Abstract
Objectives
NY-ESO-1 is a cancer testis antigen and a promising target for immunotherapy. The purpose of this study was to determine the expression frequency, immunogenicity, and clinical impact of NY-ESO-1 in ovarian cancer.
Methods
Immunohistochemistry (IHC), reverse-transcription polymerase chain reaction (RT-PCR), and quantitative-PCR (qRT-PCR) were utilized in an ovarian cancer (including Fallopian tube and primary peritoneal cancers) patient cohort; humoral responses against NY-ESO-1 were determined by ELISA. Clinicopathologic outcomes including progression-free (PFS) and overall (OS) survival were evaluated based on NY-ESO-1 expression. Cohen’s kappa (κ) tested agreement between expression tests.
Results
NY-ESO-1 expression was detected by any method in 40.7% of 1002 patients’ tumors (NY-ESO-1+) and baseline humoral response was identified in 19.0% of 689 tested patients. NY-ESO-1+ patients were older (p<0.001), higher stage (85% stage III/IV vs. 76.4%, p=0.015), less likely to have a complete response to initial therapy (53.9% vs. 68.9%, p=0.002), had more serous histotype (74.5% vs. 66.9%, p=0.011), and had more grade 3 tumors (83.7% vs. 70.8%, p<0.001). There was a trend toward shorter PFS (22.2 vs. 25.0 months, p=0.07) and significantly shorter OS (42.9 vs. 50.0 months, p=0.003) among NY-ESO-1+ patients. A subset analysis of NY-ESO-1+ patients that received immunotherapy demonstrated improved OS by more than 2 years (52.6 vs. 27.2 months, p<0.001).
Conclusions
This study is the first demonstration of an association between NY-ESO-1 expression and an aggressive cancer phenotype. The relatively high expression frequency of NY-ESO-1 in ovarian cancer patients coupled with the poor clinical outcomes in NY-ESO-1+ patients reveals an underappreciated need for targeted therapy against this antigen. In support, our study reveals that NY-ESO-1+ patients enrolled on immunotherapy trials targeting the antigen exhibited an improvement in OS.
Introduction
Ovarian cancer remains the most lethal gynecologic malignancy, more than 14,000 deaths are expected in the United States from this cancer in 2016. Despite multiple, large, Phase III trials testing multiple cytotoxic therapies [1–5], only incremental improvements have been made in survival. The addition of targeted agents initially showed promise but effect sizes were modest and short-lived [6, 7]. Thus, to overcome this chemotherapeutic plateau, novel avenues of therapies must be considered.
Cancer testis (CT) antigens have emerged as an attractive target for antigen-specific immunotherapy because of high levels of expression in the adult male germ cells, low levels of expression in normal tissues, and variable expression in cancer cells [8]. More than 100 of these antigens have been identified, the function of which is not known in the majority of cases, although some classes including MAGE, SSX, and ACAP gene families have identified functions [9]. Nevertheless, the fact that they are typically expressed in germ cells has suggested they normally play a gametogenic role and that this program is hijacked by tumor cells to facilitate progression. The ideal CT antigen is present in tumor cells, absent in normal cells, and has the capacity to elicit a CT antigen-specific immune response resulting in the cell death of tumor cells expressing the CT antigen [10].
In 2003, our group reported on the CT antigen NY-ESO-1 as a potential target for immunotherapy in epithelial ovarian cancer [11]. This initial report included a neutral effect of NY-ESO-1 expression on prognosis. Since that time we have enrolled more than 70 patients in 11 clinical trials targeting NY-ESO-1. Given the importance of NY-ESO-1 in targeted immunotherapy in ovarian cancer, we undertook the present study to: (1) provide an update on prevalence of NY-ESO-1 expression in ovarian cancers, (2) assess the association between NY-ESO-1 expression and clinicopathologic outcomes, and (3) evaluate the survival impact of targeting NY-ESO-1 with immunotherapy.
