Summary
Background
Platelets utilize proteins and pathways classically reserved for the nuclear niche.
Methods
We determined whether human platelets express retinoicacid-receptor family members, traditionally thought of as nuclear transcription factors, and deciphered the function of RARα.
Results
We found that RARα is robustly expressed in human platelets and megakaryocytes and interacts directly with actin-related protein-2/3 complex (Arp2/3) subunit 5 (Arp2/3s5). Arp2/3s5 co-localized with RARα in situ and regulated platelet cytoskeletal processes. The RARα ligand all-trans retinoic acid (atRA) disrupted RARα Arp2/3 interactions. When isolated human platelets were treated with atRA, rapid cytoskeletal events (e.g. platelet spreading) were inhibited. In addition, when platelets were cultured for 18 h in the presence of atRA, actin-dependent morphological changes (e.g. extended cell body formation) were similarly inhibited. Using in vitro actin branching assays, RARα and Arp2/3-regulated complex actin branch formation was demonstrated. Consistent with inhibition of cytoskeletal processes in platelets, atRA, when added to this branching assay, resulted in dysregulated actin branching.
Conclusion
Our findings identify a previously unknown mechanism by which RARα regulates Arp2/3-mediated actin cytoskeletal dynamics through a nongenomic signaling pathway. These findings have broad implications in both nucleated and anucleate cells, where actin cytoskeletal events regulate cell morphology, movement and division.
Keywords: blood platelets, retinoic acid receptors, actin-related protein 2–3 complex, actin, protein interaction domains and motifs
Introduction
Platelets respond to vascular damage by forming filopodia and lamellipodia that derive from a rapid remodeling of their extensive actin cytoskeleton [1–3]. This process will ultimately lead to platelet spreading over the damaged vascular surface. Platelets are anucleate and thus traditionally viewed as terminally differentiated cells incapable of cell division or fission processes. Nevertheless, recent work demonstrated that human platelets can develop extended and beaded isoforms with long extensions and two or more cell bodies, resembling dumbbells [4,5]. These platelet shape changes depend on regulated actin cytoskeletal reorganization. Therefore, platelets provide an ideal cell model to understand regulatory pathways and protein interactions that initiate and propagate actin-dependent shape changes.
Retinoids, naturally-occurring vitamin A derivatives that regulate cellular differentiation and growth [6], mediate key aspects of cellular development. The retinoid receptors, which include the retinoic-acid-receptor (RAR) and retinoid-x-receptor (RXR) family members, are classically described as nuclear receptors that regulate transcription. Human anucleate platelets are known to contain RXRs, which act in concert with peroxisome proliferator-activated receptors to mediate platelet alphagranule release and aggregation [7–10]. Nevertheless, the expression and function of RARs in platelets and megakaryocytes (MEGS) has not previously been determined. Moreover, a direct regulatory role of the retinoic acid signaling pathway for cytoskeletal reorganization has thus far not been identified.
Here we demonstrate that human platelets and MEGS endogenously express RARα and that in non-genomic fashion RARα directly interacts with the actin branching complex subunit actin-related protein-2/3 complex (Arp2/3)s5. The high-affinity RARα-ligand all-trans retinoic acid (atRA) blocked RARα Arp2/3s5 interactions and inhibited actin-dependent platelet spreading and the formation of extended platelets with two or more cell bodies [4,5]. Blocking Arp2/3 using a specific small molecule inhibitor also prevented platelet spreading, highlighting that Arp2/3 is a key regulator of actin formation in human platelets. Within an in vitro actin branching assay that requires Arp2/3, RARα enhanced actin filament formation. Consistent with inhibition of cytoskeletal processes in platelets, atRA led to dysregulated actin branching. Our findings suggest a previously unidentified, non-genomic role whereby RARα serves as a control checkpoint for actin cytoskeletal events in human platelets.
Material and methods
Reagents and antibodies
The following drugs and reagents were used freshly prepared or from stock solutions: all-trans retinoic acid (atRA; 10 pM, 10 nM, 1 or 10 μM in dimethylsulfoxide [DMSO], Sigma, St Louis, MO, USA) and CK-666 (10 or 40 μM, Merck-Millipore, Billerica, MA, USA). The following reagents and antibodies were used for microscopy, Western blot and co-immunoprecipitation (co-IP) studies: paraformaldehyde (PFA 4% [2% final]), 4′,6-diamidino-2-phenylindole (DAPI, 1: 1000), Alexa Fluor® 546 conjugate and Alexa Fluor® 633 conjugate of wheat germ agglutinin (WGA, 1: 1000), Alexa Fluor® 488 conjugate of phalloidin (1: 40) (all from Life Technologies, Eugene, OR, USA), mouse anti-RARα (EMD Millipore, Billerica, MA, USA), rabbit anti-p16ARC-Arp2/3s5 (abcam, Cambridge, MA, USA), mouse anti-β-tubulin antibody (Sigma) and goat anti-P-selectin antibody (Santa Cruz, Dallas, TX, USA).
Platelet isolation and culture
All studies were approved by the University of Utah IRB (IRB 000392) and the University Medicine of Greifswald ethics committee (BB 047/13). Platelets used for all of the described studies were freshly isolated from healthy human subjects. Leukocyte-depleted platelets were isolated as previously described [4,11,12]. Washed platelets were resuspended at 1 × 108/mL in serum-free M199 medium, placed in round-bottom polypropylene tubes (Becton Dickinson, Franklin Lakes, NJ, USA) and cultured in a 37 °C humidified incubator for different timepoints. In select studies, platelets were treated with DMSO (vehicle), atRA or CK-666. Incubation times for each specific experimental setting were based on published studies [4,5].
CD34+-derived megakaryocytes
All studies were approved by the University of Utah IRB (IRB 11919). CD34+ hematopoietic progenitor cells were isolated from human umbilical cord blood and differentiated into proplatelet-producing MEGS as previously described [11,12].
Platelet apoptosis assays
Platelets cultured ex vivo for 18 h were assessed for endogenous apoptosis using the FAM FLICA Caspase 3 and 7 Assay Kit (ImmunoChemistry Technologies, Bloomington, MN, USA). In addition, platelets were treated with 1 μM Abt-737 to induce apoptosis, as a positive control, as previously described [13] and according to the manufacturer’s protocol. Platelets were incubated with FAM FLICA for 30 min. Cells were fixed and analyzed using a single-color FACScan analyzer (BD, San Jose, CA, USA) with the system specific software.
Platelet cytoskeletal assays
For assessment of platelet spreading, platelets were placed on immobilized fibrinogen or collagen as previously described [12]. In select experiments, platelets were treated with DMSO (vehicle), atRA or CK-666. After 2 h of incubation, an appropriate time-course to allow for platelet spreading on fibrinogen, cells were fixed (PFA) and stained using WGA 546 or phalloidin 488.
For assessment of extended platelets with ≥2 cell bodies, platelets were isolated and resuspended at 1 × 108 mL−1 in serum-free M199 medium and cultured in a 37 °C humidified incubator for 6 h. The time-course for these experiments was selected based on published studies from our laboratory identifying that 6 h allows for extended cell body formation ex vivo [4]. In select experiments, platelets were treated as described above with DMSO, atRA or CK-666. Following the incubation period, cells were carefully fixed (PFA), spun on glass coverslips and stained as described above. Random fields (three for each experimental condition) were recorded using microscopy techniques. Total platelets per field were counted (average 450 cells/field). Extended platelets were defined as platelets clearly possessing an extended morphology and ≥2 distinct cell bodies as previously described by our group and others [4,5]. Changes in the formation of these proplatelet-resembling phenotypes were analyzed and compared with vehicle-treated conditions.
