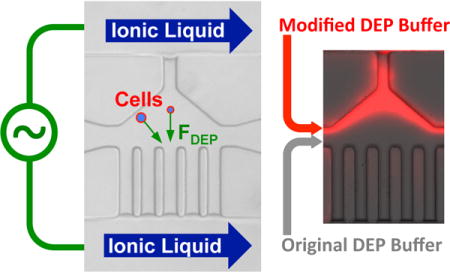
Abstract
Dielectrophoresis (DEP) has been widely explored to separate cells for various applications. However, existing DEP devices are limited by the high cost associated with the use of noble metal electrodes, the need of high-voltage electric field, and/or discontinuous separation (particularly for devices without metal electrodes). We developed a DEP device with liquid electrodes, which can be used to continuously separate different types of cells or particles based on positive DEP. The device is made of polydimethylsiloxane (PDMS) and ionic liquid is used to form the liquid electrodes, which has the advantages of low cost and easy fabrication. Moreover, the conductivity gradient is utilized to achieve the DEP-based on-chip cell separation. The device was used to separate polystyrene microbeads and PC-3 human prostate cancer cells with 94.7 and 1.2% of the cells and microbeads being deflected, respectively. This device is also capable of separating live and dead PC-3 cancer cells with 89.8 and 13.2% of the live and dead cells being deflected, respectively. Moreover, MDA-MB-231 human breast cancer cells could be separated from human adipose-derived stem cells (ADSCs) using this device with high purity (81.8 and 82.5% for the ADSC and MDA-MB-231 cells, respectively). Our data suggest the great potential of cell separation based on conductivity-induced DEP using affordable microfluidic devices with easy operation.
TOC Graphic
INTRODUCTION
The capability of efficient cell separation is significant for many applications including water analysis and diagnosis and treatment of diseases.1–6 Dielectrophoresis (DEP) is an attractive technique for particle manipulation, which may allow label-free, low-damage, high-efficiency, and easy-operation separation of cells.7,8 DEP cell separation can be achieved with direct current (DC) or alternating current (AC) electric field gradient. The DC DEP is commonly used in insulator-based dielectrophoretic (iDEP) devices and has been shown to separate particles and/or cells based on their difference in conductivity and size. For example, Lapizco-Encinas et al. developed an iDEP device with insulating posts to generate electric field gradient for separating or concentrating bacterial cells.9–11 Barbulovic-Nad et al. reported an iDEP device to sort polystyrene particles of different sizes by employing oil droplet to generate the electric field gradient.12 Kang et al. conducted a DC DEP size-dependent sorting of fixed white blood cells from breast cancer cells by using a rectangular or triangular hurdle.13 Zhu et al. fabricated an iDEP device with a spiral microchannel to separate polystyrene particles of different sizes.14,15 The iDEP devices can be easily fabricated and do not need the metal electrode. However, due to the higher conductivity of the DEP buffer than the particles or cells, a negative DEP force is usually generated in these devices. Unfortunately, a large difference of DEP response for different particles or cells usually occurs near the positive DEP region.16 This is because the electrical properties both on the surface and inside the particles or cells can be effectively utilized in this region. Additionally, the electrodes are usually placed in the inlets and outlets to reduce the negative effects of bubble formation, which requires a high DC voltage and makes the cells stay in strong damaging electric field over a long distance.
The AC electric field has also been used to separate particles or cells by utilizing the differences in the distribution of electrical properties inside the particles or cells. The electrodes used in the AC DEP devices are typically fabricated using biocompatible metals (e.g., gold and platinum). Kim et al. reported AC DEP sorting of human breast ductal carcinoma cells based on their cell-cycle phase using DEP devices with angled metal electrodes.17 Cheng et al. developed a 3D traveling-wave AC DEP device with parallel metal electrodes, which could separate red blood cells from debris-filled heterogeneous samples.18 Li et al. conducted separation of live and dead Listeria innocua cells using an AC DEP device with interdigitated metal electrodes.19 Song et al. fabricated a DEP device with angled metal electrodes to sort stem cells and their differentiation products.16 The AC DEP devices can be used to manipulate particles or cells with both negative and positive DEP forces by controlling the frequency. However, the fabrication of metal electrodes is not trivial and requires cleanroom facility, resulting in high cost. Moreover, the metal electrodes are directly bonded to the PDMS in the devices, which makes it difficult to reuse them.
More recently, contactless AC DEP is attracting more and more attention for particle or cell separation. In contactless DEP devices, the electrodes have no direct contact with the sample. This is because there are thin insulating barriers between the electrodes and sample to act as the capacitance and couple the electrodes with the sample in the main channel. For example, Shafiee et al. reported a capture-based separation of live and dead THP-1 human leukemia monocytes using a contactless DEP device with a thin PDMS insulating barriers.20 Salmanzadeh et al. fabricated a similar PDMS device, which was used to separate prostate tumor initiating cells from bulk tumor cells.21 Zellner et al. fabricated contactless DEP device to separate cells and polystyrene beads using a glass substrate as the barrier.22 The contactless DEP devices do not require metal electrodes, or the metal electrodes can be reused because they do not need to be bonded to PDMS in the device. Unfortunately, the contactless AC DEP devices require high voltage with high frequency in order to cross the insulating barrier and generate strong enough electric field gradient. Moreover, the contactless AC DEP devices typically provide discontinuous separation based on cell trapping.
In this study, a novel AC DEP approach with low-voltage and low-frequency electric field for metal-electrode-free and continuous particle or cell separation is developed. A room temperature ionic liquid (RTIL), [BMIM][PF6] that has high conductivity compared to the DEP buffer, was used to form the liquid electrode. Due to their immiscible nature, a stable interface can form between the ionic liquid and DEP buffer in the main channel. Moreover, two types of DEP buffers with different conductivities were used to couple with the ionic liquid and make full use of the electric potential applied into the main channel to generate strong enough electric field gradient for DEP manipulation of cells/particles. It is the gradient of the conductivity that induces the gradient of the electric field to generate the DEP force for manipulating particles or cells in the main channel. We tested this novel approach by using it to separate polystyrene beads versus PC-3 cells, live versus dead PC-3 cells, and human ADSCs versus MDA-MB-231 cancer cells with promising outcome. This approach may be further developed for more advanced applications, such as the separation of cancer stem-like cells from non-stem cancer cells to enrich the former, and extraction of circulating tumor cells (CTCs) from blood for cancer detection.