Methods
Patients and Specimens
All tissue specimens and health record information were accessed under an institutional review board approved protocol at the Roswell Park Cancer Institute (Buffalo, NY). All pathology specimens were reviewed by experienced gynecologic pathologists and classified according to WHO guidelines. The detailed handling protocol has been described elsewhere [11]. Briefly, formalin fixed paraffin embedded and flash frozen tumor specimens were obtained prospectively from patients diagnosed with ovarian, Fallopian tube, and primary peritoneal carcinoma (here referred to as ovarian cancer owing to their common origin of Müllerian tissues). Peripheral blood was additionally collected after surgery and serum was obtained by centrifugation. Medical records were reviewed from a prospectively maintained database to determine stage (assigned to FIGO 2014), three-tiered grade, debulking status, platinum status, and progression-free & overall survival. For patients with no disease-free interval, the date of progression was the earliest date of (a) twice the upper normal CA 125 (twice the nadir if the CA 125 never normalized), (b) biopsy proven disease at a second-look surgery, or (c) radiographic evidence of disease progression.
Total Tissue RNA Isolation
Total tissue RNA was isolated from frozen tumor tissues using the TRIReagent (Molecular Research Center Inc; Cincinnati, OH) according to the manufacturer’s protocol. Potentially contaminating DNA was removed by treating with RNase-free DNase I (Boehringer-Mannheim; Mannheim, Germany). After phenol treatment and drying, RNA was dissolved in RNase-free H2O. The resulting RNA concentration was measured spectrophotometrically using Nanodrop (GeneQuant; Amersham Pharmacia Biotech Ltd.; Cambridge, United Kingdom), and the quantity of the RNAs was checked by electrophoresis on 1% agarose gel with formamide loading buffer.
RT-PCR Analysis of NY-ESO-1 Expression
The methods for RT-PCR analysis have been described in detail elsewhere [11]. Briefly, two micrograms of each RNA sample were subjected to cDNA synthesis using the Ready-To-Go first strand synthesis kit (Pharmacia, Uppsala, Sweden). PCR was subsequently performed to analyze expression of NY-ESO-1. The primers for NY-ESO-1 were ESO1A (5’-CACACAGGATCCATGGATGCTGCAGATGCGG-3’) and ESO1B (5’-CACACAAAGCTTGGCTTAGCGCCTCTGCCCTG-3’). Amplification for both gene products was 1 min at 94°C, 1 min at 60°C, and 1.5 min at 72°C for 35 cycles. These cycles were followed by a 10-min elongation step at 72°C. Testicular tissue was used as a positive control. The PCR products were 341 bp for NY-ESO-1 and were visualized by ethidium bromide staining after separation over a 1.5% agarose gel.
qRT-PCR Analysis of NY-ESO-1 Expression
Briefly, one microgram of RNA sample was extracted by Qiagen miRNeasy (Qiagen, Hilden, Germany) and used for cDNA synthesis using the cDNA High Capacity RT Kit (Applied Biosystems, Carlsbad, CA). Real-time PCR was subsequently performed to analyze expression of NY-ESO-1 and GAPDH. The corresponding primers and probes were provided by ABI (Applied Biosystems, Carlsbad, CA). Amplification consisted of a 40-cycle amplification program with 15 seconds at 95°C and 1 minute at 60°C. A NY-ESO-1 expressing cell-line was used as a positive control and a NY-ESO-1 non-expressing cell-line was used as a negative control. The cutoffs of the qRT-PCR study for NY-ESO-1 positivity/negativity were designed to maximize the positive predictive value of the study based on paired NY-ESO-1 IHC results (IHC positivity defined as a minimum of 5% cells positive for NY-ESO-1).
IHC
The methods for immunohistochemistry have been described in detail elsewhere [11]. Briefly, Tumor specimens were fixed with buffered formalin and embedded in paraffin. Sections (5 μm) were placed on glass slides, heated at 60°C for 20 min, and then deparaffinized with xylene and ethanol. For antigen retrieval, tumor specimens mounted on glass slides were immersed in preheated antigen retrieval solution (DAKO high pH solution; DAKO, Carpinteria, CA) for 20 min and allowed to cool for 20 min at room temperature. After the inactivation of endogenous peroxidase, mAb to NY-ESO-1 (clone ES121) was then added at a concentration of 2.5 μg/mL and incubated overnight at 4°C. ES121 has been described previously [12]. The primary antibody was detected with a biotinylated antimouse IgG (DAKO). Diaminobenzidine tetrahydrochloride was then added for development for 10 min, followed by counterstaining with hematoxylin solution.
