Abstract
Pseudokinases and pseudophosphatases possess the ability to bind substrates without catalyzing their modification, thereby providing a mechanism to recruit potential phosphotargets away from active enzymes. Since many of these pseudoenzymes possess other characteristics such as localization signals, separate catalytic sites, and protein–protein interaction domains, they have the capacity to influence signaling dynamics in local environments. In a similar manner, the targeting of signaling enzymes to subcellular locations by A-kinase-anchoring proteins (AKAPs) allows for precise and local control of second messenger signaling events. Here, we will discuss how pseudoenzymes form ‘pseudoscaffolds’ and compare and contrast this compartment-specific regulatory role with the signal organization properties of AKAPs. The mitochondria will be the focus of this review, as they are dynamic organelles that influence a broad range of cellular processes such as metabolism, ATP synthesis, and apoptosis.
Introduction: protein kinase A/cyclic AMP, pseudoenzymes, and mitochondria
Pseudoenzymes as ‘pseudoscaffolds’
An emerging aspect of cell signaling is the role of pseudoenzymes as active participants in signal transduction cascades. These interesting signaling elements control the ebb and flow of metabolic information without fulfilling a catalytic function. Pseudokinases and pseudophosphatases, in particular, contain a substrate-binding domain similar enough to the active site of their relative enzymes to bind substrates, but do not typically possess detectable levels of catalytic activity [1–5]. Previous work has challenged this latter assumption by identifying catalytic activity in some pseudoenzymes, although with much lower activity than their canonical enzyme counterparts [6–9]. Another emergent role for pseudoenzymes is to act as an inhibitory anchor by recruiting substrate proteins into a pseudoenzyme scaffold, or ‘pseudoscaffold.’ This mechanism can serve to reduce the availability of free substrate protein in the vicinity of active enzymes (Figure 1A). Additionally, pseudoenzymes often possess functional domains such as localization motifs, other functional catalytic sites, and protein interaction domains (Figure 1A). Utilization of these additional features expands the repertoire of pseudokinases and pseudophosphatases as context-specific modulators of local protein phosphorylation events. Interestingly, these features of pseudoenzymes bear some resemblance to another family of non-catalytic signal organizing proteins, the A-kinase-anchoring proteins (AKAPs; Figure 1B).
Figure 1. Comparison of pseudoenzyme scaffolds and AKAPs.
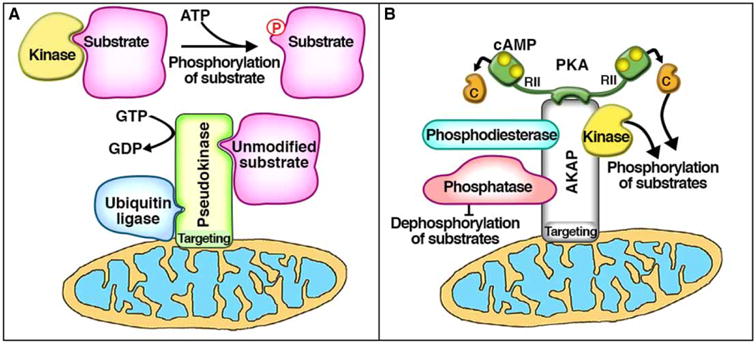
(A) Pseudoenzymes can act as an inhibitory anchor by binding and recruiting substrate protein away from active kinases and into a pseudoenzyme scaffold or ‘pseudoscaffold,’ thereby preventing substrate phosphorylation. This pseudoscaffold can possess additional features or functionalities such as (1) organelle-targeting motifs (i.e. mitochondrial, such as ADCK3), (2) separate catalytic sites (i.e. GTPase, such as MTMR5/13), or (3) other protein-protein interaction domains (i.e. heterodimerization, such as HER3; or other regulatory enzymes and their substrates, such as Trib2). Similarly, (B) AKAPs are a family of non-catalytic scaffolding proteins that, by definition, anchor the kinase PKA. In addition to PKA anchoring, AKAPs have also been shown to interact with a variety of other signaling molecules such as PDEs, phosphatases, and even other kinases. Many AKAPs also contain organelle-targeting motifs (i.e. mitochondrial, such as D-AKAP1). Through these additional features, both of these non-catalytic protein scaffolds can exhibit exquisite control of subcellular microdomains.
cAMP signaling and AKAPs
The role of signaling enzyme anchoring has been most thoroughly investigated as a means to restrict and focus the action of second messenger-regulated kinases and phosphatases [10,11]. Historically, this field was born out of an interest in how the ubiquitous second messenger cyclic AMP (cAMP) can be used to process chemical signals in parallel such that different clusters of cAMP effector enzymes can be simultaneously activated at discrete locations in the cell.