EXPERIMENTAL SECTION
Theory and Modeling
As aforementioned, there are two types of DEP forces: positive and negative. The former moves particles/cells against the electric field gradient from a weaker to stronger electric field region, which occurs if the particles/cells are more polarizable than the surrounding medium. The negative DEP force moves particles/cells from a stronger to weaker electric field region and occurs when the particles/cells are less polarizable than the medium. A detailed explanation of the DEP theory and simulation of the electric field and velocity distribution using COMSOL Multiphysics (version 5.1) is given in Supporting Information.
Materials
Fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). The F-12K and DMEM cell culture media were purchased from ATCC (Manassas, VA, USA). The ADSC base medium was purchased from Lonza (Allendale, NJ, USA). The live/dead viability/cytotoxicity assay kit was purchased from Invitrogen (Carlsbad, CA, USA). All other chemicals were purchased from Sigma (St. Louis, MO, USA) unless specifically mentioned otherwise.
Cell culture and preparation
The PC-3 human prostate cancer cells (ATCC), MDA-MB-231 human breast cancer cells (ATCC), and human adipose-derived stem cells (ADSCs, Lonza) were used in this study. The cells were cultured in 75 cm2 T-flasks at 37 °C in humidified air with 5% CO2. The medium for culturing the PC-3, MDA-MB-231, and ADSC cells was F12K, DMEM, and specialized ADSC base medium, respectively, supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The medium was refreshed every two days. After the cells were detached and removed from the culture flask, they were washed thoroughly using the original DEP buffer consisting of 10% (w/v) sucrose and 0.3% (w/v) glucose in deionized water, and re-suspended in the original DEP buffer for further use.
For separation of the 20 μm polystyrene beads from PC-3 cells, the samples washed thoroughly with the original DEP buffer were re-suspended in a modified DEP buffer made of 10% (w/v) sucrose, 0.3% (w/v) glucose, and 0.8% (v/v) PBS in deionized water. The suspensions of polystyrene beads and PC-3 cells were then mixed with a final concentration of 0.5 × 106 beads/mL and 0.5 × 106 cells/mL.
For live and dead PC-3 cell separation, the dead PC-3 cells were prepared by heating the cells in water bath at 60 °C for 30 min, and further stained with Hoechst 33342 (5 μM in medium) for 10 min at 37 °C. The live PC-3 cells were stained using the standard fluorescence-based live/dead assay kit for 10 min at 37 °C according to the manufacturer’s instructions. The two samples were then washed thoroughly with the original DEP buffer, re-suspended in the modified DEP buffer, and further mixed together with a final concentration of 0.5 × 106 cells/mL for both the live and dead cells.
For ADSC and MDA-MB-231 cell separation, the ADSCs were stained using the standard fluorescence-based live/dead assay kit for 10 min at 37 °C. Both ADSCs and MDA-MB-231 cells were washed thoroughly with the original DEP buffer, re-suspended in the modified DEP buffer, and further mixed together at a final concentration of 0.5 × 106 cells/mL for both cells.
Device fabrication
To fabricate the DEP devices, photoresist SU-8 2025 (MicroChem) was spin-coated on the surface of a 4-inch silicon wafer, and soft baked first at 65 °C for 3 min and then 95 °C for 7 min. The thickness of the photoresist was 50 μm. The photoresist was exposed to ultra violet (UV) through a mask with the designed microchannel (Figure 1), baked first at 65 °C for 1 min and then at 95 °C for 6 min, and further developed using the SU-8 developer (MicroChem) for ~5 min. The photoresist mold was then obtained by hard baking at 150 °C for 5 min. PDMS (Dow Corning) pre-polymer was fully mixed with its crosslinking agent at a 10:1 mass ratio. The mixture was poured over the photoresist mold and cured at 72 °C for 3 h. The crosslinked PDMS layer imprinted with the designed microfluidic channel was then peeled off the photoresist mold and punched with 2 mm holes at the inlets and exits of the microchannel using a puncher. Finally, the PDMS layer was bonded to a glass slide after oxygen plasma treatment for 1 min. Before use, the device was filled with 1% BSA in deionized water overnight at 4 °C to make the surface of the microfluidic channel in the PDMS layer more hydrophilic.
Figure 1.
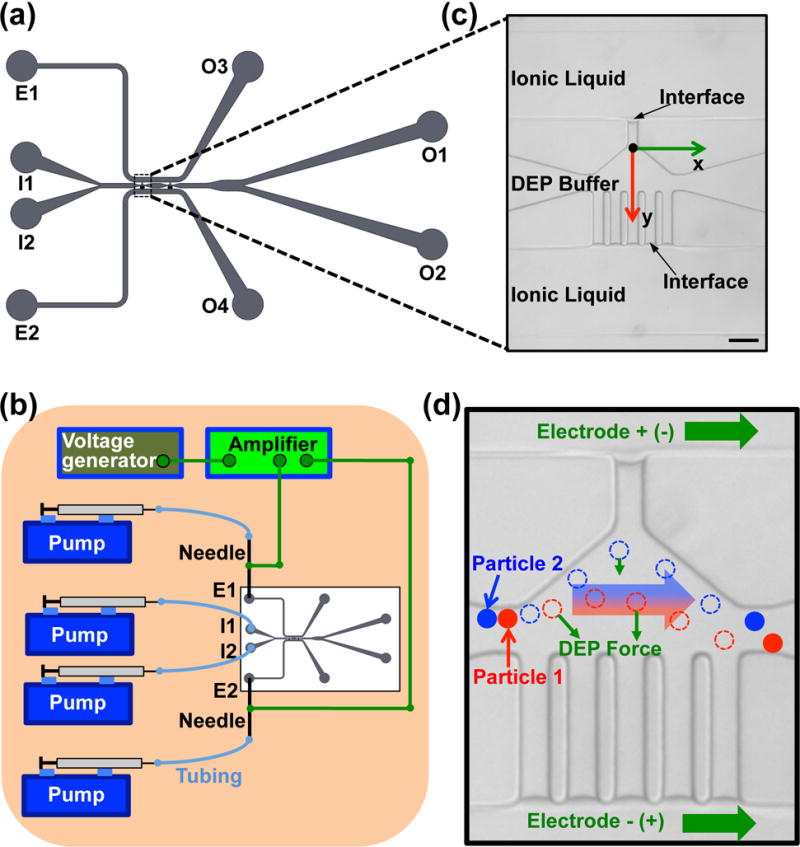
The DEP device with ionic liquid electrodes. (a) A sketch of the microfluidic channel system in the device. (b) A schematic of the experimental setup. (c) A zoom-in view of the ionic liquid electrodes and the main channel on the real image showing the stable interface formed between the ionic liquid and the DEP buffer. Scale bar: 100 μm. (d) A schematic showing the mechanism of the particle and cell separation. Particles 1 and 2 with different electrical properties and size are deflected by the DEP force differently and can be separated from each other in the electrode region. The dashed circles represent the intermediate position of the particles.