Protein co-immunoprecipitation
Platelet protein co-IP was performed using the Universal Magnetic Co-IP kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s protocol. In brief, after incubation with vehicle or atRA, platelets were washed in PBS supplemented with phosphatase and deacetylase inhibitors. Platelets were then lysed in the provided whole-cell lysis buffer and an additional protease inhibitor cocktail supplied by the manufacturer. Next, 50 μL of the harvested supernatant were incubated with the gene-specific anti-RARα or anti-p16ARC-Arp2/3s5 antibody or the isotype-matched non-immune IgG for 4 h at 4 °C on a rotator. For the IP process, 25 μL of magnetically labeled protein G beads were added to each sample and incubated for 60 min at 4 °C on a rotator. The IP complexes were isolated and cleaned by placing the samples on a magnetic stand and performing repeated washes with the provided wash-buffer. Finally, each bead pellet was resuspended in 20 μL of Laemmli-buffer and heated to 99 °C for 3 min before loading on to a 12% standard sodium dodecyl sulfate (SDS)-PAGE gel. Gels were run in duplicate. One gel was transferred on to a poly(vinylidene difluoride) (PVDF) membrane for colloidal gold total protein staining (Bio-Rad, Hercules, CA, USA), while the other gel was directly stained using colloidal-coomassie staining as previously described [14,15].
Protein mass spectrometry
Protein bands of interest were manually picked using standard techniques to ensure minimal contamination with human skin particles. Proteins were digested and analyzed by an LCQ-Deca (Thermo Fisher Scientific, Waltham, MA, USA) mass spectrometer with electrospray ionization at the University of Utah Mass Spectrometry Core. Peptides were identified using the Mascot database downloaded in October 2011 against the NCBI human database trypsin search. Analysis settings included a peptide mass tolerance of ±5 ppm, fragment ion tolerance of ±0.5 Da, monoisotopic masses and trypsin cleavage (maximum two missed cleavages). As a variable modification, oxidation was included. Only peptides with a P-value <0.05 were accepted.
Next generation RNA-sequencing
Platelets were isolated and leukocytes were removed by CD45+ selection [11,12] prior to RNA isolation [16,17]. RNA integrity was evaluated on an Agilent Bioanalyzer (Santa Clara, CA, USA) to ensure that each sample had an RNA integrity number (RIN) greater than 7.0. The same RNA isolation protocol and quality assessment applied to MEGS RNA. Poly(A)-tailed RNA was prepared and used to create cDNA libraries for deep sequencing. Samples were sequenced for 50 cycles on an Illumina GAIIx (San Diego, CA, USA) sequencer and sequence reads were processed and aligned as previously described [16,17].
Immunocytochemistry
On day 13 of the CD34 differentiation process MEGS were placed on fibrinogen-coated chamber slides to induce proplatelet formation, fixed (PFA) and subsequently incubated with IgG or an antibody against RARα, followed by co-staining using an anti-β-tubulin antibody and DAPI for nuclear localization. Platelets were either fixed immediately, to assess baseline morphology, or allowed to incubate for 18 h in suspension to allow for the development of extended cell bodies, as previously described [4]. At the end of the experimental period, PFA was added directly to the washed platelets to maintain the native morphology of the cells, as previously described [4,12]. Fixed platelets were subsequently layered onto vectabond™-coated (Vector Laboratories, Burlingame, CA, USA, USA) coverslips using a cytospin centrifuge (Shandon Cytospin, Thermo Fisher Scientific, Waltham, MA, USA). Platelets were permeabilized and counterstained with WGA 633 or phalloidin 488. RARα and Arp2/3s5 were specifically detected using the aforementioned antibodies. Specificity of the staining for anti-RARα was confirmed with isotype-matched non-immune IgG. Because the antibody concentration of the antip16ARC antibody is not known, a minus (−) primary antibody sample served as the negative control.
Actin branching assay
Non-muscle actin (#APHL99), Arp2/3 protein complex (#RP01), Wiskott-Aldrich syndrome protein-Verprolin, cofilin, acidic (WASP-VCA) domain-GST protein (#VCG03) and an actin polymerization biochem kit (#BK003) were purchased from Cytoskeleton Inc. (Denver, CO, USA). Additional reagents were: phalloidin 488 and phalloidin 546, atRA, CK-666 and recombinant human RARα(#H00005914-P01, Abnova, Taipei, Taiwan). Branching assays were performed as previously described with modifications [18,19]. Alexa 488-phalloidin (1: 10 molar ratio) and actin monomers (2 μM final) were added to polymerization buffer (0.25× final), gently mixed and allowed to polymerize into labeled mother filaments (yellow) (15 min at room temperature, protected from light). During this polymerization period the accessory protein mixtures (Arp2/3 complex 25 nM final) and WASP-VCA domain-GST protein (300 nM final) were assembled and allowed to interact. In select samples recombinant human RARα (100 nM final), atRA (10 μM final), the Arp2/3 inhibitor CK-666 (40 μM final, the IC50 of CK-666 [20]), or a combination of all components, was added to the Arp2/3 complex-WASP mixture. After mother-filament polymerization the accessory protein and treatment mixtures were added. After 10 min of incubation (room temperature in the dark) additional actin monomers (2 μM final) and Alexa 546-phalloidin (1: 10 molar ratio) were added to allow for branch formation. Finally, samples were diluted 500-fold in fresh fluorescence buffer (50 mM KCl, 1 mM MgCl2, 100 mM DTT, 10 mM imidazole pH 7.0, 0.5% methylcellulose, 20 μg mL−1 catalase, 100 μg mL−1 glucose oxidase and 3 mg mL−1 glucose). Samples (6 μL) were applied to coverslips (Corning, Corning, NY, USA #2975-246, 24 × 60 mm) previously coated with poly-L-lysine. Products of polymerization and branching were examined by fluorescence microscopy. Branches were visually inspected for their Brownian vibration to exclude artifacts of overlain filaments. Branching was quantified by counting the number of branches per μm of the mother filament for randomly selected fields. For that, three independent investigators were blinded to the experimental groups and analyzed the dataset autonomously to ensure unbiased quantification.
Microscopy
Fluorescence microscopy and high-resolution confocal reflection microscopy were performed using an Olympus IX81, FV300 (Olympus, Melville, NY, USA) confocal-scanning microscope equipped with a 60×/1.42 NA oil objective for viewing platelets. An Olympus FVS-PSU/IX2-UCB camera and scanning unit and Olympus Fluoview FV 300 image acquisition software version 5.0 were used for recording. In addition, an EVOS FL Auto Cell imaging system with an integrated dual camera system, system-specific software and equipped with a 60×/1.42 NA oil objective was used. Monochrome 16-bit images were further analyzed and changes quantified using Adobe Photoshop CS6 and ImageJ (National Institutes of Health, Bethesda, MD, USA). Super-resolution microscopy is described in a specific paragraph.
Single recognition and co-localization studies
Microscopy-based single recognition and co-localization studies were performed using the Duolink® in situ system (OLINK Bioscience, Uppsala, Sweden). Proximal ligation assay technology (PLA) was used to detect RARα, Arp2/3s5 and RARα Arp2/3s5 complexes in situ with specificity, as briefly described here. Unique short DNA strands attached to secondary antibodies against a single or two target proteins (PLA (+) and (−) probes) bind to the primary antibodies. The oligonucleotides guide the formation of circular DNA strands when bound in close proximity (< 40 nm). The DNA circles serve as templates for localized rolling-circle amplification, allowing individual interacting pairs of protein molecules to be visualized by additional hybridization techniques [21,22]. In brief, platelets were treated, cultured, fixed in suspension with paraformaldehyde (2% final) and permeabilized for microscopy as described above. The Duolink in situ kit was used as recommended by the manufacturer with the primary antibodies mouse anti-RARα and rabbit antip16ARC (Arp2/3s5). One primary antibody was omitted as a negative control. Secondary anti-mouse (+) or (−) and anti-rabbit (+) or (−) antibodies were used as PLA probes. After hybridization, ligation and amplification, a detection solution containing fluorescent probes was added. PLA signals were then detected by confocal microscopy as described above.