The second messenger cAMP is a versatile chemical signal [12] that is manufactured in response to ligand occupancy of G-protein-coupled receptors and the concomitant activation of adenylyl cyclases (ACs) [13]. The cellular accumulation of cAMP is strictly regulated by the superfamily of proteins known as phosphodiesterases (PDEs) [14]. The effects of cAMP in the cytosol have been exhaustively documented. However, distinct compartments or ‘pools’ of cAMP have also been identified at various subcellular locations, including, but not limited to, both at and within the mitochondria [15–17]. The presence of these distinct cAMP pools is indicative of tightly regulated cAMP signaling both at the mitochondrial outer membrane and within this vital organelle. A large portion of activities carried out by cAMP signaling is done so by the activation of the serine kinase protein kinase A (PKA). Four molecules of cAMP bind the regulatory subunits of the PKA holoenzyme causing the catalytic subunits to dissociate and become active, allowing the phosphorylation of PKA substrates [4,12,18–21]. In addition to PKA activation, cAMP can also activate Epacs (exchange proteins directly activated by cAMP) [22] and CNG (cyclic nucleotide-gated) channels [23].
Compartmentalization of PKA activity occurs through interactions with the large family of proteins termed AKAPs [43]. This diverse group of proteins anchors PKA via a canonical amphipathic helix and can recruit PKA to specific subcellular locations [24–26]. Interestingly, most AKAPs also create a scaffold by anchoring other important signaling molecules such as PDEs, phosphatases, and other kinases to create unique signaling microdomains that respond to cAMP and a variety of other chemical signals (Figure 1B). The clustering of enzymes with potentiating (i.e. substrates) and opposing (i.e. phosphatases) effects provides an efficient mechanism to confer bi-directional control of cell signaling at very precise locations [43,27]. Accordingly, AKAP signaling complexes and ‘pseudoscaffolds’ are both examples of macromolecular complexes that use protein–protein interactions to exert exquisite control over enzyme activity [28]. This shared mechanism affords the ability to deliver and transfer chemical information at discrete subcellular microdomains to generate local cellular responses.
Mitochondria and their disorders
Although AKAP-mediated signaling occurs at a variety of cellular organelles, the present study will focus on the role and regulation of enzymatic events, with an emphasis on cAMP-mediated phosphorylation occurring at the mitochondria. Mitochondria are often referred to as ‘the powerhouse of the cell’ because of their critical role in oxidative phosphorylation (OXPHOS) and cellular ATP production. Disorders of mitochondrial physiology, genetics, and proteins have been attributed to an array of diseases including cancer, Parkinson’s disease, Alzheimer’s disease, lung disease, and cardiomyopathy [29–33]. In addition to OXPHOS, an increasing number of reports supports the idea that the mitochondria are important signaling hubs for a variety of cellular events [34,35]. The regulation of many post-translational modifications, such as phosphorylation and ubiquitination, often occurs at the mitochondria and can have a significant impact on not only mitochondrial function, but also overall cellular health. Although both PKA and Epacs have been identified at the mitochondria, only PKA activation has been associated with changes in mitochondrial function and dynamics [36].
Part I: importance of cAMP signaling at the mitochondria
Outer mitochondrial membrane
The outer mitochondrial membrane (OMM) is known to be a hub for cAMP signaling, with multiple AKAPs responsible for anchoring PKA to the membrane, including D-AKAP1/S-AKAP84, Rab32, and WAVE-1 [37–40]. Since the OMM serves as a barrier between the cytosol and the inner workings of the mitochondria, anchored cAMP signaling molecules at this location serve to control mitochondrial function and dynamics, as well as cellular health signaling.
It has been observed that some soluble ACs (sACs) translocate to the mitochondria under ischemic conditions in cardiomyocytes [41,42]. This allows localized production of cAMP at the mitochondria, leading to the PKA-dependent phosphorylation, activation, and translocation of pro-apoptotic Bax to result in cell death [41,42]. Additionally, cAMP signaling at the OMM is responsible for the regulation of mitochondrial fission and mitochondrial membrane potential (ΔΨm; discussed further in OMM-anchored signaling) [43,44].
Intermembrane space, inner mitochondrial membrane, and matrix
The OMM is considered to be permeable to small molecules such as cAMP, allowing cytosolic cAMP to diffuse into the intermembrane space (IMS) and activate local PKA signaling. A few AKAPs have been identified in the IMS sphingosine kinase-interacting protein (SKIP) and optic atrophy 1 (OPA1), which confirm localized PKA signaling [45–47].
Conversely, the inner mitochondrial membrane (IMM) has been shown to be largely impermeable to external cAMP [16]. Therefore, any presence of cAMP within the mitochondrial matrix can probably be attributed to its production by resident sACs [48]. Interestingly, although there is some support for cAMP/PKA signaling cascade occurring in the matrix [48–50], no mechanism to import PKA has been identified to date. In fact, Lefkimmiatis et al. [16] were unable to detect the presence of endogenous mitochondrial matrix PKA activity using matrix-targeted FRET reporters. Therefore, the existence of PKA-dependent cAMP signaling within the mitochondrial matrix remains disputed.
