Abstract
We describe pharmacokinetic and pharmacodynamic properties of two novel oral drug candidates for asthma. Phenolic α4β3γ2 GABAAR selective compound 1 and acidic α5β3γ2 selective GABAAR positive allosteric modulator compound 2 relaxed airway smooth muscle ex vivo and attenuated airway hyperresponsiveness (AHR) in a murine model of asthma. Importantly, compound 2 relaxed acetylcholine contracted human tracheal airway smooth muscle strips. Oral treatment of compound 1 and 2 decreased eosinophils in bronchoalveolar lavage fluid in ovalbumin sensitized and challenged mice, thus exhibiting anti-inflammatory properties. Additionally, compound 1 reduced the number of lung CD4+ T lymphocytes and directly modulated their transmembrane currents by acting on GABAARs. Excellent pharmacokinetic properties were observed, including long plasma half-life (up to 15 hours), oral availability, and extremely low brain distribution. In conclusion, we report the selective targeting of GABAARs expressed outside the brain and demonstrate reduction of AHR and airway inflammation with two novel orally available GABAAR ligands.
Keywords: Asthma, GABAA receptor, airway hyperresponsiveness, airway inflammation, splenocytes, leukocytes, α4β3γ2, α5β3γ2
TOC image
Introduction
Asthma is a chronic inflammatory lung disorder that affects children and adults of all ages.1 The prevalence of asthma in the United States continues to rise with an estimated 39.5 million people diagnosed with asthma, including 8.7 million children.2 Characteristic features of asthma include variable and recurring symptoms of airway inflammation, mucous cell metaplasia, airflow obstruction, and airway hyperresponsiveness (AHR).3 First line therapy consists of inhaled anti-inflammatory corticosteroids and/or bronchodilators, such as long-acting beta 2 adrenergic receptor agonists, in a stepwise strategy for the long-term management of chronic persistent asthma symptoms.2 Inhalers are prone to improper use leading to imprecise dosing, oral infections and overall poor compliance.4 Severe asthma is treated with systemic corticosteroids, which are associated with adverse side effects such as adrenal suppression, growth suppression, cataract formation, thinning or bruising of the skin, increased mortality, and loss of asthma control.5–10 Furthermore, orally available leukotriene receptor antagonists are commonly used to manage asthma symptoms,11 however, up to 78% of patients do not respond to this alternative therapy.12 Thus, new oral asthma therapies are needed to overcome imprecise drug administration and reduce systemic adverse effects.
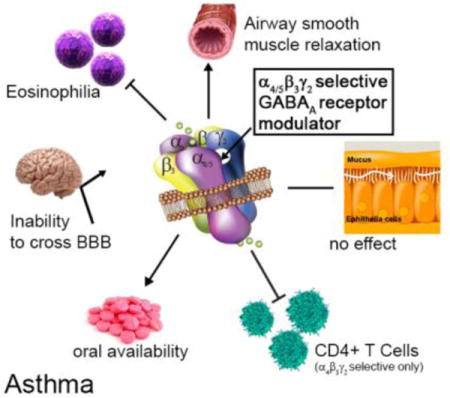
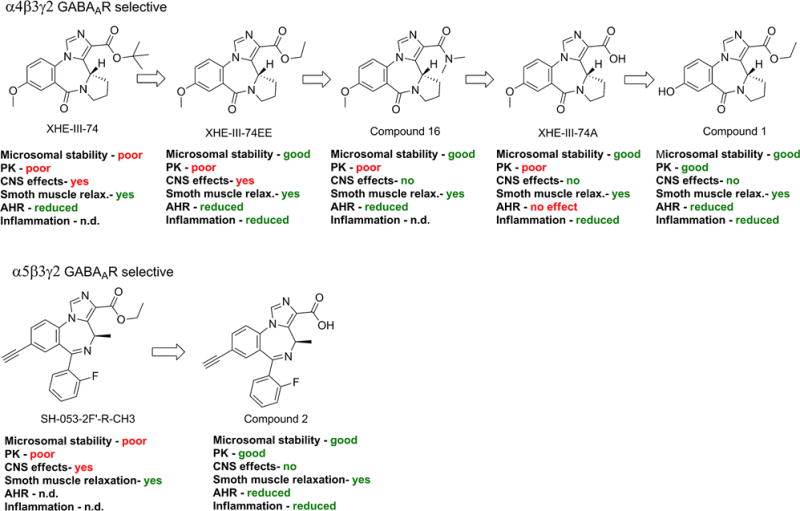
The GABAA receptors (GABAAR) are ligand-gated chloride ion channels best known for their inhibitory role in the central nervous system (CNS). GABAARs are heteropentamers assembled from 19 different subunits (α1–6, β1–3, γ1–3, δ, ε, π, θ, ρ1–3). Classical GABAARs consist of two α, two β, and one γ, δ, ε, θ, or π subunit.13, 14 GABAAR subunits have been identified in airway smooth muscle,15–18 airway epithelium,19 and immune/inflammatory cells.20–24 Evidence that GABAARs in the lungs of asthmatic mice can be targeted with small molecules was demonstrated first by Munroe, et al.25 using plant-based honokiol, a positive allosteric GABAAR modulator with selectivity towards the α1/2/3β3γ2 GABAAR subtypes.26 The compound reduced AHR and lung eosinophilia upon acute and chronic administration in an ovalbumin sensitized and challenged (ova s/c) mouse asthma model. Allergen challenge in honokiol treated mice demonstrated increased regulatory cytokine secretion and reduced pro-inflammatory cytokine release. Selective targeting of the α4β3γ2 GABAAR with XHE-III-7427 and XHE-III-74EE23 was shown to alleviate AHR in mouse asthma models (Figure 1).
Figure 1.
Development of subtype-selective GABAAR modulators as drug candidates for asthma with their most important pharmacological characteristics.
In addition, these compounds relaxed pre-contracted rodent and human airway smooth muscle ex vivo. Moreover, XHE-III-74A significantly reduced airway eosinophilia in ova s/c mice and reduced IL-2 secretion in phorbol myristate acetate (PMA) and phytohemagglutinin (PHA) activated Jurkat cells.23 In contrast, AHR alleviating properties of α5β3γ2-selective GABAAR positive allosteric modulators have not been demonstrated, although selective GABAAR ligand SH-053-2′F-R-CH3 has been shown to relax human and guinea pig smooth muscle tissue ex vivo.15, 17 XHE-III-74, XHE-III-74EE,23 and SH-053-2′F-R-CH315 can cross the blood brain barrier (BBB) and induce CNS effects at higher concentrations. In addition, limited pharmacokinetic characterization has been reported for all four compounds.23, 28 In this study, we present the pharmacological properties of compound 1 and 2 with respect to the pathognomonic features of asthma.
Experimental Section
Chemicals
Compounds 1 and 2 were synthesized using a published procedure.41, 46 The purity of both compounds (>98%) was confirmed by nuclear magnetic resonance (NMR), high resolution mass spectrometry (HRMS) and liquid chromatography (supporting information).
Experimental animals
5–10 week old male BALB/c and Swiss Webster mice (Charles River Laboratory, WIL, MA) and adult (425–450 g) male Hartley guinea pigs (Charles River Laboratory, WIL, MA) were used for the experiments. The animals were housed under specific pathogen-free conditions, under standard conditions of humidity, temperature and a controlled 12 h light and dark cycle and had free access to food and water. All animal experiments were in compliance with the University of Wisconsin, Milwaukee or Columbia University Institutional Animal Care and Use Committees (IACUC).
