Abstract
The meiotic G2/M1 transition is mostly regulated by posttranslational modifications, however, the cross-talk between different posttranslational modifications is not well-understood, especially in spermatocytes. Sumoylation has emerged as a critical regulatory event in several developmental processes, including reproduction. In mouse oocytes, inhibition of sumoylation caused various meiotic defects and led to aneuploidy. However, the role of sumoylation in male reproduction has only begun to be elucidated. Given the important role of several SUMO targets (including kinases) in meiosis, in this study, the role of sumoylation was addressed by monitoring the G2/M1 transition in pachytene spermatocytes in vitro upon inhibition of sumoylation. Furthermore, to better understand the cross-talk between sumoylation and phosphorylation, the activity of several kinases implicated in meiotic progression was also assessed upon down-regulation of sumoylation. The results of the analysis demonstrate that inhibition of sumoylation with ginkgolic acid (GA) arrests the G2/M1 transition in mouse spermatocytes preventing chromosome condensation and disassembling of the synaptonemal complex. Our results revealed that the activity of PLK1 and the Aurora kinases increased during the G2/M1 meiotic transition, but was negatively regulated by the inhibition of sumoylation. In the same experiment, the activity of c-Abl, the ERKs, and AKT were not affected or increased after GA treatment. Both the AURKs and PLK1 appear to be “at the right place, at the right time” to at least, in part, explain the meiotic arrest obtained in the spermatocyte culture.
Keywords: meiosis, phosphorylation, sumoylation, ginkgolic acid, AURKs, PLK1
Introduction
Successful progression through the G2/M1 transition in spermatocytes is a prerequisite for the formation of normal, genetically balanced gametes. Errors in meiotic recombination and chromosome segregation are the major causes of meiotic nondisjunction and aneuploidy [1]. However, the molecular mechanisms responsible for these meiotic errors are not well-understood, especially in males. Knockout mouse models arrested at the G2/M1 transition, in addition to the development of an in vitro system where pachytene spermatocytes have been induced to undergo G2/M1 transition upon treatment with okadaic acid (OA, an inhibitor of phosphatases PP1 and PP2A), have identified several important regulators of the process. For example, mice with a deletion of Cyclin A1 (CCNA1) or spermatocyte-specific deletion of cyclin dependent kinase 1 (CDK1) exhibit meiotic arrest during the exit from the meiotic prophase [2] [3]. In a similar manner, mice with meiotic expression of an inactive isoform of AURKB display abnormalities during the exit from meiosis I [4]. Events in spermatocytes during the OA-induced G2/M1 transition in vitro closely mimic the ones observed in vivo. The Initiation of desynapsis is hallmarked by the removal of the central element protein (SYCP1) of the synaptonemal complex (SC) and precedes phosphorylation of histone H3 on Serine 10 (H3SerPh). These events are followed by the re-localization of the lateral element protein of the SC (SYCP3) to the centrosomes, and the formation of condensed bivalents. Although the translation of specific proteins during the pachytene stage is a prerequisite for the successful completion of meiosis, inhibition of protein synthesis in spermatocytes at the time of G2/M1 transition does not affect the exit from meiotic prophase in vitro [5]. Therefore, the G2/M1 transition is mostly regulated by posttranslational modifications. In support of this finding, the inhibition of global tyrosine phosphorylation or the activity of specific kinases causes meiotic arrests at different stages during the OA-induced G2/M1 progression [6, 7]. For example, CDK inhibitor Butyrolactone (BLI) does not affect desynapsis or H3SerPh but does inhibit the OA-induced re-localization of SYCP3 and condensation of bivalents [8]; Interestingly, CDK inhibition also inhibits the activity of mitogen-activated protein kinases (MAPKs) through an unknown mechanism [9]. ZM447439 (ZM), an inhibitor of AURKs, did not affect initiation of desynapsis but inhibited H3SerPh (which is a direct target of AURKB) [7]. Inhibition of PLK1 with dihydropteridinone BI 2536 fully inhibited the first step of desynapsis (the removal of SYC1) and affected H3 phosphorylation to a certain degree [6]. Notably, general tyrosine kinase inhibitor completely abolishes desynapsis and chromosome condensation [5]. In addition to kinase activity, the activity of topoisomerases (TOPs, enzymes unwinding DNA) is also required for G2/M1 progression. Teniposide and ICRF-193, inhibitors of TOP2, dramatically affect the condensation of chromosomes [8].
