Abstract
Objectives
Using published, nationally-representative estimates, we calculated the total number of perinatally HIV-exposed and HIV-infected infants born during 1978–2010, the number of perinatal HIV cases prevented by interventions designed for the prevention of mother-to-child transmission (PMTCT), and the number of infants exposed to antiretroviral (ARV) drugs during the prenatal and intrapartum periods.
Design
We calculated the number of infants exposed to ARV drugs since 1994, and the number of cases of mother-to-child HIV transmission prevented from 1994 to 2010 using published data. We generated confidence limits for our estimates by performing a simulation study.
Methods
Data were obtained from published, nationally-representative estimates from the Centers for Disease Control and Prevention. Model parameters included the annual numbers of HIV-infected pregnant women, the annual numbers of perinatally infected infants, the annual proportions of infants exposed to ARV drugs during the prenatal and intrapartum period and the estimated MTCT rate in the absence of preventive interventions. For the simulation study, model parameters were assigned distributions and we performed 1,000,000 repetitions.
Results
Between 1978 and 2010, an estimated 186,157 [95% confidence interval (CI): 185,312–187,003] HIV-exposed infants and approximately 21,003 (95% CI: 20,179–21,288) HIV-infected infants were born in the United States. Between 1994 and 2010, an estimated 124,342 (95% CI: 123,651–125,034) HIV-exposed infants were born in the US, and approximately 6083 (95% CI: 5931–6236) infants were perinatally infected with HIV. During this same period, about 100,207 (95% CI: 99,374–101,028) infants were prenatally exposed to ARV drugs. As a result of PMTCT interventions, an estimated 21,956 (95% CI: 20,191–23,759) MTCT HIV cases have been prevented in the United States since 1994.
Conclusion
Although continued vigilance is needed to eliminate mother-to-child HIV transmission, PMTCT interventions have prevented nearly 22,000 cases of perinatal HIV transmission in the United States since 1994.
Keywords: HIV, perinatal, antiretroviral, prevention of mother-to-child transmission
The first cases of perinatally acquired human immunodeficiency virus 1 (HIV) in the United States probably occurred in 1978.1 In 1994, the United States Public Health Service Task Force (USPHS) recommended the use of zidovudine to reduce perinatal transmission of HIV,2 based on clinical trial results.3 In subsequent years, other interventions for prevention of mother-to-child transmission (PMTCT) were recommended, including use of combination antiretroviral (ARV) treatment in 1997,4 and elective cesarean section delivery for women with HIV viral loads greater than 1000 copies/mL.5 The recommendation of opt-out HIV testing for pregnant women was introduced in 19996 with the goal of increasing the proportion of women whose HIV status was known before delivery, allowing more HIV-infected pregnant women to receive preventive interventions during pregnancy.7 Avoidance of breastfeeding by HIV-infected women has been recommended in high-resource settings since 19858; breastfeeding by HIV-infected women has been estimated to increase the risk of mother-to-child HIV transmission by an additional 14%.9 The Enhanced Perinatal Surveillance system of the US Centers for Disease Control and Prevention (CDC) estimated the rate of MTCT in the United States as 1.7%–2.9% during 2005–2008,10,11 although by 2003 MTCT rates of less than 1% had been reported in a large European cohort.4
HIV case reporting to state surveillance systems has been conducted in some states since 1985,12 although confidential name-based HIV infection reporting was not implemented in all US states and territories until 2008.13 At present, perinatal HIV exposure reporting is conducted in only a subset of these jurisdictions. It has, therefore, been difficult to determine the annual numbers of HIV-infected women delivering infants and of perinatally infected infants.7 Reports of perinatally acquired AIDS cases have been the only data that have been consistently available from all US jurisdictions over time, from the early years of the epidemic. However, using these data to estimate the incidence of perinatal HIV infection is challenging and requires indirect estimation through statistical methods.1,14,15 Nevertheless, there have been several national estimates of the annual numbers of HIV-infected infants1,15–19 and the annual numbers of infants born to HIV-infected women (HIV-exposed infants), as well as national estimates for the annual proportions of HIV-infected pregnant women prescribed ARVs during the prenatal and intrapartum periods.7,10,20,21 Neither the annual numbers of perinatal HIV cases prevented by PMTCT interventions nor the annual numbers of infants perinatally exposed to ARV have previously been estimated for the entire United States. The latter figure is significant because it reflects the population of infants and children who may be at risk for toxicities from ARV use, prenatal ARV use in particular. Although several studies have indicated very low rates of birth defects or carcinogenicity,22–25 the possible increased risk of mitochondrial and other toxicities in infants and children who were perinatally exposed to ARV drugs26,27 warrants long-term follow-up of these infants.
The objectives of the present study were to use national estimates previously published by CDC to estimate the number of perinatally HIV-exposed and HIV-infected infants in the United States since the beginning of the HIV epidemic, the number of perinatal HIV infections prevented and the number of HIV-exposed infants perinatally exposed to ARVs in the United States during 1994–2010, a period of increasing ARV use.