Ovalbumin sensitization and challenge
Randomized male BALB/c mice in a group size of ten were sensitized three times with intra-peritoneal (i.p.) injections of 2 mg/kg/d of ovalbumin (ova) (Sigma-Aldrich, St. Louis, MO) emulsified in 2 mg of Alum (Imject Alum; Thermo Scientific, Pierce, Rockford, IL) on days 0, 7 and 14 in a total volume of 100 μL. Mice were anesthetized with isoflurane and challenged intra-nasally (i.n.) with 1 mg/kg/d ova for 5 days from days 23–27. Control mice were sensitized with ova and challenged with i.n. saline.47 The chronic effects of compound 1 and 2 for a duration of 5 days during the ova challenge period were tested in separate groups of ten ova s/c BALB/c mice. As a positive control, separate groups of ten ova s/c BALB/c mice received dexamethasone at 4 mg/kg for 8 days. Airway hyper-responsiveness parameters were assessed on day 28 and mice were sacrificed using an overdose of ketamine/xylazine i.p. on day 29 for assessment of inflammatory cells and mucus metaplasia.
Immunoblot analysis
Whole spleens from ova s/c BALB/c mice were disrupted and combined with protease inhibitor cocktail tablet (Roche Applied Sciences, Indianapolis, IN) and membrane protein extracted according to the protocol provided by Mem-PER™ Plus membrane protein extraction kit (Thermo Fisher Scientific Inc, Rockford, IL). Membrane protein concentration was determined by Pierce™ Coomassie (Bradford) protein assay kit (Thermo Fisher Scientific Inc, Rockford, IL), according to the manufacturer’s instructions. Protein extracts were subjected to SDS-PAGE using NuPAGE Novex 4–12% Bis-Tris protein gels (Thermo Fisher Scientific Inc, Rockford, IL) and transblotted onto nitrocellulose iBlot® 2 transfer stacks (Thermo Fisher Scientific Inc, Rockford, IL) using the iBlot® 2 gel transfer device. Membranes were blocked in superblock™ T20 (TBS) blocking buffer (Thermo Fisher Scientific Inc, Rockford, IL) for 1 hour at room temperature and then incubated with primary antibodies: (α1, dilution 1:100, 06-868, EMD Millipore), (α2, MABN1724, dilution 1:50, EMD Millipore), (α3, dilution 1:200, sc-133603, Santa Cruz), (α4, dilution 1:200, sc-7355, Santa Cruz), α5, dilution 1:500, sc-7348, Santa Cruz), (β3, dilution 1:500, sc-376252, Santa Cruz) and (γ2, dilution 1:100, MABN875, EMD Millipore) overnight at 4°C. This was followed by treatment for 1 hour with HRP conjugated secondary antibodies: (goat anti-mouse, dilution 1:5000, c-2031, Santa Cruz), (donkey anti-goat, dilution 1:5000, sc-2033, Santa Cruz), and (goat anti-rabbit, dilution 1:5000, sc-2030, Santa Cruz). The protein bands were developed using Pierce™ ECL western blotting substrate (Thermo Fisher Scientific Inc, Rockford, IL) according to manufacturer’s recommendations. A CCD camera connected to FluorChem HD2/ FC2 (Cell Biosciences Inc, Santa Clara, CA) was used to capture the digital images.
Automated Patch-Clamp Electrophysiology for splenocytes
Splenocytes from four ova s/c BALB/c mice were prepared following BD Biosciences instructions for preparation of murine splenocytes and red blood cell lysed using BD Pharm Lyse™ lysing solution (BD Biosciences, San Jose, CA). CD4+ T cells were isolated from splenocytes using an Affymetrix eBiosciences MagniSort® mouse CD4+ T cell enrichment kit following manufacturer’s instructions (Thermo Fisher Scientific Inc., Rockford, IL). Splenocytes were maintained in suspension in RPMI 1640 medium with L-glutamine (Thermo Fisher Scientific Inc., Rockford, IL) supplemented with 10% (v/v) fetal bovine serum, 10 μM 2-mercaptoethanol and 1% penicillin/streptomycin in the presence or absence of 100 μg/mL ovalbumin. The cells were maintained in 5% CO2, 95% humidified air at 37 °C for 48 hours. Automated patch-clamp studies were conducted as described previously.23 Briefly, the IonFlux plate layout consists of units of 12 wells: two wells contain intracellular solution (ICS containing 346 mM CsCl, 1 mM CaCl2, 1 mM MgCl2, 11 mM EGTA, 10 mM HEPES, pH 7.2 with CsOH), one contains cells diluted in extracellular solution (ECS containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM D-glucose monohydrate, and 10 mM HEPES, pH 7.4 with NaOH), eight contain the compounds of interest diluted in ECS, and one well is for waste collection. Cells are captured from suspension by applying suction to microscopic channels in ensemble recording arrays. Once the array is fully occupied, the applied suction breaks the membranes of captured cells, which establishes whole cell voltage clamp. For compound applications, pressure is applied to the appropriate compound wells, which introduces the compound into the extracellular solution rapidly flowing over the cells. For recording GABAAR induced currents, cell arrays were voltage clamped at a hyperpolarizing holding potential of −50 mV. Prior to use on the automated patch clamp, cells were centrifuged at 380g for 2 minutes and resuspended gently in ECS. This was repeated two more times before the cells were dispensed into the plate. GABA and muscimol were diluted to appropriate concentrations in ECS to appropriate concentrations before application and data was recorded from eight individual ensembles.
Drug treatment protocol
Sterile solutions of compound 1 or 2 were prepared in 2% hydroxypropyl methylcellulose solution (Sigma-Aldrich, St. Louis, MO) and 2.5% polyethylene glycol (Sigma Aldrich, St. Louis, MO) in a biological safety cabinet. A fine suspension was obtained by grinding the mixture with a mortar and pestle. Drugs were administered individually at 100 mg/kg by oral gavage with 20G gavage needles (Kent Scientific Corporation, Torrington CT) to groups of ova s/c BALB/c twice daily for 5 days during the ova challenge period. Mice received a single p.o. dose of the compounds just before airway parameter measurements. Dexamethasone (Sigma-Aldrich, St. Louis, MO) was prepared in 10% DMSO, 40% propylene glycol, and 50% PBS given as i.p injection. Dexamethasone was administered (4 mg/kg) daily for 8 days. Mice were monitored daily after drug administration.