Sumoylation is yet another type of posttranslational modification by Small Ubiquitin-like Modifiers or SUMO proteins that has been identified as an important regulatory event in several cellular processes including cell cycle progression [10–14]. Covalent conjugation of SUMO to the target protein happens through the action of SUMO activating enzyme (E1, a heterodimer of SAE1-SAE2), SUMO-conjugating enzyme UBC9 (E2), and SUMO ligases (E3). Sumoylation is reversed through the action of SENPs, which cleave the isopeptide bond between SUMO and its substrate. Four SUMO paralogs have been identified: SUMO1, 2, 3 (often termed SUMO2/3 because of their 95% sequence identity), and 4. While SUMO1, 2, and 3 are abundantly expressed in different tissues, SUMO4 is restricted to the kidneys and lymphatic tissues [15–17]. SUMO1-knockout mice show no phenotypic consequences because the protein’s function is compensated for by SUMO2 and SUMO3 [18]. SUMO2 is apparently the major SUMO isoform present during embryonic development, and as a result, SUMO2-knockout mice show early embryonic lethality [19]. In a similar manner, UBC9 (the only SUMO-conjugating enzyme)-knockout mice show early embryonic lethality with severe disruptions in mitosis; a finding that supports the indispensable role of sumoylation in cell cycle progression [20]. In mouse oocytes, sumoylation plays a crucial role in spindle organization in addition to chromosome congression and segregation. Inhibition of sumoylation by SUMO1 or UBC9 with a specific antibody or their depletion by specific si-RNA microinjection in oocytes caused defective spindle organization, misaligned chromosomes, and led to aneuploidy. In a similar manner, over-expression of SENP-2, a SUMO-specific isopeptidase, led to defects in MII spindle organization in mature eggs [21, 22]. However, the role of sumoylation in male reproduction has only begun to be elucidated. We and others have localized SUMO proteins in germ and somatic testicular cells and have obtained evidence implicating sumoylation in different aspects of normal and impaired spermatogenesis. We have also identified sumoylated targets in purified mouse spermatocytes and spermatids and in human sperm. In mouse spermatocytes, numerous proteins have been identified as SUMO targets including those regulating transcription, chromatin dynamics, and other processes. Sumoylation of several targets with potentially important roles during meiosis (such as CDK1, TOP2, RNAP II, MILI, DDX4, MDC1, KAP1, and TDP-43) were further supported by co-immunoprecipitation, co-localization, and in vitro sumoylation studies [23–28] [29]. SYCP1 and SYCP2 have also been co-immunoprecipitated with SUMO from testicular lysate, as shown by another group [23]. Interestingly, some kinases have been identified by our screen as SUMO targets [29]. This finding is consistent with growing evidence that phosphorylation and sumoylation interact at multiple levels. A phosphorylation-dependent motif has been identified [30] and inhibition of sumoylation in somatic cells by a sumoylation inhibitor (ginkgolic acid, GA) significantly affected tyrosine phosphorylation (PhosphoTyr) of multiple proteins [31].
Overall, a cross-talk between different post-translation modifications during meiotic prophase and the G2/M transition is not well-understood, especially in spermatocytes. Given the important role of several SUMO targets in meiosis, in this study, the role of sumoylation was addressed by monitoring the G2/M1 transition in pachytene spermatocytes in vitro upon inhibition of sumoylation. Furthermore, to better understand a cross-talk between sumoylation and phosphorylation, the activity of several kinases implicated in meiotic progression was also assessed upon down-regulated sumoylation.
