Abstract
Objectives
In zebrafish embryos, sprouts from the axial vein have lymphangiogenic potential, as they give rise to the first lymphatics. Here, we studied whether Notch signaling, which regulates cell fate decisions and vessel morphogenesis, controls lymphatic development.
Methods and results
Knockdown of Dll4 or its receptors Notch-1b or Notch-6 in zebrafish impaired lymphangiogenesis. Dll4/Notch silencing reduced the number of sprouts giving rise to the string of parchordal lymphangioblasts; instead, sprouts connecting to the intersomitic vessels were formed. At a later phase, Notch silencing impaired navigation of lymphatic intersomitic vessels along their arterial templates.
Conclusion
These studies imply critical roles for Notch signaling in the formation and wiring of the lymphatic network.
The lymphatic vasculature regulates interstitial fluid homeostasis, fat resorption, immune defense, inflammation and metastasis.1 In mammals, venous blood vascular endothelial cells (BECs) differentiate to lymphatic endothelial cells (LECs).1 In response to Sox18, Prox-1 induces lymphatic transdifferentiation of venous BECs.1, 2 Additional cues must regulate lymphatic development, but their nature remains unknown. Another outstanding question is how lymphatics become wired into a stereotyped network. Deep lymphatics regularly fasciculate with other vessels and track along arteries.1, 3 Similar to blood vessels,4 lymphatic sprouts have tip cells with filopodia to probe guidance cues.5 While molecules such as VEGFR-3, VEGF-C, Neuropilin-2, Ccbe1 regulate lymphatic migration,1, 6 navigation of lymphatics remains poorly understood. Thus, the mechanisms and molecules underlying lymphatic development and wiring remain largely unknown.
Intriguingly, despite the venous origin of lymph vessels, several molecules involved in arterial BEC regulation, also regulate lymphangiogenesis. For instance, EphrinB2, an initial marker of arterial BECs,7, 8 regulates lymphatics later in development.1 Sox18, together with Sox7, is required for arterial differentiation and later regulates lymphatic competence.2. This relationship between “arterial” factors and lymphangiogenesis, as well as the anatomical congruence between arteries and lymphatics8–11 prompted us to investigate whether Notch also regulates lymphatic development. Notch and its ligand Dll4 seemed intriguing candidates, given their role in vessel branching.4 Using gene silencing methods in zebrafish, we revealed novel roles for Dll4/Notch signaling at multiple steps during early lymphangiogenesis.
METHODS
Zebrafish husbandry
Transgenic zebrafish lines used were Fli1:eGFPy1,12 Flt1:YFP, kdr-l:mCherry, Stab1:YFP, Fli1:DsRed,6 Tp1bglob:eGFP,13 and intercrosses. Embryos and fish were grown and maintained as described.6, 14 All animal experimentation was approved by the institutional ethical committee.
Morpholino injection
Morpholinos (Gene Tools, LLC, Corvallis; Supplemental Table I) were injected at the indicated doses, as described.14 Phenotyping data are pooled data from at least 3 independent experiments, with analysis of 33 to 185 injected embryos per dose. Screening methods for evaluation of lymphatic development and functionality are detailed in Supplemental Methods.
RNA analysis
Whole-mount in situ hybridization of dechorionated embryos using antisense probes for the indicated genes (see Supplemental Methods) was performed as described.14 qRT-PCR was performed on whole embryo extracts or on FACS-sorted embryo cells after in vivo labeling of LECs as described in Supplemental Methods.
Cell culture
Proliferation, migration and expression analyses of LECs or HUVECs are detailed in Supplemental Methods.
Statistical analysis
Each gene-specific morpholino was always compared to a control morpholino or vehicle. To determine the penetrance of the phenotype, we counted the number of embryos, exhibiting different phenotype severities, which were analyzed by Chi-square. Pairwise comparisons were performed by two-sided t-test. Asterisks represent a significance level of P<0.05.
RESULTS
Knockdown of Dll4 and Notch-1b/6 impairs thoracic duct formation
To explore a role for Notch signaling in lymphatic development (Supplemental Note I; Supplemental Figure I), we silenced every known zebrafish orthologue of the Notch ligands (DeltaA–D, Dll4, Jagged-1a/b, Jagged-2) and receptors (Notch-1a/b, -5, -6) as well as of the Notch activating presenilins-1/2 in Fli1:eGFPy1 zebrafish embryos, in which lymphatic, arterial and venous endothelial cells (ECs) are labeled.12, 15, 16 Submaximal silencing conditions were used (Supplemental Note II), that did not affect general or blood vascular development (Supplemental Figure II, III). Development of the thoracic duct (TD), the first functional lymphatic formed in the trunk in-between the dorsal aorta (DA) and the posterior cardinal vein (PCV), was analyzed (for acronyms, Supplemental Note III).
Dll4 morpholino knockdown (Dll4KD) inhibited TD formation. Upon injection of a morpholino affecting Dll4 mRNA splicing (Dll4SPL; 10 ng), the TD failed to form at all by 6 dpf in 52% of morphant embryos, indicating that lymphatic development was completely aborted (Figure 1A,B,D). In another 27% of Dll4SPL embryos, the TD formed over only 10–30% of its normal length, while in another 15% of morphant embryos, the TD formed over 30–90% (Figure 1D). Follow-up studies at 12 dpf revealed that, in embryos with intermediate defects, the TD segments that did form failed to reconstitute the entire TD and to compensate for the lymphatic failure in nearby somites (not shown). Dll4KD embryos without TD at 6 dpf also failed to form a TD, even partially, at later stages (Supplemental Figure II G,H), indicating that lymphatic development was not simply delayed but aborted. Similarly, incomplete silencing of Notch-1b and, to a lesser extent, Notch-6, impaired TD formation (Figure 1C,E). Of note, their mammalian orthologues, Notch-1 and Notch-2, are expressed in LECs.10, 17 As Notch-1b downregulation causes more penetrant lymphatic defects, only data for Notch-1bKD are shown.