Assessment of Airway hyper-responsiveness
Airway hyper-responsiveness to methacholine in conscious, spontaneously breathing animals was measured by DSI’s Buxco® FinePointe Non-Invasive Airway Mechanics (NAM) instrument.48 Before measurements were taken, mice were acclimated to the chambers 15 minutes daily for 5 days. The chambers were also calibrated each time before data collection. Briefly, the nasal chamber in combination with the thoracic chamber allows the computation of specific airway resistance (sRaw). The FinePointe software computes specific airway resistance (sRaw) with all other ventilatory parameters derived by the NAM analyzer. Mice were exposed to aerosolized PBS (for the baseline measurement) or methacholine (1.5625–12.5 mg/mL) for 1 minute and readings were taken and averaged for 3 minutes after each nebulization. Data obtained were presented as sRaw versus the methacholine concentration (mg/mL) used to generate the aerosol.23
Eosinophil count
Immediately after assessment of airway hyperresponsiveness, mice were euthanized with a cocktail of ketamine (300 mg/kg) and xylazine (30 mg/kg) (Sigma Aldrich, St. Louis, MO) in an approximate volume of 100 μL per i.p. injection. The lungs were dissected and tracheostomized with an 18G luer stub adapter. The left lung was clamped using a hemostat and the right lung perfused with 500 μL sterile saline. Broncho-alveolar lavage fluid (BALF) was then pooled and aspirated into tubes.23, 47
BALF was spun onto positively charged slides using Thermo Shandon Cytopsin® 3 (Marshal Scientific, Brentwood, NH). Slides were stained with Wright Geimsa stain (Sigma-Aldrich, St. Louis, MO) and washed with ddH20 for 10 minutes. The slides were cover slipped with Permount™ mounting media (Fisher Scientific, Pittsburgh, PA) and the number of eosinophils counted using the Labomed™ TCM 400 inverted microscope light microscope (Labo America Inc., Freemont, CA) in four randomly chosen fields of view at 20X. Eosinophils were determined as a percentage of the total cells in each field and expressed as percent eosinophil.23, 47
Flow cytometry
Bronchoalveolar lavage (BAL) was performed with 1 ml of Ca2+ and Mg2+ free PBS. Red blood cells (RBCs) were lysed using BD red blood cell lysis buffer (BD Pharmingen, San Jose, CA). BALF was split into four different tubes and non-specific binding to Fc receptors was blocked for 5 min using 6 μg/ml of 2.4G2 mouse BD Fc Block™ (BD Pharmingen, San Jose, CA). BALF cells were stained for 30 minutes at 4 °C in the dark with 100 μl BSA stain buffer (BD Pharmingen, San Jose, CA) containing the final concentrations of the following antibodies: anti-mouse CD45 APC (1:1000, 30-F11, Affymetrix eBiosciences, San Diego, CA), FITC rat anti-mouse CD4 (1:500, RM4-5, BD Pharmingen, San Jose, CA), FITC rat anti-mouse CD11b (1:200, M1/70, BD Pharmingen, San Jose, CA), FITC rat anti-mouse Ly-6G and LY-6C (GR-1) (1:500, RB6-8C5, BD Pharmingen, San Jose, CA) and mouse CCR3 PE-conjugated antibody(1:40, 83101, R&D systems Inc, Minneapolis, MN). Flow cytometric studies were done using the BD FACS Calibur (BD Pharmingen, San Jose, CA) and data analyzed subsequently using Cell Quest pro software (BD Pharmingen, San Jose, CA).49 Gating strategies for the different markers and treatment groups are shown in supporting information (Figures S6–S9). Total inflammatory cell count was obtained by running all samples on high (60 μl/min) for 180 seconds. The gated anti-mouse CD45 positive events in the fourth channel (FL4) were used to calculate the total inflammatory cell count as cells /ml. The frequencies of CCR3+, GR1+, CD4+ and CD11b+ cell populations in their respective gates were multiplied by the total inflammatory cell count (cells/ml) to obtain the differential cell population.
Statistical analysis
Data were analyzed using GraphPad Prism 4 (GraphPad Software, San Diego, CA) and expressed as mean ± SEM. One-way analysis of variance (ANOVA) with Dunnet post hoc test or two-way ANOVA with Bonferroni post hoc test were performed for statistical difference for multiple groups. For comparison of two groups, a two-tailed unpaired Student’s t test was used. Statistical significance was defined as p < 0.05.
Results
GABAARs are expressed in splenocytes and respond to GABA and muscimol
GABAARs have been identified in multiple extra-CNS tissues such as the lungs, kidneys, endocrine organs, heart, ovary, testis, placenta, uterus, pancreas, small intestines and testis.16, 17, 23, 29, 30 Herein, we determined the expression of GABAAR subunits in immune cells collected from the spleen (Figure 2).
Figure 2. Immunoblot analysis of GABAA receptor subunits in splenocytes.
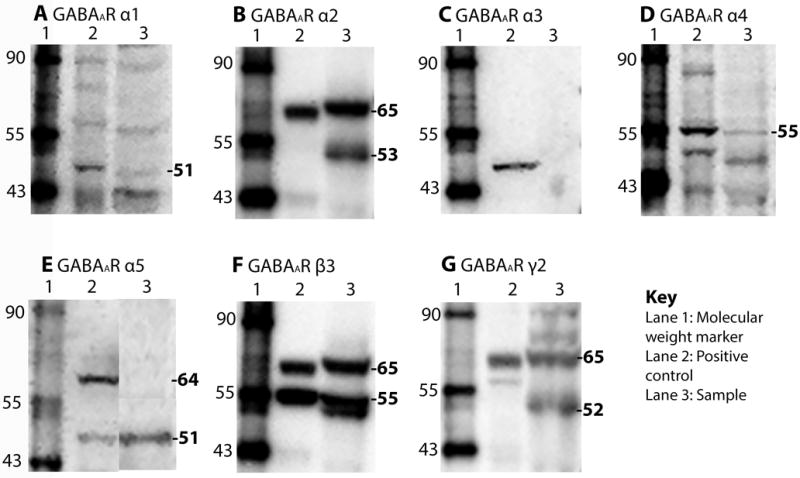
Representative immunoblots (A–G) from mouse spleen homogenates of ova s/c BALB/c mice were generated with GABAAR subunit antibodies and 50 μg of membrane protein harvested from the spleen. Lane 1, molecular weight marker (kDa); lane 2, positive controls (α1, α3, α5, β3, mouse brain extract; α4, H4 cell lysate; α2 and γ2, mouse cerebellum extract); lane 3, membrane protein extracted from spleens of ova s/c BALB/c mice.
Western blotting identified immunoreactive bands for the α1, α2, α4, α5, β3 and γ2 subunits in membrane proteins obtained from spleen tissues of ova s/c BALB/c mice. An immunoreactive band at 51 kDa for α1 was observed as reported.31 For α2, an immunoreactive band at 65 kDa was observed for splenocytes as well as mouse cerebellum extract in addition to the predicted immunoreactive band at 53 kDa. Both bands were described in an earlier report for ova s/c mouse lung extract.23 The expression of the α3 GABAAR subunit was not identified. For α5, a single molecular weight band at 51 kDa was observed for splenocyte extract (Figure 2E). An additional immunoreactive band at 64 kDa was detected for mouse brain extract and ova s/c mouse lung homogenate.23 The γ2-specific antibody produced immunoreactive bands at 65 kDa and 52 kDa in splenocytes. A single band at 65 kDa was observed in mouse cerebellum extract. Finally, the immunoreactive bands observed with β3- and α4-specific antibodies were identical in splenocyte extract, mouse cerebellum extract, and H4 cell lysate controls (Figure 2D and F). Thus, we confirmed the presence of GABAAR subunits in a heterogeneous immune cell population, which prompted us to investigate the modulation of these receptors by GABAAR agonists. Accordingly, splenocytes from ova s/c mice cultured with and without ovalbumin were investigated by patch clamp. The electrophysiological responses of both cell preparations in the presence of increasing concentration of GABA and GABAAR agonist muscimol were detected at a holding potential of −50 mV (Figure 3).
