Abstract
One begins to see improvement in glycemic measures and triglycerides with small amounts of weight loss, but with greater levels of weight loss there is even greater improvement. In fact, the relationship between weight loss and glycemia is one that is very close. This is fortunate for diabetes prevention; it takes only small amounts of weight loss to prevent progression to type 2 diabetes from impaired glucose tolerance and after the 10 kg of weight loss one cannot demonstrate much additional improvement in risk reduction. Modest weight loss (5 to 10%) is also associated with improvement in systolic and diastolic blood pressure and HDL cholesterol. With all these risk factors more weight loss produces more improvement. Further, for patients with higher BMI levels (>40 kg/m2), the ability to lose the same proportion of weight with lifestyle intervention is equal to that of those with lower BMI levels and there is equal benefit in terms of risk factor improvement with modest weight loss. For some comorbid conditions, more weight loss is needed – 10% to 15% - to translate into clinical improvement. This is true with obstructive sleep apnea, and non-alcoholic steatotic hepatitis. There is a graded improvement in improvements in measures of quality of life, depression, mobility, sexual dysfunction, and urinary stress incontinence, whereby improvements are demonstrable with modest weight loss (5–10%) and with further weight loss there are further improvements. For polycystic ovarian syndrome and infertility, modest weight loss (beginning at 2–5%) can bring improvements in menstrual irregularities and fertility Moderate weight loss (5–10%) has been shown to be associated with reduced health care costs. Reduction in mortality may take more than 10% weight loss, although definitive studies have not been done to demonstrate that weight loss per se is associated with mortality reduction. Clinicians in medical weight management should bear in mind that the target should be health improvement, rather than a number on the scale. The individual patient’s targeted health goal should be assessed for response, rather than a prescribed percentage weight loss.
Keywords: obesity, obesity comorbidity, weight loss, weight management, type 2 diabetes, dysglycemia, hypertension, dyslipidemia, obstructive sleep apnea, non-alcoholic fatty liver disease, weight loss and comorbidity improvement, polycystic ovarian syndrome, infertility
INTRODUCTION and BACKGROUND
A 5% for weight loss from baseline is generally accepted as a “clinically meaningful” amount.1 Certainly, the 2013 Obesity Guidelines recommended weight loss of 5–10% as the goal for medically supervised weight loss.2 Further, the US Food and Drug Administration Draft Guidance for medications for management of obesity has as one of the criteria for approval, that the medication achieve an average weight loss of 5% or greater than a placebo.3 But is it true that weight loss of 5% or 10% can bring health improvement for all obesity comorbidities? If not, how much weight is needed to produce clinically meaningful improvement in the various risk factors, comorbid diseases and mortality that are associated with obesity? This discussion will examine the link between excess body weight and comorbidity development and its counterpart weight loss and comorbidity improvement. The mechanisms by which excess body weight drives comorbid disease risk and by which weight loss improves pathology will also be explored. Finally, we will attempt to recommend a strategy for individual patients in selecting a target for body weight loss.
EXCESS WEIGHT AND HEALTH RISK
BMI above 25 kg/m2 is associated with increased risk for mortality and cardiometabolic diseases and the relationship demonstrates increasing risk with increasing BMI. This has been well established since the 1970’s supported by many studies of actuarial data from life insurance companies and observational studies of populations. Indeed, the recent Obesity Guidelines2 devoted a critical question and a systematic evidence review evaluating the cut point of 25 kg/m2 and 30 kg/m2 as markers of “overweight” and “obesity.” That review2 confirmed the current cut points as being valid, compared to normal weight status (BMI 18.5 <25kg/m2), for identifying increased risk for diabetes, stroke and coronary heart disease. Further, when BMI is treated as a continuous variable, the greater the BMI, the greater the risk for these conditions. As for all-cause mortality, the greater the BMI the greater the risk, but the relationship between overweight (BMI 25 <30 kg/m2) was not increased compared to normal weight.2
MECHANISMS BY WHICH EXCESS BODY FAT INCREASES HEALTH RISK