Figure 1. Role of Notch in TD formation.
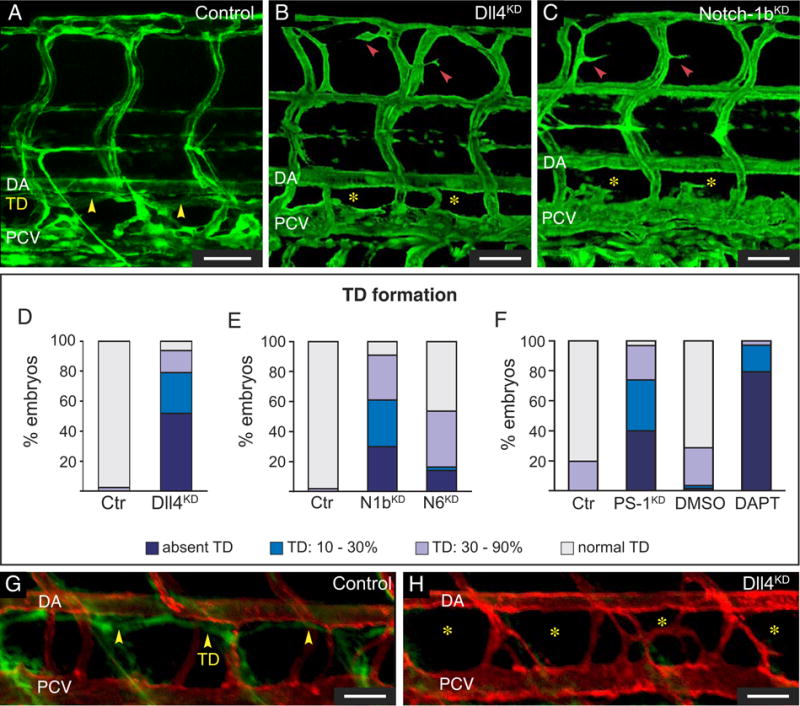
A–C, Confocal images of GFP+ vessels in Fli1:eGFPy1 embryos. Normal TD in control (yellow arrowheads; A), and absent TD in Dll4KD (B) and Notch-1bKD (C) embryos. Yellow asterisks: TD absence; red arrowheads: minimal hyperbranching of ISVs. D–F, Percentage of affected embryos in control (N=122 in D; 185 in E; 87 in F) or Dll4KD embryos (N=80; 10ng Dll4SPL; P<0.001; D); Notch-1bKD (N1bKD) embryos (N=84; 15ng Notch-1bSPL; P<0.001; E), Notch-6KD (N6KD) embryos (N=63; 15ng Notch-6SPL; P<0.001; E), PS-1KD embryos (N=65; 2.5ng PS-1ATG1; P<0.001; F), or embryos treated with DMSO (N=171; F) or DAPT (N=34; 25 μM; P<0.001; F). G,H, Lymphangiography in 7-dpf kdr-l:mCherryRed embryos revealed normal uptake and drainage of a green dye by the TD in the control (yellow arrowheads; G), but not in the Dll4KD embryo (yellow asterisks; H). Bars: 50 μm.
Similar TD defects were obtained with morpholinos, targeting the ATG of Dll4 (Dll4ATG) or Notch-1b (Notch-1bATG) (not shown), but silencing of the Notch ligands DeltaA-D, Jagged-1a/b, Jagged-2 or of Notch-1a or Notch-5 (orthologue of mammalian Notch-3), did not induce lymphatic defects (not shown). Finally, inhibition of the γ-secretase complex (which proteolytically activates Notch)18 confirmed the involvement of Notch in lymphatic development. Both morpholino knockdown of Presenilin-1 (PS-1) (but not PS-2) and pharmacological inhibition of γ-secretase activity by DAPT18 impaired TD formation (Figure 1F; Supplemental Figure IV Q,R; Supplemental Note II).
Lymphangiography in 7-dpf kdr-l:mCherryRed Dll4KD embryos (in which only blood vessels express mCherryRed) revealed no drainage of fluorescent dye in the region where the TD normally forms, confirming that the lack of a GFP+ TD in Fli1:eGFPy1 Dll4KD embryos was not due to reduced expression of GFP, but to actual absence of the vessel itself (Figure 1G,H). This assay further showed that partial TD fragments were not functional (not shown).
Notch is required for parachordal lymphangioblast string formation
We next analyzed whether silencing of Notch impaired development of the parachordal lymphangioblast (PL) cells6 at the horizontal myoseptum, as these precursors contribute to TD formation (Supplemental Note I, Supplemental Figure I). At 52 hpf, formation of the PL string was completely formed in 53% and largely completed in 40% of embryos (Figure 2A). By contrast, in Dll4SPL embryos, the PL string was completely absent in 38% and formed only in a few segments in 27% of embryos (Figure 2A). Largely comparable fractions of Dll4SPL embryos exhibited similar types of PL string and TD defects (compare Figure 1D with Figure 2A), suggesting that the TD defects were, at least in part, attributable to defects in PL string formation. Imaging of lymphangiogenic structures in Dll4SPL embryos using the Stab1:YFP line,6 which primarily visualizes venous and lymphatic ECs, confirmed these findings (Figure 2B,C). A similar absence of the PL string was observed when using the Dll4ATG morpholino (not shown), or upon knockdown of Notch-1b (Figure 2A) or Notch-6 (not shown). Since the string of PL cells forms as a result of sprouting from the PCV (Supplemental Note I; Supplemental Figure I),6 these findings suggest that Notch signaling acts in part at very early steps.