MATERIALS AND METHODS
We identified data from several published sources (Table 1, columns 1b, 1f) for the annual numbers of HIV-infected pregnant women who delivered (column 1c) and the annual numbers of HIV-infected infants born (column 1g) for 1978–2010. When available, we used published point estimates or, if only ranges were given, the midpoints of those ranges, as the best estimates of these annual numbers. When data were not available, we linearly interpolated or extrapolated the available data to obtain estimated numbers for each year (columns 1d, 1h). For the years 2007–2010, data were not available on the numbers of births to HIV-infected women, so the 2006 estimate was carried forward to be conservative in our final estimate of the number of perinatal HIV infections averted. For 1978–1987, we estimated the annual numbers of HIV-infected women (column 1d) as 4.4346 times the annual numbers of HIV-infected infants estimated by Davis et al1 (column 1g), where 4.4346 = 1/0.2255 is the reciprocal of the HIV MTCT transmission rate in the absence of ARV prophylaxis, estimated from the placebo arm of the completed AIDS clinical trials group (ACTG) 076 trial.3 Note that 1978 is the first year in which HIV-infected infants were born in the United States, according the AIDS-based back-calculation.1
TABLE 1.
Estimated Numbers of Births to HIV-infected Women, HIV-infected Infants and Perinatal HIV Cases Prevented, United States, 1978–2010*
| Number (n) of Births to HIV-infected Women
|
Number (n) of HIV-infected Infants
|
Number (n) of Perinatal HIV Cases Prevented
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Publication
|
Estimate
|
Publication
|
Estimate
|
Estimate
|
||||||
| Year | Source | n | n | (95% CI) | Source | n | n | (95% CI) | n | (95% CI) |
|
|
|
|
|
|
|
|||||
| (1a) | (1b) | (1c) | (1d) | (1e) | (1f) | (1g) | (1h) | (1i) | (1j) | (1k) |
| 1978 | – | – | 310 | (276–345) | (1) | 70 | 70 | (54–87) | NA | NA |
| 1979 | – | – | 266 | (235–298) | (1) | 60 | 60 | (45–76) | NA | NA |
| 1980 | – | – | 532 | (487–578) | (1) | 120 | 120 | (99–142) | NA | NA |
| 1981 | – | – | 843 | (787–900) | (1) | 190 | 190 | (163–217) | NA | NA |
| 1982 | – | – | 1197 | (1130–1265) | (1) | 270 | 270 | (238–303) | NA | NA |
| 1983 | – | – | 1818 | (1735–1902) | (1) | 410 | 410 | (371–450) | NA | NA |
| 1984 | – | – | 2927 | (2821–3034) | (1) | 660 | 660 | (610–711) | NA | NA |
| 1985 | – | – | 3858 | (3737–3980) | (1) | 870 | 870 | (813–928) | NA | NA |
| 1986 | – | – | 4878 | (4742–5015) | (1) | 1100 | 1100 | (1035–1165) | NA | NA |
| 1987 | – | – | 6164 | (6011–6318) | (1) | 1390 | 1390 | (1317–1464) | NA | NA |
| 1988 | (1) | 5430 | 5430 | (5286–5575) | (1) | 1360 | 1360 | (1288–1433) | NA | NA |
| 1989 | (16) | 6368 | 6368 | (6212–6525) | (1) | 1590 | 1590 | (1512–1669) | NA | NA |
| 1990 | (16) | 6770 | 6770 | (6609–6932) | (1) | 1690 | 1690 | (1610–1771) | NA | NA |
| 1991 | (16) | 7042 | 7042 | (6878–7207) | (1) | 1760 | 1760 | (1678–1843) | NA | NA |
| 1992 | (16) | 6990 | 6990 | (6827–7154) | (1) | 1750 | 1750 | (1668–1832) | NA | NA |
| 1993 | (16) | 6422 | 6422 | (6265–6580) | (1) | 1630 | 1630 | (1551–1710) | NA | NA |
| 1994 | (16) | 6145 | 6145 | (5992–6299) | – | – | 1263 | (1194–1333) | 123 | (–226 to 503) |
| 1995 | (17) | 5797 | 5797 | (5648–5947) | (17) | 895 | 895 | (837–954) | 412 | (84–770) |
| 1996 | – | – | 5887 | (5737–6038) | (17) | 480 | 480 | (438–523) | 847 | (517–1209) |
| 1997 | – | – | 5978 | (5827–6130) | – | – | 441 | (400–483) | 907 | (572–1273) |
| 1998 | – | – | 6068 | (5916–6221) | – | – | 403 | (364–443) | 965 | (626–1337) |
| 1999 | – | – | 6159 | (6006–6313) | – | – | 364 | (327–402) | 1025 | (680–1401) |
| 2000 | (14, 32) | 6249 | 6249 | (6095–6404) | (14) | 325 | 325 | (290–361) | 1084 | (734–1467) |
| 2001 | – | – | 6737 | (6577–6898) | (18) | 277 | 277 | (245–310) | 1242 | (866–1653) |
| 2002 | – | – | 7224 | (7058–7391) | (18) | 204 | 204 | (176–232) | 1425 | (1023–1864) |
| 2003 | – | – | 7712 | (7540–7885) | (18) | 167 | 167 | (142–193) | 1572 | (1143–2041) |
| 2004 | – | – | 8199 | (8022–8377) | (18) | 138 | 138 | (115–161) | 1711 | (1257–2209) |
| 2005 | (46) | 8687 | 8687 | (8505–8870) | (46) | 244 | 244 | (214–275) | 1715 | (1233–2242) |
| 2006 | (32) | 8700 | 8700 | (8518–8883) | (15) | 183 | 183 | (157–210) | 1779 | (1296–2306) |
| 2007 | – | – | 8700 | (8518–8883) | (15) | 225 | 225 | (196–255) | 1737 | (1255–2264) |
| 2008 | – | – | 8700 | (8518–8883) | (15) | 172 | 172 | (147–198) | 1790 | (1308–2316) |
| 2009 | – | – | 8700 | (8518–8883) | (15) | 151 | 151 | (127–176) | 1811 | (1329–2338) |
| 2010 | – | – | 8700 | (8518–8883) | – | – | 151 | (127–176) | 1811 | (1328–2338) |
| 1994–2010† | – | – | 124,342 | (123,651–125,034) | – | – | 6083 | (5931–6236) | 21,956 | (20,191–23,759) |
| 1978–2010 | – | – | 186,157 | (185,312–187,003) | – | – | 21,003 | (20,719–212,88) | – | – |
The columns in this table are: (1a) year; (1b) published source and (1c) estimate of the no. of HIV-infected pregnant women; (1d) point estimate and (1e) 95% confidence interval (CI) for the no. of HIV-infected pregnant women; (1f) published source and (1g) estimate of the no. of HIV-infected infants; (1h) point estimate and (1i) 95% CI for the no. of HIV-infected infants; (1j) point estimate and (1k) 95% CI for the no. perinatal HIV cases prevented. Symbol: number (n).