Protein co-localization studies and super-resolution microscopy
Resting-state human platelets were isolated, prepared and immunostained as previously described [23] with mouse anti-RARα, rabbit anti-p16 ARC (Arp2/3s5) and goat anti-P selectin as random controls, followed by AlexaFluor-conjugated secondary antibodies (Life Technologies). Stained cells were imaged via spinning-disc confocal laser fluorescence (SDF) or structured illumination microscopy (SIM, Zeiss ELYRA SIM) as previously described [24]. Deconvolved whole-cell SDF images (n = 20 cells per condition) were analyzed for signal co-localization using the Costes method for calculating the thresholded Pearson’s correlation coefficient using Volocity 6 (Perkin-Elmer, Waltham, MA, USA); images were prepared for publication using Volocity 6, Adobe Photoshop, Zen 2012 (Zeiss, Ontario, CA, USA) and Bitplane Imaris 8 (Bitplane AG Badenerstrasse, Zurich, Switzerland).
Protein expression studies
All samples were normalized for starting cell concentrations. Co-IP efficiency was determined by Western blot. IP samples were separated by SDS–polyacrylamide gel electrophoresis and examined by Western analysis for RARα and anti-p16ARC (Arp2/3s5). PVDF membranes from the same experiment were stripped and reprobed with the other primary antibody. Proteins were detected by enhanced chemiluminescence. For selected studies, platelets were cultured overnight in the presence of atRA, CK-666 or the respective vehicle, and activated with thrombin (1 U mL−1 for 10 min) or left unstimulated. The cells were subsequently incubated with fluorescein isothiocyanate (FITC)-conjugated antibodies against P-selectin (#555523, BD Pharmingen, San Diego, CA, USA), fixed and analyzed on a five-color FACScan analyzer (BD) using the system-specific software. Isotype-matched control samples were used to exclude non-specific antibody binding.
Statistical analyses
The mean ± SEM was determined for each experimental variable. ANOVA was used to identify differences that existed among multiple experimental groups. If significant differences were found, a Newman–Keuls post-hoc procedure was used to determine the location of the difference. For all datasets a two-tailed P-value of < 0.05 was considered statistically significant.
Results
CD34+-derived megakaryocytes and isolated human platelets endogenously express RARα mRNA and protein
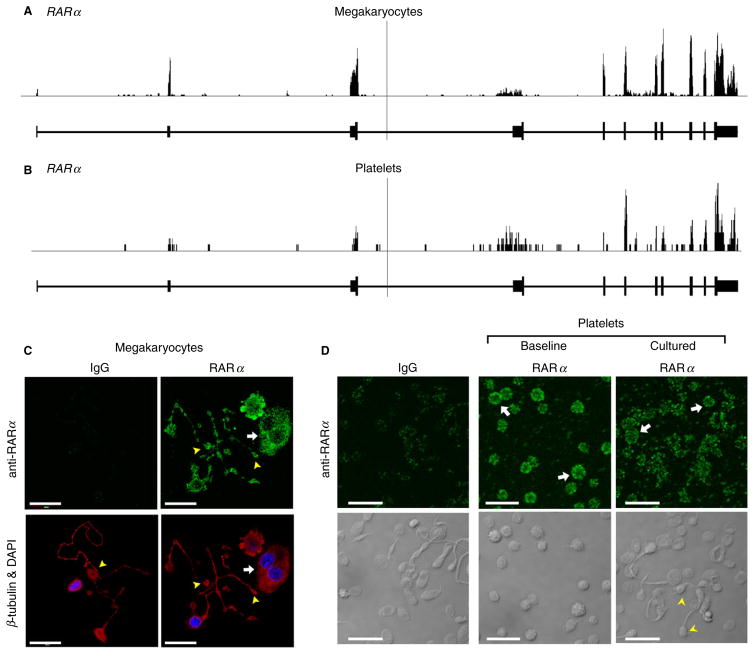
We initially sought to determine the expression patterns of RARα in CD34+ hematopoietic progenitor cell-derived MEGS and freshly isolated human platelets. Next-generation RNA deep sequencing (RNA-seq) revealed that MEGS and human platelets express mature RNA (mRNA) for RARα (Fig. 1A and B). Consistent with its expression at the mRNA level, RARα protein was expressed basally in the cytoplasm and proplatelet extensions of MEGS (Fig. 1C), freshly isolated, unstimulated human platelets at baseline, and platelets that form extended cell bodies in culture (Fig. 1D).
Fig. 1.
Megakaryocytes and human platelets express mRNA and protein for RARα. RNA-seq snapshots taken from the Integrated Genome Browser of the RARα transcript in CD34+-derived megakaryocytes (A) and human platelets (B). The height on the y-axis represents the relative accumulated number of reads spanning a particular sequence. The average of the read depths across all genomic coordinates within a transcript correlates to abundance of RNA expression. RAR gene regions are represented below the plots by thick (exon) and thin (intron) lines. (C) CD34+-derived megakaryocytes were placed on immobilized fibrinogen to induce proplatelet formation, fixed, and then incubated with an antibody against RARα (green, top right panel) or an IgG control, followed by co-staining for β-tubulin (red) and nuclei (4′,6-diamidino-2-phenylindole, DAPI, blue), as shown in the bottom panels. The white arrows point to cell bodies and yellow arrowheads highlight the proplatelet extensions. Scale bars = 20 μm. (D) RARα expression (green) at baseline and in cultured (18 h) human platelets. The bottom panels show the corresponding transmission images. The white arrows highlight RARα platelets. The yellow arrowheads point to barbell-shaped platelets that form when platelets are cultured in suspension [4]. Scale bars = 10 μm.
Retinoic acid regulates actin cytoskeletal events in human platelets
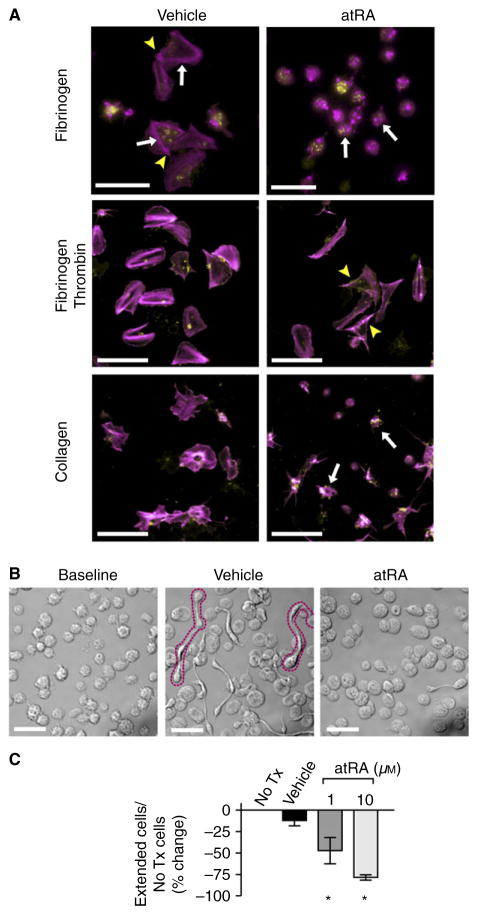
We next sought to determine the role of RARα in human platelets. As platelets are anucleate, we hypothesized that RARα would have non-genomic functions. We incubated freshly isolated human platelets with atRA, the biologically active ligand of RARα. In the presence of atRA, platelet spreading on collagen, fibrinogen and fibrinogen plus thrombin and the formation of platelets with ≥ 2 cell bodies in suspension (processes requiring regulated actin cytoskeletal assembly) were significantly inhibited (Fig. 2). Proplatelet formation by MEGS was also inhibited by atRA in a dose-dependent manner (Figure S1A). Platelet surface P-selectin expression was not inhibited by atRA, suggesting that alpha-granule release was preserved and platelets remained functional (Figure S1B). The expression of RARα protein did not significantly change during 18 h of platelet culture in the presence or absence of atRA (Figure S1C). In these 18-h culture experiments, platelet apoptosis markers (e.g. Caspase 3/7 activation) did not increase compared with baseline conditions (3.7% vs. 3.5% apoptotic cells, P = 0.93). The formation of platelets with ≥ 2 cell bodies was also inhibited by atRA when cultured in platelet-rich plasma (Figure S2). Incubating freshly isolated human platelets with the specific RARα agonist AM-580 inhibited platelet spreading, thus phenocopying the effects of atRA. In contrast, incubating platelets with Ro-41-5253, an RARα antagonist, did not inhibit platelet spreading (data not shown).