Part II: AKAP anchored cAMP/PKA signaling at mitochondria
OMM-anchored signaling
The localization of kinases and other signaling molecules at the mitochondria has been determined to play a crucial role in regulating mitochondrial physiology, health, and dynamics. One such instance is the activation of PKA by the second messenger cAMP at the mitochondria. Activation of PKA localized to the OMM by D-AKAP1 (and its isoforms AKAP149, AKAP121, and S-AKAP84) [37,38] has been attributed to an inhibitory phosphorylation of the mitochondrial fission enzyme, dynamin-like protein 1 (Drp1) [51]. This phosphorylation of Drp1 inhibits mitochondrial fission, allowing for unopposed mitochondrial fusion. Additionally, the overexpression of D-AKAP1 leads to hyperelongated mitochondria and has been attributed to protection from cell death by promoting the PKA-dependent phosphorylation and inhibition of the pro-apoptotic Bad protein [51–53]. Interestingly, the depletion or displacement of D-AKAP1 from the mitochondria has also been associated with a decrease in ΔΨm in cardiomyocytes and HEK293 cells [43,44]; however, any role of cAMP/PKA signaling remains unclear.
The mitochondrially targeted Rab32, a member of the Ras superfamily of small G-proteins, was identified as a dual-function protein that acts as both a GTPase and an AKAP [39]. Its function as a GTPase has been attributed to play a role in mitochondria-microtubule organization and the synchronization of mitochondrial fission events [39]. Interestingly, D-AKAP1 and Rab32 are localized to the OMM by different mechanisms. D-AKAP1 contains an N-terminal mitochondrial targeting motif, whereas Rab32 contains a pair of C-terminal cysteine residues that are required for mitochondrial targeting [38,54–56].
Notably, each of these mitochondrial PKA-anchoring proteins also binds a variety of other signaling molecules. For example, D-AKAP1 has been found to interact with PDE4A, PP1 (protein phosphatase 1), Drp1, and calcineurin [57–60]. The recruitment of such molecules to the OMM allows for precise control of the cAMP signaling microenvironment by localizing not only kinases, but also cAMP-degrading enzymes (PDEs), phosphatases, and important substrates to one discrete location.
IMM, IMS-anchored signaling
While the role of anchored cAMP/PKA signaling at the OMM has been broadly studied over the past few decades, signaling within the mitochondria has proved to be more difficult. PKA signaling was first proposed to occur inside the mitochondria with the observation of a PKA-dependent phosphorylation of ChChd3 (coiled-coil-helix-coiled-coil-helix domain-containing 3) [61]. This protein is a ChCh family member protein that is an important regulator in cristae maintenance and is found in the IMM, facing the IMS [61,62]. Interestingly, ChChd3 was also found to be a binding partner of the IMM protein OPA1, which was later identified as an AKAP [45,59]. PKA signaling inside the mitochondria was further confirmed by the identification of the type-I PKA-specific AKAP, SKIP, in the IMS [45,46]. Although the PKA-dependent phosphorylation of ChChd3 was critical in confirming the presence of a functional pool of PKA in the mitochondria, it remains unknown if this phosphorylation affects cristae maintenance or architecture.
Part III: pseudokinases and pseudophosphatases as localized ‘pseudoscaffolds’
General description
As mentioned earlier, pseudoenzymes can serve as an ‘inhibitory anchor’ by binding substrates, but not catalyzing their modification. This interaction can serve two purposes: (1) to reduce free substrate, preventing the substrate from being modified by active enzymes or (2) to localize substrate to particular subcellular locations. Since pseudoenzymes have the ability to carry out these actions simultaneously, they can be considered an inhibitory anchor.
In a paradigm-changing study published in 2008, Mukherjee et al. [73] found that the pseudokinase CASK ([Ca2+]/calmodulin-activated Ser-Thr kinase) was not catalytically inactive, as its classification as a pseudokinase suggested. Rather it possessed low but significant levels of kinase activity [73]. Since then, more proteins originally classified as pseudoenzymes have also been shown to possess low levels of enzymatic activity [6]. However, since their classification as pseudoenzymes often stems from their lack of important amino acid residues involved in catalysis, their enzymatic activity is often lower or distinct from their canonical enzyme counterparts [6–9]. Regardless, the implications of low-activity pseudoenzymes as inhibitory anchors represent a key cellular function that may be implicated in certain human diseases of defective cell signaling.