Experimental setup
As shown in Figure 1a–b (and Figure S1a), the modified DEP buffer with cells in 1 ml syringe was introduced from I1 at 50 μL/h via Cole-Parmer (Vernon Hills, IL, USA) plastic tubing by syringe pumps (Harvard Apparatus, Cambridge, MA, USA), the original DEP buffer without cells in 1 ml syringe was introduced from I2 at 250 μL/h, and they were collected at O1 and O2. The ionic liquid in 1 ml syringes was introduced into E1 and E2 via Cole-Parmer (Vernon Hills, IL, USA) plastic tubing by using syringe pumps (Harvard Apparatus, Cambridge, MA, USA) and exited from O3 and O4 (Figure 1a). BD (Franklin Lakes, NJ, USA) PrecisionGlide metal needles (21G) were used to connect E1 or E2 and tubing from 1 ml syringes. An Agilent (Santa Clara, CA, USA) 33500B waveform generator with TEGAM (Geneva, OH, USA) Model 2350 high-voltage amplifier was used to supply the electric voltage, which was applied to the metal needles connected to E1 and E2. The electric field was further applied to the electrode regions in the main channel via the ionic liquid electrodes. The zoom-in real image and sketch of the electrode region are shown in Figures 1c and S1b, respectively. A stable interface could form between the ionic liquid and DEP buffer at the distal end of the narrow channels when the flow rate of the ionic liquid was set as 15 μL/h (Figure 1b). As shown in Figure S1c, the interface between the ionic liquid and DEP buffer undergoes the pressures from the flows of the ionic liquid and DEP buffer , as well as the surface tension force . The ionic liquid is hydrophobic and the surface of the PDMS is hydrophilic after the BSA modification. As a result, the surface tension force trends to prevent the ionic liquid from entering the narrow channel. When the three forces reach balance as the following:
| (8) |
where θ is contact angle and A is contact area, the interface between the ionic liquid and DEP buffer can stay stable dynamically during the experiment.
As shown in Figure 1d, particles and/or cells will experience DEP force that is dependent on their electrical properties and size when they pass through the electrode region. Therefore, particles and/or cells with different electrical properties and size will experience different DEP force (e.g., particle 1 versus 2), which can be utilized to separate the particles and/or cells.
Imaging
The time-lapse images and movies were taken using a Zeiss Axio Observer.Z1 microscope equipped with a Zeiss AxioCam HSm fast-speed CCD camera. The exposure time was 5, 20, and 40 ms for taking the phase contrast, green fluorescence, and blue fluorescence images/movies, respectively. The static images were taken using the same microscope equipped with a Zeiss AxioCam MR3 CCD camera.
Measurement of cell size
After the cells were detached and removed from the culture flask, they were washed thoroughly using the original DEP buffer, and re-suspended in the original DEP buffer in a cell culture dish. After the cells settled down on the bottom surface of the dish, the diameters of cells were measured under the Zeiss Axio Observer.Z1 microscope.
Measurement of conductivity
To measure the conductivity of aqueous samples, the platinum-cured silicone tubing (Cole-Parmer) with an inner diameter of 0.51 mm was used. The tubing (10 mm long) was filled with the samples by connecting it to two BD syringe needles. The IM 3570 impedance analyzer (Hioki, Cranbury, NJ) was used to measure the total resistance of the samples in the tubing. The conductivity was calculated based on the measured resistance and sample size as: , where L is length of sample, S is cross-sectional area of sample, and Ω is resistance.
Statistical analysis
All the experiments were conducted for at least three independent runs, and all data were reported as mean ± standard deviation. Student’s two-tailed t-test assuming equal variance was calculated using JMP 10 to assess statistical significance (p < 0.05).
RESULTS AND DISCUSSION
Modeling studies on the mechanisms of cell separation
For the simulation, the conductivity of the original and modified DEP buffer was measured to be 1.0 ± 0.6 and 14.0 ± 0.5 mS/m, respectively. Additionally, all the samples are aqueous solutions with a relative permittivity of ~80. The ionic liquid, [BMIM][PF6], has a conductivity of 140 mS/m and viscosity of 450 cP (0.45 Pa s) at 25 °C.23,24 Its relative permittivity is ~12 at low frequencies (~50–100 kHz).25
As shown in Figure 2a, a conductivity gradient is established in the first and second electrodes regions in the velocity field (Figure S2), when the modified DEP buffer (14 mS/m) is introduced into I1 and the original DEP buffer (1 mS/m) is introduced into I2. It is this conductivity gradient that further generates the electric field gradient in the first and second electrodes regions (Figure 2b). The strong electric field region is close to the five-parallel electrodes. When the particles or cells pass through this region, the positive DEP force will deflect them towards this strong electric field region.
Figure 2.