Fig. 2.
Retinoic acid regulates platelet progeny formation. (A) Platelets were placed on immobilized fibrinogen (top), fibrinogen plus thrombin (middle) or collagen (bottom) for 2 h with vehicle (dimethylsulfoxide, DMSO) or in the presence of atRA (10 μM) and stained for polymerized actin (magenta) or wheat germ agglutinin (WGA) to identify sialic acids (yellow). In the presence of vehicle only, isolated human platelets demonstrate characteristic fully spread platelets and star-shaped, partially spread patterns with hallmark actin stress fibers (arrows) and actin nodules (arrowheads). Treatment with atRA inhibited full platelet spreading, although partially spread, star-shaped platelets remain visible (white arrows, scale bars = 10 μm). This figure is representative of n = 3 independent experiments. (B) Transmission microscopy of platelets following snap-fixation at baseline or following incubation (6 h) with vehicle (DMSO) or atRA (10 μM). The formation of extended platelets with ≥ 2 cell bodies (outlined in magenta dotted line) was reduced compared with baseline or vehicle-treated platelets. Scale bars = 10 μm, representative of n = 5 independent experiments. (C) Extended platelets with ≥ 2 cell bodies were quantified without treatment (no Tx) and during treatment in culture with either vehicle (DMSO) or atRA in a dose-dependent manner (1 and 10 μM). Data represent the mean ± SEM (n = 3, *P < 0.05).
RARα directly interacts with the cytoskeletal protein Arp2/3s5
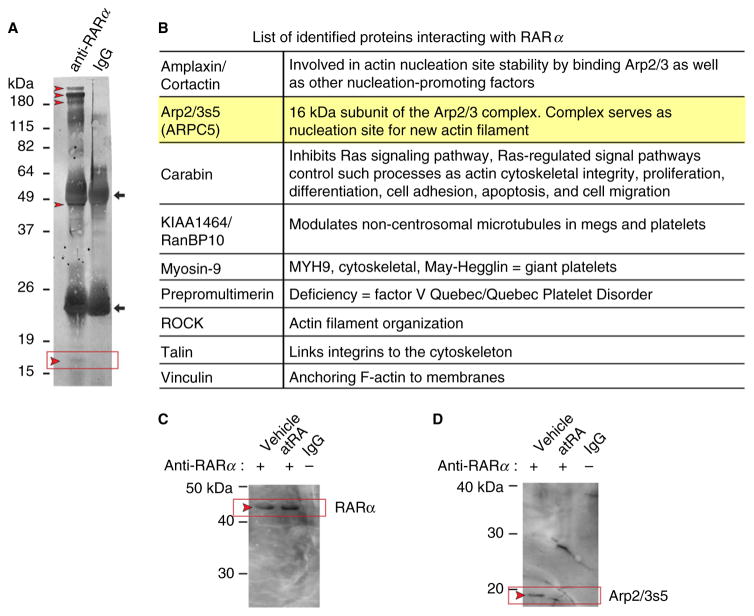
As these data suggest that platelet actin cytoskeletal events are mediated by RARα, we next hypothesized that RARα would have direct interactions with actin cytoskeletal regulatory components. To identify RARα binding partners in human platelets, we used co-IP followed by mass spectroscopy (MS) analyses of selected bands. We identified several candidate proteins that directly interacted with RARα (Fig. 3A and B). Unexpectedly, most identified proteins that directly interact with RARα are regulators of cytoskeletal formation (Fig. 3B). We chose to focus on Arp2/3s5 (also known as ARPC5), which was specifically identified by MS-based peptide sequences (Fig. 3A, red box and arrow, 3B, and Table 1). No other members of the Arp2/3 complex were identified by MS.
Fig. 3.
Arp2/3s5, a subunit of the actin-nucleating complex that regulates cytoskeletal formation, is robustly expressed in human platelets and CD34+-derived megakaryocytes (MEGS) and interacts with RARα. (A) A poly(vinylidene difluoride) (PVDF) membrane stained for total protein (colloidal gold). The corresponding sodium dodecyl sulfate (SDS) gel stained with coomassie was used for mass spectroscopy (MS)-based protein identification. The left lane shows proteins isolated after co-immunoprecipitation (co-IP) using an anti-RARα antibody. The right lane shows proteins isolated after co-IP with non-immunogenic, control IgG. The IgG heavy and light chains are indicated with black arrows. Red arrowheads mark selected protein bands subsequently used in MS analysis. The expected position of ARP2/3s5 is indicated by a black box, between 15 and 19 kDa, where a faint but visible band is present. (B) List of identified proteins interacting with RARα. Protein names are shown in the left column, known protein functions are listed in the right column. Highlighted is our primary target (Arp2/3s5). (C, D) atRA blocks RARα Arp2/3s5 interactions. Platelets were cultured overnight in the absence (vehicle, left lane) or presence of atRA (10 μM, middle lane). Platelet lysates were then analyzed by co-IP with an anti-RARα antibody followed by Western blotting. The corresponding IgG control is shown in the right lane. (C) An anti-RARα antibody was used for the detection of immuno-precipitated RARα. (D) Membranes were stripped and reprobed for the presence of Arp2/3s5. The red arrowheads indicate the expected positions of RARα and Arp2/3s5 on the membrane. (C) and (D) are representative of n = 3 independent experiments.
Table 1.