Examples of pseudoenzyme scaffolding
Interestingly, pseudoenzymes often affect more than just the localization of their trapped substrates. They have also been shown to (1) act as a scaffold for other active molecules and enzymes and (2) facilitate other protein-protein interactions, and some pseudoenzymes even (3) possess additional domains that are catalytically active. An example of this third classification of multifunctionality can be seen in the myotubularin-related (MTMR) pseudophosphatases MTMR5 and MTMR13 [3]. In addition to containing a catalytically inactive phosphatase domain, these pseudophosphatases have been found to contain DENN (‘Differentially Expressed in Neoplastic vs. Normal cells’) domains, which act as an exchange factor to activate Rab GTPases [3,63]. Both of these MTMR proteins display subcellular localization to different areas of the cell, MTMR5 to the nucleus [63] and MTMR13 to endosomes [64]. Knocking out either of these genes in mice leads to visible pathology: MTMR5 caused impaired spermatogenesis and infertility in male mice [65] and MTMR13 knockout can be used as a mouse model of Charcot-Marie-Tooth disease [66].
Another example of multifunctional pseudoenzymes is the tyrosine kinase epidermal growth factor receptor family member HER3 (human epidermal growth factor receptor 3, also known as ERBB3) [3,4,67,68]. This pseudokinase has the ability to bind ligand, but does not homodimerize, rendering it inactive. However, HER3 can heterodimerize with HER2, which itself has no known ligands, and together the heterodimer is able to function as a signaling entity [69]. This HER2/3 heterodimer signaling is said to function as an oncogenic unit [69]. Notably, HER3 also displays very low levels of catalytic activity in vitro, nearing 1000-fold less activity than HER1 [6]. However, further analyses failed to identify any significant cellular effect of this low catalytic activity [70]. HER3 displays altered expression in breast cancer and other cancers [71–74], and knockdown of HER3 in breast cancer cells decreases proliferation, migration, and invasive potential [75]. In this example, HER3 can act as a pseudokinase inhibitory anchor by binding ligand but not promoting signaling, thus reducing free ligand to also reduce the activation of other ligand-binding enzymes, such as the enzymatically active HER1. Additionally, HER3 also modulates HER-family signaling by interacting with HER2 to generate a functional heterodimer from two individually nonfunctional units.
In addition to their roles in protein phosphorylation pathways, ‘pseudoscaffolds’ can also participate in other regulatory processes. For instance, the Tribbles pseudokinase, Trib2, contains a highly unusual catalytic loop that not only abolishes kinase activity, but also plays a critical role in recruiting the COP1 ubiquitin ligase substrate C/EBP for ubiquitination [76,77]. Interestingly, C/EBP interacts with the modified amino terminal catalytic loop of Trib2, whereas COP1 interacts with a carboxy-terminal domain, thus anchoring both ligase and substrate to the same location via this Trib2 scaffold [76–78]. The disruption of this recruitment by Trib2 has been attributed to the development of acute myelogenous leukemia in mice [77]. Trib2 is a perfect example of how protein-protein interactions of a ‘pseudoscaffold’ can anchor and regulate additional cellular events separate from kinase activity.
Mitochondrial pseudoenzymes and potential significance
Since healthy mitochondrial function is crucial to cell survival, the anchoring of signaling microdomains often plays a major role in regulating their action [29–33]. Recent work has begun to highlight a potential role for pseudoenzymes at the mitochondria; however, much remains to be investigated. Protein tyrosine phosphatase non-receptor type 21 (PTPN21 or PTPD1) contains a Cys-to-Ser mutation in its catalytic motif [79], and therefore sometimes is classified as a pseudophosphatase [3], although it does not display altered levels of phosphatase activity [79]. PTPN21 was also shown to be a binding partner of the mitochondrial D-AKAP1 [44,79,80]. Interestingly, PTPN21 was found to interact with the non-receptor tyrosine kinase Src, and, through this interaction, recruit Src to the mitochondria [44]. This D-AKAP1-PTPN21-Src complex increases Src-dependent phosphorylation of mitochondrial substrates and enhances cytochrome c oxidase activity [44]. Overexpressing a D-AKAP1 mutant that cannot bind the PTPN21/Src complex led to a decrease in ΔΨm and concurrent decrease in ATP production that was comparable with a D-AKAP1ΔPKA mutant [44]. However, the mechanism of how either PKA or the PTPN21/Src complex drives ΔΨm decrease has not yet been fully elucidated.
The depletion of another pseudophosphatase, MK-STYX, in the human cervical cancer cell line HeLa was found to protect cells from initiating apoptosis with treatment by various chemotherapeutics [81]. This group identified MK-STYX as a catalytically inactive phosphatase with significant homology to the mitogen-activated protein kinase (MAPK) phosphatases [81]. However, MK-STYX-depleted HeLa cells were unable to initiate cytochrome c release by the pro-apoptotic signaling by BCL-2 family proteins (Bax, Bid, and Bim) at the mitochondria, suggesting that the activity of this pseudophosphatase is occurring at the mitochondrial outer membrane [81]. The perturbation of MK-STYX expression with RNAi did not reveal any significant effect on MAPK signaling in the present study. Therefore, it is likely that this particular pseudophosphatase is not exerting its effects as an inhibitory anchor for MAPK phosphatase substrates, but is acting through a different, unknown mechanism. Since evasion of apoptosis is a significant concern in many cancer types, the exact role of this pseudophosphatase may be interesting to follow up for the design of future cancer therapeutics.
