Abstract
A new method was developed for marine harmful algal bloom (HAB) mitigation using local beach sand or silica sand modified with chitosan and polyaluminum chloride (PAC). Untreated sand was ineffective in flocculating algal cells, but 80% removal efficiency was achieved for Amphidinium carterae Hulburt and a Chlorella sp. in 3 min (t80 = 3 min) using 120 mg L−1 sand modified with 10 mg L−1 PAC and 10 mg L−1 chitosan. After several hours 92% – 96% removal was achieved. The t80 for removing A. carterae using the modifiers only (PAC and chitosan combined) was 60 min and for Chlorella sp. 120 min, times which are much slower than with the corresponding modified sand. Sands were critical for speeding up the kinetic processes of flocculation and sedimentation of algal flocs. PAC was helpful in forming small flocs and chitosan is essential to bridge the small flocs into large dense flocs. Chitosan was also important in inhibiting the escape of cells from the flocs. Chitosan and PAC used together as modifiers make it possible to use local beach sands for HAB mitigation in seawater. Economical and environmental concerns could be reduced through the use of sands and biodegradable chitosan, but the potential impacts of PAC need further study.
Keywords: Harmful algal bloom, Seawater, Modified sands, Chitosan, Polyaluminum chloride (PAC), Synergistic effect
1. Introduction
Harmful algal blooms (HABs) pose a serious threat to public health, aquatic organisms, commercial fisheries, and the quality of freshwater lakes, rivers and reservoirs, as well as marine coastal environments. Over the past decade, there has been increasing interest in bloom mitigation strategies, though progress towards field applications has still been slow (Anderson, 1997). Significant attention has been focused on the use of clays as a means to remove HAB cells from the water column through flocculation and sedimentation. Many of these experiments were laboratory based (Beaulieu et al., 2005; Pan et al., 2006a; Pierce et al., 2004; Sengco et al., 2001; Yu et al., 1994), with some field demonstrations in Japan (Shirona, 1989), Australia(Atkins et al., 2001), China (Pan et al., 2006b) and South Korea (e.g., Lee et al., 2008). The environmental impacts of clay flocculation are generally positive, though there are studies that document negative effects. On the positive side, clay flocculation had little or no effect on marine organisms such as juvenile clams, fish, and invertebrates (Lewis et al. 2003; Archambault et al, 2004; Sengco and Anderson, 2004). In one of these studies, however, a growth effect on juvenile hard clams was observed (compared to no-clay controls) with clay maintained in suspension for two weeks. These results suggest that clay applications in the field are likely more detrimental to clams under flow conditions leading to prolonged in situ resuspension of clay than under conditions that promote rapid sedimentation. Shumway et al. (2003) also report negative impacts on filter-feeding invertebrates using relatively high levels of clay. The magnitude of impacts is thus dependent on the flow regime, duration of exposure to resuspended clay, and the total clay loading.
However, clays are not immediately available at some locations that have HAB problems, and transportation costs may render this method uneconomical. There is also a common ecological concern about the dumping of large amounts of exotic materials into aquatic systems. As an alternative strategy, the use of native ecological materials such as local beach sands or soil (that naturally enter the aquatic system through rivers or rainfall) could in principle minimize the costs and ecological risk to aquatic environments. Sands, however, have markedly different physical characteristics from clays, and by themselves, will not flocculate and remove HAB cells.
In freshwater HAB mitigation, Pan and co-workers found that local soil particles including sands can be highly effective in removing cyanobacterial cells and improving water quality, but only after modification using small amounts of a natural, biodegradable material called chitosan (Pan et al., 2006b; Zou et al., 2006; Pan et al., 2011). These authors found that the polymeric netting and bridging function of chitosan was the key mechanism that allowed local soil particles to be highly effective in flocculating HAB cells. In this approach, the chitosan made a “net” that captured the HAB cells and other particles, and the soils provided the ballast or mass to carry the aggregates to the bottom. These encouraging results in freshwater have, however, limited direct applicability in marine systems, as high ionic strength and alkalinity prevent the unfolding of the polymer chain, thereby weakening chitosan’s netting and bridging properties (Qun and Ajun, 2006; Zou et al., 2005).
