Abstract
Objective
The Veterans Affairs Rheumatoid Arthritis (VARA) registry and the VA Pharmacy Benefits Management (PBM) database were linked to determine the association of methotrexate (MTX) adherence with RA disease activity.
Methods
For each patient, the medication possession ratio (MPR) was calculated for the first episode of MTX exposure of ≥12 weeks duration for both new and established MTX users. High MTX adherence was defined as an MPR ≥0.80 and low MTX adherence <0.80. For each patient, the mean DAS28, ESR, and CRP observed during registry follow-up were compared in high versus low adherence groups.
Results
In 455 RA patients, the prescribed doses of MTX (16±4mg versus 16±4mg, p=0.6) were similar in high adherence patients (n=370) in comparison to low adherence patients (n=85). However, the actual observed MTX doses taken by patients were significantly higher in the high adherence group (16±5mg versus 11±3mg, p<0.001). DAS28 (3.6±1.2 versus 3.9±1.5, p<0.02), ESR (24±18 versus 29±24, p= 0.05) and CRP (1.2±1.3 versus 1.6±1.5, p<0.03) were lower in the high adherence group compared to those with low MTX adherence. These variances were not explained by differences in baseline demographic features, concurrent treatments, or whether MTX was initiated before or after VARA enrollment.
Conclusion
High MTX adherence was associated with improved clinical outcomes in RA patients treated with MTX. Adjustment for potential confounders did not alter the estimated effect of adherence. These results demonstrate the advantages of being able to merge clinical observations with pharmacy databases to evaluate anti-rheumatic drugs in clinical practice.
Rheumatoid arthritis (RA) is associated with significant morbidity and increased mortality (1, 2). Disease modifying anti-rheumatic drugs (DMARDs) have dramatically improved the management of RA patients (3–6). While numerous controlled clinical trials have demonstrated that methotrexate (MTX) and other DMARDs are effective in RA, controlled clinical trials are generally limited to highly selected patients often with moderate to severe disease activity and limited co-morbidities. Surveys of rheumatology clinic populations estimate that only 5–16% of patients with RA would qualify for participation in these clinical trials (7). To understand how controlled clinical trials’ estimates of DMARD effectiveness and safety translate into the clinical setting, it is critical to evaluate DMARD performance in observational studies of routine clinical practice. This work employed the detailed information provided on the medication history of US veterans collected through the Veterans Affairs (VA) Pharmacy Benefits Management (PBM) (8, 9). PBM not only lists medication dispensed, but also captures information on the specifics of each prescription including the number of tablets dispensed, expected duration of each prescription and prescribing information (“sig”). The potential to combine these detailed administrative pharmacy data sets with comprehensive clinical outcome measures provides an opportunity to evaluate DMARD therapy of RA in a “day-to-day” clinical practice setting.
The Veterans Affairs Rheumatoid Arthritis (VARA) registry prospectively records data from clinical encounters (1, 10) and has recently been merged with Veterans Affairs (VA) administrative pharmacy data collected in a centralized Pharmacy Benefits Management (PBM) program which allows for the evaluation of the use and adherence to DMARDs in clinical practice. This study was undertaken to examine the following: first, whether adherence to MTX is inversely proportional to RA disease activity measures; and second, whether other factors such as demographic and clinical diseases parameters confound this relationship. Furthermore, the study demonstrates the potential for combining data from disease registries and administrative databases such as PBM to evaluate the clinical issues in a “day-to-day” community clinical practice setting.
PATIENTS AND METHODS
Patients
The VARA multicenter observational cohort began enrollment in January 2003 and is described elsewhere (1, 10). This analysis evaluated patients enrolled through August 31, 2009 and included observation up to this date. At the time of this analysis, PBM data were only available from the Dallas, Denver, Jackson, Omaha, Salt Lake City, and Washington, DC VA medical centers, limiting the analysis to these six VARA sites. Using the PBM pharmacy database, patients were required to have recorded treatment with MTX (i.e. one or more dispensed MTX prescriptions for oral or subcutaneous MTX) prior to August 31, 2009. This analysis was limited to the first course of MTX therapy within the VA, and patients could have been either new or established (i.e. prevalent) MTX users. In addition, the analysis required at least one Disease Activity Score with 28 joint assessment (DAS28) (11) within the VARA registry occurring on or after the 90th day of MTX therapy during this first MTX course – an interval after which MTX benefit has been observed in clinical trials (4, 6). Patients contributed data to analyses specific to two of the three following groups: 1) all patients receiving MTX during VARA observation (full cohort); 2) patients initiating MTX more than 30 days prior to VARA enrollment but continuing therapy beyond enrollment (established MTX cohort); and 3) patients initiating MTX within 30 days of VARA enrollment or after VARA enrollment (incident MTX cohort).
Determination of MTX exposure
The details of MTX therapy were determined from PBM outpatient dispensing records for each patient. PBM includes the following information on each prescription including: dispense date, number of days supply, units dispensed (total number of tablets or liquid vials), unit dose, and the directions for use (“sigs”) (8). Each single prescription of MTX was defined as a dispensing episode. A drug course was defined as a period of continuous MTX treatment consisting of one or more dispensing episodes as described below and in the appendix.