Membranous or cytoplasmic was considered true staining. The extent of immunohistochemical reactivity was graded as follows: negative, (< 5% cells stained); +, 5–25% of cells stained; ++, >25–50% of cells stained; +++, >50–75% of cells stained; and ++++, >75% of cells stained. Negative control slides omitting the primary antibody were included in all assays.
ELISA
The methods for determining seroreactivity have been described in detail elsewhere [11, 13]. Briefly, recombinant NY-ESO-1 truncated proteins at a concentration of 1 μg/ml in PBS were adsorbed to 96-wellhalf area plates (Corning) at 30 μL/well overnight at 4°C. Plates were washed with PBS and blocked for 2 hrs at room temperature with 30 μL/well of 5% NF milk in PBS. After washing, 30 μL/well of serum dilutions in 5% NF milk were added and incubated overnight at 4°C. Plates were washed, and 30 μl/well diluted secondary antibody in5% NF milk were added (goat anti-human IgG-AP; Southern Biotechnology, Birmingham, AL) and incubated for 1 hr at room temperature. Plates were washed, incubated with 30 μl/well of substrate solution (Attophose substrate; JBL Scientific, San Louis Obispo, CA) for 30 min at room temperature, stop the reaction by adding 15 uL/well of 3N NaoH solution, and immediately read (Cyto-Fluor 2350; Millipore, Bedford, MA or Synergy-HT, BioTek, Winooski, VT). Sera were tested over a range of 4-fold dilutions from 1:100 to 1:100,000, as described previously.
Statistical Analysis
All statistical analyses were performed using SAS Software (Cary, NC) version 9.4 and the R 3.1.2 statistical computing language. A nominal significance threshold of 0.05 was used unless otherwise specified. A 2x2 contingency table was used to evaluate the concordance of RT-PCR and ELISA in detection of the NY-ESO-1 status.
Statistical testing included Student’s t-test, χ2, and Fisher’s exact tests, and Kaplan-Meier survival analysis with log-rank testing. The multivariate analysis included stage, categorized as early (I or II) or late (III or IV), grade (1 vs. 2/3), debulking status (optimal vs. suboptimal), and platinum status. Progression free survival (PFS) and overall survival (OS) were computed from the date of diagnosis to the date of initial recurrence for PFS and date of death for OS. Patients who did not experience a recurrence or death were censored at the date of last visit for PFS and OS, respectively.
Clinical Trials
The details of clinical trials referenced in this study have been described elsewhere [10, 14, 15]. The therapies received by patients who enrolled in clinical trials were heterogeneous and include: a peptide vaccine (LUD02-011) [15]; a recombinant vaccine with NY-ESO-1 expressing vaccinia and fowlpox vectors (NCT00112957) [10]; and epigenetic potentiation of NY-ESO-1 expression by decitabine, a DNA methyltransferase inhibitor, prior to vaccine administration (NCT00887796) [14].
Results
From January 1, 2002 to June 30, 2016, 1002 patients had tumors tested for NY-ESO-1 expression. The demographic information of these patients are represented in Table 1. The median age of diagnosis was 61.0 years (range 13.0–91.0 years). Most patients (68.2%, 95%CI: 65.4–71.2%) had stage IIIC or IV disease and serous histology (67.5%, 95CI: 64.6–70.4%).
Table 1. Patient Characteristics.