Antiretroviral prophylaxis era.
NA indicates not applicable.
In the absence of variance estimates, we assumed that the estimated annual numbers (columns 1d, 1h) represented count data and calculated exact 95% confidence intervals (CIs) using the Poisson distribution (columns 1e, 1i). We then estimated the annual numbers of HIV MTCT cases that would have occurred in the absence of ARVs as the product of the annual numbers of HIV-infected women (column 1d) times the HIV MTCT rate of 0.2255.3 In turn, we estimated the annual numbers of perinatal HIV cases prevented (column 1j) after the introduction of ARV prophylaxis in 1994 as the differences between the annual numbers of HIV MTCT cases that would have occurred in the absence of ARVs and the annual numbers of HIV-infected infants born each year (column 1h).
We performed a simulation study with 1,000,000 repetitions to obtain 95% CIs for the annual numbers of perinatal HIV cases prevented (column 1k) for 1994–2010. All simulations were performed using SAS software, version 9.3 (SAS Institute, Inc, Cary, NC). First, for each repetition, we simulated the annual numbers of HIV-infected women from Poisson distributions with mean parameters equal to (column 1d). Second, we simulated the annual numbers of HIV-infected infants from Poisson distributions with mean parameters equal to (column 1h). Third, we simulated the HIV MTCT rate as a β variable with parameters 46 and 158, corresponding to the number of transmissions and nontransmissions observed in the placebo arm of the ACTG 076 trial.3 Fourth, we simulated the annual numbers of HIV MTCT cases that would have occurred in the absence of ARVs as binomial variables with proportion parameters equal to the simulated HIV MTCT rate and sizes equal to the simulated numbers of HIV-infected women. Fifth, we computed the annual numbers of perinatal HIV cases since the 1994 USPHS recommendations to use zidovudine to reduce perinatal HIV transmission as the differences between the simulated annual numbers of HIV MTCT cases that would have occurred in the absence of ARVs and the simulated annual numbers of HIV-infected infants. Finally, we computed the 0.025 and the 0.975 percentiles over all 1,000,000 repetitions to obtain the bounds of the 95% CIs for the annual numbers of perinatal HIV cases prevented (column 1k).
We also identified data from several published sources (Table 2, column 2b) for the annual numbers of infants exposed to prenatal and intrapartum ARVs (columns 2c, 2i) among a sample of infants born to HIV-infected women (columns 2d, 2j) for 1994–2010. For 1994–1998, we obtained the unpublished data used to create the figures in the source paper.28 We calculated the annual raw proportions of infants exposed to prenatal ARVs (column 2e) as (column 2c) divided by (column 2d) and the annual raw proportions of infants exposed to intrapartum ARVs (column 2k) as (column 2i) divided by (column 2j).
TABLE 2.