Calculation of the duration and dose of MTX for each dispensing episode
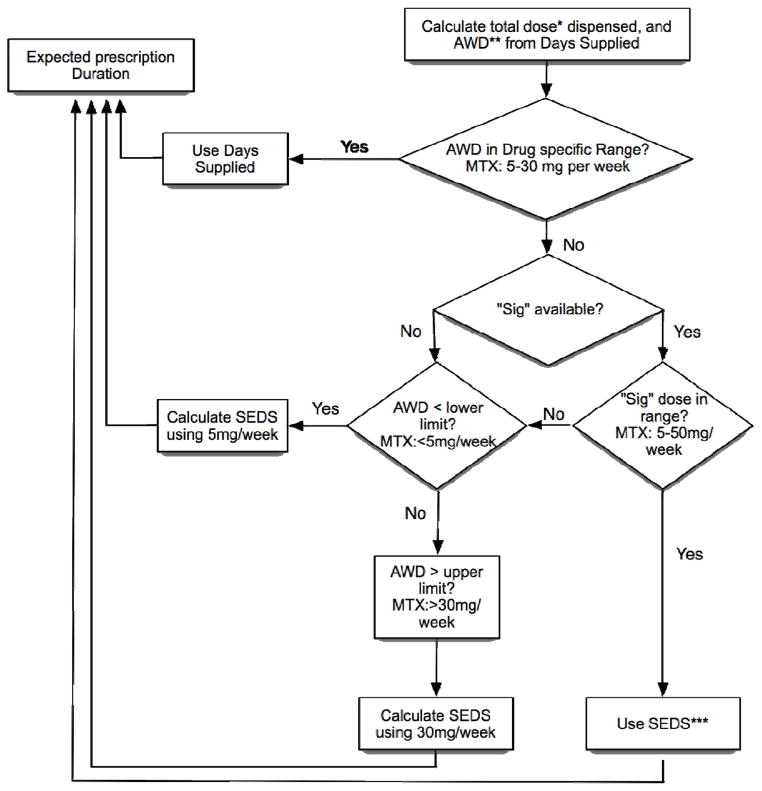
The dispense date and expected duration of each MTX prescription was needed to estimate the duration of each dispensing episode. An algorithm was used to calculate the expected duration of each dispensed prescription (Figure 1). The average weekly dose (AWD) was calculated from the days supply, unit strength and number of units dispensed. If the AWD was within the range of 5 to 30 mg per week then the number of days supply information was assumed to be accurate and reported as the expected duration of that prescription. In order to correct for rare outlier prescriptions with improbable weekly doses (e.g. greater than 50mg/week) or durations (e.g. of over 6 months of prescribed medication at a single dispensing of MTX), we employed the dispensing information in this algorithm. The application of this algorithm affected 9 (2%) of the 445 MTX courses, in which we incorporated dosing directions (i.e. “sigs”) to estimate expected dosages/durations. The lead author (G.W.C) reviewed all unique doses and “sig” combinations and from these, calculated an estimated “sig weekly dose”. If the AWD was below 5 or above 30mg, then the total amount of drug dispensed (number of units dispensed multiplied by the unit strength) was divided by the “sig weekly dose” and multiplied by seven days (to convert weeks into days) to obtain the sig estimated days supply (SEDS). The SEDS was then used to populate the expected duration. See appendix for examples and further illustration of the application of this algorithm.
Figure 1.
Algorithm for determining expected duration of each medication dispensing. The term “sig” refers to the physician prescribing instructions with the individual prescription.
*Total dose in mg dispensed = number of units (tablets, vials) × unit strength
**Average Weekly Dose (AWD) = total dose dispensed ÷ days supply
***Sig estimated days supply (SEDS) = total dose dispensed ÷ sig weekly dose × 7 days
Calculation of gaps in MTX medication dispensing and drug course
The dispensing date and expected duration were used to calculate the expected end date for each prescription dispensed. Differences between the expected end of a prescription and the dispensing date of the subsequent medication refill were considered gaps in therapy. A patient was defined as receiving continuous MTX therapy if there was less than a 90 day gap between refills of MTX which has been used in other analyses of adherence (12–14). A course ended when a 90-day gap occurred as defined above. The duration of the course was defined from the date of the first MTX dispensed prescription until the expected end date for the last prescription before a 90 day gap or end of the observation period. This study was limited to the first course of MTX identified from PBM data for each patient, whether established (beginning before VARA enrollment) or incident (occurring on or after VARA enrollment).
Calculation of MTX adherence
For the first course of MTX, we calculated a prescribed cumulative dose, course duration, average prescribed dose, prescribed duration average observed dose, and medication possession ratio (MPR). The prescribed duration of drug exposure during a course was defined as the sum of the expected durations for each prescription dispensing within a course. The average dose prescribed was calculated as the total dose dispensed divided by the prescribed duration for a course. The average dose observed was the total dose dispensed divided by the total course durations, which thereby takes into account the potential for less than full adherence. The MPR was calculated as the number of prescribed days of MTX during a course divided by the total days duration of a course (12, 14).
VARA Outcome variables
The primary outcome variable was DAS28 (11). An average DAS28 was calculated for each subject by including all DAS28s from 90 days after the MTX dispensing date (i.e. impact date) until the end date of the first MTX course. Secondary outcome variables evaluated were: tender joint count, swollen joint count, patient global disease assessment (100 mm scale), patient pain (10 point scale) physician global disease assessment (100 mm scale), Multi-dimensional Health Assessment Questionnaire (MDHAQ) (15, 16), Westergren erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Radiographic outcomes were not measured.
Covariates
At enrollment into the VARA registry, we collected: presence of RA classification criteria (e.g. nodules and radiographic erosions—based on medical documentation), medical history, smoking status (never, former, or current), sociodemographics (education, race/ethnicity, age, gender), duration of RA since initial diagnosis, and prior and current use of DMARDs (both biologic and non-biologic). A serum sample collected on enrollment was evaluated for rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (aCCP) (17). RF was determined by nephelometry (Siemens Healthcare Diagnostics, Munich, Germany) and was considered positive at levels ≥ 15 IU/ml. Standardized aCCP (IgG) was measured using a commercially available second generation ELISA (Diastat, Axis-Shield Diagnostics Ltd., Dundee, Scotland, UK) and was considered positive at levels ≥ 5 U/ml. Charlson-Deyo comorbidity scores were calculated for all subjects based on aggregated VA national administrative data (18). For the incident MTX user cohort, we also adjusted for baseline DAS28, although this was not possible for the full and established cohorts, as MTX treatment for these patients was initiated prior to VARA enrollment.
Statistical analyses
All statistical analyses were carried out using Stata v11 (StataCorp, College Station, TX). Descriptive statistics for continuous variables are summarized as means and standard deviations (SD); categorical variables are presented as percentages. Differences between high and low adherence groups were analyzed using t-tests and large sample binomial tests of proportions. To determine whether covariates were responsible for any differences in mean DAS28 between high and low adherence groups, multivariable linear regression was used. For all three cohorts (full, incident MTX, established MTX), we constructed multivariable models that included demographic characteristics and then demographic characteristics plus DMARD/biologic exposures (i.e. Full model). Because over-specification was possible, we generated propensity scores by modeling the probability of having high adherence, and used the predicted probabilities as continuous and categorical adjustment variables. Since the findings between the propensity score and standard regression models were nearly identical, only results of standard regression are shown.
Human Subjects Review
The registry has received Institutional Review Board approval at each site. This study was approved by the VARA Scientific and Ethics Advisory Committee for the VARA registry.
