Abstract
Purpose
Head and neck cancers are the third most common cancers worldwide. Oral mucositis is the most common toxicity seen in patients who receive chemoradiation to treat head and neck cancer. The aim of this study was to evaluate the efficacy and safety of oral glutamine supplementation in these patients.
Materials and Methods
From December 2013 to December 2014, we randomly assigned to two arms 162 patients who had squamous cell carcinoma of the head and neck. Patients in arm A were given oral glutamine once per day, whereas those in arm B served as negative control subjects. All patients received radiotherapy given as 70 Gy in 35 fractions over 7 weeks with an injection of cisplatin once per week. Patients were assessed once per week to evaluate for the onset and severity of mucositis, pain, use of analgesics, and for Ryle tube feeding.
Results
We observed that 53.1% of patients developed mucositis toward the fifth week in the glutamine arm compared with 55.5% of patients in the control arm at the third week. None in the glutamine arm compared with 92.35% of patients in the control arm developed G3 mucositis. Rates of adverse events like pain, dysphagia, nausea, edema, and cough, as well as use of analgesics and Ryle tube feeding, were significantly lower in the glutamine arm than in the control arm.
Conclusion
This study highlights that the onset as well as the severity of mucositis in patients receiving glutamine was significantly delayed. None of the patients receiving glutamine developed G3 mucositis. Hence, the findings emphasize the use of oral glutamine supplementation as a feasible and affordable treatment option for mucositis in patients with head and neck cancers who are receiving chemoradiation.
INTRODUCTION
Head and neck cancers are the third most common cancers worldwide, accounting for more than 550,000 cases annually.1 Radiation therapy along with chemotherapy forms the cornerstone of treatment for head and neck cancers. However, the toxicities of treatment are often severe and difficult to manage.2 Oral mucositis is the most common toxicity seen in patients with head and neck cancer who are being treated with chemoradiation, which not only impairs their function and quality of life but also affects their survival and outcomes from the disease.3 This toxicity manifests as progressive thinning of the oral mucosa to form erythematous patches and finally leads to ulceration with severe pain and swallowing disability. At this point, radiotherapy is interrupted, chemotherapy is withheld, and potent analgesics or Ryle tube feeding becomes mandatory. Although several agents have been tried, there is no standard guideline or recommendation for the treatment of chemoradiation-induced oral mucositis in head and neck cancers.
Glutamine is a conditionally essential amino acid critical to the regulation of protein synthesis, cellular energy, respiratory fueling, and signaling in cancer cells. The skeletal muscle accounts for 90% of the glutamine synthesized in the body, with the rest released by the lungs and the brain.4 Several experiments in animal species have shown that depletion of plasma glutamine levels is associated with edema, ulceration, and patchy necrosis of the intestinal mucosa.4 In catabolic states of injury or during periods of rapid growth or stress, glutamine becomes conditionally essential, and oral supplementation is necessary.5 Some studies have also highlighted the use of oral glutamine supplementation to reverse cancer-related cachexia and other debilitating conditions.6 Huang et al7 showed that oral glutamine significantly reduces the onset and severity of mucositis during radiotherapy. A review by Silverman8 showed that glutamine supplementation can reverse cellular damage caused by chemoradiation and that it thus accelerates recovery. Hence, we proceeded to conduct an institutional study to demonstrate the role of oral glutamine in the management of chemoradiation-induced mucositis in head and neck cancers.
MATERIALS AND METHODS
From December 2013 to December 2014, we enrolled 162 patients who had histopathologically proven squamous cell carcinoma of the head and neck who were treated at the Department of Radiation Oncology, A.H. Regional Cancer Centre, Cuttack, India, for the study. It was approved by our institutional ethics committee, and informed consent was obtained from all the patients. From the onset of the study, all patients who had squamous cell carcinoma of the head and neck were examined for eligibility. Patients who fulfilled the eligibility criteria were offered study participation. After we obtained consent, consecutive patients were randomly assigned one after the other into either treatment arm A, which was the glutamine arm, or arm B, which was the control arm. Because the study protocol had determined an equal number of patients for each arm of the study, a block randomization protocol (ie, AABB, ABAB, BABA, and so on) was used. Individual patients were randomly assigned to any of the preset block sequences by using a random-number table, until the total number of 162 patients were accrued. To avoid bias, the randomization sequence was obtained in sealed envelopes from a statistician.