Figure 2. Silencing of Notch blocks PL and lymphangiogenic sprout formation.
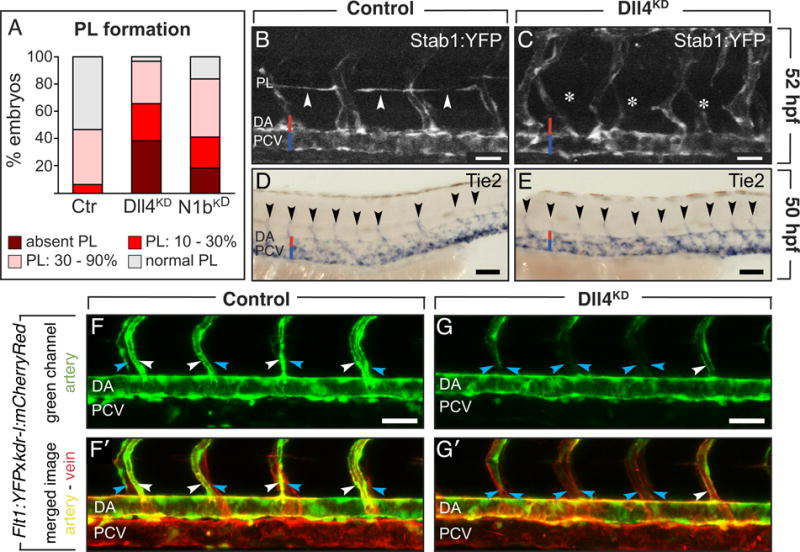
A, Percentage of 52-hpf embryos with affected PL in control (N=73), Dll4KD embryos (N=55; 10ng Dll4SPL; P<0.001) or Notch-1bKD (N1bKD) embryos (N=49; 20ng Notch-1bATG; P<0.001). B,C, Confocal images of 52-hpf Stab1:YFP embryos, showing normal PL in controls (arrowheads; B), but absence in Dll4KD embryos (asterisks; C). D,E, Whole-mount in situ Tie2 staining at 50 hpf, revealing normal numbers of secondary sprouts (arrowheads) in control (D) and Dll4KD (E) embryos. F,G, Confocal images of vessels in Flt1:YFPxkdr-l:mCherryRed embryos: kdr-l:mCherryRed marks venous and arterial vessels red (red channel not shown), Flt1:YFP labels arterial vessels green (F,G), and merged images show arterial vessels yellow and venous vessels red (F′,G′). Lateral views with left and right side ISVs partially superimposed. Imaging was at 54 hpf, when secondary angiogenic sprouts had already connected to primary ISVs, which were changing arterial to venous identity in a ventral-to-dorsal pattern. In controls (F,F′), half of the aISVs became connected by angiogenic sprouts from the PCV and acquired a venous identity, thereby losing their green arterial signal (blue arrows; F) and becoming red only (blue arrows; F′), while the other half of the ISVs remained connected to the DA and were green (white arrows; F) or yellow in the merged image (white arrows; F′). By contrast, in the Dll4KD embryo (G,G′), most ISVs lost their green arterial marker (blue arrows; G; note the single white arrow), and became marked in red only (blue arrows in G′). White arrow in G,G′ denotes a residual aISV retaining its green (G) or yellow (G′) label. Bars: 50μm (B,C,F,G); 100μm (D,E).
Dll4 silencing reduces the fraction of lymphangiogenic sprouts
We then studied whether inhibition of Notch acts during branching of PL-forming secondary sprouts from the PCV (termed “lymphangiogenic” secondary sprouts denoting that they participate in the process that leads to the formation of lymphatic structures, but not blood vessels; Supplemental Note I). Whole-mount staining for Tie2, which marks all secondary sprouts,19 showed a normal total number in Dll4KD embryos (N=20; Figure 2D,E). However, high-resolution imaging of 4-dpf Fli1:eGFPy1 embryos revealed alterations in the proportion of venous intersomitic vessels (vISVs), connected to the PCV. In control embryos, half of the ISVs were vISVs (% of total ISVs: 54 ± 1%; N=49); by contrast, in Dll4SPL embryos, 82 ± 1% of the ISVs were connected to the PCV and thus vISVs (N=97, P<0.05 versus control). Similar findings were obtained in Notch-1bSPL embryos (vISVs, % of total: 69 ± 2.7%; N=27, P<0.05 versus control). Since vISVs can only be formed via connection of a secondary “angiogenic” sprout to a primary ISV (Supplemental Note I), these findings, and the observation that silencing of Dll4, Notch-1b or Notch-6 aborted PL string formation in a substantial fraction of embryos, show that a fraction of secondary sprouts, that would normally have been lymphangiogenic, were angiogenic, thereby impairing TD formation (Figure 6A,B).
Figure 6. Schematic model of Notch in lymphatic development.