RESULTS
Patient characteristics
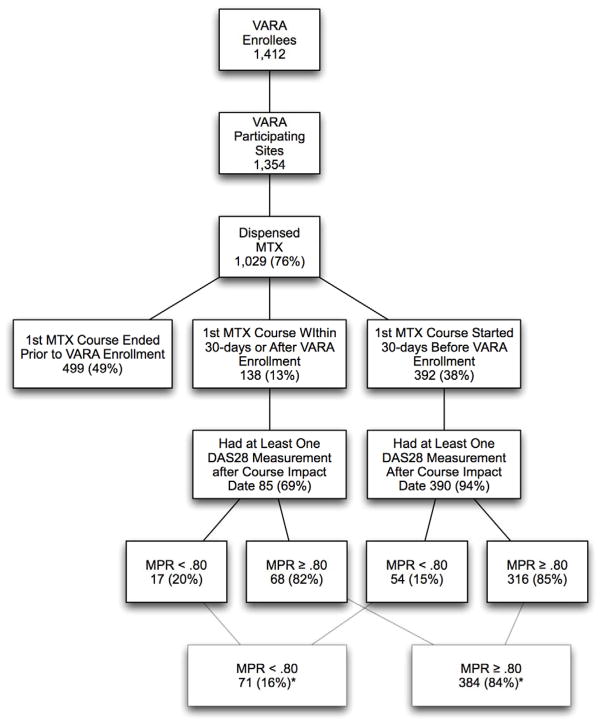
There were 1,412 enrollees in VARA prior to August 31, 2009 of which 1,354 had available PBM data (Figure 2). Of these 1,354 eligible subjects, 1,029 (76%) were dispensed at least one MTX prescription. MTX courses for 499 veterans ended before VARA enrollment making these veterans ineligible for the study. Of the 530 VARA enrollees receiving MTX, 455 veterans had at least one DAS28 more than 90 days following treatment initiation (31.5% of all VARA enrollees). In these 455 subjects, 71 (16%) had their first observed MTX course on or after their VARA enrollment date (i.e., incident MTX course) and 384 (84%) initiated their first MTX course prior to VARA enrollment (i.e., established MTX course). Of the 455 subjects, 85 (19%) had a MPR of less than 0.80 and 370 (81%) had an MPR greater than or equal to 0.80 calculated after a mean duration of follow-up of 42.7±31.2 months.
Figure 2.
Veterans Affairs Rheumatoid Arthritis (VARA) registry population with full breakdown subjects fulfilling inclusion criteria and the fraction of subjects with high (medication possession ratio (MPR) ≥0.80) and low adherence (MPR <080) to methotrexate (MTX).
*Percent of veterans from the 455 who met inclusion criteria
Patient characteristics are summarized in Table 1. Patients were predominantly men (92% male; mean age 64±11 years) and had moderate to high levels of disease activity at the time of VARA enrollment reflected by a mean DAS28 of 3.9 ±1.6. There were no differences in age, disease duration at VARA enrollment, smoking status, autoantibody status, or the use of concurrent therapies based on high versus low MTX adherence overall or in groups defined by incident or established MTX use (Table 1). Among incident MTX users, men and Caucasian race were more likely to be in the high adherence versus low adherence group. African-American patients more likely to be in the low adherence group versus the high adherence group for all cohorts. Higher adherence to MTX was also associated with lower disease activity at enrollment based on DAS28, tender and swollen joint counts, and ESR overall and in the cohort defined by established MTX use. These baseline differences were not seen in the incident user cohort.
Table 1.
Clinical features of cohort.
| Full Cohort | Incident User Cohort | Established User Cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MPR<80% (n=71) | MPR ≥80% (n=384) | p-value | MPR<80% (n=17) | MPR ≥80% (n=68) | p-value | MPR<80% (n=54) | MPR ≥80% (n=316) | p-value | |
| Demographics at time of VARA enrollment | |||||||||
| Age at enrollment, years mean(SD) | 62 (12) | 64 (11) | 0.17 | 63 (12) | 62 (12) | 0.64 | 62 (12) | 65 (11) | 0.08 |
| Disease duration, years | 9.4 (10.3) | 9.4 (11.7) | 0.94 | 6.5(7.5) | 9.4 (12) | 0.5 | 10.2 (11) | 9.5 (128) | 0.66 |
| Male n (%) | 63 (89%) | 355 (92%) | 0.37 | 14 (83%) | 66 (97%) | 0.02 | 49 (91%) | 289(91%) | 0.86 |
| Race/ethnicity | |||||||||
| Caucasian | 51 (72%) | 316 (83%) | 0.05 | 11 (65%) | 58 (85%) | 0.05 | 40 (74%) | 258 (82%) | 0.19 |
| African-American | 18 (25%) | 49 (13%) | 0.01 | 5 (29%) | 9 (13%) | 0.11 | 13 (24%) | 40 (13%) | 0.03 |
| Hispanic | 1 (1%) | 11 (1%) | 0.44 | 1 (6%) | 1 (1%) | 0.50 | 0 | 10 (3%) | 0.18 |
| Other/not specified | 1 (1%) | 8 (2%) | 0.44 | 0 | 0 | - | 1 (2%) | 8 (3%) | 0.35 |
| Ever tobacco user n (%) | 50 (70%) | 300 (78%) | 0.07 | 12 (71%) | 60 (88%) | 0.07 | 40 (74%) | 236 (75%) | 0.38 |
| Enrollment clinical measures- Mean (S.D.) | |||||||||
| DAS28 (0–28) | 4.4 (1.8) | 3.8 (1.6) | 0.02 | 4.9 (1.6) | 4.6 (1.7) | 0.45 | 4.2 (1.8) | 3.7 (1.5) | 0.03 |
| Tender joints (0–28) | 7.1 (8.0) | 5.0 (6.7) | 0.02 | 7.4 (7.6) | 7.6 (8.0) | 0.90 | 7.0 (8.2) | 4.7 (6.3) | <0.01 |
| Swollen joints (0–28) | 6.7 (7.4) | 4.2 (5.4) | <0.01 | 9.0 (7.6) | 6.2 (6.3) | 0.12 | 6.0 (7.8) | 3.8 (5.2) | <0.01 |