Polyaluminum chloride (PAC), a commonly used inorganic coagulant, is highly effective in potable water treatment where it is used routinely to flocculate and remove suspended particles. PAC has been tested in marine systems and has been shown to reduce the amount of clays needed to remove HAB organisms (Pierce et al., 2004; Sengco et al., 2001; Yu et al., 1994). The addition of PAC increases the chemical affinity of clay surfaces. According to laboratory studies, however, algal cell flocculation by clays plus PAC was temporary (Sengco et al., 2001; Sun et al., 2004). Most of the cells could escape from the flocs and resume their growth. Motile dinoflagellate species were thus more difficult to be removed permanently through flocculation compared to non-motile diatoms (Yu et al., 1994), indicating that motility was an important factor affecting bloom mitigation through clay flocculation. Furthermore, the PAC floc was light, which did not settle easily or was resuspended with only modest currents (Beaulieu et al. 2005).
No efforts have been made thus far to use local beach sands to irreversibly flocculate and sediment marine HAB cells. Here, a modification of the approach to suppress freshwater HABs using local beach sands and polymers was developed for algal bloom mitigation in seawater. The synergistic effects of chitosan and PAC (hereafter termed “modifiers”) with two types of sands were investigated for the removal of Amphidinium carterae and Chlorella sp. The results demonstrate that it is possible to use modified local or commercially available sands to irreversibly remove a high percentage of the two types of HAB cells from seawater.
2. Materials and Methods
2.1. Algal species and culture
Two algal species were used - Amphidinium carterae Hulburt, a motile dinoflagellate, and a marine Chlorella sp. which is very small, and non-motile. A. carterae is considerd a HAB species because of its production of haemolysins, and it has also been linked to fish mortalities(Hulburt, 1957; Yasumoto et al., 1987). Although Chlorella is not listed as a harmful species on some lists, it is known for its ability to produce dense blooms that can have adverse consequences, such as the decimation of the oyster industry on Long Island following eutrophication stimulated by duck farm effluents (Ryther, 1954). A. carterae was obtained from Oceanography College, Ocean University of China and Chlorella sp. was supplied by Seaweed Inheritance Breeding Center of Shandong Oriental Ocean Sci.-Tech. Co. Ltd..
The cells were grown in f/2 medium (Guillard and Hargraves, 1993) made with synthetic seawater. The synthetic seawater was composed of 23.939 g L−1 NaCl, 5.079 g L−1 MgCl2·6H2O, 3.994 g L−1 Na2SO4, 1.123 g L−1 CaCl2, 0.667 g L−1 KCl, 0.196 g L−1 NaHCO3, 0.098 g L−1 KBr, 0.027 g L−1 H3BO3, 0.003 g L−1 NaF and 0.024 g L−1 SrCl2·6H2O. The medium was adjusted to pH 8.2 before autoclaving by adding either 0.1 mol L−1 NaOH or 0.1 mol L−1 HCl solutions. Algal batch cultures were maintained at 25±1°C under continuous cool white fluorescent light of 2000–3000 lux on a 12h light and 12h darkness regimen in the illuminating incubator (LRH-250-G, Guangdong Medical Apparatus Co. Ltd., China).
2.2. Sands and modifiers
Two kinds of sand were used. One was SiO2 (silica sand) analytical grade, purchased from Sinopharm Chemical reagent Co., Ltd.. Another was local sand which collected from a Yellow Sea beach in Yantai, China. The two sands were washed with deionized water, dried at 100°C, and sieved through 180 mesh (<90 μm).
Chitosan was obtained from Qingdao Haisheng Bioengineering Co. Ltd. The chitosan flakes were dissolved by adding 100 mg chitosan to 10 mL of 0.5% HAc and stirring until all the chitosan was dissolved. This solution was diluted with deionized water to obtain a final concentration of 1mg mL−1 before use (Zou et al., 2006). PAC was supplied by Dagang Reagent Plant, Tianjin, China. The basicity (B= [OH]/[Al]) of PAC was 2.4 and its Al2O3 content was 30%. The PAC was dissolved in deionized water to obtain a solution of 1 mg mL−1. The chitosan and PAC solutions were prepared freshly before each set of experiments.