Figure 3. Electrophysiological response of splenocytes in the presence of GABAAR agonists.
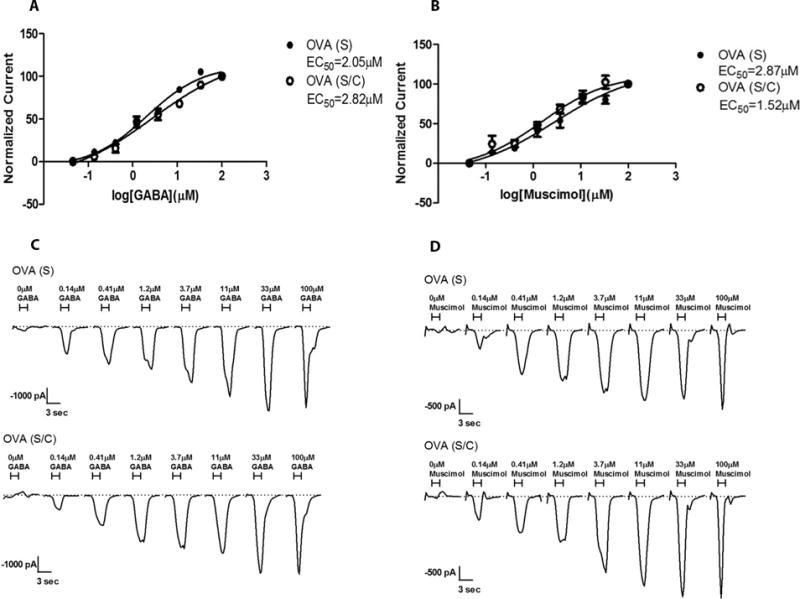
A) Concentration-dependent current responses of splenocytes from ova s/c BALB/c mice cultured with (s/c) and without (s) ovalbumin in the presence of GABA normalized to the highest current change (n = 8); B) Concentration-dependent current responses of splenocytes from ova s/c BALB/c mice cultured with (s/c) and without (s) ovalbumin in the presence muscimol normalized to the highest current change (n = 8); C) current recordings of different concentrations of GABA applied for 3 seconds using splenocytes from ova s/c BALB/c mice cultured with (s/c) and without (s) ovalbumin; D) current recordings of different concentrations of muscimol applied for 3 seconds using splenocytes from ova s/c BALB/c mice cultured with (s/c) and without (s) ovalbumin.
Patch-clamp is usually carried out on a single cell, making it difficult to investigate a heterogeneous cell population such as splenocytes. However, an automated patch clamp approach using the Ionflux system overcomes this limitation by trapping twenty cells simultaneously and recording an average change of current at a constant holding potential. Using this instrument, we were able to record a change of current at a holding potential of -50 mV for splenocytes in the presence of GABA. This current change was concentration-dependent between 0 and 33 μM of GABA with a calculated EC50 value of 2.05 μM (Figure 3A and C). Splenocytes from ova s/c mice cultured with or without ovalbumin gave similar current changes. The GABAAR agonist muscimol exhibited a similar affinity to GABAARs expressed on splenocytes based on similar EC50 values in comparison to GABA (Figure 3B). However, the induction of current alteration was only around 50% of the current change induced by GABA resulting in a lower efficacy of muscimol towards GABAARs on splenocytes (Figure 3C and D).
Compounds 1 and 2 relax airway smooth muscle
The ability of compounds 1 and 2 to relax airway smooth muscle was investigated using two ex vivo model systems (Figure 4). These include the application of substance P to guinea pig tracheal rings, causing smooth muscle constriction mediated by the Gq-coupled neurokinin receptors32 and acetylcholine applied to human tracheal airway smooth muscle strips, acting at muscarinic acetylcholine receptors that include Gi and Gq-protein coupled receptors.33
Figure 4. Smooth muscle contractile force measurement in the presence of compound 1 or 2.
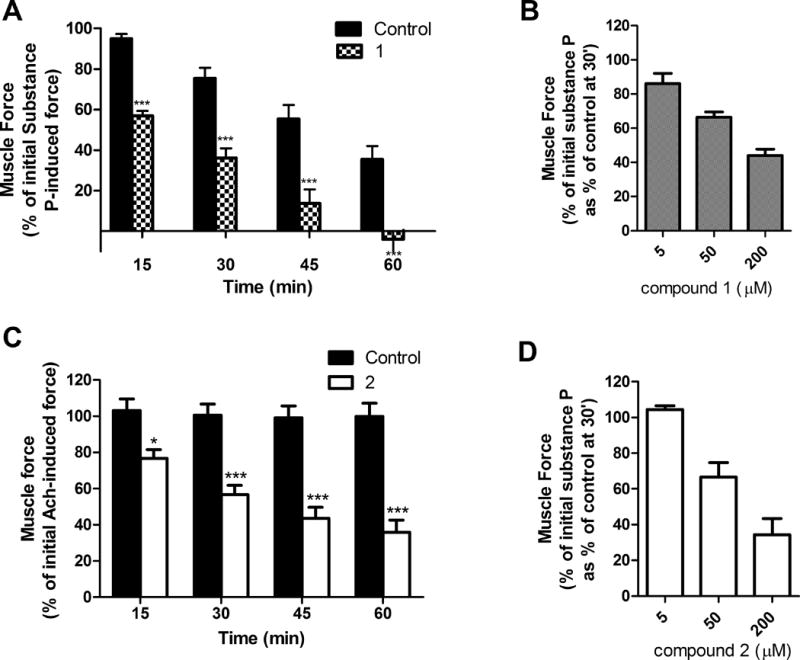
A) and B) Airway smooth muscle contractile force in guinea pig tracheal rings. Tracheal rings were contracted with 1 μM substance P and then treated with A) 50 μM of compound 1 (or the vehicle control 0.1% DMSO). The percent of remaining contractile force was measured at various time points and expressed as a percent of the initial substance P induced contractile force (N = 4); B) Tracheal rings were contracted with 1 μM substance P and then treated with compound 1 at different concentrations. The percent of remaining contractile force was measured at 30 minutes and expressed as a percent of the initial substance P induced contractile force as a percent of control (N = 3); C) Human tracheal airway smooth muscle strips were contracted with an EC50 concentration of acetylcholine (Ach) and then treated with 100 μM of compound 2, or the vehicle 0.2% ethanol. Muscle force was measured at 15, 30, 45 and 60 minutes after addition of compound 2. Data are expressed as the percent of the initial Ach-induced contractile force. Individual muscle strips from at least seven humans were used. D) Guinea pig tracheal rings were contracted with 1 μM substance P and then treated with different concentrations of compound 2. The percent of remaining contractile force was measured at 30 minutes and expressed as a percent of the initial substance P-induced contractile force as a percent of control (N = 3); * p < 0.05, ** p<0.01, *** p< 0.001 compared to the vehicle control.