Another key mitochondrial protein is the atypical pseudokinase ADCK3, also known as COQ8A [82,83]. ADCK3 is a member of the widespread but little understood UbiB protein kinase-like (PKL) family [82,83], and is localized to the matrix face of the IMM, the site of CoQ synthesis [84]. The crystal structure of ADCK3 has illuminated how the kinase activity of UbiB PKL proteins is physically self-inhibited [83], rendering ADCK3 (and therefore other related UbiB PKL proteins) an atypical pseudokinase. This structural analysis uncovered an unexpected selectivity for ADP, thus limiting the ATP binding of ADCK3 [83]. In keeping with this notion, ADCK3 knockout mice develop a slow-progressing cerebellar ataxia that closely models Purkinje cell dysfunction caused by hereditary CoQ deficiency in humans [85]. These mice also display abnormal mitochondrial morphology in skeletal muscle, although no gross changes in mitochondrial function were observed [85]. These studies went on to elucidate the mechanism of CoQ deficiency in this model, attributing it to the destabilization of the CoQ biosynthetic complex ‘complex Q’, which is normally stabilized in the presence of ADCK3 via its proposed ATPase activity [85]. This atypical pseudokinase is a great example of the importance of pseudoenzyme complexes and how their unique features can influence biological pathways.
Part IV: conclusions
It is increasingly obvious that anchoring proteins are crucial in fine-tuning localized signaling events to control a myriad of cellular functions. We have discussed the importance of anchored PKA in the mitochondria by AKAPs and attributed the precise control of cellular maintenance functions such as mitochondrial dynamics, apoptosis, ATP production, and even the concentration of small molecules to AKAP anchoring.
Pseudoenzymes, on the other hand, possess the intrinsic ability to act as an inhibitory anchor by recruiting substrates and preventing their modification by other enzymes, while tethering them to discrete locations into a unique ‘pseudoscaffold’. Furthermore, this protein scaffold can itself possess certain abilities such as (1) organelle-targeting motifs (i.e. mitochondrial, such as ADCK3), (2) catalytic activity separate from its pseudoenzyme-binding pocket (i.e. DENN domains of MTMR5/13), or (3) the ability to interact with or activate other proteins to influence signaling events (i.e. HER3 heterodimerization; Trib2-anchoring COP1 and its substrate C/EBP).
Thus, pseudoenzymes are not simply evolutionary ‘leftovers’ of functional enzymes, but a unique and emergent class of proteins united in their ability to anchor, but not modify, substrates of their enzymatic counterparts.
Acknowledgments
Funding
This work was supported by the following grants from the National Institutes of Health: R01DK105542 (J.D.S.) and P01DK05441 (J.D.S.), and PHS NRSA T32GM007270 (S.A-H.) from NIGMS.
Abbreviations
- ACs
adenylyl cyclases
- AKAPs
A-kinase-anchoring proteins
- Bcl-2
B-cell lymphoma 2
- cAMP
cyclic AMP
- C/EBP
CCAAT-enhancer-binding proteins
- ChChd3
coiled-coil-helix-coiled-coil-helixdomain-containing 3
- DENN
differentially expressed in neoplastic vs. normal cells
- Epacs
exchange proteins directly activated by cAMP
- FRET
fluorescence resonance energy transfer
- HER3
human epidermal growth factor receptor 3
- IMM
inner mitochondrial membrane
- IMS
intermembrane space
- MAPK
mitogen-activated protein kinase
- MTMR
myotubularin-related
- OXPHOS
oxidative phosphorylation
- PDEs
phosphodiesterases
- PKA
protein kinase A
- PKL
protein kinase-like
- PTPN21
protein tyrosine phosphatase non-receptor type 21
- sACs
soluble ACs
- SKIP
sphingosine kinase-interacting protein
- ΔΨm
mitochondrial membrane potential
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934 d. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Reiterer V, Eyers PA, Farhan H. Day of the dead: pseudokinases and pseudophosphatases in physiology and disease. Trends Cell Biol. 2014;24:489–505. doi: 10.1016/j.tcb.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Langeberg LK, Scott JD. Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol. 2015;16:232–244. doi: 10.1038/nrm3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyers PA, Murphy JM. The evolving world of pseudoenzymes: proteins, prejudice and zombies. BMC Biol. 2016;14:98. doi: 10.1186/s12915-016-0322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. Erbb3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA. 2010;107:7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Südhof TC, et al. CASK functions as a Mg2+-independent Neurexin Kinase. Cell. 2008;133:328–339 d. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan N, Taylor SS. Rethinking pseudokinases. Cell. 2008;133:204–205 d. doi: 10.1016/j.cell.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AS, Kornev AP, Hu J, Ahuja LG, Taylor SS. Kinases and pseudokinases: lessons from RAF. Mol Cell Biol. 2014;34:1538–1546. doi: 10.1128/MCB.00057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220–1224 d. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser IDC, Scott JD. Modulation of ion channels: a ‘current’ view of AKAPs. Neuron. 1999;23:423–426 d. doi: 10.1016/S0896-6273(00)80795-3. [DOI] [PubMed] [Google Scholar]