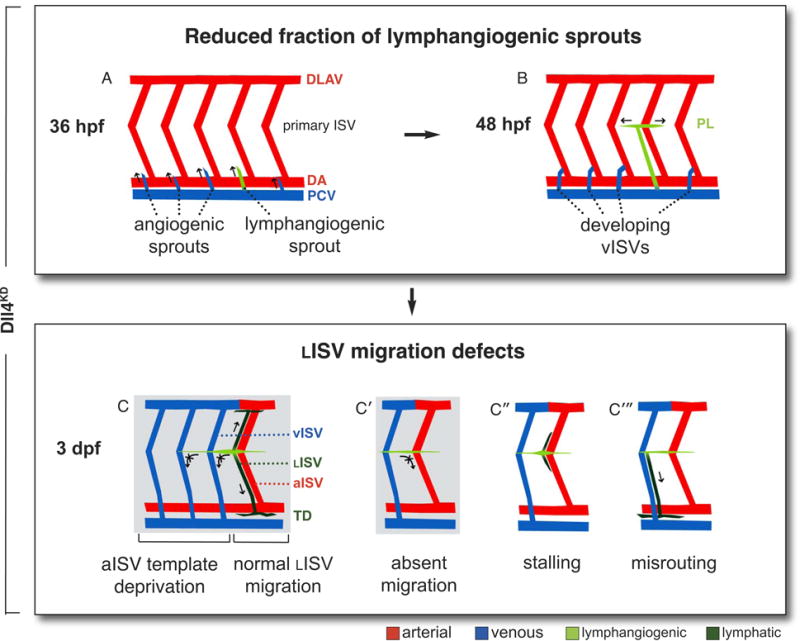
Scheme, illustrating the different lymphatic defects in Dll4KD embryos (normal lymphatic development, Supplemental Figure I). Permanent lymphatic structures (LISV, TD) are dark green; transient lymphangiogenic structures (lymphangiogenic secondary sprouts; parachordal lymphangioblasts) are light green. A,B, REDUCED FRACTION OF LYMPHANGIOGENIC SPROUTS, resulting in underdevelopment or absence of the PL string, with accompanying overrepresentation of angiogenic secondary sprouts. C–C‴, LISV MIGRATION DEFECTS: C, As a result of vISV overrepresentation, LISV-PLs are deprived of their normal aISV guidance template. C′–C‴, LISV formation is further impaired by additional navigation defects, most frequently because LISV-PLs cells bypass their turning point and never initiate ventral radial migration (C′), or occasionally make the turn but then stall (C”). More rarely, navigating LISV-PLs become misrouted along vISVs (C‴). The most frequent defects are boxed in grey.
We also used high-resolution video-imaging of the double transgenic reporter line Flt1:YFPxkdr-l:mCherryRed,6 labeling venous cells red (CherryRed+) and arterial cells yellow (YFP+CherryRed+) in merged images.6 In control embryos, half of the ISVs had a red venous color and the other half had a yellow arterial color (Figure 2F,F′). In contrast, in Dll4KD embryos with severe lymphatic defects, nearly all yellow arterial ISV (aISV) connections with the DA had disappeared (single white arrow in Figure 2G,G′; Supplemental Movies I, II). Thus, a supernumerary fraction of vISV-producing angiogenic sprouts is formed in Dll4KD embryos at the expense of lymphangiogenic sprouts, that would otherwise go on to form the PL string.
Dll4/Notch promotes lymphatic characteristics in vitro
To evaluate whether Notch activation in venous ECs could induce lymphatic properties, we co-cultured human umbilical venous ECs (HUVECs, which express NOTCH-1, but negligible levels of PROX-1; not shown), with COS cells expressing DLL4 (COSDll4) or a control vector (COSCTR), and analyzed by RT-PCR with human gene-specific primers the expression of lymphatic markers. Expression levels of the lymphatic markers PROX-1, VEGFR3, LYVE-1, and SOX18 in COSDll4-activated HUVECs were moderately to distinctly elevated (Figure 3). Notably, expression of EPHRINB2, which is regulated by Notch and has been implicated in both arterial and lymphatic processes,1, 7 and COUP-TFII which is expressed in both venous and lymphatic ECs,20 were also upregulated, but levels of other blood vessel markers (ENDOGLIN, VE-CADHERIN, CD31) were not or only minimally affected (Figure 3). The upregulation of lymphatic markers was abolished by treatment of the cells with DAPT (30 μM; not shown).
Figure 3. Notch activation by Dll4 promotes lymphatic characteristics in vitro.
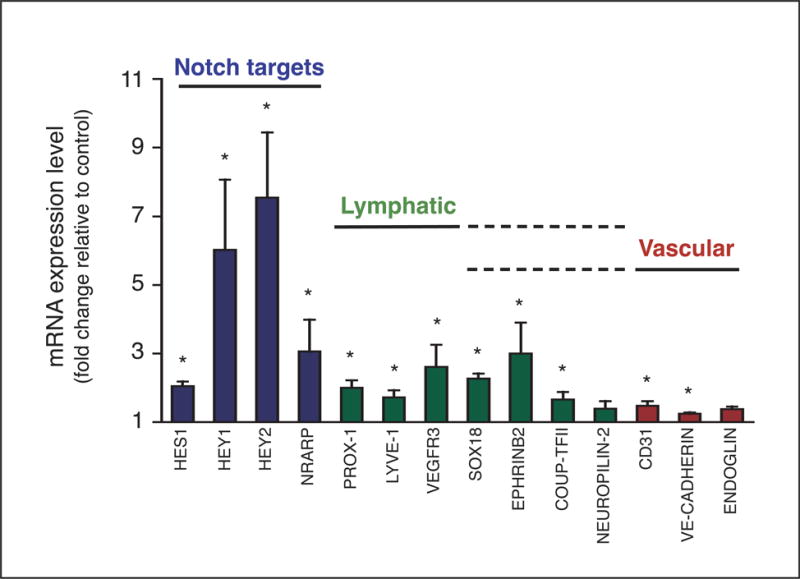
RT-PCR of HUVECs, co-cultured with COS cells expressing hDll4 (COSDll4) or control GFP (COSCTR), confirming upregulation of Notch targets (HES1, HEY1, HEY2, NRARP; blue bars) and revealing enhanced lymphatic marker expression (PROX-1, LYVE-1, VEGFR3, SOX18, EPHRINB2, green bars), while vascular genes (CD31, VE-CADHERIN; ENDOGLIN; red bars) were only minimally affected. COUP-TFII was also upregulated. NEUROPILIN-2 was not affected. Lymphatic/arterial and lymphatic/venous genes are marked by the overlapping dashed lines. Results are fold change in HUVEC/COSDll4 co-culture versus HUVEC/COSCTR. Mean±SEM; N=3–11; *, P<0.05.