Compound 1 significantly reduced substance P-induced contraction of guinea pig ASM at 15, 30, 45, and 60 minutes (Figure 4A). The contractile force induced by substance P spontaneously decreased over a period of one hour, however, significant smooth muscle relaxation was observed for 50 μM compound 1 at each time point. The response was concentration dependent (Figure 4B). In contrast, acetylcholine induced a long lasting smooth muscle constriction in human tracheal airway smooth muscle strips that did not change during one hour (Figure 4C). For compound 2 at 100 μM a time-dependent relaxation of constricted human tracheal airway smooth muscle was observed. The smooth muscle relaxation was significant after 15 minutes. In addition, the concentration of compound 2 in the human airway smooth muscle was quantified after a one hour incubation with 100 μM of compound 2. In contrast to the bath concentration of 100 μM compound 2, the concentration of compound 2 in tissue after extraction and quantification by LCMS/MS was 9.8 μM. When applying different concentrations (Figure 4D), we observed that 50 μM of compound 2 was sufficient to relax guinea pig smooth airway muscle after 30 minutes.
Compounds 1 and 2 delivered orally in mice distribute readily to lung but not brain
Pharmacokinetic analysis of drug candidates is essential to ensure pharmacological concentrations in target tissues, and enables further dosage optimization. The pharmacokinetic profiles of compounds 1 and 2 are presented in Figure 5.
Figure 5. Pharmacokinetic profile of compounds 1 and 2 in mice blood, lungs, and brain.
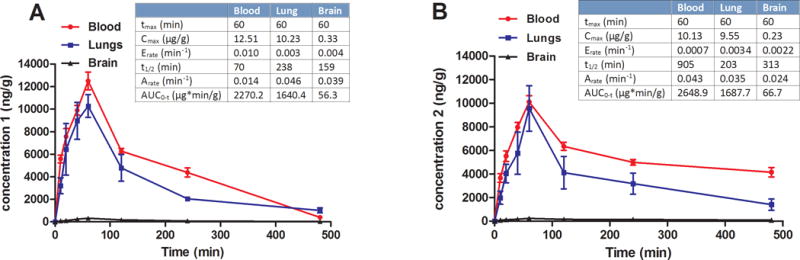
(A)Time-dependent systemic distribution of compound 1 (25 mg/kg via oral gavage) and (B) Time-dependent systemic distribution of compound 2 (25 mg/kg via oral gavage).
Pharmacokinetic analysis following oral administration of compound 1 at 25 mg/kg indicated absorption within one hour and excellent distribution (AUC = 2270.2 μg*min/g) with a Tmax of 60 minutes in blood and lungs (Figure 5A). The Cmax was highest in blood followed closely in lungs with a Cmax of 10.23 μg/g. The half-life of compound 1 was shorter in blood than in lungs (238 minutes), as compound 1 was still detected in lungs after 8 hours (Figure 5A). Importantly, extremely low amounts of compound 1 were observed in brain. Compound 2 exhibited pharmacokinetic properties similar to compound 1. The acid was well absorbed and distributed with a Tmax of 60 minutes in blood and lungs (Figure 5B). Comparable concentrations of compound 2 were observed in blood and lungs. However, compound 2 had a significantly longer half-life in the blood (~15 hours) compared to compound 1. Like compounds 1, compound 2 was still detected after 8 hours in lung tissue. Most importantly, negligible concentrations of compound 2 were detected in the brain. At a dosage of 100 mg/kg, compounds 1 and 2 were given orally to mice to investigate any CNS effect when balancing on a rotating rod (rotarod assay). As presented in the SI Figure1, no motor sensory impairment was observed. Furthermore, low efficacy toward the α1β3γ2 GABAAR, which mediates sedation and tolerance, was demonstrated for compound 1 (SI Figure 2) and compound 2.34
Compounds 1 and 2 have anti-inflammatory properties
Flow cytometry was used to quantify the changes of inflammatory cells in bronchoalveolar lavage fluid (BALF) of ova s/c BALB/c mice when treated orally with compound 1 or 2 at doses of 100 mg/kg twice daily for five days (Figure 6, A–F).
Figure 6.
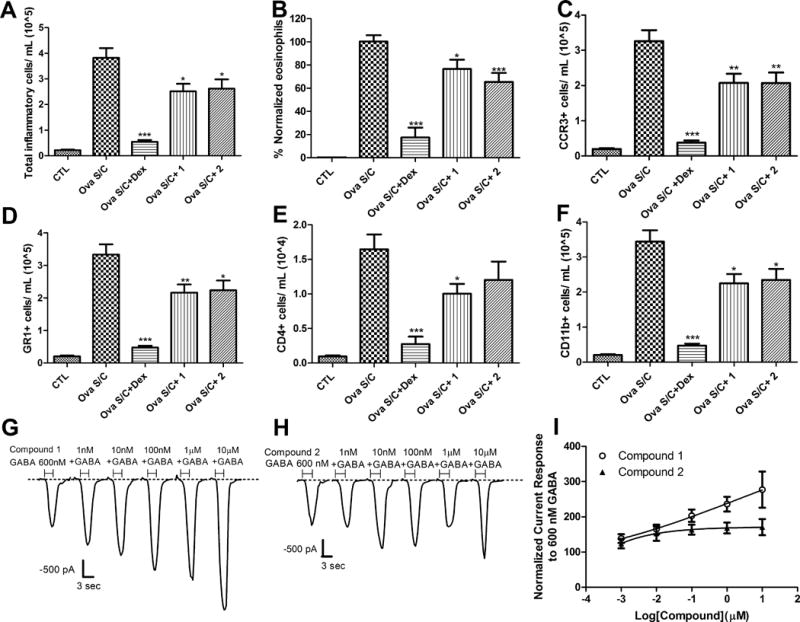
Effect of compounds 1 and 2 on inflammatory cells. Groups of 10 ova s/c BALB/c mice were administered dexamethasone i.p., 4 mg/kg daily for 8 days; compound 1 via oral gavage, 100 mg/kg twice daily for 5 days and compound 2 via oral gavage, 100 mg/kg twice daily for 5 days. BALF was harvested from each animal and used for A) Quantification of total inflammatory cells. Cells were stained with mouse CD45 APC antibody and samples were analyzed with BD FACS Calibur on high flow rate (60 μL/min) for 180 seconds. The gated positive events in the fourth channel (FL4) as depicted in the supplemental information were used to calculate the total inflammatory cell count as cells/ml. B) Quantification of Wright Giemsa stained airway eosinophils. Data represent % normalized eosinophil count relative to CTL (negative control) and ova s/c mice (positive control). Examples of captured pictures are presented in supplementary information (SI Figure 5–8). C–F) Quantification of specific leukocyte population. (C) CCR3+ (D) GR1+ (E) CD4+ and (F) CD11b+ cell populations were stained with specific antibodies and detected by flow cytometry. Data represent mean ± SEM from 10 mice in each group. *, ** and *** indicate p < 0.05, p <0.01, p <0.001significance, respectively, compared to vehicle treated ova s/c BALB/c mice; G) current recordings in the presence of 600 nM GABA and increasing concentration of compound 1 applied together for 3 seconds using CD4+ T-cells isolated from ova s/c BALB/c mice spleen; H) current recordings in the presence of 600 nM GABA and increasing concentrations of compound 2 applied together for 3 seconds using CD4+ T-cells isolated from ova s/c BALB/c mice spleen; I) Concentration-dependent current responses of CD4+ T-cells from ova s/c BALB/c mice spleen in the presence of 600 nM GABA and increasing concentration of compound 1 or 2. Current readings were normalized to 600 nM GABA response set as 100% (n = 16).