Materials and methods
Short-term culture of mouse spermatocytes; okadaic and ginkgolic acid treatment
C57BL/6NCrl mice were purchased from Charles River (Kingston, NY). The Animal Committee of Albert Einstein College of Medicine, Yeshiva University approved all animal protocols. A spermatocyte-enriched fraction was prepared as described in our recent publication. A flow-cytometry and microscopic analysis to confirm fraction purity was also performed as previously described by our group [29]. Spermatocytes were cultured according to [32]. Spermatocyte-enriched fractions were pooled together, washed three times by centrifugation at 500 g for 7 min, and resuspended in minimal essential medium (MEM) (Sigma-Aldrich, M0894) supplemented with 0.29% (v/v) DL-lactic acid, 5% (v/v) FBS, 5.9 mg/ml HEPES, 0.05 mg/ml streptomycin sulfate (Life Technologies, 11860-038), and 0.075 mg/ml penicillin G (Sigma-Aldrich, P3032) to the final concentration of 2.5 × 106 cells/ml. Next, 986.7 μl of cell suspension was added to each well of a 4-well dish (Thermo Scientific, Rockford, IL), and the cells were incubated at 32°C with 5% CO2 for 10 hours (after STA-PUT separation) or were used the same day after differential plating. The results of the experiments were similar with the both types of spermatocyte isolation, but the differential plating procedure shortened the time of the experiment by one day. Following the incubation, 1 μl of freshly prepared ginkgolic acid (GA, a specific inhibitor of sumoylation that blocks formation of the E1-SUMO intermediate [33]) or 1 μl of 100% DMSO was added to the experimental or control wells, respectively. The final concentration of GA was 10–50 μM. After one hour, G2/M transition was induced by the addition of 13.3 μl of 300 μM okadaic acid (OA, a phosphatase inhibitor [7, 34]) for an additional 4 hours. Following treatment, 100 μl of cell suspension from each well was centrifuged at 5000 rpm (Eppendorf, 5415 C, 5 min, 4°C) and resuspended in 20 μl of a 2% PFA + 0.03% SDS solution. To this, 20 μl of 0.4% Photoflo solution (Kodak Professional, #74257, Hatfield, PA) was added. For chromosome spreads, 2 μl of well-mixed cell suspension was pipetted onto each well of a Shandon™ multi-spot slide (Thermo Scientific, 9991090) and gently spread in a circular motion over the well using a tip. After a brief air drying of the cells (5–10 min), the slides were washed in a series of solutions in Coplin jars: a. 2% PFA + 0.03% SDS for 3 min; b. 2% PFA for 3 min; c. 0.4% Photoflo for 3 × 1 min. The slides were then air dried for 1 hour before being subjected to IF or storage at −20°C. In some experiments, the remaining 900 μl of cell suspension from each well was used for preparation of cell lysate. Whole cell protein lysates were prepared as previously described, using the whole cell extraction kit and protease inhibitor from Millipore (2910, Billerica MA) complemented with 2.5 mg/ml of N-ethylmaleimide (NEM (a de-sumoylation inhibitor), E3876-100G, Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitors (88667, Thermo Fisher Scientific, Rockford, IL). IF was performed as previously described. Information regarding the source, vendor, and dilution of the antibodies used in this study is summarized in Table 1.
Table 1.
Summary of the antibodies used in this study.