The inclusion criteria were the following: histopathologically proven squamous cell carcinoma of the head and neck; primary tumor in stage T2, T3, or T4; regional node of any N status; distant metastases absent; age 20 to 80 years; Eastern Cooperative Oncology Group (ECOG) performance score (PS) of 0 or 1; normal hematologic and biochemical parameters; and willingness to fulfill the study requirements and give consent.
Exclusion criteria were the following: previous surgery in the head and neck, previous chemotherapy or radiotherapy, uncontrolled systemic or widely disseminated disease, presence of a synchronous double primary malignancy, or simultaneous participation in another clinical trial. As a part of our institutional protocol, postoperative patients with head and neck cancer receive 66 Gy in 33 fractions, whereas those receiving radical chemoradiation are treated with 70 Gy in 35 fractions. In this study, all 162 patients in both the arms received exactly the same treatment. They were treated with concomitant chemoradiation, which included 70 Gy of external-beam radiotherapy in 35 fractions given with a shrinking-field technique on Monday through Friday over 7 weeks. Every Monday, they received an injection of cisplatin 40 mg/m2.
Patients in arm A were advised to swish the oral glutamine, which was given as 15 g in a glass of water, for 2 minutes and then swallow it; this was done twice per day throughout treatment. Patients in arm B served as negative or control subjects. Every Tuesday on a once-per-week basis, all patients were evaluated for the onset of mucositis; severity of mucositis; appearance of adverse events like dysphagia, nausea, edema, cough, and pain; use of analgesics to alleviate pain; and insertion of a nasogastric tube to maintain nutrition if they had severe swallowing difficulty. All patients in both arms completed chemoradiation. There were no dropouts in either arm, but a treatment delay occurred in patients who developed mucositis.
RESULTS
A total of 162 patients with squamous cell carcinoma of the head and neck were treated with radical chemoradiation and evaluated for the study. Their baseline profile including demographic parameters, clinicopathologic parameters, and PSs is shown in Table 1. Age, sex, and addictions to smoking and chewing tobacco were similar in both the arms. Diagnosis and treatment parameters like site, degree of differentiation, stage of the tumor, and histopathology of all tumors being squamous cell carcinoma were also similar in both arms. ECOG PSs in both groups of patients were also comparable. Table 2 shows factors that affected the onset of mucositis at the fourth week. Factors taken into account were sex, site, stage, habits, and PS. At the fourth week, 57% of male patients compared with 51% female patients developed mucositis. Sixty-two percent of patients with a primary site in the oropharynx developed mucositis. Sixty-one percent of the patients had disease in stage III compared with 55% who had disease in stage II. Fifty-eight percent of patients smoked and used alcohol. Fifty-seven percent of patients who had an ECOG PS of 1 compared with 51% patients who had an ECOG PS of 0 developed mucositis at the fourth week.
Table 1.