Leukocytes were incubated with fluorescent mouse CD45 antibody, which is specific for protein tyrosine phosphatase that is selectively expressed in hematopoietic cells. Ova s/c mice that exhibit asthma-like inflammation showed a significant increase of white blood cell numbers in BALF (Figure 6A). Importantly, oral administration of compounds 1 and 2 for 5 days decreased the number of leukocytes in BALF, demonstrating anti-inflammatory properties. The reduction of white blood cells was more pronounced for i.p. administration of 4 mg/kg dexamethasone over an 8 day treatment course. Eosinophilia in BALF, a hallmark of inflammation in ova s/c mice,35 was quantified by selective staining using Wright Geimsa (Figure 6B). Treatment of compound 1 (p < 0.001) or 2 (p < 0.05) for 5 days b.i.d. significantly decreased eosinophils in mouse BALF. To further differentiate the change of mouse BALF leukocyte populations, flow cytometry using cell type-specific fluorescent antibodies was carried out. Eosinophil quantification was achieved by immunostaining with a fluorescent antibody to chemokine receptor type 3 (CCR3, CD193). As demonstrated in Figure 6B, BALF from ova s/c mice contained a large number of eosinophils that were significantly reduced by oral treatment with compound 1 or 2 (Figure 6C). Dexamethasone i.p. treatment almost completely eliminated the presence of eosinophils. A similar study using fluorescent GR1 antibody, selective for mouse Ly-6G/Ly-6C myeloid differentiation antigens expressed on granulocytes and macrophages, mirrored these results (Figure 6D). Quantification of CD4+ helper T lymphocytes was achieved by fluorescent anti-CD4 immunostaining. In comparison to granulocytes, a relatively smaller number of CD4+ T cells were detected in BALF of ova s/c mice (Figure 6E). Although 5 day oral treatment of compound 2 b.i.d. significantly reduced the number of eosinophils, a similar significant reduction in CD4+ T cells was not observed. However, the same treatment regimen with compound 1 significantly reduced both of CD4+ T cell and eosinophil numbers. As expected, dexamethasone treatment at 4 mg/kg i.p. for 8 days significantly reduced the CD4+ T cell population. Finally, neutrophils, basophils, monocytes and macrophages were quantified, as detected by immunostaining with fluorescent CD11b antibody, selective for integrin alpha M (Itgam, Mac-1, CR3A, CD11b). For ova s/c mice, a significant increase in this leukocyte population was observed in BALF. Importantly, treatment with compound 1 or 2 significantly reduced their number in the BALF of ova s/c mice, as did dexamethasone treatment (Figure 6F). In addition, CD4+ T cells were isolated from spleen of ova s/c mice and their change of transmembrane currents was determined by patch clamp in the presence compounds 1 and 2 and low concentrations of GABA. Compound 1 induced a concentration-dependent increase in transmembrane current (Figure 6G). The modulation of transmembrane currents was not concentration dependent for compound 2 (Figure 6H). Overall, the electrophysiological effect on CD4+ T cells was more pronounced for compound 1, which induced large current changes at concentration higher than 1 μM (Figure 6I).
Compounds 1 and 2 reduce airway hyperresponsiveness
AHR is a cardinal feature of asthma, characterized by physiologic perturbation to cholinergic agonists.36 Pharmacodynamic properties of orally administered compound 1 or 2 were investigated with ova s/c mice in the presence of increasing concentrations of aerosolized methacholine by measuring specific airway resistance (sRaw) with a Buxco FinePointe non-invasive airway mechanics instrument (Figures 7, A and B).
Figure 7. Effect of compounds 1 and 2 on airway hyperresponsiveness.
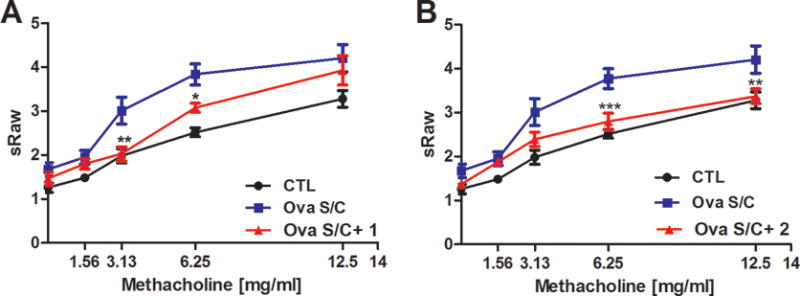
Specific airway resistance (sRaw) was measured at increasing dosages of methacholine by a DSI’s Buxco FinePointe non-invasive airway mechanics instrument. Ova s/c BALB/c mice were administered A) compound 1 via oral gavage, 100 mg/kg twice daily for 5 days or C) compound 2 via oral gavage, 100 mg/kg twice daily for 5 days. Data represent mean ± SEM from 10 mice in each group. *, **, and *** indicate p < 0.05, p < 0.01, p < 0.001 significance, respectively, compared to vehicle treated ova s/c BALB/c mice.
Significant reduction of AHR was observed at methacholine concentrations of 6.25 and 12.5 mg/mL. Administration of compound 1 (5 days, 100 mg/kg b.i.d.) including 30 minutes before the measurement caused significant reduction of sRaw values at 3.13 and 6.25 mg/mL methacholine challenge doses (Figures 7A). These results are in agreement with results published previously with α4-selective GABAAR ligand XHE-III-74EE that protected against AHR at low methacholine concentrations.23 The report also demonstrated the reduced AHR for dexamethasone treated ova s/c mice. Importantly, administration of compound 2 produced a significant downward shift of sRaw values and therefore protection of AHR at high methacholine concentrations (6.25 mg/mL and 12.5 mg/mL methacholine) (Figure 7B). The mouse lung was investigated further using periodic acid fluorescent Schiff’s stained sections to assess mucus content (Figure S4). Oral administration of compound 1 or 2 did not significantly change mucous metaplasia compared to vehicle-treated ova s/c mice.
Discussion
Inhaled corticosteroids and beta 2 adrenergic receptor (β2AR) agonists are cornerstones of the long-term management of moderate to severe persistent asthma.37, 38 Although the inhaled route of administration offers targeted drug delivery of high dosages to the lung and reduced systemic side effects (such as osteoporosis, impaired immunity, and reduced growth associated with the prolonged use of corticosteroids), it poses drug adherence and compliance issues for patients.39, 40 Inhaled asthma therapeutics can also be expensive, inconvenient, and some have to be carried at all times.39 Hence, both children and the elderly find this route of drug administration difficult. Poor inhalation technique greatly affects drug delivery to the lungs and is the principal reason for poor medication efficacy. This subsequently affects the outcome of asthma therapy resulting in increased morbidity and mortality.39, 40 Oral administration, however, is widely accepted and can be supervised by parents and caregivers. Furthermore, long-lasting drug exposure and resulting pharmacological effects enable protection during day and night.