| Antibody | Manufacturer | Catalog No. | Source | Dilution for western blot |
|---|---|---|---|---|
| Anti-SUMO1 | Abcam (Cambridge, MA) | ab32058 | Rabbit | 1:500 |
| Anti-SUMO2/3 | Abcam | ab3742 | Rabbit | 1:500 |
| Anti-phospho H3Ser10 | Millipore (Temecula CA) | 05-1336-S | Mouse | 1:1000 |
| Anti-mouse IgG | GenScript (Piscataway, NJ) | A01007 | Mouse | - |
| Anti-rabbit IgG | GenScript | A01008 | Rabbit | - |
| Anti-actin | Santa Cruz | sc-1615 | Goat | 1:1000 |
| ECL™ anti-rabbit IgG HRP linked | GE Healthcare UK Limited (Little Chalfont, Buckinghamshire, UK) | NA934V | Donkey | 1:5000 |
| Goat anti-mouse IgG (H + L) HRP | Millipore | AP308P | Goat | 1:5000 |
| Goat anti-rat IgG-HRP | Santa Cruz (Dallas, TX) | sc-2032 | Goat | 1:5000 |
| Donkey anti-goat IgG-HRP | Santa Cruz | sc-2020 | Donkey | 1:5000 |
| Anti-phosphor ERK | Sigma-Aldrich, St. Louis, MO | M8159 | Mouse | 1:1000 |
| Anti-phosphor AKT (S473) | Cell Signaling (Beverly, MA) | 4060 | Rabbit | 1:2000 |
| Anti-phosphor S6 (S235/236) | Cell Signaling | 4858 | Rabbit | 1:2000 |
| Anti-phospho Aurora A/B/C | Cell Signaling | 2914P | Rabbit | 1:1000 |
| Anti-phospho cdc2 | Cell Signaling | 9111P | Rabbit | 1:1000 |
| Anti-phospho Plk (Thr210) | Santa Cruz | sc-135706 | Rabbit | 1:100 |
| Anti-phosphotyrosine | Millipore | 16-105 | Mouse | 1:1000 |
| Phospho-c-Abl | Antibodyplus (Brookline, MA) | STJ90694 | Rabbit | 1:1000 |
Results and Discussion
Given the important roles of several SUMO targets in meiosis, we sought to examine whether sumoylation is important for the meiotic prophase I to metaphase I transition. The effect of different concentrations of a sumoylation inhibitor (GA) upon sumoylation in spermatocytes was first tested using western blot analysis. Compared with HEK cells, in which sumoylation was inhibited only by a high concentration of GA (e.g., 100–200 μM for 6 hours (in accordance with previous reports in somatic cells [33], spermatocytes were highly sensitive to GA and showed a significant inhibition of sumoylation at 20 μM after just 1 hour and at 10 μM after 3 hours (Suppl. Fig. 1A, B1, and B2). Doses of 40 and 50 μM also caused significant cell death and as such were not used for further experiments.
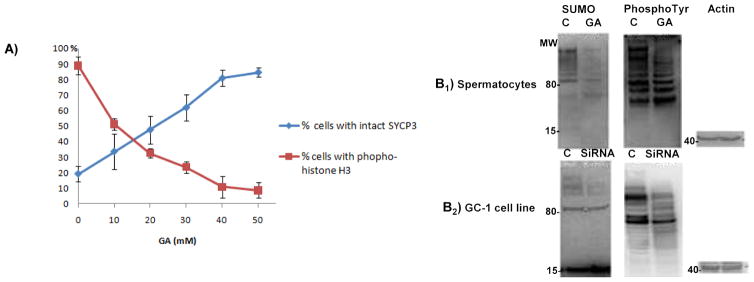
The G2/M1 transition was induced in vitro by treating the highly purified pachytene spermatocyte fraction (Fig. 1A) with the phosphatase inhibitor okadaic acid (OA) with and without a preceding addition of 30 μM GA [7, 34]. H3Ser10 phosphorylation was used as a marker of chromosome condensation initiated at the diplotene stage, and anti-SYCP3 antibody was used as a marker of the lateral element of the synaptonemal complex. The results of the analysis demonstrate that inhibition of sumoylation with GA arrests the G2/M1 transition in mouse spermatocytes. As can be seen from Fig. 1 (B, C), in the control culture (−GA, B), treatment with OA induced condensation of chromosomes accompanied by massive H3Ser10 phosphorylation (red) and full disassembly of the SC (green) as previously reported [8, 34, 35]. In contrast, the addition of GA (+GA, C)prevented chromosome condensation and disassembling of the SC. The percentage of cells arrested at the G2/M1 transition upon treatment with GA increased in a concentration-dependent manner Fig. 2 (A). Similar to the results in somatic cells, inhibition of sumoylation significantly affected the global phosphorylation (PhosphoTyr; Fig. 2B1). In a similar manner to the chemical inhibition, specific inactivation of sumoylation using UBC9 (SUMO-conjugating enzyme) si-RNA caused significant changes in PhosphoTyr of the GC-1 spermatogonia cell line (Fig. 2B2). Interestingly, the timing of the meiotic arrest was similar to the one observed upon inhibition of tyrosine phosphorylation [5].
