet Psychiatry 2015;2:909–916. [DOI] [PubMed] [Google Scholar]
- 5. Talkowski ME, Minikel EV, Gusella JF. Autism spectrum disorder genetics: diverse genes with diverse clinical outcomes. Harv Rev Psychiatry 2014;22:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr Probl Pediatr Adolesc Health Care 2014;44:277–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zerbo O, Iosif AM, Walker C, et al. Is Maternal Influenza or Fever During Pregnancy Associated with Autism or Developmental Delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) Study. J Autism Dev Disord 2013;43(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naviaux RK. Metabolic features of the cell danger response. Mitochondrion 2014;16:7–17. [DOI] [PubMed] [Google Scholar]
- 9. Silva JM, Wong A, Carelli V, Cortopassi GA. Inhibition of mitochondrial function induces an integrated stress response in oligodendroglia. Neurobiol Dis 2009;34:357–365. [DOI] [PubMed] [Google Scholar]
- 10. Nikkanen J, Forsstrom S, Euro L, et al. Mitochondrial DNA Replication Defects Disturb Cellular dNTP Pools and Remodel One‐Carbon Metabolism. Cell Metab 2016;23:635–648. [DOI] [PubMed] [Google Scholar]
- 11. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy‐inflammation‐cell death axis in organismal aging. Science 2011;333:1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naviaux JC, Wang L, Li K, et al. Antipurinergic therapy corrects the autism‐like features in the Fragile X (Fmr1 knockout) mouse model. Mol Autism 2015;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naviaux JC, Schuchbauer MA, Li K, et al. Reversal of autism‐like behaviors and metabolism in adult mice with single‐dose antipurinergic therapy. Transl Psychiat 2014;4:e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naviaux RK, Zolkipli‐Cunningham Z, Nakayama T, et al. Antipurinergic Therapy Corrects the Autism‐Like Features in the Poly(IC) Mouse Model. PLoS ONE 2013;8(3):e57380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wikoff WR, Kalisak E, Trauger S, et al. Response and recovery in the plasma metabolome tracks the acute LCMV‐induced immune response. J Proteome Res 2009;8:3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Degtyar E, Zusman T, Ehrlich M, Segal G. A Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is targeted to the host cell mitochondria. Cell Microbiol 2009;11:1219–1235. [DOI] [PubMed] [Google Scholar]
- 17. James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 2004;80:1611–1617. [DOI] [PubMed] [Google Scholar]
- 18. West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol 2011;11:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naviaux RK. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther 2012;342:608–618. [DOI] [PubMed] [Google Scholar]
- 20. Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett 2014;588:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal 2009;2:pe6. [DOI] [PubMed] [Google Scholar]
- 22. Riteau N, Baron L, Villeret B, et al. ATP release and purinergic signaling: a common pathway for particle‐mediated inflammasome activation. Cell Death Dis 2012;3:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burnstock G. The Paton Lecture: Purinergic signalling: from discovery to current developments. Exp Physiol 2014;99(1):16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imamura H, Nhat KP, Togawa H, et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer‐based genetically encoded indicators. Proc Natl Acad Sci USA 2009;106:15651–15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. G protein‐coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signalling 2012;8:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adams JB, Audhya T, McDonough‐Means S, et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab 2011;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jia M, Li MX, Fields RD, Nelson PG. Extracellular ATP in activity‐dependent remodeling of the neuromuscular junction. Dev Neurobiol 2007;67:924–932. [DOI] [PubMed] [Google Scholar]
- 28. Naviaux RK. Mitochondria and Autism In: Buxbaum JD, Hof PR, eds. The Neuroscience of Autism Spectrum Disorders. Waltham, MA: Academic Press, Elsevier, 2012:179–193. [Google Scholar]
- 29. Naviaux RK. Mitochondrial control of epigenetics. Cancer Biol Ther 2008;7:1191–1193. [DOI] [PubMed] [Google Scholar]
- 30. Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 2010;10:12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 2006;58:58–86. [DOI] [PubMed] [Google Scholar]
- 32. Stein CA. Suramin: a novel antineoplastic agent with multiple potential mechanisms of action. Can Res 1993;53(10 Suppl):2239–2248. [PubMed] [Google Scholar]
- 33. World Medical A . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 34. Schulz KF, Altman DG, Moher D, Group C . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord 2007;37:613–627. [DOI] [PubMed] [Google Scholar]
- 36. Grondhuis SN, Mulick JA. Comparison of the Leiter International Performance Scale‐Revised and the Stanford‐Binet Intelligence Scales, 5th Edition, in children with autism spectrum disorders. Am J Intellect Dev Disabil 2013;118:44–54. [DOI] [PubMed] [Google Scholar]