- 12.Glass DB, Krebs EG. Protein phosphorylation catalyzed by cyclic AMP-dependent and cyclic GMP-dependent protein kinases. Ann Rev Pharmacol Toxicol. 1980;20:363–388. doi: 10.1146/annurev.pa.20.040180.002051. [DOI] [PubMed] [Google Scholar]
- 13.Choi EJ, Xia Z, Villacres EC, Storm DR. The regulatory diversity of the mammalian adenylyl cyclases. Curr Opin Cell Biol. 1993;5:269–273. doi: 10.1016/0955-0674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 14.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 15.Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 2013;17:965–975. doi: 10.1016/j.cmet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Lefkimmiatis K, Leronni D, Hofer AM. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J Cell Biol. 2013;202:453–462. doi: 10.1083/jcb.201303159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stangherlin A, Koschinski A, Terrin A, Zoccarato A, Jiang H, Fields LA, et al. Analysis of compartmentalized cAMP: a method to compare signals from differently targeted FRET reporters. Methods Mol Biol. 2014;1071:59–71. doi: 10.1007/978-1-62703-622-1_5. [DOI] [PubMed] [Google Scholar]
- 18.Corbin JD, Soderling TR, Park CR. Regulation of adenosine 3′,5′-monophosphate-dependent protein kinase. I. Preliminary characterization of the adipose tissue enzyme in crude extracts. J Biol Chem. 1973;248:1813–1821. [PubMed] [Google Scholar]
- 19.Corbin JD, Keely SL. Characterization and regulation of heart adenosine 3':5'-monophosphate-dependent protein kinase isozymes. J Biol Chem. 1977;252:910–918. [PubMed] [Google Scholar]
- 20.Potter RL, Taylor SS. Relationships between structural domains and function in the regulatory subunit of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979;254:2413–2418. [PubMed] [Google Scholar]
- 21.Smith FD, Reichow SL, Esseltine JL, Shi D, Langeberg LK, Scott JD, et al. Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. eLife. 2013;2:e01319 d. doi: 10.7554/eLife.01319.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bos JL. Opinion: Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 23.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 24.Carr DW, Stofko-Hahn RE, Fraser ID, Bishop SM, Acott TS, Brennan RG, et al. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 25.Newlon MG, Roy M, Hausken ZE, Scott JD, Jennings PA. The A-kinase anchoring domain of type IIα cAMP-dependent protein kinase is highly helical. J Biol Chem. 1997;272:23637–23644. doi: 10.1074/jbc.272.38.23637. [DOI] [PubMed] [Google Scholar]
- 26.Newlon MG, Roy M, Morikis D, Carr DW, Westphal R, Scott JD, et al. A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. EMBO J. 2001;20:1651–1662. doi: 10.1093/emboj/20.7.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 28.Smith FD, Langeberg LK, Cellurale C, Pawson T, Morrison DK, Davis RJ, et al. AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat Cell Biol. 2010;12:1242–1249. doi: 10.1038/ncb2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 31.Chinta SJ, Andersen JK. Redox imbalance in Parkinson’s disease. Biochim Biophys Acta, Gen Subj. 2008;1780:1362–1367. doi: 10.1016/j.bbagen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cloonan SM, Choi AMK. Mitochondria in lung disease. J Clin Invest. 2016;126:809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- 34.Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640 d. doi: 10.1016/S0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- 35.Aon MA, Camara AKS. Mitochondria: hubs of cellular signaling, energetics and redox balance. A rich, vibrant, and diverse landscape of mitochondrial research. Front Physiol. 2015;6:94. doi: 10.3389/fphys.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem. 2002;277:26581–26586. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- 37.Chen Q, Lin RY, Rubin CS. Organelle-specific targeting of protein kinase AII (PKAII) Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J Biol Chem. 1997;272:15247–15257. doi: 10.1074/jbc.272.24.15247. [DOI] [PubMed] [Google Scholar]