Baseline Profile
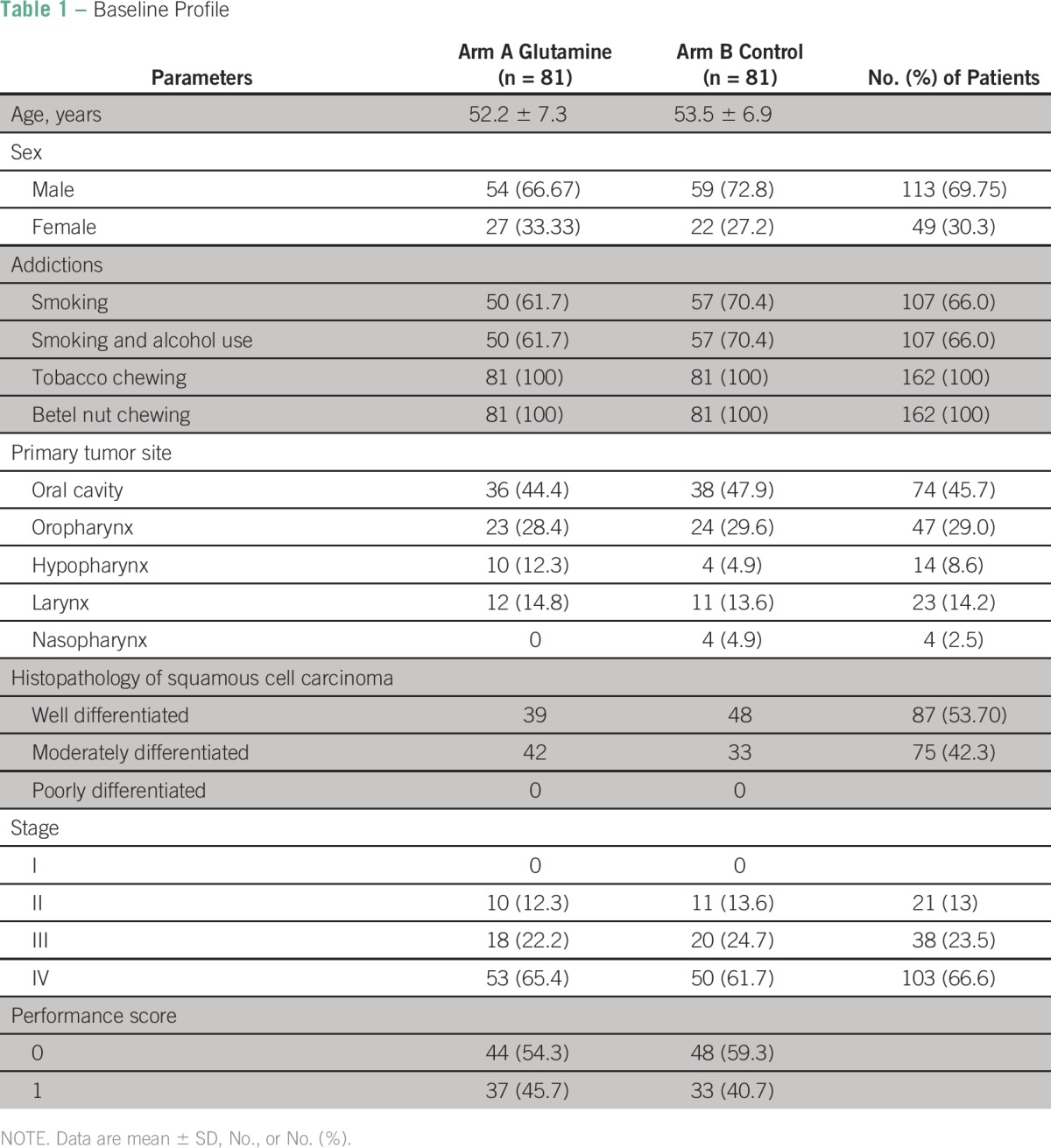
Table 2.
Demographic Factors Affecting Mucositis at the Fourth Week

Table 3 shows the number of chemotherapy cycles received and the duration of treatment in each arm. Fifty-seven percent of patients in the glutamine arm received six cycles of injected cisplatin, whereas none of the patients in the control arm could complete six cycles of chemotherapy. However, a maximum number of four cycles were completed by 56% of patients in the control arm.
Table 3.