Estimated Numbers of Infants Exposed to Prenatal and Intrapartum ARVs, United States, 1994–2010*
| Year | Source | Sample Number (n), Total (N), Raw Proportion (p) and Smoothed Proportion of Infants Exposed to Prenatal ARVs | Estimated Number (n) of Infants Exposed to Prenatal ARVs | Sample Number (n), Total (N), Raw Proportion (p) and Smoothed Proportion of Infants Exposed to Intrapartum ARVs | Estimated Number (n) of Infants Exposed to Intrapartum ARVs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| n | N | p |
|
n | (95% CI) | n | N | p |
|
N | (95% CI) | ||||
|
| |||||||||||||||
| (2a) | (2b) | (2c) | (2d) | (2e) | (2f) | (2g) | (2h) | (2i) | (2j) | (2k) | (2l) | (2m) | (2n) | ||
| 1994 | (30)† | 82 | 153 | 0.536 | 0.536 | 3293 | (2794–3784) | 49 | 153 | 0.320 | 0.320 | 1968 | (1537–2456) | ||
| 1995 | (30)† | 186 | 252 | 0.738 | 0.751 | 4353 | (4194–4510) | 150 | 252 | 0.595 | 0.629 | 3649 | (3397–3895) | ||
| 1996 | (30)† | 200 | 245 | 0.816 | 0.761 | 4477 | (4323–4631) | 175 | 245 | 0.714 | 0.667 | 3929 | (3727–4128) | ||
| 1997 | (30)† | 245 | 291 | 0.842 | 0.770 | 4603 | (4453–4753) | 218 | 291 | 0.749 | 0.701 | 4191 | (4023–4359) | ||
| 1998 | (30)† | 102 | 119 | 0.857 | 0.779 | 4728 | (4580–4875) | 87 | 119 | 0.731 | 0.731 | 4434 | (4283–4585) | ||
| 1999 | (20) | 2280 | 2970 | 0.768 | 0.788 | 4853 | (4708–4999) | 2190 | 2970 | 0.737 | 0.756 | 4659 | (4514–4804) | ||
| 2000 | (19) | 2730 | 3422 | 0.798 | 0.797 | 4978 | (4834–5123) | 1856 | 2355 | 0.788 | 0.779 | 4865 | (4720–5011) | ||
| 2001 | (19) | 2390 | 2963 | 0.807 | 0.805 | 5423 | (5274–5573) | 1812 | 2276 | 0.796 | 0.798 | 5373 | (5220–5526) | ||
| 2002 | (19) | 1627 | 1987 | 0.819 | 0.813 | 5874 | (5719–6029) | 1606 | 1938 | 0.829 | 0.814 | 5878 | (5718–6037) | ||
| 2003 | (19) | 1361 | 1636 | 0.832 | 0.821 | 6331 | (6170–6492) | 1391 | 1636 | 0.850 | 0.827 | 6379 | (6213–6545) | ||
| 2004 | – | – | – | – | 0.829 | 6793 | (6626–6961) | – | – | – | 0.839 | 6875 | (6704–7046) | ||
| 2005 | (10) | 1716 | 2098 | 0.818 | 0.836 | 7261 | (7086–7437) | 1707 | 2098 | 0.814 | 0.848 | 7366 | (7189–7542) | ||
| 2006 | (10) | 1783 | 2114 | 0.843 | 0.843 | 7334 | (7155–7512) | 1826 | 2114 | 0.864 | 0.855 | 7443 | (7264–7621) | ||
| 2007 | (10) | 1712 | 2031 | 0.843 | 0.850 | 7393 | (7211–7574) | 1738 | 2031 | 0.856 | 0.862 | 7495 | (7311–7679) | ||
| 2008 | (10)s | 1583 | 1811 | 0.874 | 0.856 | 7450 | (7265–7635) | 1588 | 1811 | 0.877 | 0.866 | 7535 | (7340–7728) | ||
| 2009 | – | – | – | – | 0.863 | 7505 | (7317–7693) | – | – | – | 0.869 | 7563 | (7350–7770) | ||
| 2010 | – | – | – | – | 0.869 | 7558 | (7366–7749) | – | – | – | 0.871 | 7580 | (7340–7810) | ||
| Total | 100,207 | (99,374–101,028) | 97,182 | (96,320–98,051) | |||||||||||
The columns in this table are: (2a) year; (2b) source for estimating the number of infants exposed to prenatal and intrapartum ARVs; (2c) number of infants exposed to prenatal ARVs out of (2d) the total number of infants in the study; (2e) raw proportion and (2f) smoothed proportion of infants exposed to prenatal ARVs; (2g) point estimate and (2h) 95% CI for the number of infants exposed to prenatal ARVs; (2i) number of infants exposed to intrapartum ARVs out of (2j) the total number of infants in the study; (2k) raw proportion and (2l) smoothed proportion of infants exposed to intrapartum ARVs; (2m) point estimate and (2n) 95% CI for the number of infants exposed to intrapartum ARVs. Symbols: number (n), total number (N), raw proportion (p) and smoothed proportion .
Estimates based on unpublished data from J Acquir Immune Defic Syndr. 2001;28:65–72.
Next, we fit a logistic regression model29 to the annual numbers of infants exposed to prenatal ARVs (column 2c) among a sample of infants born to HIV-infected women (column 2d) using year (column 2a) as a linear predictor. Similarly, we fit a logistic regression model to the annual numbers of infants exposed to intrapartum ARVs (column 2i) among a sample of infants born to HIV-infected women (column 2j) using year (column 2a) as a quadratic predictor (centered on 1990). We assumed that the true annual proportions of prenatal and intrapartum ARV usage were monotonically increasing over time. A separate term was fit for 1994, the first year ARVs were available, because the proportions for this year was so much lower than for the other years. We obtained smoothed proportions of infants exposed to prenatal and intrapartum ARVs (columns 2f, 2l). Fitting these logistic regression models allowed us to interpolate and extrapolate to years for which data were not available and gave tighter estimates of the true proportions for each year. In turn, we estimated the annual numbers of infants exposed to prenatal and intrapartum ARVs (columns 2g, 2m) as the product of the annual numbers of HIV-infected pregnant women who delivered (column 1d) and the annual smoothed proportions of infants exposed to prenatal and intrapartum ARVs (columns 2f, 2l), respectively.