Current thinking about how excess adiposity drives health risk is through several mechanistic pathways. The excess physical burden of body weight can play a role, especially in lower extremity arthritis and pain and in sleep apnea. For example, in knee osteoarthritis, every pound of excess weight exerts a four-fold burden on the knee per step in daily activities.4 Another mechanistic pathway is through biochemical products of fat tissue.5 Fat tissue itself is an active endocrine organ, secreting a number of adverse cytokines, including pro-inflammatory and pro-thrombotic molecules, among others. The “portal hypothesis” also maintains that free fatty acids released from visceral fat stores directly into the portal vein bathing the liver and contributing to the abnormal lipid profile and insulin resistance characteristic of metabolic syndrome. Circulating free fatty acids can also affect muscle insulin sensitivity. Finally, fatty infiltration of liver and muscle can contribute to pathology.6
MECHANISMS BY WHICH WEIGHT LOSS CAN IMPROVE HEALTH AND HEALTH RISK
A recent paper7 from Washington University in St Louis describes an experiment in which different levels of weight loss were assessed for their impact on metabolic function and adipose tissue biology. This experiment explored the mechanisms by which different degrees of weight loss impact a variety of advanced clinical endpoints. In the study, 40 volunteers with obesity and insulin resistance were randomly assigned to weight maintenance or to a dietary weight loss intervention which aimed for 5% weight loss, subsequently 10% weight loss and subsequently 15% weight loss. The actual mean weight losses achieved were 5.1% ± 0.9% (n = 19), 10.8% ± 1.3% (n = 9), and 16.4% ± 2.1% (n = 9). In the weight maintenance condition, 14 completed the study. Interestingly, body weight loss was associated with disproportionate loss of body fat across multiple compartments. The 5%, 11% and 16% weight loss was associated with 10%, 18% and 27% reduction in total kg fat mass, respectively and 9%, 23% and 30% reduction in intra-abdominal adipose tissue (cm3). Even more disproportionate is the reduction in intra-hepatic triglyceride, measured as percentage on Magnetic Resonance Imaging, a 13%, 52% and 65% reduction for each weight loss level, respectively. Thus, it appears that with total body fat loss, the stores of intra-abdominal and intra-hepatic fat are preferentially lost. This preferential loss of adverse fat storage sites may account for the metabolic benefits observed with 5–16% weight loss in the study.7
In addition, Magkos et al7 showed that the body composition changes were associated with a variety of improvements in clinical endpoints, but that different tissues responded to different degrees of weight loss. 5% weight loss significantly decreased the plasma concentrations of some risk factors for cardiometabolic disease (glucose, insulin, triglyceride, alanine transaminase, and leptin). but did not affect others (free fatty acids, low- and high-density lipoprotein [LDL and HDL, respectively] cholesterol, and adiponectin). Only after 16% weight loss did plasma free fatty acid and CRP concentrations decrease and plasma adiponectin concentration increase significantly. Thus, for some endpoints, greater degrees of weight loss are needed. This study7 performed sophisticated tests of multi-organ insulin sensitivity (a two-stage hyperinsulinemic euglycemic clamp with infusion of stable isotopically-labeled tracers) and demonstrated that liver and adipose tissue insulin sensitivity improved at 5% weight loss and plateaued, but that muscle insulin sensitivity continued to improve with 11% and 16% weight loss. Beta cell function also improves in a step-wise fashion with progressive weight loss. The study examined adipose tissue expression of genes involved in cholesterol flux, lipid synthesis, extracellular matrix remodeling, and oxidative stress, again with step-wise improvement in function with progressive weight loss.
For clinicians, the take-away message from the above referenced study7 is that modest weight loss (5%) has multiple metabolic and cardiovascular risk factor benefits and more weight loss (11% and 16%) has even more benefits for metabolism and cardiovascular risk factors. However, for some clinical endpoints, especially if one is seeking improvement in inflammatory markers, it may be necessary to achieve 16% weight loss or more. This may help to explain why clinically it requires more weight loss to see improvement in NASH activity scores for Non-Alcoholic Steatotic Hepatitis, and for improvement in symptoms of obstructive sleeppnea and for knee pain with osteoarthritis. This is discussed below.