Clinical and pathologic characteristics of the patient cohort.
| n | 1002 | |
| Age, Median (range) | 61.0 (13.0–91.0) | |
| Stage | ||
| I | 89 | 9.9% |
| II | 91 | 10.1% |
| IIIA/B | 35 | 3.9% |
| IIIC | 565 | 62.8% |
| IV | 119 | 13.2% |
| Grade | ||
| 1 | 81 | 8.4% |
| 2 | 133 | 13.7% |
| 3 | 754 | 77.9% |
| Histology | ||
| Serous | 676 | 67.5% |
| Other | 62 | 6.2% |
| Mixed Cell | 56 | 5.6% |
| Endometrioid | 45 | 4.5% |
| Non-epithelial | 43 | 4.3% |
| Clear Cell | 43 | 4.3% |
| Mucinous | 40 | 4.0% |
| Unknown | 37 | 3.7% |
| Debulking | ||
| Optimal | 604 | 69.8% |
| Suboptimal | 261 | 30.2% |
| Response to Therapy | ||
| Complete Response | 397 | 50.4% |
| Partial Response | 8 | 1.0% |
| Persistent/Stable Disease | 199 | 25.3% |
| Progressive Disease | 31 | 3.9% |
| Death on treatment | 152 | 19.3% |
| Unevaluated | 215 | 21.5% |
| Progression-free survival, median Months | 23.7 (21.9–25.5) | |
| Overall survival, median months | 47.5 (44.4–51.7) | |
| Survival after relapse, median months | 31.1 (28.2–33.5) | |
Clinical and pathologic characteristics of the patient cohort.
Prevalence and concordance of NY-ESO-1 expression
NY-ESO-1 was expressed by 25.9% (260/1002) of tested samples as measured by RNA (RT-PCR or qRT-PCR) and 26.5% (232/874) of tumor samples stained positive by IHC (Table 2). The concordance between RT-PCR/qRT-PCR measurements and immunohistochemistry was moderate (Table S1. 72.4% agreement, OR=3.72, 95%CI: 2.67–5.19) and semi-quantitative scoring showed a significant trend (Chi-square = 89.2, p<0.001): tissues that were not positive by immunohistochemistry were rarely RNA-measurement positive (17.1%, 110/642); intermediate cases (focal, 1+, 2+) were frequently RNA positive (33.5%, 52/155); and 3+ and 4+ cases were often RNA positive (63.6%, 49/77). We subsequently refer to any case scored positive by RT-PCR/qRT-PCR or immunohistochemistry as expression positive (40.7%, 408/1002).
Table 2.
Prevalence and concordance of NY-ESO-1expression as determined by mRNA or immunohistochemistry.
| # in Agreement | % in Agreement | ||||||
|---|---|---|---|---|---|---|---|
| Positive | Total | Percent | mRNA | Protein | mRNA | Protein | |
| mRNA Expression (RT-PCR or qRT-PCR) | 260 | 1002 | 25.9% | ||||
| Protein Expression (IHC) | 232 | 874 | 26.5% | 101 | 43.5% | ||
| Serology (ELISA) | 131 | 689 | 19.0% | 74 | 82 | 56.5% | 62.6% |
Prevalence and concordance of NY-ESO-1expression as determined by mRNA or immunohistochemistry.
Association between NY-ESO-1 Expression and clinicopathologic characteristics
Women whose tumors were NY-ESO-1 positive were an average of 3 years older (p<0.001). NY-ESO-1 positive tumors were more likely to be stage IIIC or IV (Table 3, p=0.006), higher histological grade (p<0.001), and possess a serous histology (p=0.002).
Table 3.
Comparison of demographic, clinical information, pathologic results, and survival between patients with and without NY-ESO-1 tumor expression, as well as between patients who did and did not enroll in clinical trials. Comparisons were made using Student’s t-test, χ2, and Fisher’s exact tests, and Kaplan-Meier survival analysis with log-rank testing as appropriate.