Silencing of Dll4 impairs PL cell migration along aISVs
From 60 hpf onwards, PL cells switch to radial migration, and navigate ventrally and dorsally alongside aISVs, whereby they form lymphatic intersomitic vessels (LISVs) (Supplemental Note I). Since the TD failed to form in a fraction of Dll4SPL embryos (25%) despite the presence of a partial PL string, we further explored whether Notch signaling affects LISV formation. In control embryos, LISV-PLs (PL cells that formed LISVs) migrated exclusively along aISVs, suggesting that vISVs are not permissive (Figure 4A,B). Since there were more vISVs and fewer aISVs in Dll4KD embryos, migrating LISV-PLs were deprived from their arterial template and could therefore not contribute to TD formation (Figure 4C; 6C). This was the most common migration defect. Intriguingly, even when residual aISVs formed in Dll4SPL embryos, LISV-PLs sometimes bypassed the aISV post, failing to turn and migrate along aISVs (Figure 4D; 6C′). Indeed, in Dll4SPL embryos with a nearly complete PL string (>90% of its length; N=61), 49 ± 6% of their aISVs were not accompanied by LISV-PLs, compared to only 15 ± 4% in controls (N=29; P<0.05).
Figure 4. Incomplete silencing of Notch perturbs lymphatic navigation.
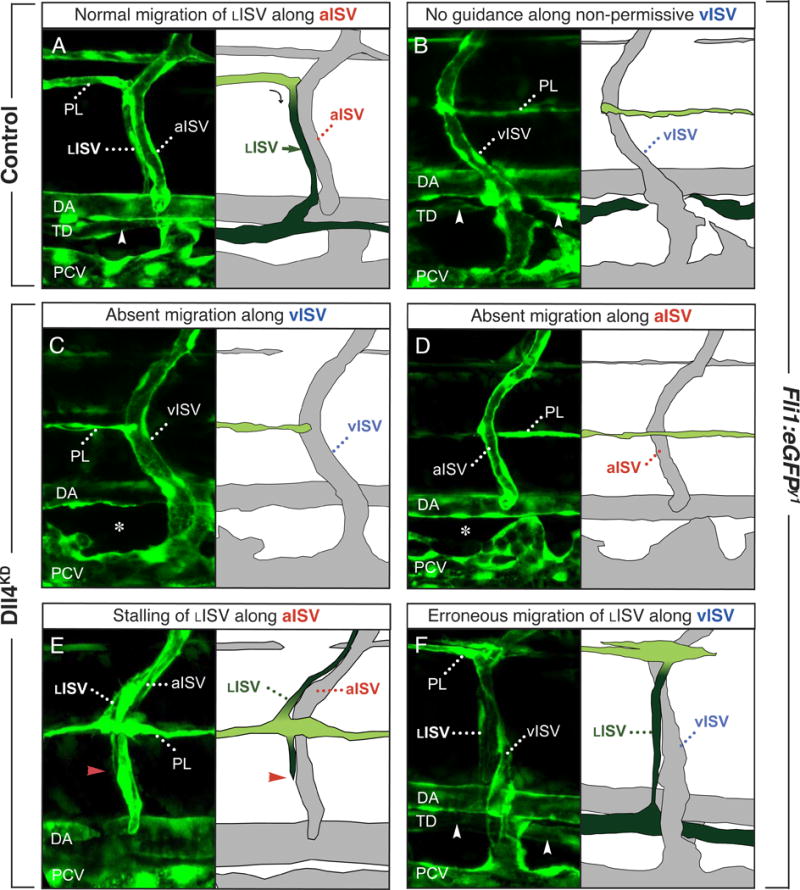
Confocal images with accompanying schematic redrawing of the navigation routes of LISVs along aISVs or vISVs in 4-dpf control (A,B) and Dll4KD (C–F) Fli1:eGFPy1 embryos. Permanent lymphatic structures (LISV, TD) are dark green; transient lymphangiogenic structures (PL) are light green. A,B, In control embryos, LISV-PLs navigate alongside aISVs and establish a continuous TD (arrowhead). Note how LISVs “creep” over their aISV guidance templates (A). LISV-PLs never navigate along vISVs in control embryos (B). C–F, Navigation defects in Dll4KD embryos. C, In a large fraction of morphant somites, LISV-PLs lack migration templates because fewer aISVs develop. As LISV-PLs do not normally migrate along vISVs, no TD was formed in these somites (asterisks). D, In other morphant somites, LISV-PLs bypassed the point of turning at the aISVs, and failed to switch to radial migration. E, In a small fraction of somites, LISV-PLs accomplished to make the turn and switched to radial migration, but then stalled (red arrowhead denotes the arrested tip of a navigating LISV). F, In most Dll4KD embryos, vISVs were not permissive to guide LISV-PLs, but, occasionally, LISV-PLs erroneously navigated alongside a vISV.
Other, much less frequent LISV defects included LISV-PLs that turned ventrally alongside the aISV, but stalled (Figure 4E; 6C”), or even, in a few cases, misrouted LISV-PLs migrating along vISVs (Figure 4F; 6C‴). In vitro studies revealed that Notch did not regulate LEC migration/motility, proliferation, or lymphatic capillary tube formation or sprouting (Supplemental Figure V; not shown).