EVIDENCE OF HEALTH BENEFITS OF DIFFERENT DEGREES OF WEIGHT LOSS
1. Modest and moderate weight loss and diabetes prevention
The health benefit of modest weight loss is best exemplified clinically in the relationship between weight loss and diabetes prevention. While an average weight loss of 6.7% reduced the incidence of diabetes by 58% in the group participating in the American Diabetes Prevention Program8 and similarly in the Finnish Diabetes Prevention Trial,9 it’s important to distinguish group benefits versus individual benefits. An analysis by Hamman, et al10 from the American Diabetes Prevention Program showed that in individuals with impaired glucose tolerance, for every kilogram of weight lost there was a 16% reduction in risk for progression to diabetes. Furthermore, after about 10 kg weight loss, there was negligible benefit, in terms of diabetes risk reduction, from further weight loss. This is illustrated in Figure 1. Clearly, even one or two kg of weight loss in persons at risk for developing type 2 diabetes (i.e. those with prediabetes) can have health benefits. In addition, this analysis of the Diabetes Prevention Program weight loss showed reduced diabetes incidence similarly across all race and ethnicity groups for both sexes, for all ages and for several levels of physical activity and regardless of the level of the initial obesity.
Figure 1.
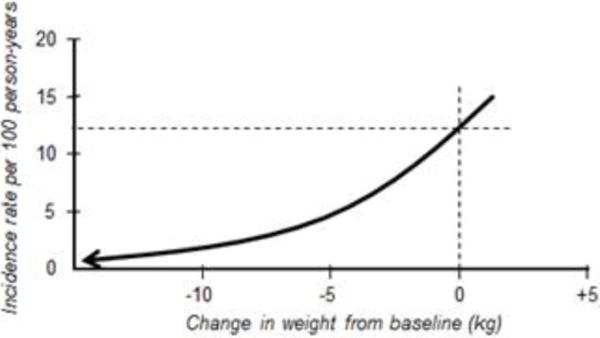
The DPP experience: Every kg of weight loss was associated with 16% reduction in risk for progression to type 2 diabetes [10].
2. Modest and moderate weight loss in established type 2 diabetes
The relationship between modest weight loss and improvement in glycemia is powerful and it is not limited to diabetes prevention. This is illustrated with analyses from the Look AHEAD study of >5000 individuals with type 2 diabetes. In one analysis,11 categories of weight loss were defined (stable weight, ≥2%<5%, ≥5%<10 %, ≥10%<15% and ≥15%). This analysis demonstrated that improvement in fasting glucose and hemoglobin A1c is observed beginning at only ≥2<5% weight loss. Of course, greater weight loss was associated with greater benefit to glycemic outcomes in a direct and linear fashion. It must be noted that these benefits to glycemic measures were achieved alongside reductions in antidiabetic medications.11
3. Modest and moderate weight loss and improvement in cardiovascular disease risk factors
Data from the Look AHEAD Study also showed that health benefits of modest weight loss are not limited to glycemic measures. The analysis cited above11 also evaluated the impact of progressive categories of weight loss on other risk factors and showed that improvement in triglycerides and systolic blood pressure begins with ≥2<5% weight loss.j For diastolic blood pressure and HDL cholesterol, improvement begins at ≥5<10% weight loss.j All of these risk factors improved in a direct and linear fashion with greater weight loss being associated with greater risk factor benefit. However, for LDL cholesterol, the relationship is less strong and in the Look AHEAD study, where baseline LDL was 100 mg/dl, there was no reduction in LDL.j However, there was a reduction in use of lipid lowering medications in the modest weight loss group (average −8.7% at year 1) in this study.11
4. Benefits of modest and moderate weight loss on cardiometabolic risk factors across all levels of obesity
Of importance is the demonstration from another analysis11 from the large Look AHEAD data set (n>5000) that baseline BMI category (Obese stage I, II or III) does not alter the benefit of modest weight loss.12 Each of the BMI categories demonstrate the same amount of mean weight loss, when expressed as a percentage from baseline, with the same lifestyle intervention. Of course, those with higher BMI category would lose more weight when expressed in kilograms; but when expressed proportionally there is no significant difference across BMI categories in weight loss. Thus, for patients with BMI 40 kg/m2 or more there was no difference in mean percentage weight loss when compared to those with BMI 35<40 or BMI 30<35. Further, the same held true for improvement in most risk factors. Except for HDL cholesterol, weight loss had the same impact across the three BMI categories with significant improvement in hemoglobin A1c, triglycerides, systolic blood pressure, and LDL cholesterol.12
5. Benefits of moderate weight loss on symptoms of obstructive sleep apnea
The Look AHEAD Study incorporated a substudy of sleep apnea, called Sleep AHEAD. More than 80% of the participants with type 2 diabetes in four sites of Look AHEAD had at least mild obstructive sleep apnea.13 With the intensive lifestyle intervention (ILI), mean weight loss at one year at these four sites was 10.8 kg vs. 0.6 kg in the diabetes support and education (DSE) group. At 1 year, remission of OSA (apnea hypopnea index, AHI, <5 events per hour) was 3 times more common in the ILI participants (13.6%) than in the DSE participants (3.5%). Further, the prevalence of severe obstructive sleep apnea among ILI participants (18.4%) was half that of the DSE group (37.9%). Participants with a weight loss of 10 kg or more had the greatest improvements. In fact, weight loss of 10 kg or more was required for significant association with AHI change. At 4 years, improvements persisted, despite some weight regain to 5.2 kg below baseline in the ILI group.14 Remission of OSA at 4 years was 5 times more common with intensive lifestyle intervention (20.7%) than diabetes support and education (3.6%).14 For clinicians, weight loss can be a major modifier of symptoms of obstructive sleep apnea as measured by the apnea hypopnea index, but 10% or more should be the goal to impact clinical symptoms. This larger amount of weight loss required for improvement may relate to the physical impingement on airway by excess body fat and it may take more proportional weight loss to impact symptoms.