We also used the above simulation study with 1,000,000 repetitions to obtain 95% CIs for the annual numbers of infants exposed to prenatal and intrapartum ARVs (columns 2h, 2n) for 1994–2010. First, for each repetition, we simulated the annual numbers of HIV-infected women from a Poisson distribution with mean parameter equal to (column 1d). Second, we simulated the annual proportions of infants exposed to prenatal and intrapartum ARVs from the logistic regression equations obtained above. Third, we simulated the annual numbers of infants exposed to prenatal and intrapartum ARVs as binomial variables with proportion parameters equal to the simulated proportions of infants exposed to prenatal and intrapartum ARVs, respectively, and sizes equal to the simulated number of HIV-infected women. Finally, we computed the 0.025 and the 0.975 percentiles over all 1,000,000 repetitions to obtain the bounds of the 95% CIs for the annual numbers of infants exposed to prenatal and intrapartum ARVs (columns 2h, 2n).
RESULTS
Using the data sources listed in the Tables 1 and 2, we estimated that between 1978 and 2010 approximately 186,157 (95% CI: 185,312–187,003) HIV-infected women gave birth in the United States (Table 1). The annual number of HIV-infected women giving birth increased approximately 28-fold during this period, from 310 in 1978 at the start of the epidemic to 8700 in 2006.30 During this period, approximately 21,003 (95% CI: 20,719–21,288) infants were perinatally infected with HIV. The estimated annual number of perinatal HIV infections peaked in 1991 at 17601 and declined steadily after 1993 to 151 (95% CI: 127–176) in 2010.16 The change in the estimated incidence of perinatal HIV infection during this period is depicted in Figure 1.
FIGURE 1.
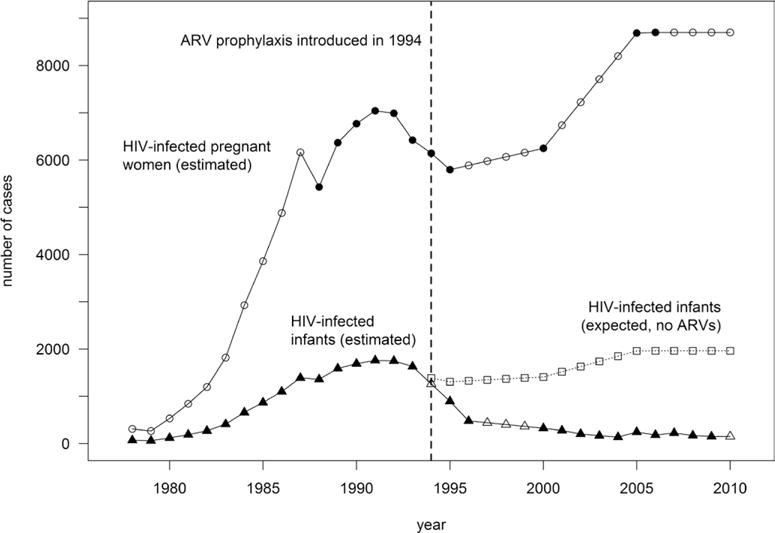
Numbers of HIV-infected pregnant women, HIV-infected infants, and expected HIV-infected infants in the absence of ARVs, United States, 1978–2010. Since the USPHS recommendation in 1994 to use zidovudine to reduce perinatal HIV transmission, the annual number of HIV-infected women giving birth (closed circles, published data; open circles, interpolated or extrapolated data) continued to increase, from approximately 6145 women in 1994, to an estimated 8700 in 2010. The dotted line indicates 0.2255 times the annual number of HIV-infected pregnant women, which estimates the annual number of HIV-infected infants that would have occurred in the absence of ARV prophylaxis (open squares), introduced in 1994.2 The annual number of perinatally HIV-infected infants (closed triangles, published data; open triangles, interpolated or extrapolated data) declined by about 88% after 1994, a decline due to increasing proportions of HIV-infected pregnant women who received ARV prophylaxis during the prenatal and intrapartum periods, in addition to other preventive interventions.
Focusing on the perinatal prophylaxis era between 1994 and 2010, an estimated 124,342 (95% CI: 123,651–125,034) HIV-infected women gave birth in the United States (Table 1). The annual number of HIV-infected women delivering increased by approximately 42%, from 6145 in 1994 to 8700 in 2010. During this same period, the annual number of HIV-infected infants decreased from 1263 (95% CI: 1194–1333) to approximately 151 (95% CI: 127–176).16 The annual MTCT rate dropped from a high of 20.6% in 1994 to a low of 1.7% in 2010. In total, approximately 6083 (95% CI: 5931–6236) infants were infected with HIV perinatally during this period. As a result of PMTCT interventions, an estimated 21,956 (95% CI: 20,191–23,759) cases of perinatal HIV were prevented between 1994 and 2010. In addition, after the introduction of ARVs, an increasing proportion of expected cases of MTCT were prevented each year; only about 9% of expected cases of MTCT were prevented in 1994, compared with 92% in 2010 (data not shown).
Both prenatal and intrapartum ARV use increased from 1994 to 2010 (Table 2). In 1994, approximately 54% of women received prenatal ARVs, resulting in 3293 (95% CI: 2794–3784) infant ARV exposures. By 2010, the proportion of HIV-infected pregnant women receiving ARVs during the prenatal period had risen to 87%, and 7558 (95% CI: 7366–7749) infants were exposed to ARVs that year. Altogether, an estimated 100,207 (95% CI: 99,374–101,028) infants were exposed to ARV drugs during the prenatal period.