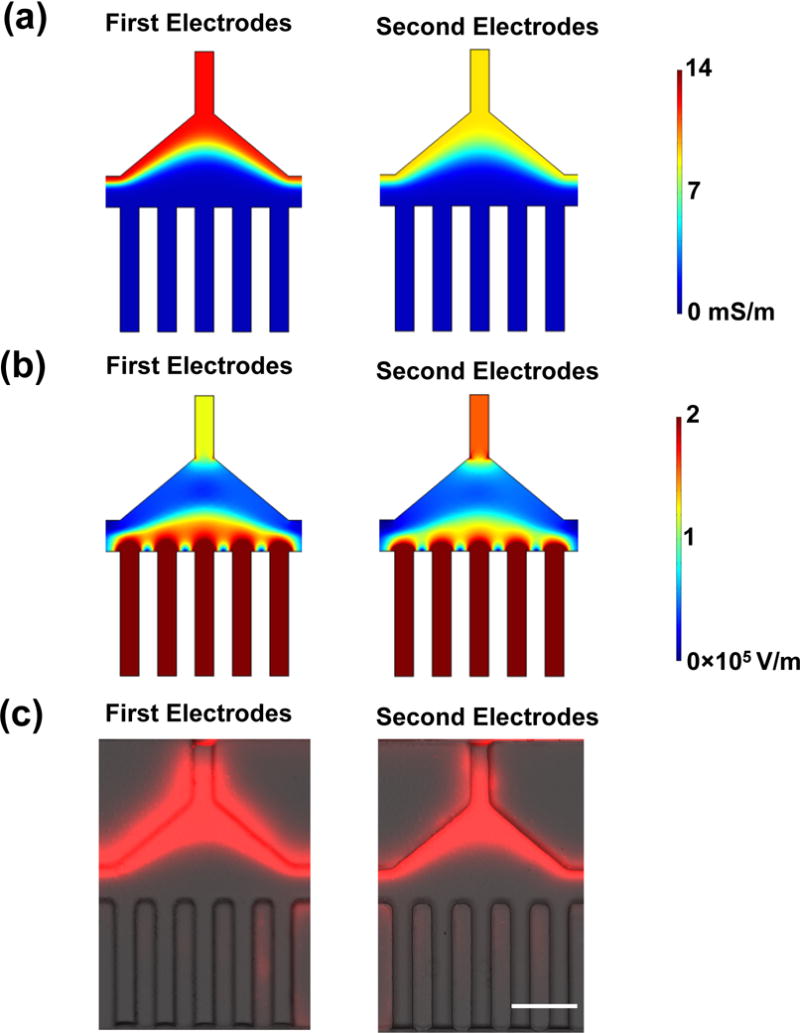
Analysis of conductivity and electric field distribution. (a) The simulated conductivity distribution in the first and second electrode regions. The conductivity of the modified DEP buffer is 14 mS/m, and the conductivity of the original DEP buffer is 1 mS/m. (b) The simulated electric field in the first and second electrode regions. The modified DEP buffer is introduced into I1 and the original DEP buffer is introduced into I2, when 88 V is applied on the device. (c) The distribution of Rhodamine B at the first and second electrodes regions without electric field. Scale bar: 100 μm. The velocity at the beginning of the main channel was set as 6 mm/s. The flow rate of the modified DEP buffer with Rhodamine B (10 μg/mL): 50 μL/hr, the flow rate of the original DEP buffer: 250 μL/hr.
In the DEP device, the electrolytes in the modified DEP buffer will move toward the original DEP buffer due to diffusion, which results in a smooth gradient of the conductivity in the main channels as shown in Figure 2a. It is the gradual gradient of the conductivity that generates the gradient of the electric field, which moves the particles or cells in the electrode regions by DEP force. To experimentally verify the simulation results, Rhodamine B was used as a fluorescent material to visualize the electrolyte movement, and was added into the modified DEP buffer (10 μg/mL). In the experiment, the modified DEP buffer with Rhodamine B was introduced into the device from I1, and the original DEP buffer was introduced from I2 (there was no cells in this experiment). Figure 2c shows the fluorescence distributions in the first and the second electrodes regions without electric field. The results show that the fluorescence distributions agree with the simulation results in Figure 2a. Figure S3 shows the fluorescence distributions in the first and the second electrodes regions under various electric field conditions, which also agrees with the simulation results in Figure 2a except the second electrodes region at 88 Vrms and 100 kHz, where the fluorescence is slightly driven to the original DEP buffer. This result indicates that the electric field moves the Rhodamine B towards the low conductivity region when the electric field is strong, which means the electrolytes are also moved by the electric field in the same direction. The gradient of conductivity in the electric field generates free charges (ρ) in the DEP buffer ( ), which undergo the Coulomb force ( ). Therefore, the DEP buffer containing these charges will undergo body force in the electric field, which is towards the low conductivity regions to minimize the electric energy of the system.26,27 Therefore, the effect of electric field on the movement of the electrolytes is in the same direction with the diffusion of the electrolytes towards the low conductivity region, which contributes to the formation of the gradual conductivity gradient in the electrodes regions and does not disturb the DEP separation.
Conductivity-induced DEP cell deflection
Based on the two-layer cell model (Equation S3), the real part of the CM factor of live PC-3 cells was calculated over frequency from 10 kHz to 104 kHz (Figure 3a). At frequencies lower than ~10 kHz, the real part of the CM factor is negative, which means that the cells experience negative DEP force and will be deflected towards the weak electric field region. At frequencies higher than ~40 kHz, the real part of the CM factor is positive, indicating that the cells will experience positive DEP force and be moved toward the strong electric field region. In this study, all the frequencies used are in the positive DEP region, and it is the positive DEP force that drives the motion of particles or cells in the microchannel.
Figure 3.