6. Benefits of modest and moderate weight loss on osteoarthritis of the knee
Osteoarthritis of the knee is closely linked to obesity as a risk factor and is quite common. Nearly half of Americans are projected to experience osteoarthritis of at least one knee in their lifetime.15 A diet and exercise intervention which achieved 5.7% weight loss on average, and compared to a control condition produced significant improvements in WOMAC (Western Ontario MacMaster University score, which measures self-reported function), the 6 minute walk distance (p<0.05), stair climb time (p<0.05) and knee pain.16 Knee joint loads were also assessed in those patients and the investigators found that each pound of weight lost resulted in a 4-fold reduction in the load exerted on the knee per step during daily activities.17 Accumulated over thousands of steps per day, a reduction of this magnitude would appear to be clinically meaningful. A subsequent study achieved average weight loss of 10.6% with diet and exercise, and compared to a control condition of exercise alone produced significant improvement in pain, function, IL-6 levels and a quality of life measure.18 However, radiographic and Magnetic Resonance Imaging outcomes did not fare as well. Despite the positive effects of weight loss in this study on symptoms as well as mechanistic outcomes (such as joint compressive force and markers of inflammation), there was no statistically significant improvement on the rate of structural progression either on X-ray or MRI over 18-months.19 Thus, if a real impact on osteoarthritis of the knee is to be achieved, one must treat before established pathology in the knee, at the stage of knee pain alone. In the Look AHEAD study of men and women with type 2 diabetes, there was 15% less incidence of knee pain at year one in lifestyle intervention group (−8.7% weight loss) than support group (−0.9% weight loss) at one year.20 However, at year 4 this difference in incidence decreased to 5% and was no longer statistically significant.20 Therefore the best strategy would be to treat early and to treat more aggressively to produce greater weight loss, thus preventing the onset of structural damage to the joint.
7. Benefits of weight loss on hepatic steatosis and non-alcoholic steatotic hepatitis (NASH)
As discussed above, in the experiment conducted by Magkos et al,7 weight loss disproportionately reduces fat from liver. In that study, 5% weight loss reduces intrahepatic triglyceride by 13%; 11% weight loss reduced it by 52% and 16% by 65%. As part of a substudy, 96 participants in Look AHEAD underwent proton magnetic resonance spectroscopy (MRS) to quantify fatty infiltration of the liver, with hepatic steatosis defined as 5.5% or higher being non-alcoholic fatty liver disease.21 In that study, the greater the weight loss the greater the reduction in hepatic steatosis. However, while there were group differences in steatosis, with the lifestyle intervention group reducing steatosis on average 50.8% (versus 22.8% in the support group; P>0.04), there were no group differences in mean ALT and ASP.21 It appears that it may take 10% or more weight loss to have an impact on NASH Activity Scores as assessed by liver biopsy.22
8. Benefits of lifestyle intervention on improvement in feeling and function (Quality of Life, Depression, Mobility, Sexual Dysfunction, and Urinary Stress Incontinence)
While reducing risks for other diseases is important, equally important is improving how patients feel and function. There is a known graded response to weight loss achieved through lifestyle intervention and improvement in quality of life as measured by the Impact of Weight – Quality of Life Assessment Tool.23 Indeed, in Look AHEAD, at year one, quality of life improved more in the group undertaking lifestyle intervention than those in the support condition.24
Also, in Look AHEAD, there were fewer patients who developed potentially significant symptoms of depression (defined as Beck Depression Inventory25 score >10) in the lifestyle intervention group as compared to the support condition.26 At 1 year, the incidence of BDI ≥10 was significantly lower in the ILI than DSE group (6.3% vs. 9.6%; P < 0.001) indicating that weight loss does not precipitate depression and may protect from it. Furthermore participants in the lifestyle intervention with and without symptoms of depression at baseline lost 7.8 ± 6.7% and 8.7 ± 6.9% of total body weight, respectively, a difference not considered clinically meaningful.