Mass spectrometry (MS) identification of Arp2/3s5. Peptides leading to Arp2/3s5 identification are listed, including scores and peptide sequences
| Observed | Mr (expt) | Mr (calc) | Miss | Score | Expect | Rank | Peptide |
|---|---|---|---|---|---|---|---|
| 559.3561 | 558.3488 | 558.3489 | 0 | 21 | 0.4 | 1 | R.VLTAR.K |
| 353.7287 | 705.4428 | 705.4425 | 0 | 30 | 0.0067 | 1 | K.VLISFK.A |
| 367.6934 | 733.3723 | 733.3719 | 0 | 39 | 0.0034 | 1 | K.NTVSSAR.F |
| 380.7136 | 759.4127 | 759.4127 | 0 | 38 | 0.0084 | 1 | K.AVQSLDK.N |
| 402.2219 | 802.4292 | 802.4297 | 1 | 37 | 0.0055 | 1 | K.SQAVKDR.A |
| 453.2419 | 904.4693 | 904.4688 | 0 | 41 | 0.0019 | 1 | K.NGVDLLMK.Y |
| 479.7882 | 957.5619 | 957.5607 | 1 | 27 | 0.043 | 1 | K.DRAGSIVLK.V |
| 535.8196 | 1069.6246 | 1069.6244 | 0 | 70 | 1.5e-006 | 1 | K.ALAAGGVGSIVR.V |
| 613.2650 | 1224.5154 | 1224.5146 | 0 | 57 | 4.8e-006 | 1 | K.VDVDEYDENK.F |
| 666.8511 | 1331.6876 | 1331.6867 | 0 | 82 | 1.2e-007 | 1 | R.QGNMTAALQAALK.N |
| 677.3125 | 1352.6104 | 1352.6096 | 1 | 79 | 8.9e-008 | 1 | R.KVDVDEYDENK.F |
| 823.9413 | 1645.8680 | 1645.8709 | 1 | 62 | 1.3e-005 | 1 | K.AVQSLDKNGVDLLMK.Y |
| 699.7080 | 2096.1022 | 2096.1048 | 1 | 72 | 1.3e-006 | 1 | R.QGNMTAALQAALKNPPINTK.S |
| 726.9977 | 2177.9711 | 2177.9688 | 0 | 50 | 6.2e-005 | 1 | K.GFESPSDNSSAMLLQWHEK.A |
Mr, average mass of protein; expt, expected; calc, calculated.
Arp2/3s5 is a subunit of the actin-nucleating complex Arp2/3, which regulates cytoskeletal formation in nucleated cells. The Arp2/3 complex, which structurally resembles monomeric actin, binds to the side of existing actin filaments, initiating growth of new filaments in a branched network formation. Because Arp2/3 is a requisite for platelet spreading and filopodia formation [25], we hypothesized that RARα Arp2/3s5 interactions would regulate platelet actin cytoskeletal events.
Next-generation RNA deep sequencing (RNA-seq) confirmed Arp2/3s5 expression in isolated human platelets and MEGS (Figure S3A). Consistent with its expression pattern at the transcript level, confocal scanning laser microscopy demonstrated robust Arp2/3s5 protein expression throughout the platelet cytoplasm (data not shown). Arp2/3s5 expression was in a localized nodular pattern (Figure S3B), consistent in appearance with platelet cytoskeletal structures called actin nodules [25–28]. Treating human platelets with atRA did not change RARα expression (Fig. 3C, and Figure S1C) but disrupted Arp2/3s5 RARα interactions (Fig. 3D).
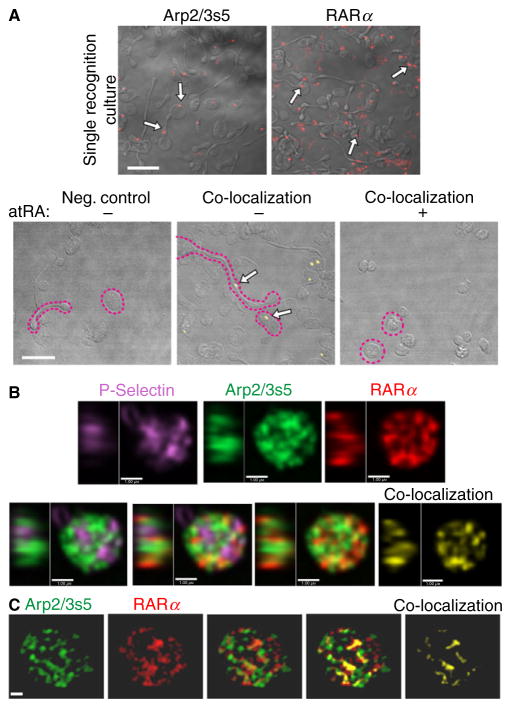
Given spatial resolution limitations in standard approaches to co-localization, we applied PLA to detect individual RARα Arp2/3s5 interactions in situ. Using this technique for co-localization studies, protein partners in a complex are detected simultaneously by the ligation of DNA-probe-labeled secondary antibodies, which only occurs when the two proteins co-localize closer than 40 nm. In addition, only proteins complexed together at the time of fixation are detected. RARα and Arp2/3s5 were readily visualized as specific, single signals within the cytoplasm of platelets (Fig. 4A). Moreover, RARα and Arp2/3s5 co-localized within platelet cell bodies. Consistent with the results of Western blot experiments shown in Fig. 3(D), atRA disrupted the co-localization of RARα and Arp2/3s5 (Fig. 4A, bottom panels).
Fig. 4.
RARα and Arp2/3s5 co-localize in situ. (A) Platelets were cultured overnight, fixed and spun down. Single recognition studies were performed using the Duolink® in situ system. RARα and Arp2/3s5 protein were detected using specific primary antibodies. Single recognition signals for either RARα or Arp2/3s5 (red, top panels) are highlighted in each panel with white arrows, demonstrating that both RARα and Arp2/3s5 are present within human platelets. Co-localization studies were performed using the Duolink® in situ system. RARα-Arp2/3s5 complexes were detected by using specific primary antibodies and matching (−) and (+) PLA probes. Co-localization signals for RARα Arp2/3s5 complexes (yellow, bottom panels) are identified by white arrows. The negative control (left panel) demonstrates specificity of the signal. Examples of discoid and extended cells are outlined (magenta dotted line). Scale bars = 10 μm. Images in (A) are representative of n = 3 independent experiments. (B and C) Human platelets were isolated, prepared and immunostained using primary antibodies: mouse anti-RARα (red), rabbit anti-Arp2/3s5 (green) and goat anti-P selectin (magenta). Stained cells were imaged via spinning disc confocal laser fluorescence microscopy (B) or structured illumination microscopy (SIM) (C). The top row panels in (B) and the two left side panels in (C) show single channel images for each protein indicated. Merged images used for co-localization studies are shown in the bottom row (B) and middle panels in (C). Co-localization pixels only for RARα and Arp2/3s5 are shown in the far right panels (B, C) in yellow. In (B) the left side in each panel shows the central YZ profile of the Z-section shown at right, in (C) images are snapshots of the 3D render shown in Movie S1 (B, bars = 1 μm, representative of three independent experiments; C, bars = 0.5 μm, representative of three independent experiments).
To better visualize Arp2/3s5 RARα interactions we used high-resolution spinning disc laser fluorescence (SDF) and structured illumination microscopy (SIM). Imaging of immunostained platelets (Fig. 4B and C, Movie S1) showed Arp2/3s5 and RARα localized to an intracellular compartment distinct from the alpha granular system defined by P-selectin. Co-localization analysis of SDF and SIM images gave a Pearson’s correlation coefficient of 0.50 for RARα and Arp2/3s5, while the value for P-selectin and Arp2/3s5 was 0.29 (P < 0.0001).