Here, we demonstrate the pharmacological effects of novel GABAAR positive allosteric modulators that, unlike commonly known ligands with such activity, have extremely low distribution in brain. Low brain exposure was confirmed by no detectable adverse CNS effects, as measured with a murine sensorimotor assay at high oral dosages (Figure S1). The innovative chemical compositions include hydrophilic groups such as phenolic hydroxyl (compound 1) and carboxylic acid (compound 2) attached to well-known imidazobenzodiazepine scaffolds. Although different in their acidity (pKA), both changes almost completely reduced brain exposure. Importantly, compounds 1 and 2 exhibited excellent pharmacokinetics with very high concentrations in blood and lung. A long in vivo half-life, especially observed for compound 2 (15 hours) would ensure protection against asthma exacerbation during the day and when taken again during the night. This feature is similar to long acting β-agonists. We observed that oral b.i.d. administration of compound 1 for 5 days during the ovalbumin challenge period significantly attenuated AHR at low concentrations of methacholine. This result is in agreement with previously investigated α4-selective positive allosteric GABAAR modulator XHE-III-74EE administered for 7 days via osmotic pump.23 In addition, chronic i.p. administration of 20 mg/kg XHE-III-74EE or 40 mg/kg of its dimethylamide analog b.i.d. for 5 days reduced sRaw values at the highest methacholine concentration used.23, 41 Similar results were observed in a house dust mite model with C57BL/6 mice with XHE-III-74 when administered as aerosol 10 minutes prior to the AHR measurement.27 Thus, GABAAR ligands derived from the same scaffold with significant selectivity towards the α4β3γ2 GABAAR have been shown to attenuate AHR when administered by different routes and variable dosages. Furthermore, we demonstrated for the first time that compound 2, an α5β3γ2 GABAAR selective positive modulator given orally b.i.d. at 100 mg/kg, protected against AHR.
The in vivo effects of compounds 1 and 2 on airway smooth muscle relaxation mimicked the ex vivo airway smooth muscle effects. In organ bath experiments, compounds 1 and 2 significantly relaxed substance P mediated pre-contraction of guinea pig airway smooth muscle. Similar pharmacological effects of selective α4β3γ2 or α5β3γ2 positive allosteric GABAAR modulators have been reported previously.15, 17, 23, 27, 34 Importantly, compound 2 was additionally efficacious in a clinically relevant model where human tracheal smooth muscle was exposed to the contractile mediator acetylcholine, which in addition to muscle constriction mediates airway inflammation and mucus hypersecretion.42 The actual concentration of compound 2 in the tissue was 9.8 μM although the concentration in the organ bath was 100 μM. Thus, single digit micromolar concentrations of GABAAR modulators enabled airway muscle relaxation.
Another important pharmacological property of the selective positive allosteric GABAAR modulators tested herein is their absence of mucogenic properties (Figure S4). A recent study has shown an excitatory rather than an inhibitory GABAergic system in the epithelium, whose inhibition decreased mucous production.19 However, compounds that specifically positively modulate the α4β3γ2 or α5β3γ2 GABAAR subtype did not increase mucus production in ova s/c mice.23
Airway inflammation is one of the hallmarks of asthma. It can be treated successfully with inhaled corticosteroids such as fluticasone,43 resulting in fewer side effects than oral corticosteroids such as dexamethasone or prednisolone, causing among others cardiovascular44 and CNS effects.45 To develop a single compound to combat AHR and airway inflammation, we target GABAAR proteins expressed on immune/inflammatory cells that respond to GABA concentrations similar to GABAARs expressed in the brain. Comparable effects of GABA and muscimol have been reported in activated human peripheral lymphocytes where both have elicited macroscopic currents.22 In whole cell clamp studies, tonic currents were observed in GABA activated encephalitogenic T lymphocytes.21 Importantly, we showed for the first time that CD4+ T cell responded directly to selective GABAAR modulators in the presence of GABA with a change of current response. The lung eosinophilic inflammatory response to ovalbumin allergen was attenuated with orally administered positive allosteric GABAAR modulators. Further investigation of different leukocyte populations demonstrated that at least compound 1 affected the number of CD4+ T cells in BALF and changed concentration-dependently their transmembrane currents. The effect on T cells was not significant for α5β3γ2 selective GABAAR ligand compound 2 as reflected in both the CD4+ T cell population in BALF and change of current response ex vivo. Evidence for GABAAR subunit expression for this cell type include reports of splenic CD4+ T cells obtained from NOD mice that expressed the α1 subunits but not the α5 subunits.24 In addition, encephalitogenic T cells from mice have been reported to express the α1, α4, β3, and δ subunits under resting and activated conditions.21 Still, the exact mechanism by which changes in T cell transmembrane currents reduces inflammation remains to be elucidated.
In summary, we have demonstrated that splenocytes express functional GABAARs that can be modulated with small molecules. In addition, oral administration of compounds 1 and 2 relaxed guinea pig airway smooth muscle ex vivo and alleviated AHR in murine model of asthma without increasing mucous metaplasia. Thus, we demonstrated for the first time a new oral asthma drug strategy based on effective prototype ligands that can be optimized further to combat both AHR and airway inflammation.
Supplementary Material
Acknowledgments
We thank Dr. Beryl R. Forman and Jennifer L. Nemke (Animal Facility at UWM) for their guidance and support.
Funding Sources: This work was supported by the University of Wisconsin-Milwaukee, the National Institutes of Health R03DA031090 (L.A.A.), R01NS076517 (J.M.C., L.A.A.), R01HL118561 (J.M.C., L.A.A., C.W.E., D.C.S.), R01MH096463 (J.M.C.,.A.A.), R01GM065281 (C.W.E., G.T.Y., J.M.C.), and R01HL122340 (C.W.E.) as well as the University of Wisconsin Milwaukee Research Foundation (Catalyst grant), the Lynde and Harry Bradley Foundation, the Richard and Ethel Herzfeld Foundation, the Stony Wold-Herbert Fund (G.T.Y.), the Foundation for Anesthesia Education and Research (G.T.Y.).