Look AHEAD also assessed functionality. For participants in the lifestyle intervention, compared to the support condition, there was attenuation in the decline in mobility that occurs with aging.27
In overweight and obese women with type 2 diabetes participating in Look AHEAD, urinary stress incontinence improved in those who were randomized to the lifestyle intervention as compared to the control condition.28 Look AHEAD demonstrated the same finding in men.29 Sexual dysfunction was also studied in Look AHEAD and there was improvement in measures of sexual function for participants in the lifestyle intervention compared to the support condition. There was improvement in erectile function for men30 and sexual dysfunction in women.31
9. Benefits of weight loss in Polycystic Ovarian Syndrome and infertility in women
A hallmark of women with PCOS is menstrual irregularities and its resulting infertility in addition to androgen excess and metabolic dysfunction. Most of the evidence points to improvement in ovulatory cycles and subsequent pregnancy with weight loss in obese women with PCOS. Furthermore, even a minimal weight loss of only 2–5% of total body weight improves ovulatory function and is more likely to result in spontaneous pregnancy.32 There is more robust evidence to support improved outcomes from ovulatory cycles and pregnancy at higher rates of 5 and 10% of total body weight loss.33 The return of normal menstrual function and decreased hirsutism are thought to be due to improved insulin sensitivity, decreased Luteinizing Hormone levels, and lower androgenemia.33,34 Not only is pregnancy easier to achieve after modest weight loss it is also more likely to result in a successful live birth-miscarriage rates are lower at lower BMIs.35
10. Benefits of weight loss on health care costs and mortality
In an analysis from Look AHEAD36 the impact of the lifestyle intervention on use and costs of medical services, with the support condition as comparator, showed that in the lifestyle group, annual hospitalizations were reduced by 11% (P=0.004) and hospital days by 15% (p=0.01). The cost savings for hospitalizations were 10% less in the lifestyle group (p=0.04). Medication cost savings were 7% less in the lifestyle group compared to the support group (P<0.001). Over 10 years, the relative cost savings per person in the lifestyle group were $5280 (95% CI = $3385-$7175). However, there were no differences in outpatient costs and the savings were not observed in those with a history of cardiovascular disease. The costs of conducting the Look AHEAD intervention have not been reported so cost effectiveness cannot yet be calculated.36
11. Weight Loss and Mortality Reduction
The Swedish Obese Subjects Study37 provides a relevant paradigm for assessing the impact of weight loss per se on mortality and cardiovascular disease mortality, because while surgery was the method of obtaining weight loss, the procedure done in >80% of participants was the vertical banded gastroplasty. This would not great physiologic changes in gut signals as the Roux-en-Y-Bypass does that might have independent effects on mortality. The results from the Swedish Obese Subjects study, showed that surgical treatment which produces on average 16% weight loss, compared with a matched but un-operated control group without weight loss, showed a 29% reduction in overall mortality after ~ 20 years.37 In the Look AHEAD study, participants were followed for 13 years. The mean initial weight loss at 1 year was 8.7% but half of the weight was regained. At the end of the trial there was no significant difference in the incidence of a composite end-point for major cardiovascular end-points for the intensive lifestyle intervention compared to the diabetes support and education condition.38 However, a subsequent analysis from Look AHEAD39 where individuals who lost at least 10% of their bodyweight in either arm of the study, in the first year of the study, had a 21% lower risk of the primary outcome of major cardiovascular events (p=0·034) and a 24% reduced risk of the secondary cardiovascular disease outcome (p=0·003) compared with individuals with stable weight or weight gain. Granted, this is not a randomized comparison, but a post hoc analysis, but it suggests that more than 10% weight loss may be needed to achieve reduction in cardiovascular events and mortality.