The proportion of women receiving ARVs during the intrapartum period increased substantially during this period, from 32% in 1994 to 87% in 2010 (Table 2). The estimated annual number of infants exposed to ARVs during the intrapartum period grew from 1968 (95% CI: 1537–2456) in 1994 to 7580 (95% CI: 7340–7810) in 2010. In total, an estimated 97,182 (95% CI: 96,320–98,051) infants were exposed to intrapartum ARVs from 1994 to 2010.
DISCUSSION
PMTCT interventions—including HIV testing for pregnant women, ARV prophylaxis, avoidance of breastfeeding and elective cesarean section—have dramatically reduced the rates of MTCT of HIV in the United States. However, during the years of this study, a lack of national HIV infection and perinatal HIV exposure reporting has made it difficult to determine the full impact of these interventions. Strategies such as opt-out HIV testing for pregnant women, rapid testing at labor and delivery and improved ARV drugs have played significant roles in identifying HIV infections in pregnant women and preventing perinatal transmission. We estimate that nearly 22,000 perinatal HIV infections were prevented between 1994 and 2010 as a result of PMTCT interventions, suggesting that by 2010 the number of infants in whom HIV infection was prevented in the United States had exceeded the number of cases that have occurred. A growing share of perinatal HIV cases was prevented each year after the introduction of ARV prophylaxis.
We estimate that approximately 21,000 infants have been perinatally infected with HIV between 1978 and 2010. As of 2010, the CDC National HIV Surveillance System reported approximately 10,500 persons living with diagnoses of perinatally acquired HIV infection,13 of whom 7959 were ≥13 years of age and 2541 were <13 years of age. The implication of these figures is that, overall, approximately half (10,500/21,003) of the perinatally infected persons born within the United States were living as of 2010; of those born before 1999 (ie, ≥13 years old in 2011), approximately 43% (7959/18,402) were living, and of those born since 1998 (ie, < 13 years old in 2011), approximately 98% (2541/2601) were living.
An increasing proportion of infants born to HIV-infected women were exposed to ARV drugs during the prenatal or intrapartum periods. By 2010, more than 100,000 infants had been exposed to ARV prenatally. While widespread ARV prophylaxis has produced significant PMTCT successes, challenges remain. Although the number of HIV-infected pregnant women receiving prenatal ARV prophylaxis has increased annually, the proportion of women receiving ARVs during the prenatal period appears to have remained relatively stable since 2004, when an estimated 83% received prenatal ARVs.7 We estimated that approximately 87% of HIV-infected pregnant women received prenatal ARV prophylaxis in 2010.
Other studies have highlighted the role of missed HIV prevention opportunities, including late or no maternal HIV testing, inadequate ARV prophylaxis, breastfeeding or low maternal CD4 cell counts.11 Addressing these missed prevention opportunities is crucial to the elimination of perinatal HIV transmission. Efforts to identify HIV infections and provide timely treatment for women before or early in pregnancy need continued support.
Whereas ARV drugs have played an important role in reducing the MTCT rate, recent research has also explored potential toxicities associated with fetal/infant ARV drug exposure, including low birth weight, congenital abnormalities, childhood cancers and mitochondrial dysfunction.23–25,27,31–36 While a number of observational studies have found an association between maternal combination ARV therapy (now simply known as ARV therapy) and infant prematurity,35,37–39 this relationship has not been consistently observed.40–42 A recent meta-analysis (including studies from the United States), however, found that exposure to ARV therapy during pregnancy did not increase the odds of premature birth.36 Other adverse outcomes such as mitochondrial dysfunction are thought to be rare.43 Estimates of the number of individuals exposed to these drugs during the prenatal or intrapartum periods may help investigators plan future research on potential adverse effects of perinatal ARV drug exposure.
Preventing cases of MTCT directly through ARV prophylaxis affects the health of perinatally exposed infants by eliminating HIV-associated morbidity and mortality. Reducing rates of perinatal HIV transmission likely also leads to decreased healthcare utilization costs that are associated with HIV testing, treatment, in-patient care and outpatient care, and laboratory monitoring,44 and reductions in the levels of overall HIV-associated health system spending.45,46 In addition to health system costs, perinatal HIV infections also have economic ramifications for individuals, families and society. Preventing perinatal HIV reduces annual expenditures on HIV care and treatment for people living with HIV, their families and the wider US healthcare system. Calculating the cost-effectiveness of perinatal HIV transmission prevention interventions may be an avenue for future research.
Limitations
This analysis was based on the best available nationally representative estimates. However, a lack of national, HIV infection (during the time of the study) and perinatal HIV exposure reporting made the annual numbers of HIV-infected pregnant women and infants difficult to determine with precision. Several of the published estimates by CDC involved modeling, including “back-calculations” from numbers of AIDS cases. The modeled data were limited to numbers of HIV-infected women delivering, and the numbers of HIV-exposed and HIV-infected infants. This analysis was limited by missing data for several years during our period of interest. Our method of estimation assumed a constant rate of change for unknown years, when the actual rate of change may have been substantially more variable. Notably, we assumed no change in the annual number of HIV-infected women delivering after 2006, whereas that number might have increased in that period. The annual number of infected infants was last modeled for 2009, and we assumed that the number did not change in 2010. Estimates for the annual number of HIV-infected pregnant women and their infants who were exposed to ARV drugs are subject to the same limitations. For several years of ARV data, we had to use raw data, as opposed to that which was published. In the absence of consistent nationwide data on the number of HIV-infected infants, we relied on published numbers derived from models; for consistency’s sake, we used only CDC data.