2.3. Algal flocculation
Flocculation experiments were conducted using a jar test apparatus (ZR3-6, Zhongrun Water Industry Technology Development Co. Ltd., China) using cultures in mid- to late-exponential growth phase. The initial cell concentrations of A. carterae and Chlorella sp. were 3.25 – 3.42×105 cells mL−1 and 6.65 – 6.82×106 cells mL−1, respectively. Two hundred milliliters of experimental culture were transferred into a 250 mL beaker, stirred at 200 rpm for 2 min, followed by 30 rpm for another 5 min. Chitosan alone, PAC alone, chitosan plus PAC together, and chitosan plus PAC plus sands were added to the algal culture in different flocculation experiments. The control culture was run without adding any sands or modifiers.
Samples from 2 cm below the surface of the experimental beaker were collected after sedimentation at different times and the cells enumerated in a counting chamber under an electromotive microscope (Axioskop 2 mot plus, Carl ZEISS, Germany) after being fixed by Lugol solution. The removal efficiency of cells was calculated as (initial cell concentration - sample cell concentration)/initial cell concentration × 100%. Algal flocs were collected by pipette and observed under the microscope.
Algal floc size and size distribution during the flocculation process were monitored with a laser particle size analyzer Mastersizer 2000 (Malvern Co. United Kingdom). The culture was drawn into the Mastersizer and back to the jar by a peristaltic pump (BT00-300M, Baoding Longer Precision Pump Co. Ltd., China) at a flow rate of 34 mL min−1 (Zhang et al., 2007). Samples were at the same position in the jar, which was located between the impeller and the top of suspension. Algal floc size was denoted by the measured mean diameter (d50).
2.4. Viability and growth of algae after flocculation
The effect of PAC or chitosan with PAC on the viability and the growth of A. carterae after flocculation was investigated using two strategies. In the first experiment, fresh f/2 medium was added to the supernatant without disturbing the algal flocs (Sengco et al., 2001; Sun and Choi, 2004). This flask was maintained in an illuminated incubator, and viability and growth of the cells were monitored by measuring the cell concentrations in the supernatant after 24 and 48 hours. In the second experiment, flocs were maintained in the incubator without fresh f/2 medium or light.
3. Results
3.1. Algal flocculation using modified sands
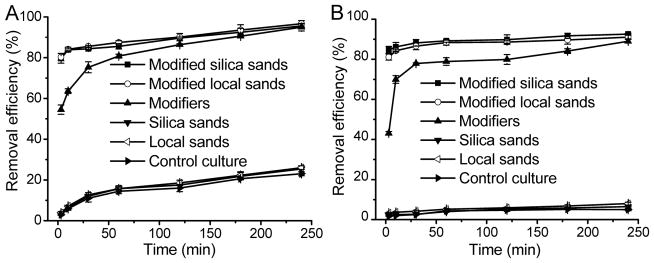
Compared with control experiments, 100 mg L−1 silica sand or local sand was ineffective in removing A. carterae and Chlorella sp. (Fig. 1). However, sands modified using chitosan and PAC combined were highly efficient in flocculating and sinking algal cells. The removal efficiency with 120 mg L−1 modified sands containing 10 mg L−1 chitosan and 10 mg L−1 PAC reached 80% for the two algal species within 3 min (t80=3 min), whereas the removal efficiencies of only 10 mg L−1 chitosan plus 10 mg L−1 PAC on A. carterae (Fig. 1A) and Chlorella sp. (Fig. 1B) were 54% and 43%, respectively. The t80 of the modifiers alone for A. carterae removal was 60 min and that for Chlorella sp. was 120 min. Using only sands, the removal efficiencies of A. carterae and Chlorella sp. after 240 min were 26% and 7% (Figs. 1A, 1B). This increased to 96% and 92% when the chitosan and PAC modifiers were added with the sand. The results in Fig. 1 also demonstrate that there was no large difference between silica sand and local beach sand on HAB cell removal if the modifiers chitosan and PAC were present.
Fig. 1.
Algal removal efficiency of 100 mg L-1 local sands, 100 mg L-1 silica sands, modifiers (10 mg L-1 chitosan plus 10 mg L-1 PAC), modified local sands (10 mg L-1 chitosan plus 10 mg L-1 PAC plus 100 mg L-1 local sands) and modified silica sands (10 mg L-1 chitosan plus 10 mg L-1 PAC plus 100 mg L-1 silica sands) at different time. (A) A. carterae, (B) Chlorella sp.