No. of Chemotherapy Cycles and Total Duration of Radiotherapy
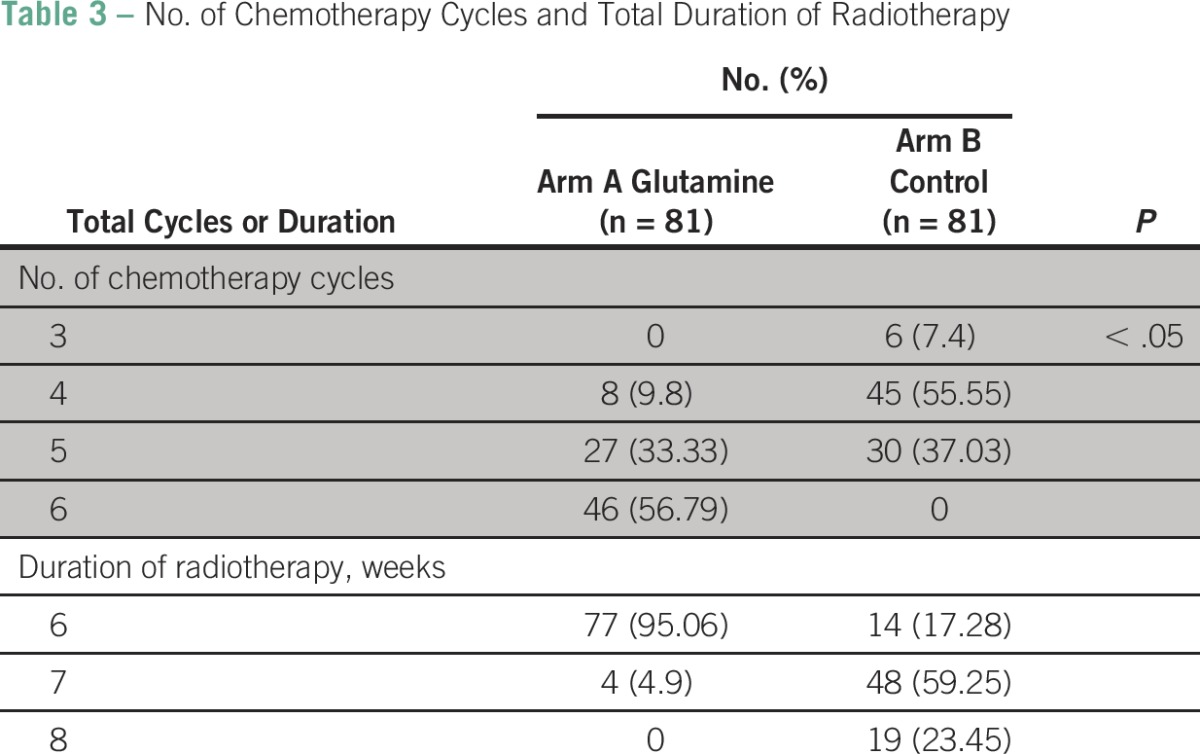
Ninety-five percent of patients in the glutamine arm completed treatment within the stipulated period of 7 weeks, whereas only 17% patients in the control arm completed treatment within 7 weeks. Twenty-four percent of patients in the control arm continued treatment beyond 8 weeks.
The onset and development of mucositis after various weeks of radiation as well as the severity of mucositis in both the arms are shown in Table 4. Fifty-five percent of patients in the glutamine arm developed mucositis at the fifth week, whereas 55% of patients in the control arm developed mucositis at the third week.
Table 4.
Onset and Development of Mucositis at Various Weeks of Radiotherapy
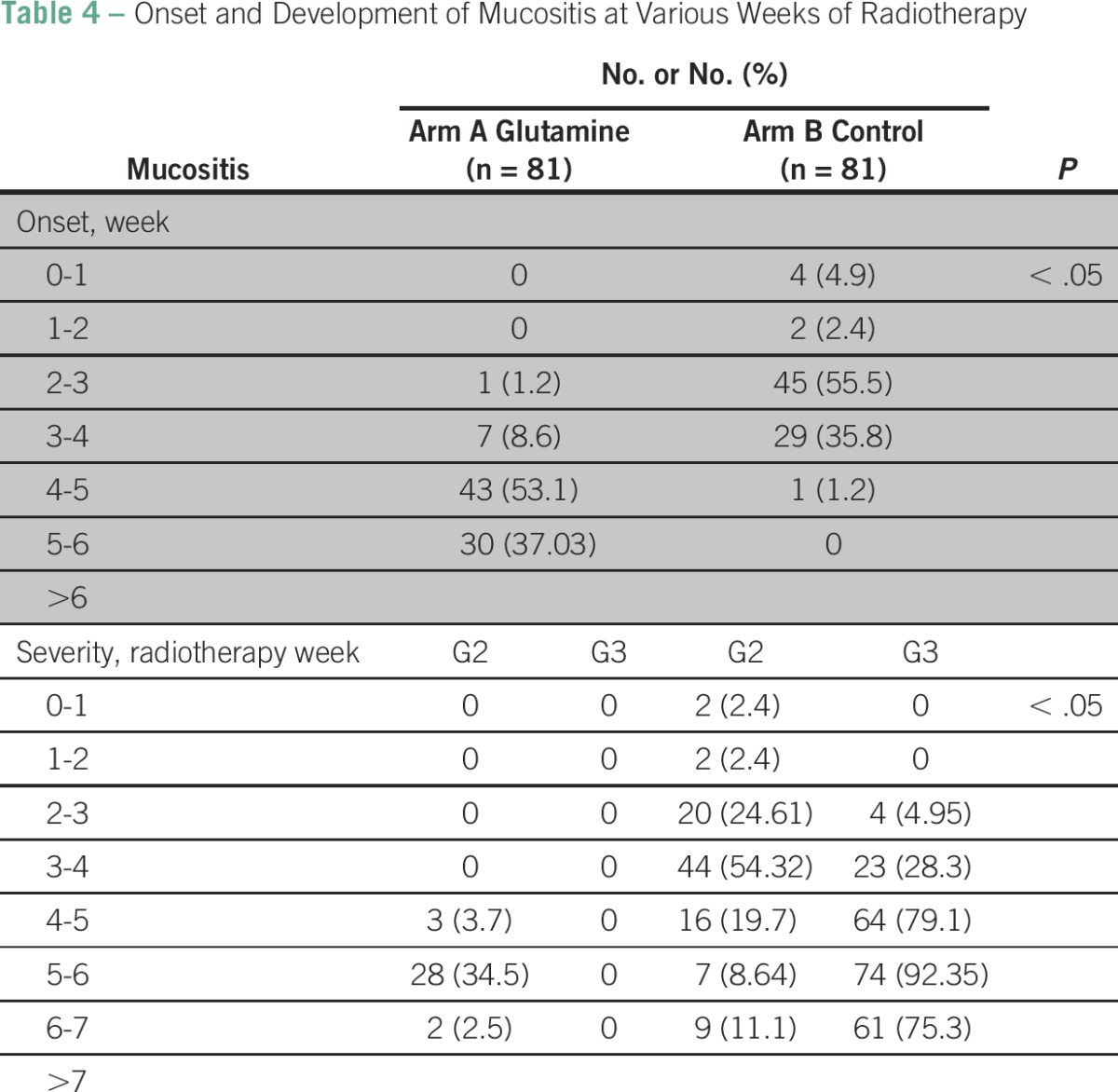
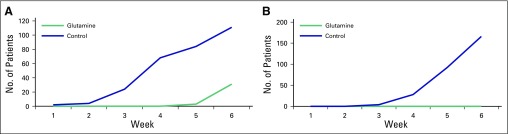
Thirty-five percent of patients in the glutamine arm developed mucositis at the sixth week, whereas 54% patients in the control arm developed G2 mucositis as early as the fourth week. None of the patients in the glutamine arm developed G3 mucositis at any point, whereas 92% of patients in the control arm developed mucositis at the sixth week. Figure 1 demonstrates the cumulative incidence rate of G2 and G3 mucositis in both the arms.
Fig 1.
Cumulative incidence rate of (A) G2 and (B) G3, or severe, mucositis.
The appearance of adverse events like pain, dysphagia, nausea, edema, and cough, as seen in the sixth week is shown in Table 5. Although 68% patients in the glutamine arm developed pain, 100% of patients in the control arm developed pain. Sixty-three percent of patients developed dysphagia in the glutamine arm, whereas 100% of patients developed dysphagia in the control arm.
Table 5.