| MDHAQ (0–3.0) | 0.9 (0.7) | 0.9 (0.6) | 0.98 | 1.0 (0.7) | 1.0 (0.5) | 0.79 | 0.9 (0.7) | 0.9 (0.6) | 0.99 |
| Pain (0–10) | 4.7 (3.2) | 4.4 (2.8) | 0.42 | 4.6 (3.3) | 4.5 (2.8) | 0.90 | 4.7 (3.2) | 4.3 (2.8) | 0.41 |
| Patient global (0–100) | 41 (29) | 40 (26) | 0.88 | 46 (20) | 46 (26) | 0.99 | 34 (24) | 35 (22) | 0.76 |
| ESR, mm/hr | 32 (27) | 24 (21) | 0.02 | 36 (24) | 27.1 (21) | 0.15 | 30.3 (28.1) | 24.5 (21.5) | 0.04 |
| Rheumatoid factor positive | 61 (86%) | 298 (78%) | 0.13 | 12 (715%) | 55 (81%) | 0.35 | 49 (91%) | 243 (77%) | 0.02 |
| aCCP positive | 53 (75%) | 290 (76%) | 0.85 | 13 (76%) | 54 (79%) | 0.97 | 04 (74%) | 236 (75%) | 0.90 |
| Deyo Index | 1.6 (2.1) | 1.5 (1.8) | 0.67 | 1.8 (1.7) | 1.2 (1.6) | 0.20 | 1.6 (2.2) | 1.6 (1.8) | 0.94 |
| Concurrent therapy with MTX – n (%) | |||||||||
| Traditional DMARDs † | 39 (55%) | 226 (59%) | 0.54 | 7 (41%) | 33 (49%) | 0.59 | 32 (59%) | 193 (61%) | 0.80 |
| TNF alpha blockers ‡ | 29 (41%) | 143 (37%) | 0.56 | 7 (41%) | 12 (18%) | 0.04 | 22 (41%) | 131 (41%) | 0.92 |
| Other Biologic DMARSs # | 1 (1%) | 12 (3%) | 0.43 | 1 (6%) | 0 (0%) | 0.00 | 0 (0%) | 12 (4%) | 0.16 |
Traditional DMARDs included auranofin, azathioprine, cyclosporine, injectable gold, hydroxychloroquine, leflunomide, minocycline, sulfasalazine.
TNF blockers included adalimumab, etanercept, and infliximab
Other Biologic agents were abatacept and rituximab
= P <0.05 and marked in bold
Methotrexate therapy and adherence
Prescribed weekly MTX doses were similar in high and low adherence groups across cohorts (Table 2). However, observed weekly doses and duration of use were both greater in the high adherence group compared to the low adherence group. These differences were statistically significant in the overall group and in the group defined by established MTX use.
Table 2.
Summary of Drug Course Information - Mean (S.D.)
| Course | Full Cohort | Incident User Cohort | Established User Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MPR<80% (n=71) | MPR ≥80% (n=384) | p-value | MPR<80% (n=17) | MPR ≥80% (n=68) | p-value | MPR<80% (n=54) | MPR ≥80% (n=316) | p-value | |
| Average prescribed weekly dose, (mg/wk)† | 16 (4) | 16 (4) | 0.6 | 15 (3) | 16(4) | 0.44 | 16 (4) | 16 (4) | 0.95 |
| Average observed weekly dose, (mg/wk)‡ | 11 (3) | 16 (5) | <0.001 | 10 (2) | 16 (6) | <0.001 | 12 (3) | 16 (5) | <0.001 |
| Total Prescribed duration, days¥ | 654 (599) | 1345 (975) | <0.001 | 350 (320) | 605 (382) | 0.02 | 749 (634) | 1504 (1395) | <0.01 |
| Observed Course Duration, days¶ | 902 (783) | 1370 (980) | <0.001 | 497 (410) | 592 (374) | 0.36 | 1029 (803) | 1536 (14727) | <0.01 |
| Course total dose, (mg)§ | 1492 (1425) | 3154 (2490) | <0.001 | 768 (770) | 1421 (1079) | 0.02 | 1720 (1307) | 3527 (3245) | <0.01 |
= P <0.05 and marked in bold
Average prescribed dose is the course total dose (mg) during the course divided by the total prescribed duration (weeks).
Average observed dose is the course total dose (mg) during the course divided by the total course duration.
Total prescribed duration was the sum of all the expected prescription durations for each prescription over the course.
The observed course duration was the time from the date of the first prescription to the end of the final prescription in a course.
The course total dose was the total number of mgs dispensed with each prescription over the course.
Clinical outcomes during methotrexate therapy
Mean (± SD) disease activity values during VARA follow-up are summarized in Table 3. In these unadjusted analyses high MTX adherence was associated with a significantly lower mean DAS28 score compared to low MTX adherence across study groups. Higher adherence to MTX was also associated with improvements in secondary outcomes including ESR (borderline in the established use cohort), CRP, tender, and swollen joints in the overall group and in the established use group.
Table 3.
Clinical Outcome Measures in Full, Incident, and Established MTX User Cohorts - Mean (S.D.)