CONCLUSION
This study provides the first national estimates of the number of HIV-infected pregnant women, HIV-infected infants and the number of perinatal HIV cases prevented by PMTCT interventions in the United States. Using these estimates, we calculated the number of prenatal and intrapartum ARV exposures among infants born to HIV-infected mothers. These estimates will be important for policy-making and provide reference data for future studies, including those on ARV toxicities and those on net-savings associated with PMTCT programs. Great progress has been made in the prevention of MTCT of HIV since the introduction of ARVs and other preventive interventions; PMTCT interventions have prevented nearly 22,000 cases of MTCT of HIV between 1994 and 2010. However, continued vigilance to prevent, identify and treat HIV infections in women must be undertaken to eliminate perinatal HIV in the United States.
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Footnotes
K.M.L. performed the literature review, created tables and wrote the manuscript. A.W.T. assisted with the study design and manuscript writing and editing. C.B.B. performed the principal statistical analysis, wrote the methods section and assisted with table and figure preparation. M.C.B.M. assisted with the statistical analysis, methods writing and manuscript review. M.A.L. assisted with study design and manuscript preparation and editing. P.J.W. participated in the study design and analysis, and assisted in manuscript preparation and editing. S.R.N. was primarily responsible for study design, assisted with manuscript preparation and coordinated manuscript editing.
The authors have no conflicts of interest or funding to disclose.
References
- 1.Davis SF, Byers RH, Jr, Lindegren ML, et al. Prevalence and incidence of vertically acquired HIV infection in the United States. JAMA. 1995;274:952–955. [PubMed] [Google Scholar]
- 2.Recommendations of the U.S. Public Health Service Task Force on the use of zidovudine to reduce perinatal transmission of human immunodeficiency virus. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep Cent Dis Control. 1994;43:1–20. [PubMed] [Google Scholar]
- 3.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335:1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 4.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40:458–465. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 5.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2014 Available at: http://aidsinfo.nih.gov/con-tentfiles/lvguidelines/PerinatalGL.pdf. Accessed February 26, 2015.
- 6.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Joint Statement of ACOG/AAP on Human Immunodeficiency Virus Screening. Washington, DC: College Executive Board; 1999. Available at: https://www.acog.org/-/media/Statements-of-Policy/Public/sop075.pdf?dmc=1&ts=20160106T1441231646. Accessed January 31, 2016. [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Achievements in public health. Reduction in perinatal transmission of HIV infection–United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–597. [PubMed] [Google Scholar]
- 8.Centers for Disease Control (CDC) Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1985;34:721–726. 731–732. [PubMed] [Google Scholar]
- 9.Dunn DT, Newell ML, Ades AE, et al. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340:585–588. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Enhanced perinatal surveillance–15 areas, 2005–2008. HIV Supplemental Report. 2011;16(2) Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/resports/. Accessed March 2016. [Google Scholar]
- 11.Whitmore SK, Taylor AW, Espinoza L, et al. Correlates of mother-to-child transmission of HIV in the United States and Puerto Rico. Pediatrics. 2012;129:e74–e81. doi: 10.1542/peds.2010-3691. [DOI] [PubMed] [Google Scholar]
- 12.Glynn MK, Lee LM, McKenna MT. The status of national HIV case surveillance, United States 2006. Public Health Rep. 2007;122(suppl 1):63–71. doi: 10.1177/00333549071220S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Atlanta, GA: CDC; 2013. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Accessed March 2016. [Google Scholar]
- 14.Byers RH, Jr, Caldwell MB, Davis S, et al. Projection of AIDS and HIV incidence among children born infected with HIV. Stat Med. 1998;17:169–181. doi: 10.1002/(sici)1097-0258(19980130)17:2<169::aid-sim759>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Fleming P, Lindegren M, Byers R, et al. Estimated number of perinatal HIV infections, U.S., 2000. Barcelona, Spain: 2002. Abstract No. TuPeC4773. Available at: http://www.iasociety.org/Default.aspx?pageId=11&abstractId=7403. Accessed March 2016. [Google Scholar]
- 16.Taylor A, Little K, Zhang X, et al. Estimated Perinatal Antiretroviral Exposures, Cases Prevented and Infected Infants in the Era of Antiretroviral Prophylaxis in the United States. Seattle, Washington: CROI foundation; 2012. Poster 1000. [Google Scholar]
- 17.Davis SF, Rosen DH, Steinberg S, et al. Trends in HIV prevalence among childbearing women in the United States, 1989–1994. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:158–164. doi: 10.1097/00042560-199810010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Lindegren ML, Byers RH, Jr, Thomas P, et al. Trends in perinatal transmission of HIV/AIDS in the United States. JAMA. 1999;282:531–538. doi: 10.1001/jama.282.6.531. [DOI] [PubMed] [Google Scholar]