Adverse Events
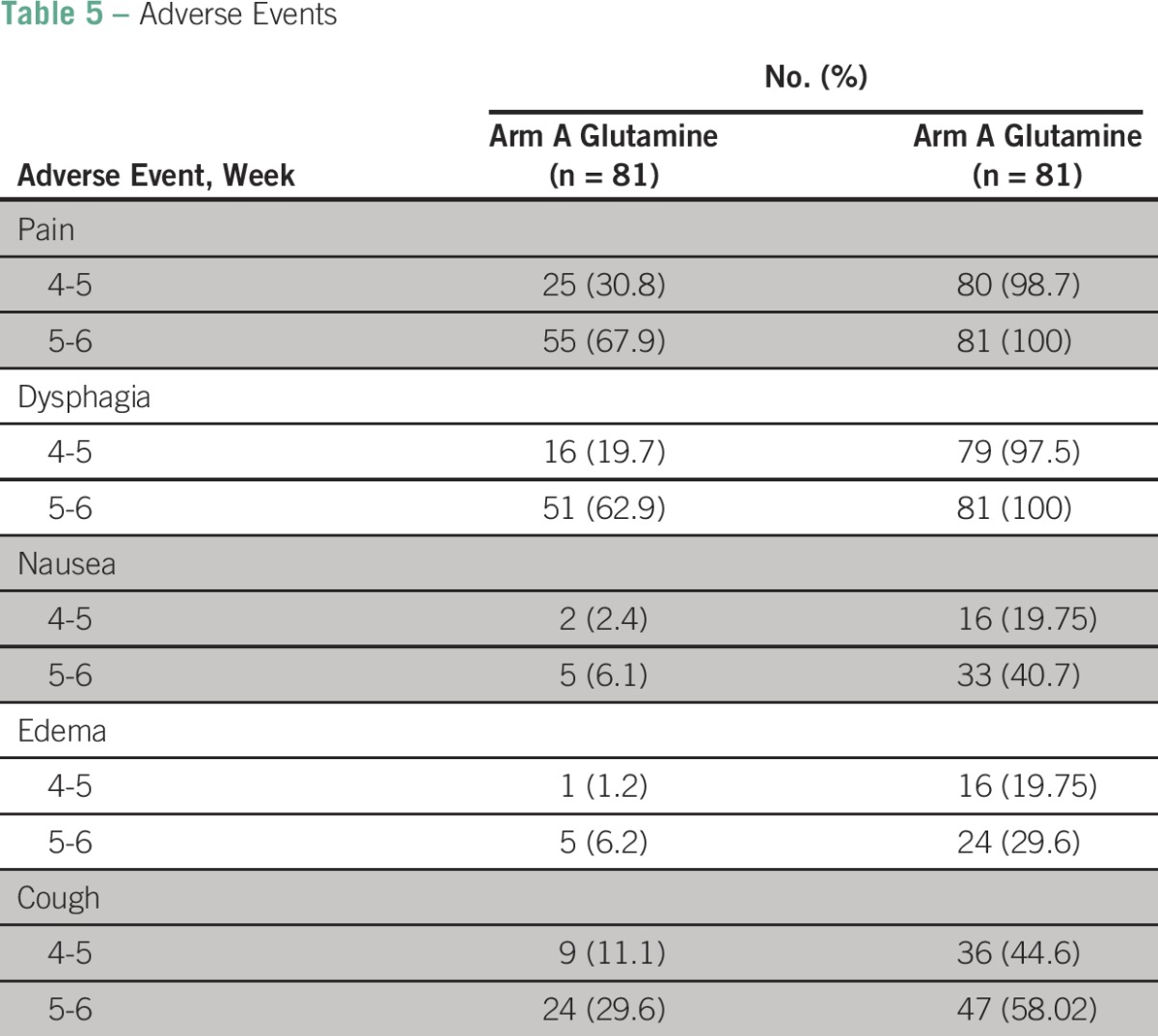
In the sixth week, 6.1% patients developed nausea in the glutamine arm, whereas 40.7% of patients developed nausea in the control arm. Similarly, 6.2% patients developed edema in the glutamine arm, whereas 30% of patients developed edema in the control arm. Thirty percent of patients developed cough in the glutamine arm at the sixth week, whereas 58% of patients developed cough in the control arm.