3.2. Synergistic effect of chitosan and PAC on algal cell removal
When chitosan was used alone, cell removal efficiencies increased with increasing dosage of chitosan (0 – 20 mg L−1 for A. carterae and 0 – 50 mg L−1 for Chlorella sp.; Fig. 2). However, the removal efficiency of A. carterae (Fig. 2A) was maximally 71% at 20 mg L−1 chitosan and that of Chlorella sp. (Fig. 2B) was only 51% at 50 mg L−1, which suggests that chitosan is not as efficient at removing algal cells from seawater as it is in fresh water (Pan et al., 2006b; Zou et al., 2006).
Fig. 2.
Synergistic effect of chitosan and PAC on algae removal. (A) A. carterae, (B) Chlorella sp.
Cell removal efficiency for both species increased when PAC and chitosan were used together (Fig. 2). After the addition of 5 mg L−1 PAC with 10 mg L−1 chitosan, the removal efficiency of A. carterae and Chlorella sp. increased to 92% and 62% from 68% and 11%, respectively. When 10 mg L−1 PAC was added with 10 mg L−1 chitosan, the A. carterae removal efficiency increased by an additional 28% over that with chitosan alone, and that of Chlorella sp. increased by 78%.
3.3. Synergistic effect of chitosan and PAC on algal floc formation
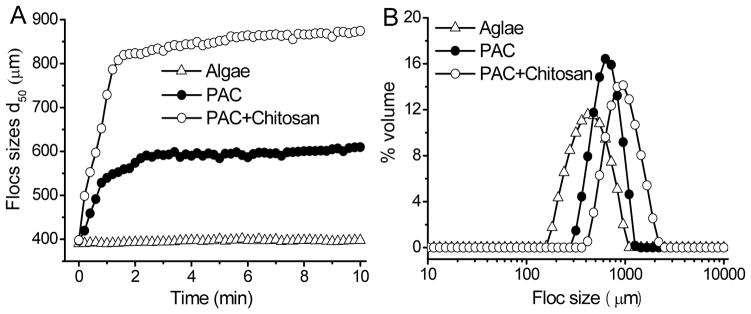
The formation and development of algal flocs using 10 mg L−1 PAC or PAC with 10 mg L−1 chitosan were investigated using Chlorella sp. as the target species. The floc size (Fig. 3A) and size distributions (Fig. 3B) were monitored. Compared with PAC alone, the algal flocs of PAC plus chitosan increased in size much faster in the first two minutes. During the slow stir phase, algal floc size increased to a plateau. The floc size of PAC plus chitosan increased to 860 μm, compared to that of PAC alone, for which the size was approximately 600 μm. The floc produced by chitosan and PAC appeared rapidly and quickly increased in size to form larger particles than with PAC only.
Fig. 3.
Synergistic effect of chitosan and PAC on algal flocs. (A) Floc size, (B) Floc size distributions at 7 min
At 7 min, the stir was over and floc size distribution curves were shown in Fig. 3B. The floc size distribution of PAC alone ranged between 316 μm and 1259 μm, with the highest peak at 631 μm. The size distribution of PAC plus chitosan was between 417 μm and 2188 μm, with the highest peak at 955 μm.
3.4. Synergistic effect of chitosan and PAC on cell viability
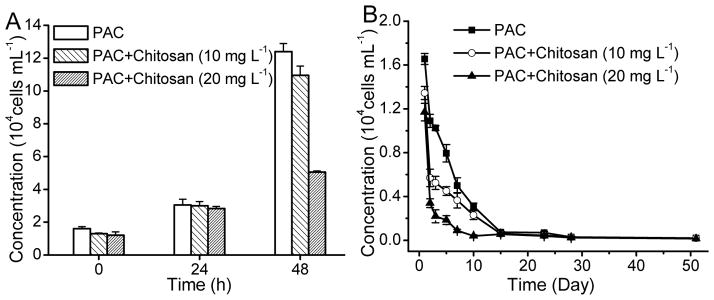
An experiment examining the synergistic effect of chitosan and PAC on the viability and growth of A. carterae was divided into three treatments: (1) 10 mg L−1 PAC only, (2) 10 mg L−1 PAC plus 10 mg L−1 chitosan, (3) 10 mg L−1 PAC plus 20 mg L−1 chitosan. After these flocculation experiments, the residual cell concentration in the supernatant of the three treatments was 1.2 – 1.6×104 cells mL−1, approximately 4% of the original concentration prior to the treatment. The cell concentration for all the treatments roughly doubled to 2.8 – 3.0×104 cells mL−1 after 24 hours of incubation in an incubator with light and added nutrients (Fig. 4A). After another 24 hours, the cell concentration with PAC only increased dramatically to 12.4 ×104 cells mL−1, while the concentration in the treatments of PAC plus 20 mg L−1 chitosan rose to 5.05 ×104 cells mL−1, approximately half of the concentration with PAC only.