Expression of Dll4 and Notch
Whole-mount in situ hybridization (ISH) in control embryos at 30 hpf, when secondary sprout formation starts, showed that Dll4 was detectable in the DA but not in the PCV (Figure 5A,B), in line with previous reports.19, 21, 22 Notch-1b was strongly expressed in the DA (Figure 5C,D), while a much weaker signal appeared dispersed in certain endothelial cells of the dorsal part of the PCV, though the low Notch-1b signal approached the detection limit of available techniques (Supplemental Figure VI).
Figure 5. Expression of Dll4./.Notch-1b.
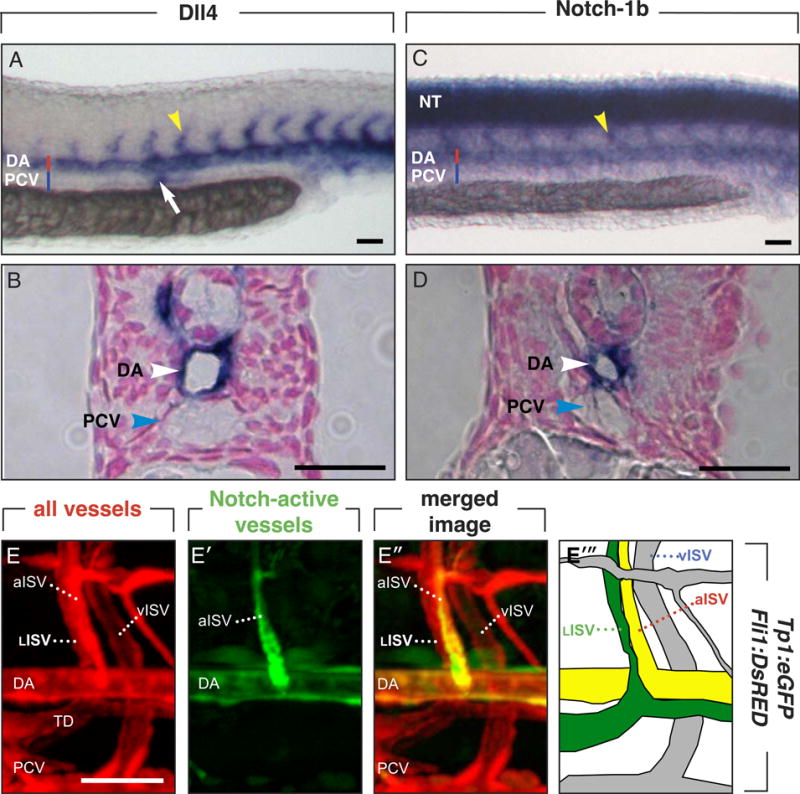
A–D, Whole-mount embryos at 30 hpf, when lymphangiogenic sprouting occurs, stained for Dll4 (A,B) or Notch-1b (C,D); panels B,D show cross-sections of the respective embryos. Primary ISVs are indicated by yellow arrowheads (A,C). A,B, Dll4 expression was detected in the DA and primary aISVs, pronephric duct (white arrow; A). C,D, Notch-1b is strongly expressed in the neural tube (NT), DA and primary aISVs. E, Confocal images of Tp1bglob:eGFPxFli1:DsRed embryos, in which Fli1:DsRed marks blood and lymph vessels in red (E) and Tp1bglob:eGFP labels cells with activated canonical Notch activity in green (E′). The merged image shows arterial vessels (DA; aISV) with active Notch in yellow (green-red), while the LISV are only red (E”). In the schematic representation, the lymphatic structures are indicated in green, Notch-activated vessels in yellow, and other vessels in grey (E‴). Representative images of arterial activation of Notch in a 6-dpf embryo are shown (for technical reasons), but similar data were obtained at 60 hpf. Bars: 50 μm.
We also developed a new technique to isolate LECs from zebrafish embryos. When TRITC-dextran dye is injected intramuscularly in 4 week old Fli1:eGFPy1 embryos, the red dye is taken up by LECs but not BECs via pinocytosis, allowing FACS sorting of red/green LECs. By RT-PCR, low Notch-1b transcript levels were detected in these LECs (copies Notch-1b/105 copies β-actin: 6.9 ± 0.77, N=4). However, TRITC-dextran ‘LEC labeling’ is only feasible in large 4 week-old embryos, but not in small early stage embryos, precluding us from quantifying Notch-1b expression in early lymphatic development.
Dll4 and Notch-1b were also detected by ISH in primary ISVs at 30 hpf (Figure 5A–C).21, 22 As ISH is technically challenging in embryos beyond 2 dpf, we analyzed Notch expression during LISV-PL migration in Tp1bglob:eGFPxFli1:DsRed fish, in which all ECs are red, and cells with canonical Notch activity are green (GFP driven by a promotor containing 12 Su(H) binding sequences13). Imaging at the time when PL cells turn and switch to radial ventral migration revealed that the DA and aISVs are yellow in the merged image, indicating that canonical Notch signaling was active in arterial vessels, but not in LISVs or vISVs (Figure 5E).
DISCUSSION
The key finding of this study is that incomplete silencing or pharmacological inhibition of Notch impaired lymphatic development in zebrafish. Phenotypic analysis indicates that Notch signaling regulates the formation of lymphangiogenic sprouts and their descendent PL cells, which give rise to the TD (Figure 6A,B). At a later stage, Notch is required for guided migration of LISV-PLs along aISVs (Figure 6C–C‴).