| Course | Full Cohort | Incident User Cohort | Established User Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MPR<80% (n=71) | MPR ≥80% (n=384) | p-value | MPR<80% (n=17) | MPR ≥80% (n=68) | p-value | MPR<80% (n=54) | MPR ≥80% (n=316) | p-value | |
| Primary Outcome Measure | |||||||||
| DAS28 | 3.9 (1.5) | 3.6 (1.2) | 0.02 | 4.2 (1.4) | 3.6 (1.1) | 0.10 | 3.8 (1.5) | 3.5 (1.2) | 0.09 |
| Secondary Outcome Measures | |||||||||
| Erythrocyte sedimentation rate mm/hr | 29 (24) | 24 (18) | 0.05 | 35.2 (26) | 25 (17) | 0.05* | 27.4 (23.0) | 24.2 (18.4) | 0.26 |
| C-reactive Protein mg/dl | 1.6 (1.5) | 1.2 (1.3) | 0.03 | 1.8 (1.6) | 1.4 (1.9) | 0.51 | 1.6 (1.5) | 1.2 (1.2) | 0.03 |
| Tender Joints (0–28) | 5.4 (6.2) | 3.6 (4.5) | <0.01 | 4.9 (4.7) | 3.6 (4.4) | 0.28 | 5.6 (6.71) | 3.7 (4.6) | 0.02 |
| Swollen Joints (0–28) | 4.3 (4.8) | 3.2 (3.3) | 0.02 | 4.6 (4.6) | 3.2 (3.5) | 0.19 | 4.3 (4.9) | 3.2 (3.2) | 0.03 |
| Health Assessment Questionnaire (0–3.0) | 0.9 (0.56) | 0.9 (0.52) | 0.93 | 1.0 (0.45) | 0.9 (0.54) | 0.66 | 0.9 (0.6) | 0.9 (0.9) | 0.75 |
| Visual Analogue Scale | 38 (24) | 39 (20) | 0.63 | 37 (21) | 32 (19) | 0.27 | 35 (28) | 39 (20) | 0.22 |
| Patient Global | 37 (24) | 39 (20) | 0.30 | 41 (23) | 41 (21) | 0.975 | 34 (24) | 33 (17) | 0.40 |
| Pain (0–10) | 4.4 (2.7) | 4.2 (2.2) | 0.71 | 3.8 (2.9) | 4.4 (2.1) | 0.33 | 4.5 (2.6) | 4.21 (2.2) | 0.34 |
= P <0.05 and marked in bold
Multivariable Models for DAS28
Multivariable linear regression was then used to examine whether the association of higher MTX adherence with improved DAS28 scores was independent of potentially confounding variables (Table 4). Differences in mean DAS28 based on MTX adherence remained statistically significant across cohorts after multivariable adjustments, although this difference was greater in magnitude among incident MTX users compared to established users. An analysis limited to male subjects produced very similar results to those presented. The number of female subjects was too small for a separate analysis.
Table 4.
Mean difference in DAS28 based on adherence to MTX and other covariates; calculated using univariate (Crude) and multivariable linear regression.
| Covariates | Full User Cohort | Incident User Cohort | Established User Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude (95%CI) |
Demographic Adjusted |
Full Model | Crude (95%CI) |
Demographic Adjusted |
Full Model | Crude (95%CI) | Demographic Adjusted |
Full Model | |
| MPR≥80% | −0.34 (−0.68, −0.06)* |
−0.35 (−0.66, −0.04)* |
−0.37 (−0.67, −0.07)* |
−0.54 (−1.18, 0.11) |
−0.56 (−1.2, 0.08) |
−0.40 (−1.11, 0.30) |
−0.38 (−0.67, −0.05)* |
−0.29 (−0.65, −0.07) |
−0.37 (−0.72, −0.02)* |
| Age at enrollment | 0 (−0.01, 0.01) | 0.01 (0.00, 0.02) | −0.01 (−0.04, 0.01) | 0.01 (−0.03, 0.01) | 0.01 (−0.01, 0.02) | 0.01* (0.00, 0.02) | |||
| Disease duration Q2 | −0.12 (−0.43, 0.20) | −0.36 (−0.68, −0.04) | −0.13 (−0.04, 0.01) | −0.15 (−0.88, 0.59) | −0.05 (−0.41, 0.31) | −0.17 (−0.52, 0.17) | |||
| Disease duration Q3 | 0.08 (−0.24, 0.40) | −0.13 (−0.45, 0.20) | 0.13 (−0.59, 0.85) | −0.01 (−0.75,0.74) | 0.03 (−0.38, 0.33) | −0.17 (−0.52, 0.17) | |||
| Disease duration Q4 | −0.11 (−0.43, 0.20) | −0.24 (−0.56, 0.07) | 0.33 (−0.4, 1.08) | 0.26 (−0.50, 1.03) | −0.07 (−0.42, 0.29) | −0.11 (−0.46, 0.22) | |||
| Male | −0.16 (−0.61, 0.28) | −0.18 (−0.61, 0.25) | 0.88 (−0.31, 2.07) | 0.68 (−0.55, 1.91) | −0.40 (−0.88, 0.09) | −0.41 (−0.87, 0.06) | |||
| African American Race | 0.08 (−0.24, 0.40) | 0.09 (−0.22, 0.41) | .10 (−0.59, 0.79) | −0.17 (−0.53, 0.87) | 0.02 (−0.34, 0.39) | 0.1 (−0.34, 0.36) | |||
| Ever tobacco user | 0.31 (0.03, 0.59)* | 0.30 (0.03,0.57)* | 0.08 (−0.66, 0.82) | 0.08 (−0.68, 0.85) | 0.32 (0.02, 0.62)* | 0.32 (0.03, 0.61)* | |||
| Rheumatoid factor positive | 0.27 (−0.04, 0.59) | 0.26 (−0.05,0.50) | −0.43 (−1.19, 0.34) | −0.45 (−1.21, 0.32) | 0.42 (0.07, 0.77)* | 0.40* (0.02, 0.61) | |||
| ACCP positive | 0.22 (−0.08, 0.52) | 0.21 (−0.08,0.50) | 0.36 (−0.39, 1.11) | −0.34 (−0.41, 1.10) | 0.16 (−0.16, 0.49) | 0.14 (−0.17, 0.46) | |||
| BaselineDAS28 | -------- | -------- | 0.28 (0.52,5.1)* | 0.26 (0.21,4.89)* | -------- | -------- | |||
| Concurrent therapy with MTX | |||||||||
| Traditional DMARDs | 0.28 (0.06, 0.50)* | 0.07 (−0.57, 0.72) | 0.43 (0.17, 0.69)* | ||||||
| TNF alpha blockers | 0.39 (0.15, 0.63)* | −1.76 (−0.75, 4.2) | 1.18 (0.45, 1.91)* | ||||||
| Other Biologic DMARDs | 1.37 (0.62,2.02)* | 1.73 (−0.74,4.21) | 2.38 (1.43, 3.33)* | ||||||
= P <0.05 and marked in bold
Quartile disease duration cut points: Full Cohort 0.05, 2.3, 9.9, 11.1; Incident cohort 0.06, 1.1, 5.4, 15.7; Established cohort 0.02, 0.47, 4.1, 13.4
DISCUSSION
In this investigation, high adherence to prescribed MTX was associated with improved clinical outcomes in both new and established MTX users. Our analysis was limited to the initial course of MTX during VARA observation to provide a more homogenous cohort. We found that the majority of US veterans showed high levels of medication adherence during their first course of therapy, with 84% having a MPR ≥ 0.8. This is similar to prior reports of MTX adherence in other RA populations receiving MTX (13, 19) but higher than that seen with other medications commonly used in RA, including NSAIDs (20). This relatively favorable rate of treatment adherence is also higher than commonly reported in patients receiving medications for chronic conditions such as hypertension (21), hyperlipidemia (21, 22), and diabetes(23). Our observation of a relatively high adherence may have been in part a result of our large established cohort, although the percent of subjects with high adherence was similar in the incident and established cohorts. While the observations were very similar in the incident and established groups, the power to confirm that the differences observed in the incident group was limited by the small sample size in this sub-population.