Fig. 4.
Synergistic effect of chitosan and PAC on algae viability. (A) with light and added nutrients, (B) with no light or added nutrients
The results shown in Fig. 4B demonstrate that the cell concentration in the supernatant of the three treatments in the incubator with no light or added nutrients decreased gradually throughout the study interval. However, the algal cell concentrations of PAC plus chitosan used together were less than that of PAC alone and the cell concentration was inversely related to the chitosan dosage. After 28 days, the concentration of algal cells in supernatant was only 300 cells mL−1, indicative of almost no recovery of A. carterae cells under conditions similar to those found near bottom sediments.
4. Discussion
In this study, a method was developed that uses sands or local soils that could be collected from the immediate vicinity of a HAB, and used in conjunction with small amount of chitosan and PAC to flocculate and effectively remove cells from the water column. Our results demonstrate that PAC was needed to maintain the netting and bridging function of chitosan in seawater and to form small flocs, while chitosan was essential in bridging the small flocs into large and dense flocs that hindered the escape of cells from the flocs. As the safe and cheap carrier of these modifiers, sand was critical for speeding up sedimentation. This approach, which was a modification of the one used successfully for HAB removal in freshwater systems (Pan et al., 2006b; Pan et al., 2011), greatly minimizes environmental concerns for mitigation of HABs in seawater using clays since the use of native beach sands has few environmental concerns. As discussed below, however, there are still some issues that need to be addressed if this method is used for field applications on natural blooms.
4.1. Synergistic effects of chitosan plus PAC
The flocculation of algal cells in natural waters occurs as a result of attractive anion-cation interactions, as well as hydrophobic or polymer interactions (Divakaran and Pillai, 2001; Strand et al., 2002). Sands alone are much less efficient in flocculating algal cells compared to clays such as kaolinite, montmorillonite, and sepiolite (Pan et al., 2006a; Pan et al., 2006b; Pierce et al., 2004; Sengco et al., 2001; Yu et al., 1994). Chitosan and PAC as modifiers increase the surface charge of sands and enhance the netting and bridging interactions with algal cells. Sands also provide the mass or ballast to carry flocs to bottom sediments.
Chitosan, a cellulose-like polyelectrolyte biopolymer, is derived from the alkaline deacetylation of crustacean chitin, which possesses several intrinsic characteristics of coagulants and flocculants, i.e., high cationic charge density, long polymer chains, bridging of aggregates and precipitation (Renault et al., 2009; Rinaudo, 2006). Chitosan, by itself, does not flocculate effectively in seawater (Fig. 2). This is because its molecular structure includes abundant amino groups (−NH2) and hydroxyl groups (−OH) on the chain. The active amine group (−NH2) of chitosan is easily protonated as −NH3+ in dilute acidic solutions, and there is a strong electrostatic repulsion force within and between molecules (Rinaudo, 2006). The high content of positively charged amine groups in the chitosan structure facilitates electrostatic interactions between polymer chains and negatively charged contaminants (Huang et al., 2000; Renault et al., 2009). However, in high ionic strength solutions such as seawater, counter-ions accumulate near the −NH3+ group, which would screen the protonated amine groups and decrease the electrostatic repulsion among them (Qun and Ajun, 2006; Schatz et al., 2003). This prevents the unfolding of the molecular chain, thereby weakening its netting and bridging properties (Zou et al., 2005).
In contrast to chitosan, the high ionic strength of seawater is beneficial to PAC flocculation due to the reduction of the thickness of the electrical double layer which enhances the collision probability of granules. PAC supplies cationic hydrolysis products that are strongly adsorbed on negative particles and can give effective destabilization, leading to the formation of micro-flocs (Renault et al., 2009). Particles with thinner electrical double layers are easier to coagulate because of reduced repulsion. With the high salinity of seawater, flocculation of particles is increased because the thickness of the electrical double layer is decreased due to the compression of the electrolytes (Han and Kim, 2001; Pan et al., 2006b). This explains why PAC is effective in flocculating HAB cells in seawater and why the algal cell removal efficiencies of chitosan are increased remarkably with the addition of PAC. PAC cannot be used by itself in seawater, however, since, discussed by Beaulieu et al. (2005), PAC flocs are light and fluffy and do not settle even in light flow regimes. If these small flocs can be combined and form a stronger, larger, and heavier flocs, then the limitations of PAC flocs can be overcome.