Retinoic acid receptor-α and atRA are required for complex actin branching
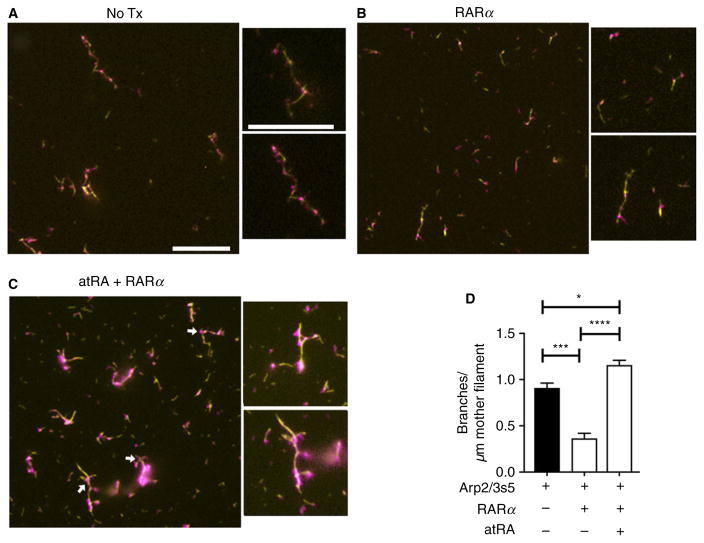
We next hypothesized that RARα and atRA are both required for complex actin branching, a process also dependent on Arp2/3. To confirm this we used an in vitro Arp2/3-dependent actin branching assay previously described by Amann and others [18,19,29]. In this assay actin, WASP-VCA protein and Arp2/3 are all required components for actin branching [18,19].
Compared with standard conditions usually used for branching assays, which include Arp 2/3 (Fig. 5A), addition of recombinant human RARα reduced actin branch complexity, as indicated by a significantly fewer number of actin side-branches per mother filament (Fig. 5B and D). This condition most likely reflects Arp2/3 and RARα interactions, which based on our findings occur basally in quiescent human platelets (Fig. 3). The addition of both RARα and atRA led to deregulated actin branch formation when compared with the native state, resulting in a significant increase in the number of actin side-branches per mother filament (Fig. 5C and D). In control experiments where the actin nucleation initiating complex Arp2/3 was omitted, atRA alone, RARα alone, or the combination of atRA and RARα, resulted in the complete absence of actin branch formation (data not shown). These results indicate that RARα and Arp2/3s5 interact closely to specifically control Arp2/3-mediated actin branch formation in quiescent, discoid platelets, and that RARα does not regulate actin nucleation independent of Arp2/3.
Fig. 5.
Retinoic acid receptor-α and atRA are required for complex actin branching in the presence of Arp2/3. An actin branching assay was performed as described in ‘Materials and methods’. Simple actin mother filaments are shown in yellow while complex actin branching is shown in magenta. (A) In the presence of the Arp2/3 complex and WASP-VCA alone (control), actin polymerization and actin branching were detectable. (B, D) When recombinant human RARα (100 nM final) was added, actin branching formation and the number of actin side-branches per μM of actin mother filament were significantly reduced. (C, D) In comparison, atRA significantly rescued actin branch formation, even in the presence of RARα (magenta, white arrows). Scale bars = 10 μM; these figures are representative of n = 3 independent experiments.
Arp2/3 regulates complex actin branch formation and platelet cytoskeletal events
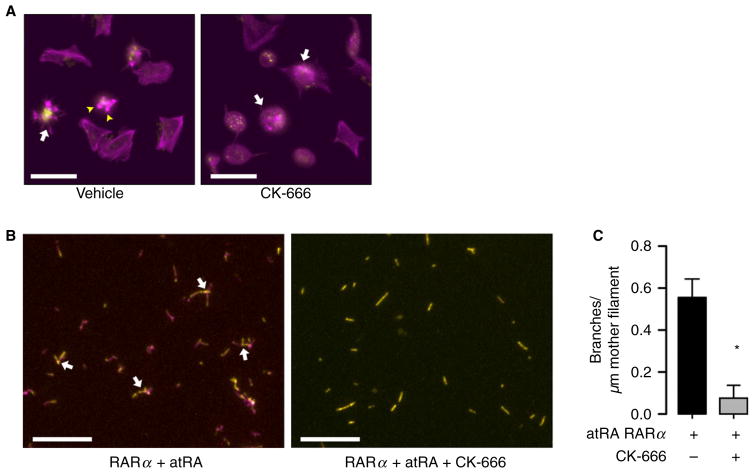
Our findings demonstrate that RARα interacts directly with Arp2/3s5 in platelets, that atRA disrupts RARα Arp2/3s5 interactions and inhibits platelet spreading and the formation of extended platelets with ≥ 2 cell bodies, and that atRA promotes dysregulated complex actin branching in the presence of atRA. We next blocked Arp2/3 directly, hypothesizing that this inhibition would reduce complex actin branching and platelet spreading.
Arp2/3 inhibition by CK-666 decreased the ability of platelets to spread on immobilized fibrinogen (Fig. 6A) and completely abolished complex actin branch formation (Fig. 6B and C). Platelets treated with CK-666 retained their ability to express surface P-selectin after stimulation with thrombin, demonstrating that inhibition of Arp2/3 does not block platelet activation responses (Figure S4A). The formation of platelets with extended cell bodies was significantly increased in the presence of CK-666, indicating that actin polymerization, but not branching, is necessary for this process (Figure S4B and C). Consistent with this hypothesis, treating platelets with cytochalasin D, which globally blocks actin polymerization [30], completely inhibited platelet spreading and extended cell body formation (data not shown).
Fig. 6.
Actin cytoskeletal dynamics in human platelets is dependent on Arp2/3s5. (A) Platelets were placed on immobilized fibrinogen for 2 h with vehicle (dimethylsulfoxide, DMSO) or in the presence of CK-666, a specific Arp2/3s5 inhibitor, and stained for polymerized actin (magenta) or wheat germ agglutinin (WGA) to identify sialic acids (yellow). In the presence of vehicle only, isolated human platelets demonstrate characteristic fully spread platelets and star-shaped (arrows), partially spread patterns with hallmark actin stress fibers and focal adhesion points (arrowheads). Treatment with CK-666, in contrast, inhibited full platelet spreading, although partially spread, star-shaped platelets remain visible (white arrows, scale bars = 10 μm). This figure is representative of n = 3 independent experiments. (B, C) When an actin branching assay was supplemented in control conditions [18,19] with bovine Arp2/3 complex in the presence of recombinant human RARα and atRA, actin filaments were visible and complex actin branching occurred. In the presence of the Arp2/3 inhibitor CK-666, actin filaments were visible but complex actin branching was inhibited, consistent with the dependency of the Arp2/3 complex on actin branch formation. Boxes indicate the origin of inserts. Scale bars = 10 μm. This figure is representative of n = 3 independent experiments.
Discussion
Recent studies demonstrate that human platelets, although anucleate, possess unexpected and dynamic functions [4,5]. While activation-dependent changes in platelet adhesion, activation and spreading have been observed for decades, many of the regulatory mechanisms underlying these actin-dependent events remain incompletely understood. As retinoids regulate peripheral blood platelet counts in AML patients [31], induce megakaryopoiesis and platelet-like particle formation in a MEG-O1 cell line [32] and reactivate transduction pathways to promote differentiation [33], we hypothesized that retinoids would also play integral roles in actin cytoskeletal events in human platelets.
In the present study, we demonstrate that human platelets and CD34+-derived MEGS express RARα mRNA and protein. Moreover, we show that the high-affinity RARα ligand, atRA [34,35], inhibits platelet spreading and the formation of platelets with ≥ 2 cell bodies. We hypothesized that RARα regulates actin cytoskeletal dynamics in platelets through interactions with non-traditional (and in non-genomic fashion) partner protein interactions. Consistent with this hypothesis, we found that Arp2/3s5, a subunit of the Arp2/3 complex [36], is expressed in human platelets and MEGS in a nodular pattern that co-localizes with actin. Furthermore, using a co-IP approach, we identified for the first time that RARα directly interacts with Arp2/3s5 in human platelets under basal conditions. In the presence of atRA protein interactions between RARα and Arp2/3s5 were disrupted, indicating that these two interacting partners are actin cytoskeletal regulators.