Figure 1. Inhibition of sumoylation blocks the G2/M1 progression in spermatocytes.
A highly purified population of spermatocytes (A) was treated with the phosphatase inhibitor okadaic acid (OA) with (B) and without (C) a preceding addition of 30 μM of a sumoylation inhibitor (ginkgolic acid, GA). Color-coding is indicated for each image group. Nuclei are stained by DAPI (blue). In a control culture, OA treatment induced massive H3Ser10 phosphorylation (which hallmarks chromosome condensation) and disassembly of the synaptonemal complex (B); In contrast, neither disassembly of the synaptonemal complex nor H3Ser10 phosphorylation were detectable in GA-treated spermatocytes (C).
Figure 2.
A) Effect of increasing concentration of sumoylation inhibitor ginkgolic acid (GA) on disassembly of synaptonemal complex and appearance of H3Ser10 phosphorylation. Fifty cells were scored for each condition and the experiments were repeated three times. Data are presented and mean ± standard deviation.
B1) The effect of inactivation of sumoylation by ginkgolic acid (GA) on tyrosine phosphorylation in spermatocytes.
Spermatocytes were treated with 30 μM of GA for 1 hour followed by a preparation of the whole cell lysate and western blot analysis with anti-SUMO or anti-phospho-tyrosine antibody. Anti-actin antibody was used to confirm equal loading.
B2) The effect of specific inactivation of sumoylation on tyrosine phosphorylation in GC-1 spermatogonia cell lines.
Si-RNA mediated inactivation of UBC9 (SUMO-conjugating enzyme) decreased sumoylation and caused significant changes in tyrosine phosphorylation of the GC-1 spermatogonia cell line.
The role of tyrosine phosphorylation and its regulation by specific tyrosine kinases or phosphatases is not well understood in spermatocytes. One exception is a study of c-Abl kinase, which was shown to be highly expressed in spermatocytes and required for normal spermatogenesis [36]. Based on these data, we used an antibody against an activated form of c-Abl to examine changes in its activity upon inhibition of sumoylation. Our data suggest that addition of OA did not change the level of activated c-Abl, and inhibition of sumoylation did not inhibit (but rather slightly activated) c-Abl (Fig. 3, Ph-Abl), suggesting that other unknown tyrosine kinases or phosphatases contributed to significant changes (mostly decreases) in the level of global tyrosine phosphorylation observed after GA treatment (Fig. 2. B1).
Figure 3. The effect of inhibition of sumoylation during the G2/M transition on the activity of several kinases implicated in the regulation of meiosis.
A purified population of spermatocytes was treated with okadaic acid (+OA) with (+GA) and without (−GA) a preceding addition of 30 μM ginkgolic acid (GA).
Specific antibodies (Table 1) against either activating (c-Abl, PLK1, AUR, ERK, AKT) or inhibitory (CDC2) phosphorylation on several kinases were used to examine changes in their activity upon addition of OA and inhibition of sumoylation.