- 19.McKenna MT, Hu X. Recent trends in the incidence and morbidity that are associated with perinatal human immunodeficiency virus infection in the United States. Am J Obstet Gynecol. 2007;197(3 suppl):S10–S16. doi: 10.1016/j.ajog.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Enhanced perinatal surveillance–15 areas, 2000–2003. HIV Surveill Suppl Rep. 2008;13:1–33. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Enhanced perinatal surveillance–15 areas, 1999–2001. HIV Surveill Suppl Rep. 2004;4:1–20. [Google Scholar]
- 22.Brogly S, Williams P, Seage GR, et al. In utero nucleoside reverse transcriptase inhibitor exposure and cancer in HIV-uninfected children: an update from the pediatric AIDS clinical trials group 219 and 219C cohorts. J Acquir Immune Defic Syndr 1999. 2006;41:535–536. doi: 10.1097/01.qai.0000194735.66322.d9. [DOI] [PubMed] [Google Scholar]
- 23.Hankin C, Lyall H, Peckham C, et al. Monitoring death and cancer in children born to HIV-infected women in England and Wales: use of HIV surveillance and national routine data. AIDS. 2007;21:867–869. doi: 10.1097/QAD.0b013e3280b01822. [DOI] [PubMed] [Google Scholar]
- 24.Benhammou V, Warszawski J, Bellec S, et al. ANRS-Enquête Périnatale Française. Incidence of cancer in children perinatally exposed to nucleoside reverse transcriptase inhibitors. AIDS. 2008;22:2165–2177. doi: 10.1097/QAD.0b013e328311d18b. [DOI] [PubMed] [Google Scholar]
- 25.Ivy W, III, Nesheim SR, Paul SM, et al. Cancer among children with perinatal exposure to HIV and antiretroviral medications–New Jersey, 1995–2010. J Acquir Immune Defic Syndr 1999. 2015;70:62–66. doi: 10.1097/QAI.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barret B, Tardieu M, Rustin P, et al. French Perinatal Cohort Study Group Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Brogly SB, Ylitalo N, Mofenson LM, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 28.Orloff SL, Bulterys M, Vink P, et al. Perinatal AIDS Collaborative Transmission Study Maternal characteristics associated with antenatal, intrapartum, and neonatal zidovudine use in four US cities, 1994–1998. J Acquir Immune Defic Syndr. 2001;28:65–72. doi: 10.1097/00042560-200109010-00010. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer DW, Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 30.Whitmore SK, Zhang X, Taylor AW, et al. Estimated number of infants born to HIV-infected women in the United States and five dependent areas, 2006. J Acquir Immune Defic Syndr 1999. 2011;57:218–222. doi: 10.1097/QAI.0b013e3182167dec. [DOI] [PubMed] [Google Scholar]
- 31.Benhammou V, Tardieu M, Warszawski J, et al. Clinical mitochondrial dysfunction in uninfected children born to HIV-infected mothers following perinatal exposure to nucleoside analogues. Environ Mol Mutagen. 2007;48:173–178. doi: 10.1002/em.20279. [DOI] [PubMed] [Google Scholar]
- 32.Alimenti A, Burdge DR, Ogilvie GS, et al. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr Infect Dis J. 2003;22:782–789. doi: 10.1097/01.inf.0000086400.93257.74. [DOI] [PubMed] [Google Scholar]
- 33.Bae WH, Wester C, Smeaton LM, et al. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. AIDS. 2008;22:1633–1640. doi: 10.1097/QAD.0b013e328307a029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotter AM, Garcia AG, Duthely ML, et al. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193:1195–1201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 35.European Collaborative S. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. JAIDS. 2003;32:380–387. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kourtis AP, Schmid CH, Jamieson DJ, et al. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS. 2007;21:607–615. doi: 10.1097/QAD.0b013e32802ef2f6. [DOI] [PubMed] [Google Scholar]
- 37.Lorenzi P, Spicher VM, Laubereau B, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS. 1998;12:F241–F247. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
- 38.European Collaborative Study, Swiss Mother and Child HIV Cohort Study. Combination antiretroviral therapy and duration of pregnancy. AIDS Lond Engl. 2000;14:2913–2920. doi: 10.1097/00002030-200012220-00013. [DOI] [PubMed] [Google Scholar]
- 39.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS. 2004;18:2337–2339. doi: 10.1097/00002030-200411190-00019. [DOI] [PubMed] [Google Scholar]
- 40.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346:1863–1870. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 41.Schulte J, Dominguez K, Sukalac T, et al. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: pediatric spectrum of HIV disease, 1989–2004. Pediatrics. 2007;119:e900–e906. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 42.Szyld EG, Warley EM, Freimanis L, et al. NISDI Perinatal Study Group Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS. 2006;20:2345–2353. doi: 10.1097/01.aids.0000253362.01696.9d. [DOI] [PubMed] [Google Scholar]
- 43.Thorne C, Newell ML. Safety of agents used to prevent mother-to-child transmission of HIV: is there any cause for concern? Drug Saf. 2007;30:203–213. doi: 10.2165/00002018-200730030-00004. [DOI] [PubMed] [Google Scholar]
- 44.Sansom SL, Anderson JE, Farnham PG, et al. PSD Consortium Updated estimates of healthcare utilization and costs among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2006;41:521–526. doi: 10.1097/01.qai.0000191286.70331.7b. [DOI] [PubMed] [Google Scholar]
- 45.Mauskopf JA, Paul JE, Wichman DS, et al. Economic impact of treatment of HIV-positive pregnant women and their newborns with zidovudine. Implications for HIV screening. JAMA. 1996;276:132–138. [PubMed] [Google Scholar]
- 46.Gorsky RD, Farnham PG, Straus WL, et al. Preventing perinatal transmission of HIV–costs and effectiveness of a recommended intervention. Public Health Rep. 1996;111:335–341. [PMC free article] [PubMed] [Google Scholar]