Arp2/3 is present in platelets and regulates actin filament formation and platelet spreading [2]. Nevertheless, a regulatory function for RARα in mediating the actions of Arp2/3 has not previously been identified. Using an in vitro actin branching assay, we demonstrated that RARα mediated Arp2/3-dependent actin filament formation. Consistent with inhibition of cytoskeletal processes in platelets, atRA disrupted RARα-Arp2/3-dependent actin filament formation, resulting in a phenotypic shift towards side branching rather than filament elongation. These findings demonstrate that RARα, in a non-genomic fashion, directly interacts in situ with Arp2/3s5 in human platelets and regulates actin cytoskeletal events.
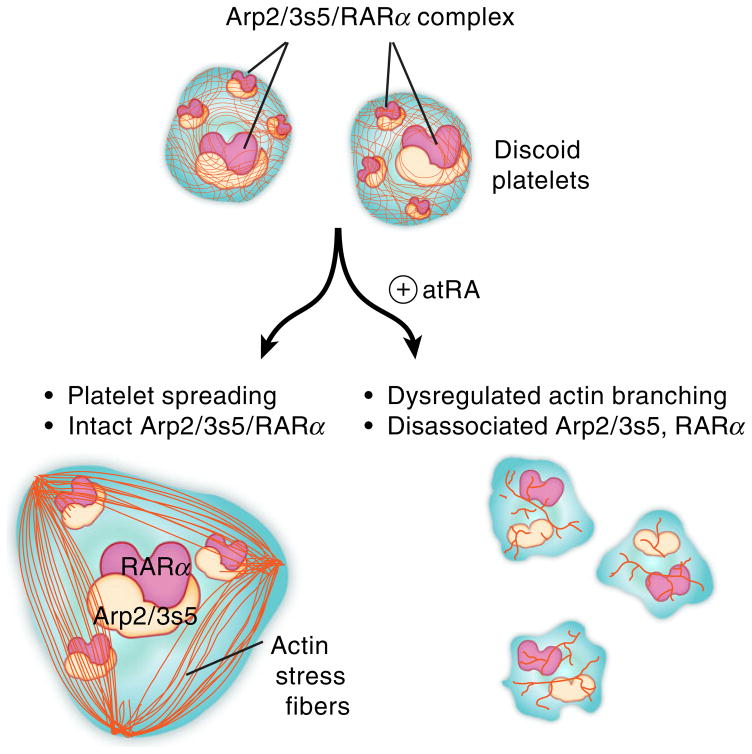
Taken together, these results provide new evidence for a regulatory model of platelet actin cytoskeletal processes. We propose a model whereby under basal conditions in human platelets, RARα Arp2/3 interactions control actin formation and regulate platelet spreading (Fig. 7). Disrupting these interactions, as with atRA, however, leads to deregulated actin filament formation, impaired platelet spreading and formation of barbell-shaped platelets. As our studies were done in primary human cells isolated from healthy donors, we postulate that RARα and its natural ligand atRA serve as rheostats in Arp2/3-mediated actin cytoskeletal organization.
Fig. 7.
Proposed mechanisms by which retinoic acid regulates complex actin cytoskeletal dynamics in human platelets. Basally, in unstimulated human platelets, RARα is bound to Arp2/3s5 and these direct interactions mediate platelet shape change and spreading, processes dependent on regulated actin cytoskeletal events. These interactions are disrupted by the binding of retinoic acid to its receptor, leading to dysregulated actin cytoskeletal rearrangement and blocking of platelet shape change and spreading.
While we observed a direct RARα Arp2/3s5 interaction, we cannot completely dismiss other possible binding partners of RARα forming heterodimers, which could interact with RXR family members or other actin cytoskeleton regulatory proteins [37]. In addition, subordination is a common feature of retinoid receptor signaling. This means that an RARα binding partner can autonomously bind its agonist, but signal-transduction only occurs if both subunits are stimulated when a heterodimer is formed [35,38,39]. Therefore, a synergistic action could potentially be observed when RARα and other binding partners besides Arp2/3s5 are simultaneously bound to agonists [40]. Additional studies are needed to further dissect the complex pathways involved in retinoid biology in human platelets.
In conclusion, our data identify a previously unrecognized regulatory signaling pathway of RARα in human platelets. Because RARα is classically considered a nuclear receptor, our findings also demonstrate that in anucleate human platelets, RARα functions in a nongenomic fashion. Finally, we provide new mechanisms of how actin cytoskeletal events are regulated via RARα interactions with Arp2/3 in human platelets.
Supplementary Material
Fig. S1. Demonstrates that atRA does influence proplatelet formation but does not influence platelet alphagranule release. Furthermore, atRA does not alter RARα protein expression.
Fig. S2. Demonstrates that the regulatory effect of atRA on the formation of extended barbell-shaped platelets is preserved when incubated in PRP.
Fig. S3. Shows that Arp2/3s5 is present in human platelets and CD34+-derived megakaryocytes.
Fig. S4. Shows that the inhibition of Arp2/3s5 does not influence platelet alpha-granule release but does effect the formation of extended platelets with ≥ 2 cell bodies.
Movie S1. Represents a human platelet demonstrating RARα-Arp2/3s5 co-localization.
Essentials.
Platelets employ proteins/signaling pathways traditionally thought reserved for nuclear niche.
We determined retinoic-acid-receptor alpha (RARα) expression and function in human platelets.
RARα actin-related protein-2/3 complex (Arp2/3) interact via non-genomic signaling in platelets.
RARα regulates Arp2/3-mediated actin cytoskeletal dynamics and platelet spreading.
Acknowledgments
We thank C. K. Rodesch and K. R. Carney of the University of Utah Cell Imaging Core for technical assistance, C. Nelson and K. Parsawar of the University of Utah Mass Spectrometry Core and K. Schwertz for critical data analysis. We also thank D. Lim for preparation of the figures, critical comments and consultation regarding effective display of the images. This work was funded by the following grants: HL066277 and HL112311 (A. S. Weyrich), HL044525 (G. A. Zimmerman), AG040631, HL126547 and HL092161 (M. T. Rondina), GM103806 (J.W.R.) and HL075507 (L. W. Kraiss). W. A. H. Kahr was supported by operating grants from the Canadian Institutes of Health Research (CIHR; MOP-81208 and MOP-259952). H. Schwertz was supported by a post-doctoral fellowship (0625098Y), a Beginning-Grant-in-Aid (09BG1A 2250381) from the American Heart Association Western States Affiliate and a Lichtenberg-Professorship from the Volkswagen Foundation.