In all groups, the mean MTX dose prescribed, 16 mg per week, was within the established standard of care and consistent with doses employed in recent RA clinical trials (6). In the high MTX adherence groups, the observed dose (the estimated dose that the patient actually received), approximated the prescribed dose of 16 mg per week. The low adherence group, however, had an estimated dose of 11 mg per week, which is substantially lower than the prescribed dose and generally not considered to be as effective as the higher MTX dose (24). The lower adherence group also exhibited longer delays in refilling their MTX during their first MTX course which may have additionally affected clinical outcomes. These combined observations may explain the association between MTX adherence and disease outcome measures, with lower adherence associated with higher DAS28, ESR, and CRP values in patients with RA.
A similar association of MTX adherence with CRP level was recently reported in a Danish population of RA patients (25). Using administrative databases, this study did not examine clinical outcome measures other than laboratory values. In that study, the reported MPR (87.3%) was very similar to the adherence observed in our study. These investigators also noted that in patients initiating MTX strong perceptions of “a personal need for the treatment” were associated with higher treatment adherence (19). Consistent with findings from our study, other investigators have measured persistence on MTX and demonstrated that patients with higher persistence had improved DAS28, CRP levels, and patient reported outcomes (26).
Several factors could serve to confound the relationship of treatment adherence with outcomes. Results from our multivariable analyses showed that some factors such as smoking were significantly associated with disease activity but did not significantly confound the relationship between MTX adherence and DAS28 relationship. Furthermore, the effect estimates in each cohort were not highly influenced after accounting for these potential confounders. It is important to note that enrollment clinical measures are a mix of baseline and intermediate variables for the full cohort, necessitating the evaluation of adherence in the established and incident MTX user subsets. For the established cohort (and therefore, the full cohort), we were unable to adjust for baseline DAS28, as MTX treatment was initiated prior to VARA registry enrollment. At enrollment, clinical disease activity measures were lower in the high adherence groups in the full and established groups, which likely reflect the beneficial effects of higher adherence and more optimal MTX use even prior to VARA enrollment. Of note, there were no differences in baseline DAS28 at the time of enrollment for the incident cohort, suggesting a similar clinical disease state at the time of enrollment for patients that ultimately demonstrated either high or low adherence. This observation also suggests that baseline disease activity does not predict subsequent adherence; therefore, it is unlikely to confound the relationship between adherence and post-treatment average DAS28. However, it is recognized that unmeasured confounding could impact our findings.
Estimation of days supply was a key component to inferring the duration of drug courses, observed weekly dose, and MPR. While VA administrative pharmacy databases have been used for evaluation of medication adherence in other clinical settings (22, 27), medications for RA patients present special challenges related to complex dosing schedules, varying formulations, and different routes of administration. Because of this complexity, errors in data input for days supply and units dispensed can occur and result in improbable estimates of exposure duration and weekly dosing. These problems are particularly relevant to MTX, the most commonly used DMARD in RA, because of its varying routes of administration and sometimes complex dosing schedules. To address these problems, we employed an algorithm that added information from the dispensing instructions (“sig”) to the calculation of prescription duration, thus improving the accuracy of the estimated course duration and measure of adherence (28).
Our study has the additional strengths of the prospective data collection in VARA and the documented “gold-standard” diagnosis of RA by a rheumatologist in all study subjects. In contrast, other studies of this nature have frequently relied on administrative data to establish the diagnosis of RA (13, 19, 25), a method prone to misclassification (29). Other strengths include the large number of comprehensive clinical outcome measures and other potential confounders available for analysis, in addition to the collection of these data by clinicians who were not specifically informed about the patient medication adherence history.
In contrast to other RA populations, VARA is composed of predominantly older men with substantial co-morbidity (1), potentially limiting the generalizability of our results. While most enrolled veterans received their comprehensive care within VA, the potential for concurrent medication from non- VA sources cannot be excluded. Additionally, this study included many patients for whom disease activity measures were not available for much of their MTX course before entry into the VA system, preventing adjustment for baseline disease activity among these established MTX users. An analysis of incident and established users, however, showed that higher adherence was associated with lower disease activity in both groups. The effect estimates were lower in the established cohort, a result which likely reflects early unmeasured changes in DAS28, since VARA enrollment occurred after initiation of MTX in this group. The limitation of small sample size in the incident cohort is also noted and the impact on statistical analysis in this subgroup because of limited power.
Our study does not specifically address the reasons for high or low adherence in our patients. Future work will be directed at this issue specifically. There are reports that high medication adherence is associated with “healthy behaviors” which could more globally impact clinical outcomes. For example, high adherence to placebo in osteoporosis intervention trials has been associated with a decrease in fracture rate (30). Treatment adherence has been associated with several other factors including cognitive (31, 32) and psychological impairment (31, 33, 34), complex dosing schedules (33), treatment of an asymptomatic disease (35), medication side effects (34), substance abuse (27, 36), barrier to obtaining medication (including costs) (34, 37–39), consistency of follow-up (35, 40), ethnicity (41), and provider-patient communication difficulties. Further investigations will be needed to identify the reasons for poor adherence to medications in this population and potentially develop strategies to improve patient compliance with prescribed therapies.
In summary, this investigation demonstrates that high adherence with prescribed MTX is associated with improved clinical outcome measures in RA, an effect that is independent of other important demographic and treatment-related clinical factors. These data support the clinical benefit of MTX therapy in “day-to-day” clinical practice and imply that strategies focused on optimizing MTX adherence may have the important potential to improve patient outcomes. Moreover, these findings demonstrate that VA administrative pharmacy databases can be combined with our observational cohort data to evaluate clinical outcomes during DMARD therapy. This methodology has the potential for expanded applications for the continued evaluation of community practice DMARD experience.
Significance and Innovation.