Onset of pain was assessed by using a patient-reported pain on a numeric rating scale. None of the patients in the glutamine arm developed pain until the end of the third week, and 63% of patients developed mild pain toward the end of the sixth week. On the contrary, 48% of patients developed mild pain as early as the third week, and 59.2% of patients developed severe pain toward the end of the sixth week in the control arm.
Only 18.5% of patients in the glutamine arm required analgesics, whereas 93.8% of patients in the control arm did so. For G1 mucositis, local anesthetics like oxethazaine, aluminum hydroxide, and magnesium hydroxide gel as well as benzalkonium chloride and lidocaine hydrochloride gel were used. For G2 mucositis, oral paracetamol tablets 500 mg were given three times per day. For G3 mucositis, tramadol 100 mg was given twice per day. None of the patients in the glutamine arm required Ryle tube feeding, whereas 8.51% of patients in the control arm required Ryle tube feeding toward the sixth week.
DISCUSSION
Radiotherapy plays a pivotal role in the treatment of head and neck cancers, and concomitant chemotherapy is frequently indicated for locally advanced head and neck cancers. Oral mucositis is by far the most common toxicity of chemoradiation in head and neck cancers. Not only does it cause severe pain and dysfunction but also it causes severe swallowing defects, which lead to frequent treatment interruptions and delay. This, finally, adversely affects the outcome of the disease.
To date, there are no established guidelines or recommendations for the treatment of oral mucositis induced by cancer treatment. Several guidelines including those of the National Comprehensive Cancer Network recommend basic oral care as a standard practice to prevent infections and alleviate mucositis.9 Although basic oral care maintains mucosal health, little evidence suggests that it can reduce the onset and severity of mucositis.10 Agents like N-acetyl cysteine, amifostine, and systemic or topical antimicrobial formulations have been formulated for oral mucositis, though without encouraging results.11 A phase III Radiation Therapy Oncology Group double-blind study revealed that subcutaneous use of granulocyte macrophage colony-stimulating factor failed to reduce oral mucositis.12 The recombinant form of fibroblast growth factor called keratinocyte growth factor, or palifermin, has been studied in a phase III study. In a group of patients with hematologic malignancies who required total-body irradiation with high-dose chemotherapy and blood stem cell support, palifermin reduced the onset and severity of oral mucositis. Therefore, the US Food and Drug Administration has approved palifermin for this particular indication alone.13
Glutamine is a conditionally essential amino acid that is critical for the regulation of protein synthesis, respiratory fueling, cellular energy, and cancer cell-signal pathways. Glutamine reserve is depleted when the body is under great stress or during chemotherapy or radiotherapy. During this time, glutamine supplementation prevents mucosal damage and helps to maintain the metabolic milieu. A study conducted by Huang et al7 demonstrated that oral glutamine supplementation decreases both the onset and severity of mucositis during radiation.
In another study conducted by Savarese et al14, glutamine was combined with an advanced drug-delivery system involving a swish-and-swallow technique. The drug protected the mucosa from damage caused by chemotherapy or radiotherapy. Cockerham et al15 used oral glutamine supplementation in 21 women with metastatic breast cancer who were treated with high-dose paclitaxel and melphalan. Patients who used glutamine experienced a decrease in the severity and duration of oral mucositis.
In this study, we evaluated 162 patients with locally advanced head and neck cancer treated with concomitant chemoradiation. From our analysis, 1.2% of patients in the glutamine arm versus 55.5% of patients in the control arm developed mucositis at the end of the third week. At the sixth week, 34.5% of patients in the glutamine arm developed G2 mucositis, and none of the patients developed G3 mucositis. However, in the control arm, 54.32% of patients had G2 mucositis as early as the fourth week, and 92.35% of patients had G3 mucositis by the sixth week.
Rates of adverse events like pain, dysphagia, nausea, edema, and cough were significantly higher in the control arm than in the glutamine arm. Pain was assessed by the Patient Reported Pain Scale (NRS -11). None of the patients in the glutamine arm developed pain until the third week, and 63.4% of patients developed mild pain in the sixth week. Forty-eight percent of patients in the control arm developed mild pain at the third week, and 59% of patients developed severe pain from the fifth week onward. The use of analgesics in was significantly higher in the control arm than in the glutamine arm. None of the patients in the glutamine arm required Ryle tube feeding, whereas 8.5% of patients needed it at the sixth week.