Role of Notch in lymphangiogenic secondary sprout formation
Our results reveal that Notch, in addition to its role in blood vessel morphogenesis and arterial development,4, 9 also regulates lymphatic development. Half of the Notch hypomorphant embryos failed to form a TD without later rescue, indicating lymphatic abortion rather than delay. The earliest identifiable abnormality, the increased fraction of venous ISVs, indicated a defect at the level of the secondary sprouts from the PCV, where fewer lymphangiogenic but more angiogenic sprouts developed (Figure 6A,B). Also, most embryos, surviving DAPT treatment at stages when lymphangiogenic sprouting was initiated, did not form a TD, further suggesting an early role for Notch in lymphatic development (not shown). The hypomorphant phenotypic change correlated with defective formation of the PL and TD, and could result from defects in LEC fate acquisition, migration, proliferation, survival and/or other cellular processes contributing to sprout formation and maintenance.
How Notch signaling regulates lymphatic development remains unresolved. Based on the present and recent other studies, three possible (non-exclusive) models can be considered to explain our findings. A first explanation is that Notch silencing altered blood vessel development and, secondarily, influenced lymphatic development. Previous studies documented that arterial differentiation is impaired by inhibition of multiple Notch signaling pathways (for instance by a dominant negative Su(H)),7 but not by selective silencing of Dll4 21, 22. Our imaging and marker expression analyses are consistent with these findings and reveal that initial formation and differentiation of the PCV, DA and primary ISVs all occurred normally upon incomplete silencing of Notch signaling. Thus, at least by generally accepted criteria of arterial and venous identity, these blood vessels developed normally in Dll4KD and Notch-1bSPL embryos. Nonetheless, we do not exclude the possibility that subtle alterations in arterial characteristics of the primary ISVs might have favored supernumerary connections with secondary sprouts, thereby “entrapping” sprouts that would otherwise have remained lymphangiogenic. Also, Notch silencing resulted in a greater fraction of venous than arterial ISVs; since arterial ISVs act as guidance templates for LISVS, impaired migration of the latter was indeed attributable to such a change in arterial morphogenesis. However, an outstanding question is whether the aISV changes themselves were in fact not caused by defective formation of the lymphangiogenic sprouts in the first instance. Indeed, precisely because lymphangiogenic branches failed to develop in Notch morphants, venous angiogenic sprouts formed instead, which then connected to the primary ISVs and converted them to vISVs.
A second model is that Dll4 and Notch are expressed by the same or adjacent arterial ECs within the DA and that this cis signaling induces the release of paracrine lymphangiogenic factors (such as EphrinB2, VEGF-D1, 23 or an unknown signal), that indirectly instruct venous ECs of the nearby PCV to induce lymphangiogenic sprout formation in a cell non-autonomous manner. A similar indirect model was proposed to explain segregation of the DA from PCV in zebrafish.8 Likewise, during LISV migration, release of a guidance signal from aISVs in response to Dll4/Notch signaling in arterial cells could assist navigation of LISVs to their target projection.
Finally, a third and perhaps the most appealing, but at this stage still speculative, explanation for our data is that arterial Dll4 in the DA signals in trans to Notch on ECs in the PCV, which lies in close juxtaposition at the time of lymphangiogenic sprouting. There are arguments in support and against this model. An argument in favour for a cell autonomous role of Notch in PCV cells is that activation of Notch by Dll4 upregulated several LEC-specific markers in venous ECs in vitro. Expression analysis experiments in vivo yielded inconclusive results. Notch-1b expression was weakly detectable in dispersed dorsal PCV cells, but only at a very low level that approached the detection limit of the techniques used. Notch-1b was also measurable by RT-PCR in isolated LECs in older embryos, but this technique could not be used during early lymphatic development. We therefore acknowledge that the Notch-1b expression results represent a limitation of this study, which precludes us from drawing firm conclusions regarding a cell-autonomous role for Notch in lymphangiogenic sprouting.
Another recent study also documented a cell autonomous role for Notch,24 while a second did not.25 In LEC cultures, Notch signaling reprogrammed lymphatic to arterial cell fate, while Prox1 counteracted this force, thereby allowing fine-tuning of the LEC fate in a delicately balanced feedback.24 These findings should not necessarily be in contradiction to our findings, as they analyzed reprogramming of fully differentiated LECs away from their lymphatic fate, while we used venous BECs to study programming towards the LEC fate in vitro. As the authors of this study mention,24 “LEC-fate may not be governed by a two-way turn ON-OFF switch, but rather by a dial switch that allows a gradient increase or decrease in the lymphatic cell fate force”. Reconciling these and our findings, it seems that Notch levels must be tightly controlled to induce and maintain LEC fate. Low levels of Notch signaling might be required to induce lymphatic fate in venous BECs and, once differentiated into LECs, Prox1 would then secure lymphatic fate by preventing overexpression of Notch, as this would promote arterial cell fate.24 The lower expression of Notch-1 in LECs (this study and 10, 24) than in arterial ECs8–11 supports this model and could also explain why incomplete Notch silencing sufficed to abrogate lymphatic but not arterial development. However, in the absence of more conclusive evidence that Notch silencing abrogates Prox1 induction in PCV cells in the zebrafish model in vivo, a role for Notch in programming LEC fate remains unproven. Also, Notch may regulate other processes than LEC specification in lymphangiogenic sprouting.
A recent study in mice further adds complexity to this model. Indeed, conditional inactivation of RbpJ, a mediator of canonical Notch signaling, in ECs did not alter the expression of lymphatic markers in venous ECs.25 While these data may suggest that Notch signaling is redundant for LEC specification in mammals in vivo, an alternative interpretation is that Notch regulates this process via non-canonical signaling. This might also explain why we could not detect a robust signal in LECs or their precursors in the Tp1bglob:eGFPxFli1:DsRed line. Also, species-specific differences between mammals and zebrafish could account for some of the observations. Overall, whether Notch signaling regulates lymphatic development in a cell-autonomous manner remains to be further elucidated in the future.