Global changes in serine/threonine (Ser/Thr) phosphorylation were less prominent, probably due to the poor quality of available antibodies (not shown). Therefore, we used specific antibodies against either activating (PLK1, AUR, ERK, AKT) or inhibitory (CDC2) phosphorylation on several Ser/Thr kinases implicated in the regulation of meiosis. Our results revealed that the activity of PLK1 and the Aurora kinases increased during the G2/M1 meiotic transition (+OA), but was negatively regulated by the inhibition of sumoylation (+30 μM of GA). In the same experiment, the activity of the ERKs and AKT were not affected or increased after GA treatment (Fig. 3). The results for the CDC2 experiment were inconclusive because of low signal intensity (Fig. 3); however, our recently published data in cell lines suggests that inhibition of sumoylation by different means activates CDC2, so such activation would probably not be a contributing factor to the meiotic arrest [37]. Both the AURKs and PLK1 appear to be “at the right place, at the right time” to at least, in part, explain the meiotic arrest obtained in the spermatocyte culture. AURKB is responsible for phosphorylation of H3 on Ser10 [8] and PLK1 is responsible for the disassembling of the SC [6]. As discussed above, an inhibitor of the AURKs prevented H3Ser phosphorylation during the G2/M1 transition in spermatocytes [7]. In a similar manner, mice with expression of an inactive isoform of AURKB display meiotic abnormalities [4]. Inhibition of PLK1 activity fully inhibited the first step of desynapsis (the removal of SYC1) and affected H3 phosphorylation to certain degree [6]. In addition to regulating kinase activity, sumoylation may regulate other proteins required for meiotic progression such as TOP2a (involved in meiotic chromosome condensation), or directly affect the assembly of the SC through modification of SC proteins. Indeed, the fact that SYCPs were found to be sumoylated [23] and interact with UBC9 (the SUMO-conjugating enzyme) [38] supports an important role for SUMO at the time of the observed spermatocyte arrest. Further studies are required to understand how sumoylation regulates events responsible for the disassembly of the SC.
In other studies, La Salle et al. [39] showed that upon the transition from pachytene to diplotene (the time when meiotic arrest occurs upon inhibition of sumoylation), both SUMO1 and SUMO2/3 remained localized to the XY body, but the centromeric signal was significantly diminished. We also failed to observe differences in the localization of SUMO1 and SUMO2/3 upon transition from pachytene to diplotene. Furthermore, western blots of OA-induced spermatocytes with and without the addition of GA showed very similar patterns for SUMO1 and SUMO2/3 (Supplementary Figure 2). It can therefore be suggested that GA-induced inhibition of SUMO-conjugating machinery affects SUMO1 and SUMO2/3 conjugation to a similar degree. However, La Salle et al. observed a different pattern of SUMO1 and SUMO2/3 in MI spermatocytes, as determined by IF with the specific antibodies used in that study. Differences in the functions of SUMO1 and SUMO2/3 and the effect of GA on MI spermatocytes should be further evaluated (this may prove to be difficult because the GA would hypothetically need to be added immediately after the disassembly of the synaptonemal complex). However, possible differences in the specificity of the antibodies used for IF and the absence of a fertility phenotype in SUMO1-knockout mice (suggesting possible overlapping functions of SUMO1 and SUMO2/3) should be taken into account.
Based on the fact that kinases were identified as SUMO targets in spermatocytes, our discussion mostly focused on kinases; however, the inhibition of sumoylation may also affect the activity of phosphatases. Nevertheless, in somatic cells, when compared with cells treated with pervanadate, a strong pan-tyrosine phosphatase inhibitor, GA-treated cells showed different patterns of changes in protein tyrosine phosphorylation [31]. These results implied that SUMO regulation of protein tyrosine phosphorylation is different from the phosphatase-dependent alteration and suggest that kinases may be the major target of SUMO. Whether these conclusions are relevant to germ cells remains to be determined.
Supplementary Material
Highlights.
Inhibition of sumoylation arrests the G2/M1 transition in mouse spermatocytes
Inhibition of sumoylation significantly affects global phosphorylation
PLK1 and the AURKs are negatively regulated by inhibition of sumoylation
Acknowledgments
Grant support: This study was supported by the NIH, NICHD, and Academic Research Enhancement Award 1R15HD067944-01A1 (MV, PI).