In conclusion, all patients with head and neck cancer who received chemoradiation developed mucositis. However, the onset of mucositis as well as the severity of mucositis in patients receiving glutamine supplementation was significantly delayed. None of the patients receiving glutamine developed G3 mucositis during treatment. Hence, the current study emphasizes the use of oral glutamine supplementation as a feasible and affordable option for treatment of oral mucositis in patients with head and neck cancers who are receiving chemoradiation.
Footnotes
Authors' disclosures of potential conflicts of interest and contributions are found at the end of this article
AUTHOR CONTRIBUTIONS
Conception and design: Lucy Pattanayak, Sumita Mohanty
Administrative support: Lucy Pattanayak
Collection and assembly of data: Lucy Pattanayak, Niharika Panda, Sagarika Samantaray
Data analysis and interpretation: Lucy Pattanayak, Manoj Kumar Dash
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Management of Chemoradiation-Induced Mucositis in Head and Neck Cancers with Oral Glutamine
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Lucy Pattanayak
No relationship to disclose
Niharika Panda
No relationship to disclose
Manoj Kumar Dash
No relationship to disclose
Sumita Mohanty
No relationship to disclose
Sagarika Samantaray
No relationship to disclose
REFERENCES
- 1.Jamal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Dose AM. The symptom experience of mucositis, stomatitis, and xerostomia. Semin Oncol Nurs. 1995;11:248–255. doi: 10.1016/s0749-2081(05)80005-1. [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Jansma J, Spijkervet FK, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 4.Newsholme P, Lima MMR, Procopio J, et al. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36:153–163. doi: 10.1590/s0100-879x2003000200002. [DOI] [PubMed] [Google Scholar]
- 5. University of Maryland Medical Center: Glutamine. Medical Reference Guide. https://umm.edu/health/medical/altmed/supplement/glutamine.
- 6.May PE, Barber A, D’Olimpio JT, et al. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479. doi: 10.1016/s0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 7.Huang EY, Leung SW, Wang CJ, et al. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535–539. doi: 10.1016/s0360-3016(99)00402-2. [DOI] [PubMed] [Google Scholar]
- 8.Silverman S., Jr Diagnosis and management of oral mucositis. J Support Oncol. 2007;5:13–21. (2 suppl 1) [PubMed] [Google Scholar]
- 9.Keefe DM, Schubert MM, Elting LS, et al. Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–831. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 10.Sonis ST, Elting LS, Keefe D, et al. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer, International Society for Oral Oncology Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. (9 suppl) [DOI] [PubMed] [Google Scholar]
- 11.Symonds RP, McIlroy P, Khorrami J, et al. The reduction of radiation mucositis by selective decontamination antibiotic pastilles: A placebo-controlled double-blind trial. Br J Cancer. 1996;74:312–317. doi: 10.1038/bjc.1996.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rovirosa A, Ferre J, Biete A. Granulocyte macrophage-colony-stimulating factor mouthwashes heal oral ulcers during head and neck radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:747–754. doi: 10.1016/s0360-3016(98)00109-6. [DOI] [PubMed] [Google Scholar]
- 13.Antunes HS, de Azevedo AM, da Silva Bouzas LF, et al. Low-power laser in the prevention of induced oral mucositis in bone marrow transplantation patients: A randomized trial. Blood. 2007;109:2250–2255. doi: 10.1182/blood-2006-07-035022. [DOI] [PubMed] [Google Scholar]
- 14.Savarese DM, Savy G, Vahdat L, et al. Prevention of chemotherapy and radiation toxicity with oral glutamine. Cancer Treat Rev. 2003;29:501–513. doi: 10.1016/s0305-7372(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 15.Cockerham MB, Weinberger BB, Lerchie SB. Oral glutamine for the prevention of oral mucositis associated with high dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann Pharmacother. 2000;34:300–303. doi: 10.1345/aph.19168. [DOI] [PubMed] [Google Scholar]