The amino groups (−NH2) and hydroxyl groups (−OH) in chitosan’s molecular structure contain single-pair electrons that can offer the electron pair to empty trajectories of metal ions; they then chelate into a complex compound (Bassi et al., 2000). It was reported that there was a positive correlation between chitosan and PAC and the effect of chitosan adsorbing Al3+ in solution was very obvious (Zeng et al., 2008). The cationic hydrolysis products of PAC that are adsorbed on the molecule chain of chitosan might increase electrostatic repulsion between them and protonated groups (−NH3+), which would in turn be beneficial to the unfolding of chitosan’s molecular chain and weaken the negative effect of high ionic strength on chitosan’s netting and bridging properties in seawater. Therefore, PAC and chitosan are complementary in flocculating HAB cells in seawater. Larger and denser algal flocs are formed by the compression of electrical double layer, charge neutralization, adsorption, and netting interactions to bind and bridge cells tightly.
4.2. Cell escape from flocs
As shown in Figure 4, with light and nutrients provided to cells flocculated using PAC and chitosan alone, cell concentrations in the supernatant doubled in 24 hours, and then doubled again 24 hours later. Amphidinium can grow rapidly, with growth rates as high as 2.7 divisions per day (Ismael et al., 1999), so the cell increase in the supernatant of the chitosan plus PAC treatment could be explained entirely by growth with little or no contribution from cells escaping from the flocs. The much larger increase in cell abundance in the PAC only treatment suggests that a significant number of cells escaped into the supernatant.
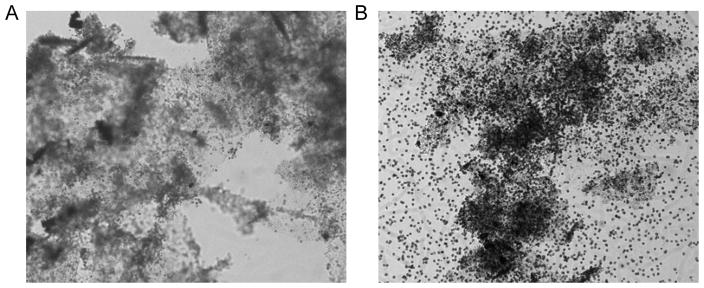
Chitosan flocs were fibrous and formed large entangled masses resembling cobwebs by bridging mechanisms (Fig. 5A). The protonated amine group of chitosan attract negatively charged algal cells to produce large and complex flocs that help to prevent the escape of motile cells. In contrast, the flocs of PAC alone were small and there were large numbers of cells around the flocs (Fig. 5B). This implies that PAC does not bridge the algal cells firmly nor bind them as strongly as chitosan does. Overall, the number of cells escaping from the PAC plus chitosan flocs was small, and the method appeared promising for bloom mitigation. The addition of sand would make cell escape even more difficult.
Fig. 5.
Algal flocs micrographs with the magnification of 50 times. (A) Chitosan and A. carterae, (B) PAC and A. carterae
4.3 Environmental impacts
One of the challenging and controversial aspects of HAB research relates to methods to directly control or suppress blooms (Anderson 1997). Of the many methods that have been proposed, removal of HAB cells through clay flocculation is seen by some as promising in terms of efficiency, cost, and environmental impacts (e.g., Sengco and Anderson, 2004; Lee et al. 2008). There are, however, those who feel that the environmental impacts of this approach are unacceptable, or poorly understood. In addition to the possible adverse ecological impact caused by the addition of large amount of exotic materials (Shumway et al, 2003), other concerns expressed relates to the constituents in the clay, which might include nutrients such as phosphorus, or toxic or harmful metals and radioactive materials bound to the clay. As an alternative to clays, sands are relatively inert or refractory and thus may minimize these impacts. Most importantly, as a native part of the ecosystem, beach sand is ecologically safe to the marine system which may avoid the fundamental concern associated with clays. Large-scale dredging and beach nourishment projects abound in nearshore waters worldwide, suggesting that environmental opposition to HAB mitigation efforts using local sands might be minimal. In cases where beach sands need to be conserved, commercially available sands may also be safe, cheap and easily available to be used.