This study was supported by the NIH, NICHD, and Academic Research Enhancement Award 1R15HD067944-01A1 (MV, PI). Undergraduate student research was supported by Selma and Jacques H. Mitrani Foundation.
Abbreviations
- GA
ginkgolic acid
- OA
okadaic acid
- SUMO
Small Ubiquitin-like Modifiers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–91. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 2.Liu D, et al. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20(4):377–80. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- 3.Clement TM, et al. Disrupting Cyclin Dependent Kinase 1 in Spermatocytes Causes Late Meiotic Arrest and Infertility in Mice. Biol Reprod. 2015;93(6):137. doi: 10.1095/biolreprod.115.134940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimmins S, et al. Differential functions of the Aurora-B and Aurora-C kinases in mammalian spermatogenesis. Mol Endocrinol. 2007;21(3):726–39. doi: 10.1210/me.2006-0332. [DOI] [PubMed] [Google Scholar]
- 5.Tarsounas M, Pearlman RE, Moens PB. Meiotic activation of rat pachytene spermatocytes with okadaic acid: the behaviour of synaptonemal complex components SYN1/SCP1 and COR1/SCP3. J Cell Sci. 1999;112(Pt 4):423–34. doi: 10.1242/jcs.112.4.423. [DOI] [PubMed] [Google Scholar]
- 6.Jordan PW, Karppinen J, Handel MA. Polo-like kinase is required for synaptonemal complex disassembly and phosphorylation in mouse spermatocytes. J Cell Sci. 2012;125(Pt 21):5061–72. doi: 10.1242/jcs.105015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun F, Handel MA. Regulation of the meiotic prophase I to metaphase I transition in mouse spermatocytes. Chromosoma. 2008;117(5):471–85. doi: 10.1007/s00412-008-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb J, et al. Meiotic events at the centromeric heterochromatin: histone H3 phosphorylation, topoisomerase II alpha localization and chromosome condensation. Chromosoma. 1999;108(7):412–25. doi: 10.1007/s004120050393. [DOI] [PubMed] [Google Scholar]
- 9.Inselman A, Handel MA. Mitogen-activated protein kinase dynamics during the meiotic G2/MI transition of mouse spermatocytes. Biol Reprod. 2004;71(2):570–8. doi: 10.1095/biolreprod.104.027938. [DOI] [PubMed] [Google Scholar]
- 10.Ayaydin F, Dasso M. Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol Biol Cell. 2004;15(12):5208–18. doi: 10.1091/mbc.E04-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasso M. Emerging roles of the SUMO pathway in mitosis. Cell Div. 2008;3:5. doi: 10.1186/1747-1028-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18(17):2046–59. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- 13.Muller S, Ledl A, Schmidt D. SUMO: a regulator of gene expression and genome integrity. Oncogene. 2004;23(11):1998–2008. doi: 10.1038/sj.onc.1207415. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J. Sumoylation regulates diverse biological processes. Cell Mol Life Sci. 2007;64(23):3017–33. doi: 10.1007/s00018-007-7137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohren KM, et al. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem. 2004;279(26):27233–8. doi: 10.1074/jbc.M402273200. [DOI] [PubMed] [Google Scholar]
- 16.Dohmen RJ. SUMO protein modification. Biochim Biophys Acta. 2004;1695(1–3):113–31. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Li M, et al. SUMO wrestling with type 1 diabetes. J Mol Med. 2005;83(7):504–13. doi: 10.1007/s00109-005-0645-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhang FP, et al. Sumo-1 function is dispensable in normal mouse development. Mol Cell Biol. 2008;28(17):5381–90. doi: 10.1128/MCB.00651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, et al. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep. 2014;15(8):878–85. doi: 10.15252/embr.201438534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nacerddine K, et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell. 2005;9(6):769–79. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZB, et al. The SUMO pathway functions in mouse oocyte maturation. Cell Cycle. 2010;9(13):2640–6. doi: 10.4161/cc.9.13.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan YF, et al. SUMO-1 plays crucial roles for spindle organization, chromosome congression, and chromosome segregation during mouse oocyte meiotic maturation. Mol Reprod Dev. 2014;81(8):712–24. doi: 10.1002/mrd.22339. [DOI] [PubMed] [Google Scholar]