- 38.Huang LJ-s, Wang L, Ma Y, Durick K, Perkins G, Deerinck TJ, et al. NH2-terminal targeting motifs direct dual specificity A-kinase—anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. J Cell Biol. 1999;145:951–959. doi: 10.1083/jcb.145.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westphal RS, Soderling SH, Alto NM, Langeberg LK, Scott JD. Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. EMBO J. 2000;19:4589–4600. doi: 10.1093/emboj/19.17.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S, Kostin S, Flacke JP, Reusch HP, Ladilov Y. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem. 2009;284:14760–14768. doi: 10.1074/jbc.M900925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appukuttan A, Kasseckert SA, Micoogullari M, Flacke JP, Kumar S, Woste A, et al. Type 10 adenylyl cyclase mediates mitochondrial Bax translocation and apoptosis of adult rat cardiomyocytes under simulated ischaemia/reperfusion. Cardiovasc Res. 2012;93:340–349. doi: 10.1093/cvr/cvr306. [DOI] [PubMed] [Google Scholar]
- 43.Perrino C, Feliciello A, Schiattarella GG, Esposito G, Guerriero R, Zaccaro L, et al. AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction, and increases oxidative stress. Cardiovasc Res. 2010;88:101–110. doi: 10.1093/cvr/cvq155. [DOI] [PubMed] [Google Scholar]
- 44.Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, et al. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol Biol Cell. 2006;17:263–271 d. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovanich D, van Der Heyden MAG, Aye TT, Van Veen TAB, Heck AJR, Scholten A. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. ChemBioChem. 2010;11:963–971 d. doi: 10.1002/cbic.201000058. [DOI] [PubMed] [Google Scholar]
- 46.Means CK, Lygren B, Langeberg LK, Jain A, Dixon RE, Vega AL, et al. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc Natl Acad Sci USA. 2011;108:E1227–E1235. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pidoux G, Witczak O, Jarnæss E, Myrvold L, Urlaub H, Stokka AJ, et al. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011;30:4371–4386. doi: 10.1038/emboj.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G, et al. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sardanelli AM, Signorile A, Nuzzi R, De Rasmo D, Technikova-Dobrova Z, Drahota Z, et al. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006;580:5690–5696. doi: 10.1016/j.febslet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Affaitati A, Cardone L, De Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, et al. Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. J Biol Chem. 2003;278:4286–4294. doi: 10.1074/jbc.M209941200. [DOI] [PubMed] [Google Scholar]
- 53.Merrill RA, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, et al. Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin RY, Moss SB, Rubin CS. Characterization of S-AKAP84, a novel developmentally regulated A kinase anchor protein of male germ cells. J Biol Chem. 1995;270:27804–27811. doi: 10.1074/jbc.270.46.27804. [DOI] [PubMed] [Google Scholar]
- 55.Glomset JA, Farnsworth CC. Role of protein modification reactions in programming interactions between Ras-related GTPases and cell membranes. Annu Rev Cell Biol. 1994;10:181–205. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- 56.Bui M, Gilady SY, Fitzsimmons REB, Benson MD, Lynes EM, Gesson K, et al. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285:31590–31602. doi: 10.1074/jbc.M110.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asirvatham AL, Galligan SG, Schillace RV, Davey MP, Vasta V, Beavo JA, et al. A-kinase anchoring proteins interact with phosphodiesterases in T lymphocyte cell lines. J Immunol. 2004;173:4806–4814. doi: 10.4049/jimmunol.173.8.4806. [DOI] [PubMed] [Google Scholar]
- 58.Bridges D, MacDonald JA, Wadzinski B, Moorhead GBG. Identification and characterization of D-AKAP1 as a major adipocyte PKA and PP1 binding protein. Biochem Biophys Res Commun. 2006;346:351–357. doi: 10.1016/j.bbrc.2006.05.138. [DOI] [PubMed] [Google Scholar]
- 59.Kim H, Scimia MC, Wilkinson D, Trelles RD, Wood MR, Bowtell D, et al. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol Cell. 2011;44:532–544 d. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abrenica B, AlShaaban M, Czubryt MP. The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2009;46:674–681. doi: 10.1016/j.yjmcc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 61.Schauble S, King CC, Darshi M, Koller A, Shah K, Taylor SS. Identification of ChChd3 as a novel substrate of the cAMP-dependent protein kinase (PKA) using an analog-sensitive catalytic subunit. J Biol Chem. 2007;282:14952–14959. doi: 10.1074/jbc.M609221200. [DOI] [PubMed] [Google Scholar]
- 62.Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins GA, et al. Chchd3, an inner mitochondrial membrane protein, is essential for maintaining Crista integrity and mitochondrial function. J Biol Chem. 2011;286:2918–2932. doi: 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Firestein R, Cleary ML. Pseudo-phosphatase Sbf1 contains an N-terminal GEF homology domain that modulates its growth regulatory properties. J Cell Sci. 2001;114:2921–2927. doi: 10.1242/jcs.114.16.2921. [DOI] [PubMed] [Google Scholar]