Abbreviation
- GABAAR
GABAA receptor
- AHR
airway hyperresponsiveness
- CNS
central nervous system
- PMA
phorbol myristate acetate
- PHA
phytohemagglutinin
- NMR
nuclear magnetic resonance
- HRMS
high resolution mass spectrometry
- BBB
blood brain barrier
- ECS
external cell solution
- ICS
internal cell solution
- DMSO
dimethylsulfoxide
- BALF
bronchoalveolar lavage fluid
- GABA
gamma aminobutyric acid
- sRaw
specific airway resistance
- β2AR
beta 2 adrenergic receptor
Footnotes
Supporting Information. The supporting information includes Supplemental Experimental Procedures and twelve figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Trends in Asthma Morbidity and Mortality. American Lung Association Epidemiology & Statistics Unit Research and Program Services [Google Scholar]
- 2.National Heart, L., and Blood Institute. Guidelines for the Diagnosis and Management of Asthma. Full Report 2007 Aug; [Google Scholar]
- 3.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116:477–486. doi: 10.1016/j.jaci.2005.07.011. quiz 487. [DOI] [PubMed] [Google Scholar]
- 4.Everard ML. Aerosol therapy past, present, and future: a clinician’s perspective. Respir Care. 2000;45:769–776. [PubMed] [Google Scholar]
- 5.Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2008:CD006363. doi: 10.1002/14651858.CD006363.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2012:CD006923. doi: 10.1002/14651858.CD006923.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–1317. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, Raissy HH, Van Natta ML, Tonascia J, Strunk RC, Group, C. R. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904–912. doi: 10.1056/NEJMoa1203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999;159:941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database Syst Rev. 2014:CD009471. doi: 10.1002/14651858.CD009471.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma treatment. Clinical and Experimental Allergy. 2010;40:1732–1741. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- 12.Lima JJ, Zhang S, Grant A, Shao LH, Tantisira KG, Allayee H, Wang LW, Sylvester J, Holbrook J, Wise R, Weiss ST, Barnes K. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. American Journal of Respiratory and Critical Care Medicine. 2006;173:379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mortensen M, Patel B, Smart TG. GABA Potency at GABA(A) Receptors Found in Synaptic and Extrasynaptic Zones. Front Cell Neurosci. 2011;6:1. doi: 10.3389/fncel.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. Update. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayton T, Poe MM, Rallapalli S, Biawat P, Savic MM, Rowlett JK, Gallos G, Emala CW, Kaczorowski CC, Stafford DC, Arnold LA, Cook JM. A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model. Int J Med Chem. 2015;2015:430248. doi: 10.1155/2015/430248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, Gerthoffer WT, Emala CW., Sr Targeting the restricted alpha-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2012;302:L248–256. doi: 10.1152/ajplung.00131.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW., Sr Selective targeting of the alpha5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am J Physiol Lung Cell Mol Physiol. 2015;308:L931–942. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA, Jr, Yang J, Emala CW., Sr GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1206–1216. doi: 10.1152/ajplung.00287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O’Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 20.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjurstom H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Dionisio L, Jose De Rosa M, Bouzat C, Esandi Mdel C. An intrinsic GABAergic system in human lymphocytes. Neuropharmacology. 2011;60:513–519. doi: 10.1016/j.neuropharm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Forkuo GS, Guthrie ML, Yuan NY, Nieman AN, Kodali R, Jahan R, Stephen MR, Yocum GT, Treven M, Poe MM, Li G, Yu OB, Hartzler BD, Zahn NM, Ernst M, Emala CW, Stafford DC, Cook JM, Arnold LA. Development of GABAA Receptor Subtype-Selective Imidazobenzodiazepines as Novel Asthma Treatments. Mol Pharm. 2016;13:2026–2038. doi: 10.1021/acs.molpharmaceut.6b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 25.Munroe ME, Businga TR, Kline JN, Bishop GA. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma. J Immunol. 2010;185:5586–5597. doi: 10.4049/jimmunol.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ai J, Wang X, Nielsen M. Honokiol and magnolol selectively interact with GABAA receptor subtypes in vitro. Pharmacology. 2001;63:34–41. doi: 10.1159/000056110. [DOI] [PubMed] [Google Scholar]
- 27.Yocum GT, Gallos G, Zhang Y, Jahan R, Stephen MR, Varagic Z, Puthenkalam R, Ernst M, Cook JM, Emala CW. Targeting the gamma-Aminobutyric Acid A Receptor alpha4 Subunit in Airway Smooth Muscle to Alleviate Bronchoconstriction. Am J Respir Cell Mol Biol. 2016;54:546–553. doi: 10.1165/rcmb.2015-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamenic TT, Poe MM, Rehman S, Santrac A, Divovic B, Scholze P, Ernst M, Cook JM, Savic MM. Ester to amide substitution improves selectivity, efficacy and kinetic behavior of a benzodiazepine positive modulator of GABAA receptors containing the alpha5 subunit. Eur J Pharmacol. 2016;791:433–443. doi: 10.1016/j.ejphar.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akinci MK, Schofield PR. Widespread expression of GABA(A) receptor subunits in peripheral tissues. Neurosci Res. 1999;35:145–153. doi: 10.1016/s0168-0102(99)00078-4. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi N, Lominadze D, Gillespie W, Moshal KS, Sen U, Rosenberger DS, Steed M, Tyagi SC. Differential expression of gamma-aminobutyric acid receptor A (GABA(A)) and effects of homocysteine. Clin Chem Lab Med. 2007;45:1777–1784. doi: 10.1515/CCLM.2007.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J. Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol Pharmacol. 2011;79:432–442. doi: 10.1124/mol.110.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Recio S, Gascon P. Biological and Pharmacological Aspects of the NK1-Receptor. Biomed Res Int. 2015;2015:495704. doi: 10.1155/2015/495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roffel AF, Elzinga CR, Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm Pharmacol. 1990;3:47–51. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- 34.Puthenkalam R, Treven M, Ramerstorfer J, Steudle F, Scholze P, Gallos G, Poe MM, Methuku KR, Li G, Arnold LA, Sieghart W, Santrac A, Savic MM, Emala CW, Cook JM, Ernst M. Benzodiazepine ligands with improved α5-selectivity and their airway smooth muscle relaxant effect. Mol Pharmacol. 2016 submitted. [Google Scholar]
- 35.Henderson WR, Jr, Lewis DB, Albert RK, Zhang Y, Lamm WJ, Chiang GK, Jones F, Eriksen P, Tien YT, Jonas M, Chi EY. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996;184:1483–1494. doi: 10.1084/jem.184.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014:CD003137. doi: 10.1002/14651858.CD003137.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson HS, Nathan RA, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. MedGenMed. 2001;3:3. [PubMed] [Google Scholar]
- 39.Cohn RC. A review of the effects of medication delivery systems on treatment adherence in children with asthma. Curr Ther Res Clin Exp. 2003;64:34–44. doi: 10.1016/S0011-393X(03)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabbri LM, Piattella M, Caramori G, Ciaccia A. Oral vs inhaled asthma therapy. Pros, cons and combinations, Drugs. 1996;52(Suppl 6):20–28. doi: 10.2165/00003495-199600526-00005. [DOI] [PubMed] [Google Scholar]
- 41.Jahan R, Stephen MR, Forkuo GS, Kodali R, Guthrie ML, Nieman AN, Yuan N, Zahn NM, Poe MM, Li G, Yu OB, Yocum GT, Emala CW, Stafford DC, Cook JM, Arnold LA. Optimization of Substituted Imidazobenzodiazepines as Novel Asthma Treatments. Eur J Med Chem. 2016;126:550–560. doi: 10.1016/j.ejmech.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF, Jr, Strunk RC, Allen DB, Bloomberg GR, Heldt G, Krawiec M, Larsen G, Liu AH, Chinchilli VM, Sorkness CA, Taussig LM, Martinez FD. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 44.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16:505–511. [PubMed] [Google Scholar]
- 45.Ciriaco M, Ventrice P, Russo G, Scicchitano M, Mazzitello G, Scicchitano F, Russo E. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013;4:S94–98. doi: 10.4103/0976-500X.120975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook JM, Clayton T, Hiteshkumar D, Rallapalli S, Johnson YT, Yang J, Poe MM, Namjoshi OA, Wang ZJ. US 20150258128 GABAergic receptor subtype selective ligands and their uses. 2012
- 47.Forkuo GS, Kim H, Thanawala VJ, Al-Sawalha N, Valdez D, Joshi R, Parra S, Pera T, Gonnella PA, Knoll BJ, Walker JK, Penn RB, Bond RA. PDE4 Inhibitors Attenuate the Asthma Phenotype Produced by beta2-adrenoceptor Agonists in PNMT-KO Mice. Am J Respir Cell Mol Biol. 2016 doi: 10.1165/rcmb.2015-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glaab T, Taube C, Braun A, Mitzner W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res. 2007;8:63. doi: 10.1186/1465-9921-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods. 2004;288:111–121. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