Role of Notch in lymphatic migration from the PL
Notch signaling also regulated the formation of LISVs, which arise from the PL cells. Most frequently, the LISV was absent but in other rarer cases, migrating LISV-PLs stalled or became misrouted (Figure 6C–C‴). Our findings suggest that lymphangiogenic EC migration per se (motility) was normal. Also, we did not detect signs of lymphatic regression or retraction (not shown). It is therefore tempting to speculate that LISV defects in Notch-silenced embryos reflect impaired lymphangiogenic cell pathfinding. LISV-PLs navigated in close association along aISV templates, raising the question whether aISVs act as guidance templates for LISV-PLs, reminiscent of how follower axons navigate along a pioneer axon’s pathway or how autonomic nerves use arterial tracks to reach their target.26, 27 Hence, as fewer aISVs are present in Notch morphants because of lymphangiogenic sprouting defects, PL cells are deprived of navigation templates and therefore cannot form LISVs normally (Figure 6C). Other observations that LISV-PLs failed to switch from tangential to radial migration or, more rarely, stalled or selected incorrect paths (Figure 6C′–C‴), are reminiscent of classic neuronal guidance defects. That arteries may act as navigation templates is evidenced by reports that autonomic nerves stall or become misrouted, when these arteries do not produce appropriate guidance cues.27 Su(H)-dependent Notch activity was detectable in aISVs at the time when PL cells switch from tangential to radial migration alongside aISV, indicating that lymphatic navigation is regulated either non-cell autonomously or via non-canonical Notch signaling. Whether and how Notch regulates the production of turning and guidance cues for LISV-PL cells by aISVs or nearby (somitic) cells remains to be determined. Other morphant and mutant zebrafish phenotypes also suggest that LISV development requires arterial-lymphatic congruence 28.
In conclusion, this study revealed a role of Notch in lymphatic development, in part by regulating the initial steps of lymphangiogenic sprouting and PL formation. Moreover, the navigation defects of LISV-PL cells along aISVs suggest that Notch also regulates lymph vessel pathfinding along arteries.
Supplementary Material
Condensed abstract.
Dll4 and its receptors Notch-1b and Notch-6 are involved in the formation and wiring of the lymphatic network in zebrafish. Silencing of Dll4/Notch reduced the number of sprouts giving rise to the string of parachordal lymphangioblasts, and impaired navigation of lymphatic intersomitic vessels along arterial templates.
Acknowledgments
The authors thank AL Harris for LZRSpBMN-WT and LZRSpBMN-DLL4, H Pendeville for Tbx20, and J den Hertog for Dab2 probe.
Sources of funding: IG, FD, KH and WV are sponsored by the IWT-Vlaanderen, Belgium; IS by the EU FP7; CRA by FEBS; JB by Vrienden Hubrecht Stichting; BMH by CJ Martin fellowship; SL by Deutsche Krebshilfe; SSM by the KNAW. This work is supported by the Belgian State – Federal Science Policy Office (IUAP), EU FP6 grant (#LSHG-CT-2004-503573), and Methusalem Grant to PC; VIDI grant to HJD; CARIPLO N.O.B.E.L funding to FC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no competing financial interests.
References
- 1.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Francois M, Koopman P, Beltrame M. SoxF genes: Key players in the development of the cardio-vascular system. Int J Biochem Cell Biol. 2010;42:445–448. doi: 10.1016/j.biocel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 4.De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 5.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 6.Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte-Merker S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41:396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- 7.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 8.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 10.Shawber CJ, Kitajewski J. Arterial regulators taken up by lymphatics. Lymphat Res Biol. 2008;6:139–143. doi: 10.1089/lrb.2008.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emuss V, Lagos D, Pizzey A, Gratrix F, Henderson SR, Boshoff C. KSHV Manipulates Notch Signaling by DLL4 and JAG1 to Alter Cell Cycle Genes in Lymphatic Endothelia. PLoS Pathog. 2009;5:e1000616. doi: 10.1371/journal.ppat.1000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 13.Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell. 2006;10:783–795. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 16.Kuchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16:1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Ota H, Katsube KI, Ogawa JI, Yanagishita M. Hypoxia/Notch signaling in primary culture of rat lymphatic endothelial cells. FEBS Lett. 2007;581:5220–5226. doi: 10.1016/j.febslet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan BM, Herpers R, Witte M, Helotera H, Alitalo K, Duckers HJ, Schulte-Merker S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009;136:4001–4009. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]
- 20.Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai YT. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010 doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 22.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill CF, Urs S, Cinelli C, Lincoln A, Nadeau RJ, Leon R, Toher J, Mouta-Bellum C, Friesel RE, Liaw L. Notch2 signaling induces apoptosis and inhibits human MDA-MB-231 xenograft growth. Am J Pathol. 2007;171:1023–1036. doi: 10.2353/ajpath.2007.061029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J, Yoo J, Lee S, Tang W, Aguilar B, Ramu S, Choi I, Otu HH, Shin JW, Dotto GP, Koh CJ, Detmar M, Hong YK. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010 Mar 29; doi: 10.1182/blood-2009-11-252270. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MPR, Lagutin O, Oliver G. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bak M, Fraser SE. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development. 2003;130:4999–5008. doi: 10.1242/dev.00713. [DOI] [PubMed] [Google Scholar]
- 27.Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bussmann J, Bos LF, Urasaki A, Kawakami K, Duckers HJ, Schulte-Merker S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development. doi: 10.1242/dev.048207. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