Merging of clinical information in the Veterans Affairs Rheumatoid Arthritis (VARA) registry with the VA Pharmacy Benefits Management (PBM) database
Development of methods for measuring methotrexate (MTX) adherence
Correlation of MTX adherence with clinical outcome measures of RA disease activity with the control for potential confounders
Demonstration of the association of MTX adherence with improvement for RA patients in clinical practice
Acknowledgments
Support: This project was supported by VA HSR&D grant (SHP 08-172). Drs. Sauer and Caplan are each supported by a VA Career Development Award (RCD 06-300-2 and CDA 07-221). Dr. Curtis receives support from the NIH (AR053351). Dr. Mikuls is supported by a VA CSR&D Merit.
Appendix to Methods section
Sample applications of algorithm
For example, the combination of product name “METHOTREXATE NA 2.5MG TAB” and sig “TAKE 8 TABLETS ORAL QWEEK” was standardized to 20mg per week. If a patient received 24 tablets (2.5 mg MTX tablet) and the days supply was listed as 21 days, the AWD would be calculated as 20 mg per week. Because this AWD is within the expected range of 5–30 mg per week, the days supply of 21 would be used as the expected prescription duration.
Modifying the example above for a patient with a sig of 20 mg per week on a prescription of 24 (2.5 mg MTX tablets - total dose 60 mg), if the days supply listed as 3 days (which could occur if the number weeks supplied was incorrectly entered as the days supply), the AWD is calculated at 140 mg per week. This weekly dose of 140 mg per week is outside the expected range of 5–30 mg per week. In this case, the sig of 20 mg per week is then used in conjunction with the known total dose dispensed of 60 mg to calculate a SEDS of 3 weeks (21 days). This calculated SEDS of 21 days would replaced the recorded days supply (3 days) as the expected prescription duration
Continuing the above example, if no sig information was available and the AWD was outside the expected range, the expected prescription duration was calculated using a presumed sig dose of 5 mg per week if the calculated AWD dose was lower than 5mg per wk or 30mg per wk if the AWD was greater than 30mg per wk. In the current example, with a total dose dispensed of 60 mg per week and a calculated AWD of 140 mg per week, an assumed dose of 30 mg per week would be used to calculate estimated expected prescription duration of 2 weeks (14 days). This estimate would be substituted for the days supply as the expected prescription duration.
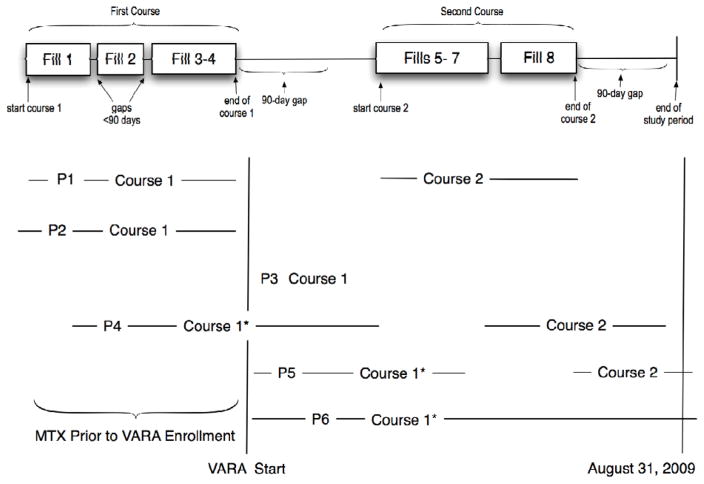
Appendix Figure. Illustration of Drug Course and Study Inclusion Criteria.
This figure demonstrates the experience with six different patients. Patient 1 (P1). The exposure experience at the top of the figure represents a magnified dispensing history for Patient 1 (P1) who had two courses of MTX therapy. The subject had four medication dispensings (fills) during the first course of therapy. The first two boxes, fill 1–2, represents a dispense date and the expected duration for that dispensing. The gaps between fills 1 & 2 indicate a brief duration where the subject was not expected to have access to the medication if the medication was used as prescribed. The third box includes two dispensings since no gap occurred in the patients expected drug supply, i.e., they refilled the medication early or on the day the estimated days supply ended. After the first four fills there is a period (gap) of greater than 90 days. Thus, the second course begins with fill #5 and continues through the expected end date for fill #8 which was also followed by a great than 90 day gap. Our analysis was limited to the first course of MTX. Because the first course of MTX for P1 ended before VARA enrollment, the patient is excluded from the analysis.
Other examples. Patient 2 (P2) represents a patient with a single course of MTX that ended before VARA enrollment and therefore was not included in the study. Patient 3 (P3) represents a patient with an initial course of MTX therapy that did not reach the 90-day in duration and/or did not have a DAS28 measurement after the impact date. This P3 course would not be included in the analysis. The initial course of therapy for patient 4 (P4), patient 5 (P5), and patient 6 (P6) would be selected because these patient had a course of at least 90 days duration and had at least one DAS28 recorded in the VARA database during the initial MTX course and after 90 days of treatment. P4 would be included in the established user group. P5 and P6 would in the incident user group. Only the first course would be included for P5.
References
- 1.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology (Oxford, England) 2010 Jul 21; doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clinical and experimental rheumatology. 2008 Sep-Oct;26(5 Suppl 51):S35–61. [PubMed] [Google Scholar]
- 3.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis and rheumatism. 2008 Jun 15;59(6):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 4.Katchamart W, Trudeau J, Phumethum V, Bombardier C. Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Cochrane Database Syst Rev. 2010;(4):CD008495. doi: 10.1002/14651858.CD008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Annals of the rheumatic diseases. 2010 Jun;69(6):964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suarez-Almazor ME, Belseck E, Shea B, Wells G, Tugwell P. Methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2000;(2):CD000957. doi: 10.1002/14651858.CD000957. [DOI] [PubMed] [Google Scholar]
- 7.Sokka T, Pincus T. Eligibility of patients in routine care for major clinical trials of anti-tumor necrosis factor alpha agents in rheumatoid arthritis. Arthritis and rheumatism. 2003 Feb;48(2):313–8. doi: 10.1002/art.10817. [DOI] [PubMed] [Google Scholar]
- 8.Pharmacy Benefits Management Services of the Department of Veterans Affairs. Available from: http://www.pbm.va.gov/Default.aspx.