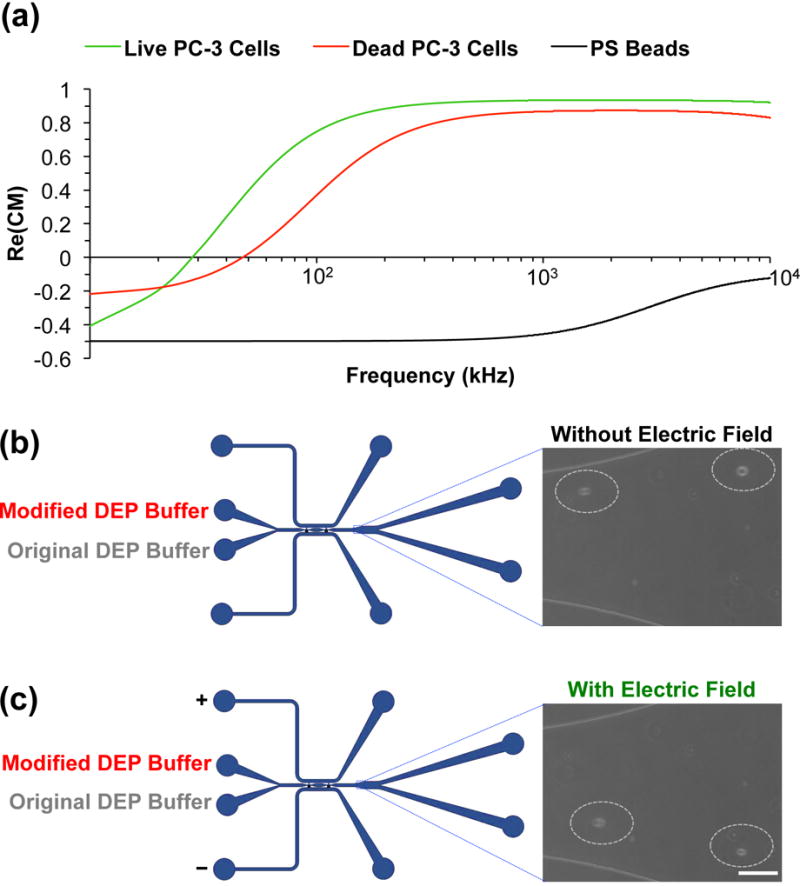
Mechanism analysis of cell deflection. (a) The real parts of the CM factors of live PC-3 cells, dead PC-3 cells, and 20 μm Polystyrene beads calculated with the two-layer model. The conductivity of the surrounding medium is set as 14 mS/m and its relative permittivity is set as 80. The conductivity of cell membrane and cell cytoplasm, the relative permittivity of cell membrane and cell cytoplasm of live PC-3 cells are set as 1×10−4 mS/m, 1000 mS/m, 20, and 60, respectively. The conductivity and relative permittivity of the polystyrene beads are set as 0.05 mS/m and 60, respectively. The conductivity of cell membrane and cell cytoplasm, the relative permittivity of cell membrane and cell cytoplasm of dead PC-3 cells are set as 1×10−2 mS/m, 500 mS/m, 10, and 60, respectively. The diameter of the live and dead PC-3 cells is 16 μm. (b) The cells move out of the device without deflection, when modified DEP buffer is introduced from I1 (50 μL/hr) and original DEP buffer is introduced from I2 (250 μL/hr), and no electric field is applied. (c) The cells move out of the device with deflection, when modified DEP buffer is introduced from I1 (50 μL/hr) and original DEP buffer is introduced from I2 (250 μL/hr), and electric field is applied. Scale bar: 100 μm.
The real part of the CM factor of 20 μm polystyrene beads is also shown in Figure 3a. For the separation of live PC-3 cells and 20 μm polystyrene beads, the difference in conductivity of the two different particles is the dominating factor. The conductivity of the beads is as low as 0.05 mS/m.28–32 The relative permittivity of the polystyrene beads is ~60 in aqueous solution.29,33,34 From the CM factor data, when the frequency is 100 kHz, the real part of the CM factor of polystyrene beads is negative and it is positive for the PC-3 cells, which indicates that the beads experience negative DEP force while the PC-3 cells experience positive DEP force at this frequency. Therefore, when they pass through the electrodes regions, the PC-3 cells will be deflected to the strong electric field region, while the polystyrene beads will stay the same position where the electric field is weak.
Compared to the live cells, the dead cells have a plasma membrane with higher conductivity and lower permittivity and a cytoplasm with lower conductivity.19,35–38 As shown in Figure 3a, at low frequency below 10 kHz, both the live and dead cells experience negative DEP force. When the frequency increases to 50 kHz, the live cells experience strong positive DEP force, and will be deflected to the high electric field region, while the dead cells experience weak positive or negative DEP force due to the conductivity and permittivity changes of the cell membrane and cytoplasm, and will be slightly deflected or stay the same position.
ADSCs are adult human stem cells and MDA-MB-231 cells are human cancer cells. The conductivity and permittivity of their cell membrane, cytoplasm and nucleus are different.16,21,39 In addition, the ADSCs are larger than the MDA-MB-231 cells. As a result, the effective conductivity and permittivity of the cells in the DEP environment are different.39 The influence of size on the DEP deflection includes not only the effective conductivity and permittivity, but also the relationship between the DEP force and drag force. Assuming the cell is a small spherical particle, and in the y direction, the cell experiences both the DEP force (Equation S1) and Stoke’s drag force that is linearly related to the diameter of the cell. Therefore, large cells are more likely to have a large acceleration and deflected more than small cells.
Further experiments were conducted to confirm the mechanism of the conductivity-induced DEP cell deflection revealed by the aforementioned modeling studies. As shown in Figure 3b, when the modified DEP buffer with PC-3 cells was introduced from I1 and the original DEP buffer introduced from I2, the PC-3 cells were not deflected and moved out from O1 in the absence of an external electric field. In contrast, when an electric field (88 Vrms at 100 kHz) was applied, the PC-3 cells were deflected by the DEP force and moved out from O2 (Figure 3c). However, when the modified (or original) DEP buffer with PC-3 cells was introduced from I1 and the modified (or original) DEP buffer without cells was introduced from I2, there was no deflection of the cells, and all the cells moved out from O1, as shown in Figure S4a (or Figure S4b).