| Expression | ESO Positive Trial patients | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Positive | Negative | % (Positive) | Trial | Not | |||
| n | 1002 | 408 | 594 | 41% | 68 | 340 | ||
| Age, Median (range) | 61.0 (13.0–91.0) | 62.0 (22.0–91.0) | 59.0 (13.0–89.0) | p<0.001 | 54.5 (36.0–89.0) | 64.0 (22.0–91.0) | p=0.003 | |
| Stage | ||||||||
| I | 89 | 26 | 63 | 29% | p=0.006 | 3 | 23 | p=0.795 |
| II | 91 | 31 | 60 | 34% | 7 | 24 | ||
| IIIA/B | 35 | 10 | 25 | 29% | 1 | 9 | ||
| IIIC | 565 | 256 | 309 | 45% | 46 | 210 | ||
| IV | 119 | 55 | 64 | 46% | 9 | 46 | ||
| Grade | ||||||||
| 1 | 81 | 17 | 64 | 21% | p<0.001 | 3 | 14 | p=0.432 |
| 2 | 133 | 47 | 86 | 35% | 11 | 36 | ||
| 3 | 754 | 340 | 414 | 45% | 54 | 286 | ||
| Histology | ||||||||
| Serous | 676 | 298 | 378 | 44% | p=0.002 | 53 | 245 | p=0.739 |
| Other | 62 | 23 | 39 | 37% | 3 | 20 | ||
| Mixed Cell | 56 | 25 | 31 | 45% | 5 | 20 | ||
| Endometrioid | 45 | 11 | 34 | 24% | 1 | 10 | ||
| Non-epithelial | 43 | 21 | 22 | 49% | 3 | 18 | ||
| Clear Cell | 43 | 12 | 31 | 28% | 0 | 12 | ||
| Mucinous | 40 | 10 | 30 | 25% | 1 | 9 | ||
| Unknown | 37 | 8 | 29 | 22% | 2 | 6 | ||
| Debulking | ||||||||
| Optimal | 604 | 251 | 353 | 42% | p=0.688 | 46 | 205 | p=0.208 |
| Suboptimal | 261 | 113 | 148 | 43% | 14 | 99 | ||
| 69.0% | 70.5% | |||||||
| Response to Therapy | ||||||||
| Complete Response | 397 | 145 | 252 | 37% | p=0.002 | 41 | 104 | p<0.001 |
| Partial Response | 8 | 4 | 4 | 50% | 0 | 4 | ||
| Persistent/Stable Disease | 199 | 103 | 96 | 52% | 8 | 95 | ||
| Progressive Disease | 31 | 17 | 14 | 55% | 1 | 16 | ||
| Death on treatment | 152 | 67 | 85 | 44% | 5 | 62 | ||
| Unevaluated | 215 | 72 | 143 | 33% | 13 | 59 | ||
| Progression-free survival, median Months | 23.7 (21.9–25.5) | 22.2 (18.8–24.5) | 25.0 (22.3–27.9) | p=0.009 | 23.0 (18.7–38.5) | 22.2 (17.7–25.3) | p=0.239 | |
| Overall survival, median months | 47.5 (44.4–51.7) | 42.9 (38.5–50.1) | 50.0 (45.2–59.3) | p=0.002 | 75.3 (60.9–117.0) | 38.0 (31.0–43.0) | p<0.001 | |
| Survival after relapse, median months | 31.1 (28.2–33.5) | 29.4 (22.4–34.6) | 32.0 (28.2–34.1) | p=0.490 | 42.9 (34.7–62.5) | 22.4 (19.6–30.2) | p=0.006 | |
Comparison of demographic, clinical information, pathologic results, and survival between patients with and without NY-ESO-1 tumor expression, as well as between patients who did and did not enroll in clinical trials. Comparisons were made using Student’s t-test, χ2, and Fisher’s exact tests, and Kaplan-Meier survival analysis with log-rank testing as appropriate.
Impact of NY-ESO-1 expression on clinical outcomes
While it was not associated with the ability to achieve optimal cytoreduction (69.0% vs 70.5%, p=0.688), NY-ESO-1 expression was associated with shorter progression-free survival (22.2 versus 25.0 months, p=0.009) and overall survival (42.9 versus 50.0 months, p=0.002). The association between NY-ESO-1 expression and survival was robust to adjustment for stage, grade, and residual disease the differences in overall survival (Cox-model p=0.013) but not PFS (p=0.088). Even so, the PFS effect was similar before adjustment (HR=1.21, 1.05–1.40) and after (HR=1.14, 0.98–1.33).