- 23.Brown PW, et al. Small ubiquitin-related modifier (SUMO)-1, SUMO-2/3 and SUMOylation are involved with centromeric heterochromatin of chromosomes 9 and 1 and proteins of the synaptonemal complex during meiosis in men. Hum Reprod. 2008;23(12):2850–7. doi: 10.1093/humrep/den300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzler-Guillemain C, et al. In human pachytene spermatocytes, SUMO protein is restricted to the constitutive heterochromatin. Chromosome Res. 2008;16(5):761–82. doi: 10.1007/s10577-008-1225-7. [DOI] [PubMed] [Google Scholar]
- 25.Rogers RS, et al. SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma. 2004;113(5):233–43. doi: 10.1007/s00412-004-0311-7. [DOI] [PubMed] [Google Scholar]
- 26.Vigodner M. Sumoylation precedes accumulation of phosphorylated H2AX on sex chromosomes during their meiotic inactivation. Chromosome Res. 2009;17(1):37–45. doi: 10.1007/s10577-008-9006-x. [DOI] [PubMed] [Google Scholar]
- 27.Vigodner M, et al. SUMO-1, human male germ cell development, and the androgen receptor in the testis of men with normal and abnormal spermatogenesis. Am J Physiol Endocrinol Metab. 2006;290(5):E1022–33. doi: 10.1152/ajpendo.00527.2005. [DOI] [PubMed] [Google Scholar]
- 28.Vigodner M, Morris PL. Testicular expression of small ubiquitin-related modifier-1 (SUMO-1) supports multiple roles in spermatogenesis: silencing of sex chromosomes in spermatocytes, spermatid microtubule nucleation, and nuclear reshaping. Dev Biol. 2005;282(2):480–92. doi: 10.1016/j.ydbio.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Y, et al. Identification of cell-specific targets of sumoylation during mouse spermatogenesis. Reproduction. 2016;151(2):149–66. doi: 10.1530/REP-15-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hietakangas V, et al. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci U S A. 2006;103(1):45–50. doi: 10.1073/pnas.0503698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Q, et al. SUMOylation-regulated protein phosphorylation, evidence from quantitative phosphoproteomics analyses. J Biol Chem. 2011;286(31):27342–9. doi: 10.1074/jbc.M111.220848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.La Salle S, Sun F, Handel MA. Isolation and short-term culture of mouse spermatocytes for analysis of meiosis. Methods Mol Biol. 2009;558:279–97. doi: 10.1007/978-1-60761-103-5_17. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda I, et al. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol. 2009;16(2):133–40. doi: 10.1016/j.chembiol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Wiltshire T, et al. Induced premature G2/M-phase transition in pachytene spermatocytes includes events unique to meiosis. Dev Biol. 1995;169(2):557–67. doi: 10.1006/dbio.1995.1169. [DOI] [PubMed] [Google Scholar]
- 35.Cobb J, Cargile B, Handel MA. Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol. 1999;205(1):49–64. doi: 10.1006/dbio.1998.9101. [DOI] [PubMed] [Google Scholar]
- 36.Kharbanda S, et al. Functional role for the c-Abl tyrosine kinase in meiosis I. Oncogene. 1998;16(14):1773–7. doi: 10.1038/sj.onc.1201934. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y, et al. Inhibition of CDK1 activity by sumoylation. Biochem Biophys Res Commun. 2016;478(2):919–23. doi: 10.1016/j.bbrc.2016.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarsounas M, et al. Protein-protein interactions in the synaptonemal complex. Mol Biol Cell. 1997;8(8):1405–14. doi: 10.1091/mbc.8.8.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Salle S, et al. Developmental control of sumoylation pathway proteins in mouse male germ cells. Dev Biol. 2008;321(1):227–37. doi: 10.1016/j.ydbio.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.