- 64.Ng AA, Logan AM, Schmidt EJ, Robinson FL. The CMT4B disease-causing phosphatases Mtmr2 and Mtmr13 localize to the Schwann cell cytoplasm and endomembrane compartments, where they depend upon each other to achieve wild-type levels of protein expression. Hum Mol Genet. 2013;22:1493–1506. doi: 10.1093/hmg/dds562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Firestein R, Nagy PL, Daly M, Huie P, Conti M, Cleary ML. Male infertility, impaired spermatogenesis, and azoospermia in mice deficient for the pseudophosphatase Sbf1. J Clin Invest. 2002;109:1165–1172. doi: 10.1172/JCI0212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robinson FL, Niesman IR, Beiswenger KK, Dixon JE. Loss of the inactive myotubularin-related phosphatase Mtmr13 leads to a Charcot-Marie-Tooth 4B2-like peripheral neuropathy in mice. Proc Natl Acad Sci USA. 2008;105:4916–4921. doi: 10.1073/pnas.0800742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:E1–E13. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149 d. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 69.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novotny CJ, Pollari S, Park JH, Lemmon MA, Shen W, Shokat KM. Overcoming resistance to HER2 inhibitors through state-specific kinase binding. Nat Chem Biol. 2016;12:923–930. doi: 10.1038/nchembio.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441 d. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043 d. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 73.Erjala K, Sundvall M, Junttila TT, Zhang N, Savisalo M, Mali P, et al. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin Cancer Res. 2006;12:4103–4111. doi: 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Linn D, Liu Z, Melamed J, Tavora F, Young CY, et al. EBP1, an ErbB3-binding protein, is decreased in prostate cancer and implicated in hormone resistance. Mol Cancer Ther. 2008;7:3176–3186 d. doi: 10.1158/1535-7163.MCT-08-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J, Jeong H, Lee Y, Kim C, Kim H, Kim A. HRG-p1-driven ErbB3 signaling induces epithelial-mesenchymal transition in breast cancer cells. BMC Cancer. 2013;13:383 d. doi: 10.1186/1471-2407-13-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy JM, Nakatani Y, Jamieson SA, Dai W, Lucet IS, Mace PD. Molecular mechanism of CCAAT-enhancer binding protein recruitment by the TRIB1 pseudokinase. Structure. 2015;23:2111–2121 d. doi: 10.1016/j.str.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 77.Keeshan K, Bailis W, Dedhia PH, Vega ME, Shestova O, Xu L, et al. Transformation by Tribbles homolog 2 (Trib2) requires both the Trib2 kinase domain and COP1 binding. Blood. 2010;116:4948–4957 d. doi: 10.1182/blood-2009-10-247361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eyers PA, Keeshan K, Kannan N. Tribbles in the 21st century: the evolving roles of tribbles pseudokinases in biology and disease. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Møller NP, Møller KB, Lammers R, Kharitonenkov A, Sures I, Ullrich A. Src kinase associates with a member of a distinct subfamily of protein-tyrosine phosphatases containing an ezrin-like domain. Proc Natl Acad Sci USA. 1994;91:7477–7481. doi: 10.1073/pnas.91.16.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cardone L, Carlucci A, Affaitati A, Livigni A, deCristofaro T, Garbi C, et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol. 2004;24:4613–4626. doi: 10.1128/MCB.24.11.4613-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niemi NM, Lanning NJ, Klomp JA, Tait SW, Xu Y, Dykema KJ, et al. MK-STYX, a catalytically inactive phosphatase regulating mitochondrially dependent apoptosis. Mol Cell Biol. 2011;31:1357–1368. doi: 10.1128/MCB.00788-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leonard CJ, Aravind L, Koonin EV. Novel families of putative protein kinases in bacteria and archaea: evolution of the ‘eukaryotic’ protein kinase superfamily. Genome Res. 1998;8:1038–1047. doi: 10.1101/gr.8.10.1038. [DOI] [PubMed] [Google Scholar]
- 83.Stefely JA, Reidenbach AG, Ulbrich A, Oruganty K, Floyd BJ, Jochem A, et al. Mitochondrial ADCK3 employs an atypical protein kinase-like fold to enable coenzyme Q biosynthesis. Mol Cell. 2015;57:83–94 d. doi: 10.1016/j.molcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhee HW, Zou P, Udeshi ND, Martell JD, Mootha VK, Carr SA, et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013;339:1328–1331 d. doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stefely JA, Licitra F, Laredj L, Reidenbach AG, Kemmerer ZA, Grangeray A, et al. Cerebellar ataxia and coenzyme Q deficiency through loss of unorthodox kinase activity. Mol Cell. 2016;63:608–620 d. doi: 10.1016/j.molcel.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