Impact of Clinical Trial enrollment on survival
During the study period we offered 11 vaccine trials [10, 14–18]. A total of 68 patients with NY-ESO-1 positive tumors enrolled in these trials. Compared with those NY-ESO-1 positive patients who did not enroll in trials, patients enrolling in trials were younger (p=0.002), had similar stage, grade and histology of disease. There was no difference in PFS (p = 0.239). Only 13 vaccine trial patients had tumors that did not express NY-ESO-1; too few to analyze for specific differences.
The availability of clinical trials for patients with NY-ESO-1 expressing tumors allowed definition of three independent groups: Group 1, those patients with NY-ESO-1 expressing tumors who were enrolled in cancer vaccine trials; Group 2, those patients with NY-ESO-1 expressing tumors who did not enroll in cancer vaccine trials; and Group 3, those patients without NY-ESO-1 expressing tumors.
Survival characteristics of these groups are compared in Figure 1. Group 1 patients had significantly improved OS when compared with both Group 2 (75.3 vs. 38.0 median months, p<0.001) and Group 3 (75.3 versus 50.0 months, p=0.046). Adjusting for time to trial, the directions of these associations persisted: expression positive patients had improved survival on trial (Group 1 42.9 versus Group 2 22.4 months, p=0.006) and Group 1 patients trended towards overcoming the aggressive phenotype versus Group 3 patients (42.9 versus 32.0 months, p=0.07).
Figure 1.
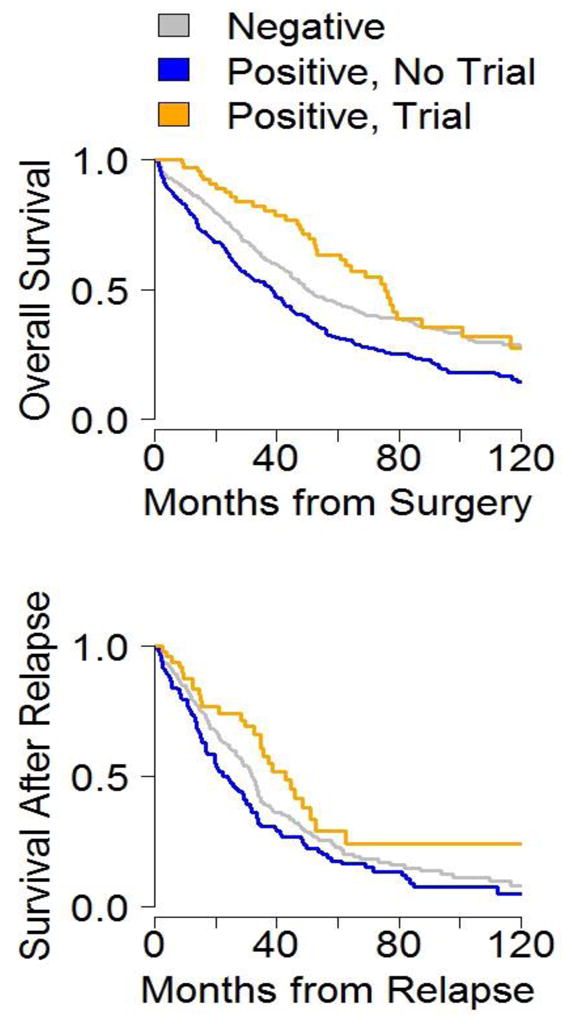
Post-relapse and overall survival for patients in Group 1 (yellow), Group 2 (blue), and Group 3 (gray).
Correlation of spontaneous seroreactivity with clinicopathologic outcomes
Among the 689 patients tested by ELISA, 302 had NY-ESO-1 expressing tumors (by PCR and/or IHC). NY-ESO-1 antibody was present in 104 of 302 (34.4%) of patients with NY-ESO-1 expressing tumors. Additionally, there were 27 patients who had demonstrable NY-ESO-1 antibodies despite having tumors that did not express the antigen. Analysis of seropositive patients, those with spontaneous antibody response to NY-ESO-1, showed they were more likely to have serous histology (p = 0.006) compared with seronegative patients with NY-ESO-1 expressing tumors (those without a spontaneous NY-ESO-1 antibody response), but there was no difference in stage (p = 0.33), grade (p = 0.43), or platinum status (p = 0.45). There was a trend towards worse median overall survival in seropositive patients, 39.2 vs. 48.3 months (p = 0.27). The difference in median progression free survival also approached statistical significance and favored seronegative patients (23.8 vs. 16.9 months, p = 0.11).