GUIDELINE RECOMMENDATIONS FOR WEIGHT LOSS
The concept that we do not need to normalize weight or achieve major weight loss to obtain health benefits has been reinforced in recent Obesity Guidelines.2 In 2013, an expert panel formed by the NIH conducted an evidence-based review2 around 5 critical questions. Critical Question 1 addressed the health benefits of weight loss: What amount (shown as percent lost, pounds lost, etc.) of weight loss is necessary to achieve benefit with respect to CVD risk factors, morbidity, and mortality? The graded evidence statements that resulted from this effort provide the strongest support for weight loss beginning at 3% (for glycemic measures and triglycerides) and 5% (for blood pressure, HDL and LDL cholesterol) to be considered clinically meaningful.2 The Expert Panel went on to observe that increased weight loss amounts provide even greater benefits. Still, the clinical practice recommendation, based on expert opinion, was to set an initial goal of 10% weight loss. Clinical Practice Guidelines for Comprehensive Care of Patients with Obesity issued by the American Society of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) take a different approach.40 That approach is more complications centric. Obesity disease stage is based on ethnic-specific BMI along with assessment cutoffs for adiposity-related complications. Stage 0 is assigned to individuals who are overweight or obese by BMI classification but have no complications, whereas Stage 1 and 2 are defined as individuals who are overweight or obese by BMI classification and having 1 or more mild-moderate complications (Stage 1) or at least 1 severe complication (Stage 2). For patients with Stage 2, 10% or more weight loss is recommended. The American College of Obstetrics and Gynecology provides an Obesity Tool Kit41 for practitioners and relies on the 2013 Obesity Guidelines,2 as well as other sources to inform their recommendations. The Tool Kit does not specifically address weight loss goals.41
CONCLUSIONS
This brief review addresses the approach to weight loss for different obesity-related comorbidities. Because different tissues respond differently to weight loss, only modest amounts of weight loss may be needed for diabetes prevention, but moderate or more weight loss may be needed, especially where the goal is to reduce inflammation (as in NASH) or to reduce fat burden (as in obstructive sleep apnea and knee pain and osteoarthritis. These concepts are broadly applicable. But where individual patients are concerned, we must judge not just success at achieving a weight loss goal, but also success in reaching targeted health outcome goals. Targeted health outcome goals may be reached by an individual with weight loss of less than 5% or more than 10%. What is meaningful for our patients is the message that patients need not reach a BMI <25 kg/m2 in all instances, but can be healthier at any weight, as long as it is a reduced weight.
Table 1.
Relationship with amount of weight loss and various comorbidities
| Condition | Amount of weight loss needed to effect improvement | References |
|---|---|---|
| Glycemic Improvement–Diabetes prevention in impaired glucose tolerance | 2.5% weight loss or more; maximal impact at 10% | 2,8,9,10 |
| Glycemic improvement–Type 2 diabetes | 2.5% to >15%; greater weight loss associated with greater glycemic improvement; true for all BMI classes | 11,12 |
| Triglyceride reduction | 2.5% to >15%; greater weight loss associated with greater glycemic improvement; true for all BMI classes; | 11,12 |
| HDL increase | 5% to >15%; greater weight loss associated with greater glycemic improvement; not true for BMI >40 kg/m2 | 11,12 |
| Apnea Hypopnea Index Improvement in Obstructive Sleep Apnea | 10%+ weight loss required for significant improvement | 13,14 |
| Knee pain and function in persons with osteoarthritis | 5–10% improves knee functionality, speed, walk distance and pain; 10%+ required to improve IL-6 and CRP levels; knee MRI and X-ray findings do not change | 16–19 |
| Emergent knee pain prevalence | 5–10% weight loss, with persistent maintenance required to prevent knee pain in individuals with obesity | 20 |
| Hepatic steatosis reduction | 5–15%+; greater weight loss associated with greater improvement | 21 |
| Non-alcoholic steatotic hepatitis activity score | 10%+ weight loss required for significant improvement | 22 |
| Impact of Weight on Quality of Life score | 5%–15%+; greater weight loss associated with greater improvement | 23 |
| Depression | 5–10% may reduce risk for emergent depression; individuals with depression lose as much weight as non-depressed individuals. | 26 |
| Mobility | 5–10% loss attenuates mobility decline with aging | 27 |
| Urinary Incontinence | 5–10% improves symptoms in men and women | 28,29 |
| Sexual Function | 5–10% improves erectile function in men and sexual dysfunction in women | 30,31 |
| Polycystic Ovarian Syndrome and infertility | Improvement in ovulatory cycles and subsequent pregnancy with 2–5% weight loss, with more weight loss producing more robust effect. | 32–34 |
| Health care costs | In persons with diabetes 5–10% weight loss associated with reduction in hospitalization and medication costs, but not outpatient costs. | 36 |
| Mortality | 16% weight loss (vertical banded gastrectomy) associated with reduction in all cause and cardiovascular mortality. 5–10% weight loss with lifestyle intervention had no effect on major cardiovascular outcomes, but in those with 10%+ weight loss, there was a reduction in those outcomes. | 37,38,39 |
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Donna H. Ryana and Sarah Ryan Yockey declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Donna H. Ryan, Professor Emerita, Pennington Biomedical Research Center 625 St. Charles Avenue #10B, New Orleans, LA 70130, 225 229 3909.