These results show that the DEP force that the cells experience is dependent on the distribution of the conductivity in the main channel. When the modified (or original) DEP buffer is introduced from both I1 and I2, there is no gradient of the conductivity and the strong electric field gradient in the electrodes regions. Therefore, there is no deflection of cells in this condition. On the contrary, when the modified DEP buffer and the original DEP buffer are introduced, the gradient of the conductivity induces a gradient of the electric field and the positive DEP force will deflect the cells to strong electric field region that is close to the parallel electrodes (Figure 2b), and drive the cells towards O2.
Conductivity-induced DEP Cell and particle separation
The separation of 20 μm polystyrene beads from PC-3 cells was conducted first. As shown in Figure S5 and Movie S1, the beads and cells pass through the main channel and come out from O1 without electric field. When an electric field is applied to electrodes E1 and E2, the cells are deflected and move out from O2 while the beads remain in the original direction and still move out from O1 (Movie S2). These results indicate that the PC-3 cells experience strong positive DEP force in the positive y direction (Figure 1b) while the DEP response of the beads is negative, which agrees with the modeling analysis discussed above. When the cells and beads pass through the electrode regions, the DEP force deflects the PC-3 cells towards the strong electric field regions while the polystyrene beads experience negative DEP force and move without any deflection. The separation efficiency of the 20 μm polystyrene beads and PC-3 cells was measured when the applied voltage is 88 Vrms and the frequency is 100 kHz (Figure 4a). Approximately 94.7% of the PC-3 cells are deflected and move toward O2, while ~99% of the polystyrene beads move out from O1 without deflection.
Figure 4.
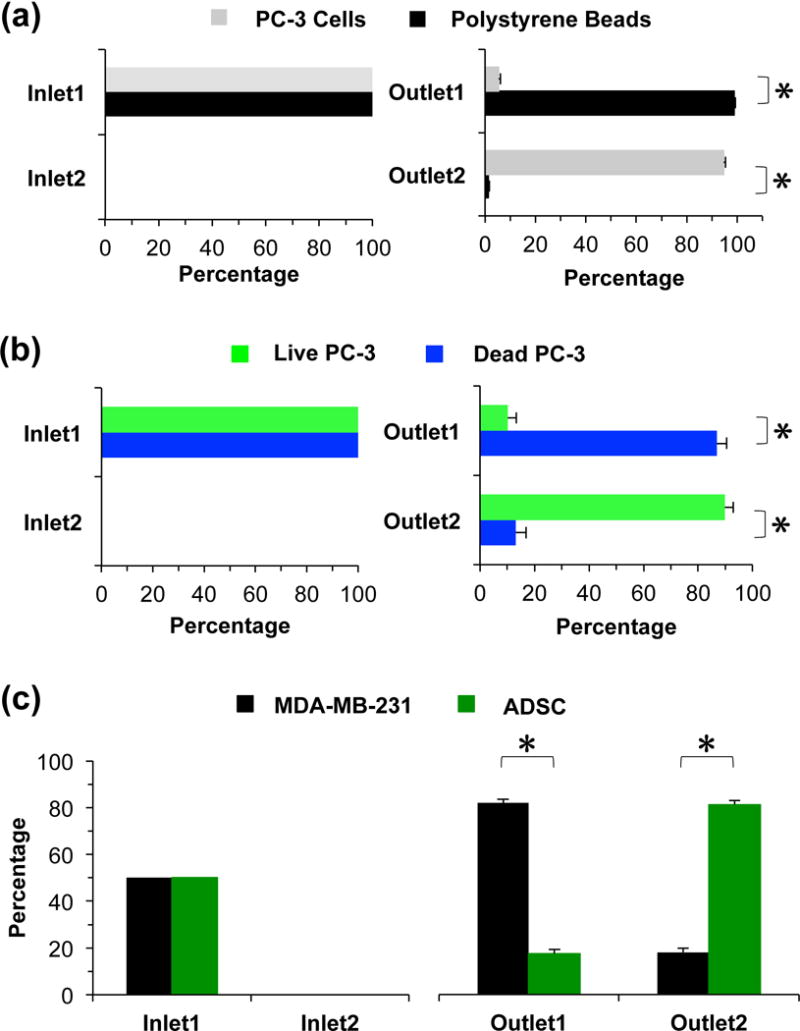
The separation of PC-3 cells and 20 μm polystyrene beads, live and dead PC-3 cells, and ADSCs and MDA-MB-231 cells. (a) The separation efficiency of PC-3 cells and 20 μm polystyrene beads when the voltage is 88 Vrms and the frequency is 100 kHz. (b) The separation efficiency of live and dead PC-3 cells when the voltage is 53 Vrms and the frequency is 50 kHz. (c) Purity of ADSCs and MDA-MB-231 cells in the outlet when the initial cell number ratio is 1:1. The voltage applied is 35 Vrms and the frequency is 50 kHz. Ncell > 500. Flow rates: 50 and 250 μL/hr for the modified and original DEP buffers, respectively.
Separation of live and dead PC-3 cells was tested using this device. As shown in Figure S6a, there is no significant difference between the live and dead cells in terms of size and the average diameter is ~16 μm. Figure S6b shows the fluorescence images of the movement of the live and dead PC-3 cells near the outlets after passing the main channel without and with electric field, where the green and blue fluorescence traces are due to the live and dead PC-3 cells, respectively. When there is no electric field, both the live and dead cells move towards O1 without deflection (Movies S3 and S4). When the external electric field is applied, most of the live cells are deflected and move towards the O2 while most of the dead cells still move towards O1 (Movies S5 and S6). These results indicate that the live PC-3 cells experience positive DEP force in the positive y direction, while the dead PC-3 cells experience weak positive DEP force or negative DEP force, which is in agreement with the modeling analysis discussed above. When the cells pass through the electrode regions, the live PC-3 cells are deflected more by the positive DEP force than the dead PC-3 cells. As shown in Figure 4b, when the electric field of 53 Vrms and 50 kHz is applied to the device, ~89.8% of the live cells are deflected to O2, while only 13.2% of the dead cells are deflected and come out from O2.