Discussion
Over the last decade, NY-ESO-1 has emerged as an immunogenic tumor antigen with promise in antigen-specific immunotherapy. The lackluster performance of combination chemotherapy [2–5], anti-angiogenesis therapies [6, 7], and immune-modulating therapies [19, 20] has accentuated the urgency to identify alternative strategies to treat ovarian cancers. To confirm the utility of NY-ESO-1 as a target for antigen-specific immunotherapy the present analysis was undertaken on a large cohort of ovarian cancer patients. To our knowledge, the current study represents the largest cohort of patients who have undergone CT antigen analysis in any tumor type. Our results indicate expression of NY-ESO-1 in 40.7% of tumors. The reported prevalence of expression remains higher than in most other solid tumors [12, 21], but consistent with our initial report on NY-ESO-1 in ovarian cancer [11].
In that report, expression of the CT antigen had a neutral effect on prognosis [11]. Additionally there was a lack of correlation with clinical parameters such as stage and grade, a finding contrary to reports from other tumors [22, 23]. The contrast with other tumors was explained by most ovarian cancers being diagnosed at advanced stage. A second possibility, however, was that the 2003 study was simply underpowered to identify differences in survival [11]. With more than five times the original patient population size, the present analysis identified NY-ESO-1 expression as a biomarker of an aggressive clinical phenotype, consistent with other tumor types [22, 23]. Patients with NY-ESO-1 expressing tumors had higher stage, higher grade, more serous histology, and fewer complete responses to primary therapy. Patients with NY-ESO-1 expressing tumors had profoundly worse outcomes, with median survival approaching just 2 years (27.2 months). However, when patients who received antigen-specific immunotherapy were analyzed separately, it appeared to rescue these patients from early death and thus reverse the dismal clinical trajectory experienced in the absence of immunotherapy.
The subpopulation of patients who exhibited spontaneous immune response to NY-ESO-1 were more likely to have serous histotype, but there was no difference in stage, grade, or treatment response. An exciting area of investigation will be to determine if patients who exhibit a spontaneous immune response to cancer testis antigens will be sensitive to immunomodulating therapies such as checkpoint inhibitors. Studies in other solid tumors identified somatic mutational load, a surrogate for a tumor’s potential to generate neoantigens, as a predictor of response to anti-PD-L1 therapy [24]. To date only 7 of the patients in this cohort have been treated with checkpoint inhibitors and the length of follow-up is too short to infer associations between NY-ESO-1 seroreactivity and response to checkpoint blockade. Although there have been negative checkpoint inhibitor studies in the past [25], perhaps patients with tumors expressing CT antigens and demonstrating spontaneous seroreactivity to those antigens will define a subset of patients who will benefit from such therapies [26, 27].
There are several limitations with the current study, including (1) the multiple clinical trials these patients were enrolled in were all small; (2) patients received heterogeneous therapies and there is the possibility of selection bias for those seeking care at a cancer center instead of in a community setting, and (3) some of the subgroups of patients, including those who had spontaneous immune responses to NY-ESO-1 and subsequently received checkpoint inhibitors, are quite small. Nevertheless, the results of the present analysis support NY-ESO-1 as a target for immunotherapy.
Homologous repair deficiency (HRD), tumor mutation burden (TMB), and loss of heterozygosity (LOH) are identified predictors of response to either targeted therapies or treatment with immunoregulatory molecules [25, 28, 29]. Identification of NY-ESO-1 expressing tumors is not expected to replace any of these other biomarkers; however, NY-ESO-1 is a shared CT antigen with tissue restricted expression in ovarian cancer and other tumor types [8]. NY-ESO-1 expression may play a role as an adjunct to these other biomarkers to help stratify their risk of recurrence and early death.