- 9.Sales MM, Cunningham FE, Glassman PA, Valentino MA, Good CB. Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005 Feb;11(2):104–12. [PubMed] [Google Scholar]
- 10.Mikuls TR, Kazi S, Cipher D, Hooker R, Kerr GS, Richards JS, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. The Journal of rheumatology. 2007 Jul;34(7):1480–4. [PubMed] [Google Scholar]
- 11.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis and rheumatism. 1995 Jan;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 12.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006 Aug;15(8):565–74. doi: 10.1002/pds.1230. discussion 75–7. [DOI] [PubMed] [Google Scholar]
- 13.Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF, Jr, Griffin MR. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care. 2007 Oct;45(10 Supl 2):S66–76. doi: 10.1097/MLR.0b013e318041384c. [DOI] [PubMed] [Google Scholar]
- 14.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997 Jan;50(1):105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 15.Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. The Journal of rheumatology. 2005 Aug;32(8):1432–9. [PubMed] [Google Scholar]
- 16.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis and rheumatism. 1999 Oct;42(10):2220–30. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Miriovsky BJ, Michaud K, Thiele GM, O’Dell JR, Cannon GW, Kerr G, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Annals of the rheumatic diseases. 2010 Jul;69(7):1292–7. doi: 10.1136/ard.2009.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.de Thurah A, Norgaard M, Harder I, Stengaard-Pedersen K. Compliance with methotrexate treatment in patients with rheumatoid arthritis: influence of patients’ beliefs about the medicine. A prospective cohort study. Rheumatol Int. 2010 Sep;30(11):1441–8. doi: 10.1007/s00296-009-1160-8. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Inui TS, Sullivan B. Noncompliance with arthritis drugs: magnitude, correlates, and clinical implications. The Journal of rheumatology. 1981 Nov-Dec;8(6):931–6. [PubMed] [Google Scholar]
- 21.Chapman RH, Benner JS, Petrilla AA, Tierce JC, Collins SR, Battleman DS, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Archives of internal medicine. 2005 May 23;165(10):1147–52. doi: 10.1001/archinte.165.10.1147. [DOI] [PubMed] [Google Scholar]
- 22.Doshi JA, Zhu J, Lee BY, Kimmel SE, Volpp KG. Impact of a prescription copayment increase on lipid-lowering medication adherence in veterans. Circulation. 2009 Jan 27;119(3):390–7. doi: 10.1161/CIRCULATIONAHA.108.783944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittdiel JA, Uratsu CS, Fireman BH, Selby JV. The effectiveness of diabetes care management in managed care. Am J Manag Care. 2009 May;15(5):295–301. [PubMed] [Google Scholar]
- 24.Furst DE, Koehnke R, Burmeister LF, Kohler J, Cargill I. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. The Journal of rheumatology. 1989 Mar;16(3):313–20. [PubMed] [Google Scholar]
- 25.de Thurah A, Norgaard M, Johansen MB, Stengaard-Pedersen K. Methotrexate compliance among patients with rheumatoid arthritis: the influence of disease activity, disease duration, and co-morbidity in a 10-year longitudinal study. Scandinavian journal of rheumatology. 2010 May;39(3):197–205. doi: 10.3109/03009740903251318. [DOI] [PubMed] [Google Scholar]
- 26.Contreras-Yanez I, Cabiedes J, Villa AR, Rull-Gabayet M, Pascual-Ramos V. Persistence on therapy is a major determinant of patient-, physician- and laboratory- reported outcomes in recent-onset rheumatoid arthritis patients. Clinical and experimental rheumatology. 2010 Sep-Oct;28(5):748–51. [PubMed] [Google Scholar]
- 27.Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Annals of internal medicine. 2008 Dec 2;149(11):795–804. doi: 10.7326/0003-4819-149-11-200812020-00004. [DOI] [PubMed] [Google Scholar]
- 28.Hayden C, Sauer B, Cannon G. Impact of Including Physician’s Prescribing Directions on Calculations of Medication Possession Ratios International Society for Disease Surveillance. 9th Annual Conference Enhancing the Synergy Between Research, Informatics, and Practice in Public Health; 2010. [Google Scholar]
- 29.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis and rheumatism. 2004 Dec 15;51(6):952–7. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 30.Curtis JR, Delzell E, Chen L, Black D, Ensrud K, Judd S, et al. The relationship between bisphosphonate adherence and fracture: Is it the behavior or the medication? results from the placebo arm of the fracture intervention trial. J Bone Miner Res. 2010 Oct 11; doi: 10.1002/jbmr.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuno J, Yanagi H, Tomura S. Is cognitive impairment a risk factor for poor compliance among Japanese elderly in the community? Eur J Clin Pharmacol. 2001 Oct;57(8):589–94. doi: 10.1007/s002280100347. [DOI] [PubMed] [Google Scholar]
- 32.Stilley CS, Sereika S, Muldoon MF, Ryan CM, Dunbar-Jacob J. Psychological and cognitive function: predictors of adherence with cholesterol lowering treatment. Ann Behav Med. 2004 Apr;27(2):117–24. doi: 10.1207/s15324796abm2702_6. [DOI] [PubMed] [Google Scholar]
- 33.Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002 Dec 15;31( Suppl 3):S123–7. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- 34.van Servellen G, Chang B, Garcia L, Lombardi E. Individual and system level factors associated with treatment nonadherence in human immunodeficiency virus-infected men and women. AIDS Patient Care STDS. 2002 Jun;16(6):269–81. doi: 10.1089/10872910260066705. [DOI] [PubMed] [Google Scholar]
- 35.Sewitch MJ, Abrahamowicz M, Barkun A, Bitton A, Wild GE, Cohen A, et al. Patient nonadherence to medication in inflammatory bowel disease. Am J Gastroenterol. 2003 Jul;98(7):1535–44. doi: 10.1111/j.1572-0241.2003.07522.x. [DOI] [PubMed] [Google Scholar]
- 36.Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, Chesney M, et al. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care. 2009 Feb;21(2):168–77. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998 Jul-Aug;20(4):764–71. doi: 10.1016/s0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 38.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004 Jun;19(6):638–45. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry. 2002 Dec;63(12):1121–8. doi: 10.4088/jcp.v63n1206. [DOI] [PubMed] [Google Scholar]
- 40.Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002 Oct;63(10):892–909. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- 41.Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to cardiovascular disease medications: does patient-provider race/ethnicity and language concordance matter? J Gen Intern Med. 2010 Nov;25(11):1172–7. doi: 10.1007/s11606-010-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]