The modification technique using chitosan and PAC can not only turn local beach sands or local soils into highly effective flocculants in the mitigation of HABs in seawater, but is also useful in reducing the loading of sands/soils required for effective cell removal, which is crucial for large scale field applications. Chitosan, a commercially available product of edible food additives, is known to be a biodegradable and non-toxic natural polymer. Compared with other chemical reagents, chitosan is environmental friendly, but it might be a source of oxygen demand as it decays. The amount of chitosan used is, however, much less than the amount of algal biomass being sedimented, so this is not a serious concern. Nevertheless, it may be worthwhile to develop techniques that could carry and release oxygen with the flocs to combat this potential problem (Pan et al., 2009). In some coastal areas, it is also possible to sink the algal blooms into the bottom and cover them using a second layer of sands or local soils so that the cells can be permanently buried and sealed in the sediment and turned into fertilizers for the growth of seaweeds, as Pan et al (2011) demonstrated in shallow lakes. By decomposing the algal cells and the modifiers and converting them into the biomass of seaweeds, the harmful blooms may be turned into useful resources for the improvement of the ecosystem. However, this possibility needs further study in marine systems affected by HABs. Although PAC (a compound used in drinking water treatment) was needed to maintain the netting and bridging function of chitosan in seawater, the adverse ecological effects of this compound in seawater remain a concern. More research is needed in this area before larger-scale applications can be undertaken. Similarly, efforts are needed to identify new, environmentally benign modifiers that could replace PAC in this bloom control strategy.
5. Conclusion
Dispersal of sands or local soils modified with chitosan and PAC achieved high removal efficiency of marine HAB cells in a short time and prevented the escape of significant numbers of motile organisms from the algal flocs. This method greatly reduces potential environmental impacts by using relatively inert or refractory sand or local and by using a biodegradable polymer such as chitosan, but there may be environmental concerns about the use of PAC. With some additional studies, this approach shows great promise to become an effective and environmentally acceptable strategy for HAB mitigation.
Acknowledgments
The research was funded by the National Key Project for Basic Research (2008CB418105, 2010CB933600), for which the authors are grateful. Support for DMA was provided by GOMTOX program through NOAA Grant NA06NOS4780245. Additional support came from NSF grant OCE-0430724, DMS-0417769 and NIEHS grant 1P50-ES01274201 (Woods Hole Center for Oceans and Human Health).
References
- Anderson DM. Turning back the harmful red tide. Nature. 1997;388:513–514. [Google Scholar]
- Archambault MC, Bricelj VM, Grant J, Anderson DM. Effects on juvenile hard clams, Mercenaria mercenaria, from the application of phosphatic clays to mitigate harmful algal blooms. Mar Biol. 2004;144:553–565. [Google Scholar]
- Atkins R, Rose T, Brown RS, Robb M. The Microcystis cyanobacteria bloom in the Swan River - February 2000. Water Sci Technol. 2001;43(9):107–114. [PubMed] [Google Scholar]
- Bassi R, Prasher SO, Simpson BK. Removal of selected metal ions from aqueous solutions using chitosan flakes. Sep Sci Technol. 2000;35(4):547–560. [Google Scholar]
- Beaulieu SE, Sengco MR, Anderson DM. Using clay to control harmful algal blooms: deposition and resuspension of clay/algal flocs. Harmful Algae. 2005;4(1):123–138. [Google Scholar]
- Divakaran R, Pillai VNS. Flocculation of kaolinite suspensions in water by chitosan. Water Res. 2001;35(16):3904–3908. doi: 10.1016/s0043-1354(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Guillard RRL, Hargraves PE. Stichochrysis-immobilisis is a diatom, not a chyrsophyte. Phycologia. 1993;32(3):234–236. [Google Scholar]