Although all testing was performed at a single institution, the present study did include tumor specimens sent from multiple hospitals around the United States. We acknowledge the possibility of selection bias may exist because patients with aggressive tumors could be differentially likely to seek out clinical trials and this cohort included people referred to our institution for additional therapeutic options. With respect to clinical outcomes, patients in this cohort had heterogeneous exposure to chemotherapy, targeted therapy, and immunotherapy.
In this study we identified a population of ovarian cancer patients at high risk for aggressive disease and poor clinical outcomes. Those who received antigen-directed therapy were able to change clinical trajectories to be more similar to NY-ESO-1 non-expressers. Because of the high prevalence of expression in ovarian cancer, the association of expression with adverse clinical outcomes, and these early successes at overcoming a poor clinical outcome, we suggest that NY-ESO-1 should be a high-priority target for future immunotherapy studies.
Supplementary Material
Highlights.
NY-ESO-1 is a common cancer-testis antigen expressed in ovarian cancer.
NY-ESO-1 expressing tumors portend an aggressive clinical course and early cancer-related death.
Antigen-targeted immunotherapy can overcome a harsh clinical phenotype.
Acknowledgments
This work was supported by the Cancer Vaccine Collaborative Grant for Immunological Monitoring and the Ovarian Cancer Working Group grant from the Cancer Research Institute/Ludwig Institute for Cancer Research Cancer Vaccine Collaborative Grant, an Anna-Maria Kellen Clinical Investigator Award of the Cancer Research Institute, the Ovarian Cancer Research Fund, the Roswell Park Alliance Foundation, NIH grants 1R01CA158318–01A1, 1K01LM012100, T32CA108456, and RPCI-UPCI Ovarian Cancer SPORE P50CA159981–01A1. This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s shared resources including the Immune Analysis Facility, Pathology Resource Network, and Clinical Data Network. Dr. Gnjatic acknowledges grant support from the Cancer Research Institute and from NIH NCI P01CA190174.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberts DS, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(4):1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 4.McGuire WP, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 5.Ozols RF, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 6.Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 7.Pignata S, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16(5):561–8. doi: 10.1016/S1470-2045(15)70115-4. [DOI] [PubMed] [Google Scholar]
- 8.Almeida LG, et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37(Database issue):D816–9. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YT, et al. Identification of the MAGE-1 gene product by monoclonal and polyclonal antibodies. Proc Natl Acad Sci U S A. 1994;91(3):1004–8. doi: 10.1073/pnas.91.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odunsi K, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci U S A. 2012;109(15):5797–802. doi: 10.1073/pnas.1117208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odunsi K, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63(18):6076–83. [PubMed] [Google Scholar]
- 12.Jungbluth AA, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92(6):856–60. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 13.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–9. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 14.Odunsi K, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res. 2014;2(1):37–49. doi: 10.1158/2326-6066.CIR-13-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odunsi K, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(31):12837–42. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berinstein NL, et al. First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients. J Transl Med. 2012;10:156. doi: 10.1186/1479-5876-10-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa H, et al. Influence of CD4+CD25+ regulatory T cells on low/high-avidity CD4+ T cells following peptide vaccination. J Immunol. 2006;176(10):6340–6. doi: 10.4049/jimmunol.176.10.6340. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji T, et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011;186(2):1218–27. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- 19.Hamanishi J, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2015;33(34):4015–22. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashino K, et al. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85(5):713–20. doi: 10.1054/bjoc.2001.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jager E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187(2):265–70. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurashige T, et al. Ny-ESO-1 expression and immunogenicity associated with transitional cell carcinoma: correlation with tumor grade. Cancer Res. 2001;61(12):4671–4. [PubMed] [Google Scholar]
- 24.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract. 2016;3:11. doi: 10.1186/s40661-016-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 27.Webb JR, et al. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141(2):293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Martin SD, et al. Low Mutation Burden in Ovarian Cancer May Limit the Utility of Neoantigen-Targeted Vaccines. PLoS One. 2016;11(5):e0155189. doi: 10.1371/journal.pone.0155189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong ZY, et al. Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumour Biol. 2016;37(4):4251–61. doi: 10.1007/s13277-016-4812-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