- 37. Adams‐Chapman I, Bann C, Carter SL, et al. Language outcomes among ELBW infants in early childhood. Early Hum Dev 2015;91:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaat AJ, Lecavalier L, Aman MG. Validity of the aberrant behavior checklist in children with autism spectrum disorder. J Autism Dev Disord 2014;44:1103–1116. [DOI] [PubMed] [Google Scholar]
- 39. Geier DA, Kern JK, Geier MR. A Comparison of the Autism Treatment Evaluation Checklist (ATEC) and the Childhood Autism Rating Scale (CARS) for the quantitative evaluation of autism. J Mental Health Res Intellect Disabil 2013;6:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rimland B, Edelson S. Autism treatment evaluation checklist. Autism Research Institute 2000. https://www.autism.com/ind_atec [cited 2016 June 15]. [Google Scholar]
- 41. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 42. Honey E, McConachie H, Turner M, Rodgers J. Validation of the repetitive behaviour questionnaire for use with children with autism spectrum disorder. Res Autism Spectr Disord 2012;6:355–364. [Google Scholar]
- 43. Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. II. Biexponential model and experimental pharmacokinetic data. J Pharmacokinet Biopharm 1981;9:635–651. [DOI] [PubMed] [Google Scholar]
- 44. Naviaux RK, Naviaux JC, Li K, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA 2016;113(37):E5472–E5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0‐making metabolomics more meaningful. Nucleic Acids Res 2015;43(W1):W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hawking F. Suramin: with special reference to onchocerciasis. Advances Pharmacol Chemother 1978;15:289–322. [DOI] [PubMed] [Google Scholar]
- 47. Pickles A, Le Couteur A, Leadbitter K, et al. Parent‐mediated social communication therapy for young children with autism (PACT): long‐term follow‐up of a randomised controlled trial. Lancet 2016;388:2501–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hawking F. Concentration of Bayer 205 (Germanin) in human blood and cerebrospinal fluid after treatment. Trans R Soc Trop Med Hyg 1940;34:37–52. [Google Scholar]
- 49. Siso S, Jeffrey M, Gonzalez L. Sensory circumventricular organs in health and disease. Acta Neuropathol 2010;120:689–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Single‐dose suramin pharmacokinetics.
Table S2. Suramin pharmacometabolomics. Pathways changed at 2 days.
Table S3. Suramin pharmacometabolomics. Metabolites changed at 2 days.
Table S4. Suramin pharmacometabolomics. Metabolites changed at 6 weeks.
Figure S1. Storyboard illustration of each step of the infusion day visit.
Figure S2. Suramin pharmacometabolomics. Rank order of metabolites and pathways that were changed by suramin at 2 days after treatment.
Figure S3. Suramin pharmacometabolomics pathway visualization. (A) After 2 days. (B) After 6 weeks. Metabolites indicated in red were increased, and those in green were decreased compared to controls (see z‐score scale in upper right).
Figure S4. Outcomes. (A) 6 Weeks ADOS comparison scores by two‐way ANOVA. (B) 6 Weeks ADOS comparison score improvement after suramin. (C) 6 Weeks ADOS social affect score improvement after suramin. (D) 6 Weeks ADOS restricted and repetitive behavior score improvement after suramin. (E) 2 days ADOS comparison scores were not changed. (F) no change in 6 weeks ADOS scores in subjects receiving saline placebo. (G) no change in 6 weeks ADOS social affect scores in subjects receiving placebo. (H) no change in 6 weeks ADOS restricted and repetitive behavior scores in subjects receiving placebo. (I) no change in 6 weeks Expressive One‐Word Picture Vocabulary scores. (J) 7‐day improvement in ABC stereotypy scores after suramin. (K) 6‐week Improvement in ABC stereotypy scores after suramin. (L) 7‐day Improvement in ATEC total scores after suramin. (M) no change in 6 weeks EOWPVT scores after saline. (N) no change in 7 days ABC stereotypy scores after saline. (O) no change in 6 weeks ABC stereotypy scores after saline. (P) no change in 7 days ATEC total scores after saline. (Q) improved ATEC speech, language, and communication scores 7 days after suramin. (R) improved ATEC sociability scores 7 days after suramin. (S) improved ATEC speech, language, and communication scores 6 weeks after suramin. (T) improved ADOS comparison scores after dropping a subject who missed the 6‐week visit (N = 4). (U) no change in 7 days ATEC speech, language, and communication after saline. (V) no change in 7 days ATEC sociability after saline. (W) no change in 6 weeks ATEC speech, language, and communication scores 6 weeks after saline (X) no change in EOWPVT scores after dropping subject who missed the 6‐week visit (N = 4). (Y) no change in 2 days ADOS scores after suramin. (Z) no change in 6 weeks RBQ total scores after suramin. (aa) improved core symptoms of ASD and other behaviors by CGI at 6 weeks after suramin. P values: *0.05, **0.01, ***0.001. (bb) Top 3, most changed symptoms named by parents in the 6‐week CGI. (cc) no change in 2 days ADOS scores after saline. (dd) no change in 6 weeks RBQ total scores after saline.
Data S1. Clinical Global Impression (CGI) questionnaire.
Data S2. Social Stories to Accompany the Storyboard Panels Describing Each Step of the Infusion Day Visit.