We further tested the separation of ADSCs from MDA-MB-231 cancer cells using the DEP device. As shown in Figure S7a, the ADSCs (~22 μm on average) are larger than MDA-MB-231 cells (~12 μm on average). The voltage applied to the electrodes was 35 Vrms and the frequency was 50 kHz. Figure S7b show the florescence and phase images of the cell sample before separation, collection from O1, and collection from O2, respectively (from left to right), where the green-fluorescence cells are the ADSCs and the rest are the MDA-MB-231 cells. In the cell sample before separation, the number ratio of ADSCs to MDA-MB-231 cells is 1:1. As shown in Figure 4c, the percentage of the ADSCs in the collection from O1 is only about 17.5%, while its percentage in the collection from O2 is as high as 81.8%. These results indicate that both ADSCs and MDA-MB-231 cells experience positive DEP force in the positive y direction. However, the deflection of ADSCs is much more than that of MDA-MB-231 cells. These experimental results also agree with the discussion above.
Of note, switching the two DEP buffers in the microfluidic channel could compromise the separation of cells and beads. If the two DEP buffer solutions of unequal conductivities are switched, there are two cases: (1) the particles and cells are still suspended in the modified DEP buffer and (2) the particles and cells are suspended in the original DEP buffer. We conducted further simulation and experimental studies to understand the electric field distribution and the capability of particle and cell separation for both cases The simulation of the conductivity and electric field distributions in the electrode regions for these two cases was conducted in the same way as that for obtaining Figure 2 and the results are shown in Figure S8 (a–b for case 1 and c-d for case 2). For case 1, the beads and/or cells were suspended in the modified DEP buffer and introduced into I2 at 50 μL/hr, and the original DEP buffer was introduced into I1 at 250 μL/hr (please see Figure 1a for the location of I1 and I2). Separation experiments of PC-3 cells and polystyrene beads were conducted under the same conditions as that for obtaining the data shown in Figure 4a except switching the two DEP buffers. No separation of the beads and cells was observed. The cells were only partially deflected even when a much higher voltage (400 Vpp) was applied to the electrodes. This is because the initial position of the beads and cells are close to the parallel electrodes, where the electric field (Figure S8b) is much weaker than that shown in Figure 2b (without switching buffer: the modified DEP buffer was introduced into I1 at 50 μL/hr, and the original DEP buffer was introduced into I2 at 250 μL/hr). For this case 1, the strong electric field is close to the single electrode while the electric field is very weak in the region of modified DEP buffer with particles and cells next to parallel electrodes, which compromises the capability of separating particles and cells using the device. For case 2, the cells were suspended in the original DEP buffer and introduced into I1 at 50 μL/hr, and the modified DEP buffer was introduced into I2 at 250 μL/hr. Experiments on deflection of live PC-3 cells were conducted in the same way as that for obtaining the data shown in Figure 3b (where live PC-3 cells were deflected when electric field was applied) except switching the two DEP buffers. No deflection of the live PC-3 cells was observed even when 400 Vpp was applied to the electrodes. Again, the strong electric field is close to the single electrode under this case (Figure S8d). As the cells experience positive DEP force that is towards the strong electric field region, but they are already in the strong electric field regions and cannot be deflected. Therefore, no cell separation could be achieved under this case either.
Lastly, it is worth noting that the cell viability after separation under the aforementioned conditions is high for all the three types of cells (Figure S9). In other words, the electric field and DEP device do little harm to the cells during the experiments. Ionic liquid, [BMIM][PF6], was used as the liquid electrodes in this study. When its concentration is low, such as at 0.1 mM, [BMIM][PF6] does no harm to human cells, even after 48 h of incubation at 37 °C.40,41 In this study, there is no direct contact between the cells and the ionic liquid. In addition, the cells were collected in cell culture medium after separation, and then cultured or for further use after washing with culture medium. All these contribute to the high cell viability shown in Figure S9.
CONCLUSION
In this study, a novel DEP device with self-assembled liquid electrodes (ionic liquid) was developed to separate particles or human cells continuously by positive DEP. This device is very affordable and can be fabricated easily, as no expensive metal electrodes are needed. With proper flow rate conditions, stable interface between the ionic liquid and DEP buffer was established dynamically. Moreover, the conductivity-induced electric field gradient was used to achieve efficient DEP separation of cells and particles in the device. Our data suggest the great potential of cell separation based on the conductivity-induced DEP using affordable microfluidic devices without any metal electrode.
Supplementary Material
Acknowledgments
This work was partially supported by grants from American Cancer Society (ACS) (120936-RSG- 11-109-01-CDD) and NIH (R01EB012108 and R01CA206366).