Footnotes
Addendum
H. Schwertz and A. S. Weyrich conceived and designed the experiments. H. Schwertz, M. T. Rondina, W. A. H. Kahr and F. G. Pluthero performed the experiments. H. Schwertz, M. T. Rondina, M. Freitag, J. W. Rowley, W. A. H. Kahr, F. G. Pluthero and A. S. Weyrich analyzed the data. H. Schwertz, G. A. Zimmerman, L. W. Kraiss, W. A. H. Kahr, F. G. Pluthero and A. S. Weyrich contributed reagents, materials or analysis tools. H. Schwertz, M. T. Rondina, J. W. Rowley, M. Freitag, L. W. Kraiss, G. A. Zimmerman and A. S. Weyrich wrote and reviewed the paper.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–42. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartwig JH. The platelet: form and function. Semin Hematol. 2006;43:S94–100. doi: 10.1053/j.seminhematol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.White JG. An overview of platelet structural physiology. Scanning Microsc. 1987;1:1677–700. [PubMed] [Google Scholar]
- 4.Schwertz H, Koster S, Kahr WH, Michetti N, Kraemer BF, Weitz DA, Blaylock RC, Kraiss LW, Greinacher A, Zimmerman GA, Weyrich AS. Anucleate platelets generate progeny. Blood. 2010;115:3801–9. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thon JN, Montalvo A, Patel-Hett S, Devine MT, Richardson JL, Ehrlicher A, Larson MK, Hoffmeister K, Hartwig JH, Italiano JE., Jr Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191:861–74. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Napoli JL. Retinoic acid biosynthesis and metabolism. FASEB J. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 7.Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–8. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 8.Ali FY, Davidson SJ, Moraes LA, Traves SL, Paul-Clark M, Bishop-Bailey D, Warner TD, Mitchell JA. Role of nuclear receptor signaling in platelets: antithrombotic effects of PPARbeta. FASEB J. 2006;20:326–8. doi: 10.1096/fj.05-4395fje. [DOI] [PubMed] [Google Scholar]
- 9.Moraes LA, Swales KE, Wray JA, Damazo A, Gibbins JM, Warner TD, Bishop-Bailey D. Nongenomic signaling of the retinoid X receptor through binding and inhibiting Gq in human platelets. Blood. 2007;109:3741–4. doi: 10.1182/blood-2006-05-022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray DM, Spinelli SL, Pollock SJ, Murant TI, O’Brien JJ, Blumberg N, Francis CW, Taubman MB, Phipps RP. Peroxisome proliferator-activated receptor gamma and retinoid X receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. doi: 10.1160/TH07-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–91. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenecity of human platelets. J Exp Med. 2006;203:2433–40. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–62. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 15.Schwertz H, Carter JM, Abdudureheman M, Russ M, Buerke U, Schlitt A, Muller-Werdan U, Prondzinsky R, Werdan K, Buerke M. Myocardial ischemia/reperfusion causes VDAC phosphorylation which is reduced by cardioprotection with a p38 MAP kinase inhibitor. Proteomics. 2007;7:4579–88. doi: 10.1002/pmic.200700734. [DOI] [PubMed] [Google Scholar]
- 16.Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–11. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahr WH, Hinckley J, Li L, Schwertz H, Christensen H, Rowley JW, Pluthero FG, Urban D, Fabbro S, Nixon B, Gadzinski R, Storck M, Wang K, Ryu GY, Jobe SM, Schutte BC, Moseley J, Loughran NB, Parkinson J, Weyrich AS, et al. Mutations in NBEAL2, encoding a BEACH protein, cause gray platelet syndrome. Nat Genet. 2011;43:738–40. doi: 10.1038/ng.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amann KJ, Pollard TD. The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat Cell Biol. 2001;3:306–10. doi: 10.1038/35060104. [DOI] [PubMed] [Google Scholar]
- 19.Amann KJ, Pollard TD. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proc Natl Acad Sci USA. 2001;98:15009–13. doi: 10.1073/pnas.211556398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetrick B, Han MS, Helgeson LA, Nolen BJ. Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem Biol. 2013;20:701–12. doi: 10.1016/j.chembiol.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weibrecht I, Leuchowius KJ, Clausson CM, Conze T, Jarvius M, Howell WM, Kamali-Moghaddam M, Soderberg O. Proximity ligation assays: a recent addition to the proteomics toolbox. Expert Rev Proteomics. 2010;7:401–9. doi: 10.1586/epr.10.10. [DOI] [PubMed] [Google Scholar]
- 22.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 23.Begonja AJ, Pluthero FG, Suphamungmee W, Giannini S, Christensen H, Leung R, Lo RW, Nakamura F, Lehman W, Plomann M, Hoffmeister KM, Kahr WH, Hartwig JH, Falet H. FlnA binding to PACSIN2 F-BAR domain regulates membrane tubulation in megakaryocytes and platelets. Blood. 2015;126:80–8. doi: 10.1182/blood-2014-07-587600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurak Begonja A, Pluthero FG, Suphamungmee W, Giannini S, Christensen H, Leung R, Lo RW, Nakamura F, Lehman W, Plomann M, Hoffmeister KM, Kahr WH, Hartwig JH, Falet H. FlnA binding to PACSIN2 F-BAR domain regulates membrane tubulation in megakaryocytes and platelets. Blood. 2015;126:80–8. doi: 10.1182/blood-2014-07-587600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Kim ES, Bearer EL. Arp2/3 complex is required for actin polymerization during platelet shape change. Blood. 2002;99:4466–74. doi: 10.1182/blood.v99.12.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calaminus SD, Thomas S, McCarty OJ, Machesky LM, Watson SP. Identification of a novel, actin-rich structure, the actin nodule, in the early stages of platelet spreading. J Thromb Haemost. 2008;6:1944–52. doi: 10.1111/j.1538-7836.2008.03141.x. [DOI] [PubMed] [Google Scholar]
- 27.Falet H, Hoffmeister KM, Neujahr R, Hartwig JH. Normal Arp2/3 complex activation in platelets lacking WASp. Blood. 2002;100:2113–22. [PubMed] [Google Scholar]
- 28.Falet H, Hoffmeister KM, Neujahr R, Italiano JE, Jr, Stossel TP, Southwick FS, Hartwig JH. Importance of free actin filament barbed ends for Arp2/3 complex function in platelets and fibroblasts. Proc Natl Acad Sci USA. 2002;99:16782–7. doi: 10.1073/pnas.222652499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith BA, Daugherty-Clarke K, Goode BL, Gelles J. Pathway of actin filament branch formation by Arp2/3 complex revealed by single-molecule imaging. Proc Natl Acad Sci USA. 2013;110:1285–90. doi: 10.1073/pnas.1211164110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–8. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stapnes C, Ryningen A, Gjertsen BT, Bruserud O. Treatment with valproic acid, all-trans retinoic acid (ATRA) and theophyllamine for 9 days caused a persistent increase in peripheral blood platelet counts for a patient with acute myelogenous leukemia. Acta Oncol. 2006;45:346–9. doi: 10.1080/02841860500482233. [DOI] [PubMed] [Google Scholar]
- 32.Schweinfurth N, Hohmann S, Deuschle M, Lederbogen F, Schloss P. Valproic acid and all trans retinoic acid differentially induce megakaryopoiesis and platelet-like particle formation from the megakaryoblastic cell line MEG-01. Platelets. 2010;21:648–57. doi: 10.3109/09537104.2010.513748. [DOI] [PubMed] [Google Scholar]
- 33.Lengfelder E, Saussele S, Weisser A, Buchner T, Hehlmann R. Treatment concepts of acute promyelocytic leukemia. Crit Rev Oncol Hematol. 2005;56:261–74. doi: 10.1016/j.critrevonc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–54. [PubMed] [Google Scholar]
- 35.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–25. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 36.Firat-Karalar EN, Welch MD. New mechanisms and functions of actin nucleation. Curr Opin Cell Biol. 2011;23:4–13. doi: 10.1016/j.ceb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochim Biophys Acta. 2012;1821:57–69. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58:760–72. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 39.Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;415:187–92. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- 40.Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, Hong WK. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–10. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Demonstrates that atRA does influence proplatelet formation but does not influence platelet alphagranule release. Furthermore, atRA does not alter RARα protein expression.
Fig. S2. Demonstrates that the regulatory effect of atRA on the formation of extended barbell-shaped platelets is preserved when incubated in PRP.
Fig. S3. Shows that Arp2/3s5 is present in human platelets and CD34+-derived megakaryocytes.
Fig. S4. Shows that the inhibition of Arp2/3s5 does not influence platelet alpha-granule release but does effect the formation of extended platelets with ≥ 2 cell bodies.
Movie S1. Represents a human platelet demonstrating RARα-Arp2/3s5 co-localization.