Sarah Ryan Yockey, Assistant Professor, Department of Obstetrics and Gynecology, LSU School of Medicine, 1542 Tulane Avenue, New Orleans, LA 70112.
References
- 1.Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 2015 Dec;23(12):2319–20. doi: 10.1002/oby.21358. [DOI] [PubMed] [Google Scholar]
- 2**.Jensen MD, Ryan DH, Donato KA, et al. Guidelines 2013 for managing overweight and obesity in adults. Obesity. 2014;22:S1–S410. doi: 10.1002/oby.20819. These guidelines are based on a systematic evidence review around 5 critical questions (benefits of weight loss, risks of excess body weight, best diet for weight loss, weight loss with comprehensive lifestyle intervention and role of bariatric surgery). [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDEER) Guidance for Industry Developing products for Weight Management. Draft Guidance. 2007 Feb; Revision 1. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071612.pdf Accessed 1/7/2017.
- 4.Messier RP, Gutekunst DJ, Davis C, DeVita P. ARTHRITIS & RHEUMATISM. 2005;52(7):2026–32. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 5.Sikaris KA. The Clinical Biochemistry of Obesity. Clin Biochem Rev. 2004 Aug;25(3):165–181. [PMC free article] [PubMed] [Google Scholar]
- 6.Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014 Oct;21(5):345–51. doi: 10.1097/MED.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de las Fuentes K, Sonbing H, Okunade AL, Patterson BW, Klein S. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metabolism. 2016;23:1–11. doi: 10.1016/j.cmet.2016.02.005. This paper measures advanced clinical endpoints and performs measurements at baseline, after 5% weight loss, after 11% weight loss and after 16% weight loss and compares to a control, stable-weight condition. It provides insight into the mechanisms by which weight reduction produces effects on different tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes Prevention Program Research Group. Reduction in the incidence of Type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuomilheto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 10.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unick JL, Beavers D, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, Wing RR, the Look AHEAD Research Group Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes. Diabetes Care. 2011;34:2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX, Reboussin D, Kuna ST, Sleep AHEAD Research Group of the Look AHEAD Research Group A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with Type 2 Diabetes: The Sleep AHEAD Study. Arch Intern Med. 2009 Sep;169(17):28. 1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuna ST, Reboussin DM, Borradaile KE, Sanders MH, Millman RP, Zammit G, Newman AB, Wadden TA, Jakicic JM, Wing RR, Pi-Sunyer FX, Foster GD, Sleep AHEAD Research Group Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. SLEEP. 2013;36(5):641–649. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/arthritis/resources/spotlights/kneereplacements.htm.