References
- 1.Armstrong DW, Schneiderheinze JM, Kullman JP, He LF. Fems Microbiol Lett. 2001;194:33–37. doi: 10.1111/j.1574-6968.2001.tb09442.x. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera CR, Yager P. Electrophoresis. 2001;22:355–362. doi: 10.1002/1522-2683(200101)22:2<355::AID-ELPS355>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Fizazi K, Morat L, Chauveinc L, Prapotnich D, De Crevoisier R, Escudier B, Cathelineau X, Rozet F, Vallancien G, Sabatier L, Soria JC. Ann Oncol. 2007;18:518–521. doi: 10.1093/annonc/mdl419. [DOI] [PubMed] [Google Scholar]
- 4.Girod M, Armstrong DW. Electrophoresis. 2002;23:2048–2056. doi: 10.1002/1522-2683(200207)23:13<2048::AID-ELPS2048>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LWWM. Clin Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 6.Naoe M, Ogawa Y, Morita J, Omori K, Takeshita K, Shichijyo T, Okumura T, Igarashi A, Yanaihara A, Iwamoto S, Fukagai T, Miyazaki A, Yoshida H. Cancer. 2007;109:1439–1445. doi: 10.1002/cncr.22543. [DOI] [PubMed] [Google Scholar]
- 7.Bhagat AA, Bow H, Hou HW, Tan SJ, Han J, Lim CT. Med Biol Eng Comput. 2010;48:999–1014. doi: 10.1007/s11517-010-0611-4. [DOI] [PubMed] [Google Scholar]
- 8.Haishui Huang MS, Heisler-Taylor Tyler, Kiourti Asimina, Volakis John, Lafyatis Gregory, He Xiaoming. Small. 2015;11:5369–5374. doi: 10.1002/smll.201501388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapizco-Encinas BH, Davalos RV, Simmons BA, Cummings EB, Fintschenko Y. J Microbiol Meth. 2005;62:317–326. doi: 10.1016/j.mimet.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y. Electrophoresis. 2004;25:1695–1704. doi: 10.1002/elps.200405899. [DOI] [PubMed] [Google Scholar]
- 11.Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y. Anal Chem. 2004;76:1571–1579. doi: 10.1021/ac034804j. [DOI] [PubMed] [Google Scholar]
- 12.Barbulovic-Nad I, Xuan XC, Lee JSH, Li DQ. Lab Chip. 2006;6:274–279. doi: 10.1039/b513183a. [DOI] [PubMed] [Google Scholar]
- 13.Kang YJ, Li DQ, Kalams SA, Eid JE. Biomed Microdevices. 2008;10:243–249. doi: 10.1007/s10544-007-9130-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhu JJ, Tzeng TRJ, Xuan XC. Electrophoresis. 2010;31:1382–1388. doi: 10.1002/elps.200900736. [DOI] [PubMed] [Google Scholar]
- 15.Zhu JJ, Xuan XC. J Colloid Interf Sci. 2009;340:285–290. doi: 10.1016/j.jcis.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Song HJ, Rosano JM, Wang Y, Garson CJ, Prabhakarpandian B, Pant K, Klarmann GJ, Perantoni A, Alvarez LM, Lai E. Lab Chip. 2015;15:1320–1328. doi: 10.1039/c4lc01253d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim U, Shu CW, Dane KY, Daugherty PS, Wang JYJ, Soh HT. P Natl Acad Sci USA. 2007;104:20708–20712. doi: 10.1073/pnas.0708760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng IF, Froude VE, Zhu YX, Chang HC, Chang HC. Lab Chip. 2009;9:3193–3201. doi: 10.1039/b910587e. [DOI] [PubMed] [Google Scholar]
- 19.Li HB, Bashir R. Sensor Actuat B-Chem. 2002;86:215–221. [Google Scholar]
- 20.Shafiee H, Sano MB, Henslee EA, Caldwell JL, Davalos RV. Lab Chip. 2010;10:438–445. doi: 10.1039/b920590j. [DOI] [PubMed] [Google Scholar]
- 21.Salmanzadeh A, Romero L, Shafiee H, Gallo-Villanueva RC, Stremler MA, Cramer SD, Davalos RV. Lab Chip. 2012;12:182–189. doi: 10.1039/c1lc20701f. [DOI] [PubMed] [Google Scholar]
- 22.Zellner P, Shake T, Sahari A, Behkam B, Agah M. Anal Bioanal Chem. 2013;405:6657–6666. doi: 10.1007/s00216-013-7123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanayakkara YS, Perera S, Bindiganavale S, Wanigasekara E, Moon H, Armstrong DW. Anal Chem. 2010;82:3146–3154. doi: 10.1021/ac9021852. [DOI] [PubMed] [Google Scholar]
- 24.Singh G, Kumar A. Indian J Chem A. 2008;47:495–503. [Google Scholar]
- 25.Stoppa A, Hunger J, Buchner R, Hefter G, Thoman A, Helm H. J Phys Chem B. 2008;112:4854–4858. doi: 10.1021/jp800852z. [DOI] [PubMed] [Google Scholar]
- 26.Morgan H, Green NG, Ramos A, Garcia-Sanchez P. Appl Phys Lett. 2007:91. [Google Scholar]
- 27.Ren YK, Jiang HY, Yang HK, Ramos A, Garcia-Sanchez P. J Electrostat. 2009;67:372–376. [Google Scholar]
- 28.Ermolina I, Morgan H. J Colloid Interf Sci. 2005;285:419–428. doi: 10.1016/j.jcis.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Kuokkanen MH. Cent Eur J Phys. 2010;8:178–183. [Google Scholar]
- 30.Paul R, Kaler KVIS, Jones TB. J Phys Chem-Us. 1993;97:4745–4755. [Google Scholar]
- 31.Tsukahara S, Sakamoto T, Watarai H. Langmuir. 2000;16:3866–3872. [Google Scholar]
- 32.Wenfeng Liang SW, Qu Yanli, Dong Zaili, Lee Gwo-Bin, Li Wen J. Nano/Micro Engineered and Molecular Systems. 2011 [Google Scholar]
- 33.Mikko Haapalainen AM. Instrumentation and Measurement Technology Conference. 2011 [Google Scholar]
- 34.Watarai H, Sakamoto T, Tsukahara S. Langmuir. 1997;13:2417–2420. [Google Scholar]
- 35.Davey C, Markx GH, Kell DB. Pure Appl Chem. 1993;65:1921–1926. [Google Scholar]
- 36.Patel PM, Bhat A, Markx GH. Enzyme Microb Tech. 2008;43:523–530. [Google Scholar]
- 37.Patel S, Showers D, Vedantam P, Tzeng TR, Qian SZ, Xuan XC. Biomicrofluidics. 2012:6. doi: 10.1063/1.4732800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahey MD, Voldman J. Anal Chem. 2008;80:3135–3143. doi: 10.1021/ac7020568. [DOI] [PubMed] [Google Scholar]
- 39.Pethig R, Menachery A, Pells S, De Sousa P. J Biomed Biotechnol. 2010 doi: 10.1155/2010/182581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cvjetko M, Radosevic K, Tomica A, Slivac I, Vorkapic-Furac J, Srcek VG. Arh Hig Rada Toksiko. 2012;63:15–20. doi: 10.2478/10004-1254-63-2012-2132. [DOI] [PubMed] [Google Scholar]
- 41.Stepnowski P, Skladanowski AC, Ludwiczak A, Laczynska E. Hum Exp Toxicol. 2004;23:513–517. doi: 10.1191/0960327104ht480oa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