- 16.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH, Pahor M, Williamson JD. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis. the Arthritis, Diet, and Activity Promotion Trial. ARTHRITIS & RHEUMATISM. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 17.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. ARTHRITIS & RHEUMATISM. 2004;52(7):2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 18.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013 Sep;310(12):25. 1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Hunter DJ, Beavers DP, Ekstein F, Guermazi A, Loeser RF, Nicklas BJ, Mihalko SL, Miller GD, Lyles M, DeVita P, Legault C, Carr JJ, Williamson JD, Messier SP. The Intensive Diet and Exercise for Arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis and Cartilage. 2015;23:1090–1098. doi: 10.1016/j.joca.2015.03.034. This paper demonstrates that despite significant weight loss and improvements in symptoms and function, there are no changes in MRI or X-ray changes with knee osteoarthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.White DK, Neogi T, Rejeski WJ, Walkup MP, Lewis CE, Nevitt MC, Foy CG, Felson DT, Look ARG. Can an intensive diet and exercise program prevent knee pain among overweight adults at high risk? Arthritis Care Res (Hoboken) 2015;67(7):965–971. doi: 10.1002/acr.22544. This paper demonstrates that a lifestyle intervention producing moderate weight loss (5–10%), compared to a control condition, was associated with a reduction in emergence of knee pain over one year in persons with obesity and type 2 diabetes. However, over the next three years with 50% of the lost weight being regained, there was no difference in emergent rates of knee pain among study groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazo M, Solga S, Horska A, et al. Fatty Liver Subgroup of the Look AHEAD Research Group Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33(10):2156–63. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001 Sep;9(9):564–71. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]
- 24.Williamson D, Rejeski J, Lang W, et al. Look AHEAD Research Group Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169(2):163–71. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Faulconbridge L, Wadden T, Rubin R, et al. Look AHEAD Research Group One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity. 2012;20(4):783–93. doi: 10.1038/oby.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–17. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelan S, Kanaya AM, Subak LL, et al. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urol. 2012;187:939–44. doi: 10.1016/j.juro.2011.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breyer BN, Phelan S, Hogan PE, et al. Look AHEAD Research Group Intensive lifestyle intervention reduces urinary incontinence in overweight/obese men with type 2 diabetes: results from the Look AHEAD trial. J Urol. 2014 doi: 10.1016/j.juro.2014.02.036. EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing R, Rosen R, Fava J, Bahnson J, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. Journal of Sexual Medicine. 2010;7(1 Pt 1):156–65. doi: 10.1111/j.1743-6109.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing RR, Bond DS, Gendrano IN, et al. Sexual Dysfunction Subgroup of the Look AHEAD Research Group Effect of intensive lifestyle intervention on sexual dysfunction in women with type 2 diabetes: results from an ancillary Look AHEAD study. Diab Care. 2013;36:2937–2944. doi: 10.2337/dc13-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiddy DS, Hamilton-Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;36:105. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 33.Crosignani PG, Colombo M, Vegetti W, et al. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18:1928. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- 34.Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1470. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- 35.Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med. 2011;29:507. doi: 10.1055/s-0031-1293204. [DOI] [PubMed] [Google Scholar]
- 36*.Look AHEAD Research Group. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with Type 2 diabetes. Diabetes Care. 2014 Sep;37(9):2548–2556. doi: 10.2337/dc14-0093. This paper shows that modest weight loss (mean 8.7% weight loss at one year, with regain of 50% over the next 3 years) was associated with reduction in costs for hospitalization and in medication costs, but not outpatient medical care costs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C. Effects of bariatric surgery on mortality in Swedish obese subjects. New England journal of medicine. 2007 Aug;357(8):23. 741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 38.The Look AHEAD Research Group. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. New Engl J Med. 2013 Jul;369(2):11. 145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Look AHEAD Research Group. Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, Evans M, Foreyt J, Foster G, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jeffery RW, Johnson KC, Kitabchi AE, Knowler WC, Kriska A, Lang W, Lewis CE, Montez MG, Nathan DM, Neiberg RH, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Redmon B, Regensteiner J, Rejeski J, Ribisl PM, Safford M, Stewart K, Trence D, Wadden TA, Wing RR, Yanovski SZ. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016 Nov;4(11):913–921. doi: 10.1016/S2213-8587(16)30162-0. For patients who lost 10% or more in the first year of the study, there was a reduction in major cardiovascular events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R, reviewers of the AACE/ACE Obesity Clinical Practice Guidelines American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for Comprehensive Medical Care of Patients with Obesity – Executive Summary. Endocr Pract. 2016 Jul;22(7):842–84. doi: 10.4158/EP161365.GL. New guidelines from the AACE organization emphasize complications-centric, rather than BMI-centric treatment decisions for patients with overweight and obesity. [DOI] [PubMed] [Google Scholar]
- 41.http://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Obesity-Toolkit accessed 2/22/2017.