- Han MY, Kim W. A theoretical consideration of algae removal with clays. Microchem J. 2001;68(2–3):157–161. [Google Scholar]
- Huang C, Chen S, Ruhsing Pan J. Optimal condition for modification of chitosan: a biopolymer for coagulation of colloidal particles. Water Res. 2000;34(3):1057–1062. [Google Scholar]
- Hulburt EM. The taxonomy of unarmored Dinophyceae of shallow embayments off Cape Cod, Massachusetts. Biol Bull. 1957;112(2):196–219. [Google Scholar]
- Ismael AAH, Halim Y, Khalil AG. Optimum growth conditions for Amphidinium carterae Hulburt from eutrophic waters in Alexandria (Egypt) and its toxicity to the brine shrimp Artemia salina. Grana. 1999;38(2–3):179–185. [Google Scholar]
- Lee YJ, Choi JK, Kim EK, Youn SH, Yang EJ. Field experiments on mitigation of harmful algal blooms using a Sophorolipid--Yellow clay mixture and effects on marine plankton. Harmful Algae. 2008;7(2):154–162. [Google Scholar]
- Lewis MA, Dantin DD, Walker CC, Kurtz JC, Greene RM. Toxicity of clay flocculation of the toxic dinoflagellate, Karenia brevis to estuarine invertebrates and fish. Harmful Algae. 2003;2(4):235–246. [Google Scholar]
- Pan G, Yang B, Li L, Liao L, Ding C, Wang D. A method for anaerobic sediments remediation and ecological restoration in eutrophic shallow lakes. 200910080563.5 Chinese Patent Application No. 2009
- Pan G, Zhang M, Chen H, Zou H, Yan H. Removal of cyanobacterial blooms in Taihu Lake using local soils. I Equilibrium and kinetic screening on the flocculation of Microcystis aeruginosa using commercially available clays and minerals. Environ Pollut. 2006a;141(2):195–200. doi: 10.1016/j.envpol.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Pan G, Zou H, Chen H, Yuan X. Removal of harmful cyanobacterial blooms in Taihu Lake using local soils III. Factors affecting the removal efficiency and an in situ field experiment using chitosan-modified local soils. Environ Pollut. 2006b;141(2):206–212. doi: 10.1016/j.envpol.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Pan G, Yang B, Wang D, Chen H, Tian BH, Zhang ML, Yuan XZ, Chen J. In-lake algal bloom removal and submerged vegetation restoration using modified local soils. Ecol Eng. 2011;37:302–308. [Google Scholar]
- Pierce RH, Henry MS, Higham CJ, Blum P, Sengco MR, Anderson DM. Removal of harmful algal cells (Karenia brevis) and toxins from seawater culture by clay flocculation. Harmful Algae. 2004;3(2):141–148. [Google Scholar]
- Qun G, Ajun W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydr Polym. 2006;64(1):29–36. [Google Scholar]
- Renault F, Sancey B, Badot PM, Crini G. Chitosan for coagulation/flocculation processes - An eco-friendly approach. Eur Polym J. 2009;45(5):1337–1348. [Google Scholar]
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006;31(7):603–632. [Google Scholar]
- Ryther JH. The Ecology of Phytoplankton Blooms in Moriches Bay and Great South Bay, Long Island, New York. Biol Bull. 1954;106(2):198–209. [Google Scholar]
- Schatz C, Viton C, Delair T, Pichot C, Domard A. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromolecules. 2003;4(3):641–648. doi: 10.1021/bm025724c. [DOI] [PubMed] [Google Scholar]
- Sengco MR, Anderson DM. Controlling harmful algal blooms through clay Flocculation. J Eukaryot Microbiol. 2004;51(2):169–172. doi: 10.1111/j.1550-7408.2004.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Sengco MR, Li A, Tugend K, Kulis D, Anderson DM. Removal of red- and brown-tide cells using clay flocculation. I. Laboratory culture experiments with Gymnodinium breve and Aureococcus anophagefferens. Mar Ecol Prog Ser. 2001;(210):41–53. [Google Scholar]
- Shirona A. Red tide problem and countermeasures. Int J Aquat Fish Technol. 1989;1:195–223. [Google Scholar]
- Shumway SE, Frank DM, Ewart LM, EvanWard J. Aquaculture Res. 2003;34:1391–1402. [Google Scholar]
- Strand SP, Nordengen T, Ostgaard K. Efficiency of chitosans applied for flocculation of different bacteria. Water Res. 2002;36(19):4745–4752. doi: 10.1016/s0043-1354(02)00173-2. [DOI] [PubMed] [Google Scholar]
- Sun XX, Choi JK. Recovery and fate of three species of marine dinoflagellates after yellow clay flocculation. Hydrobiologia. 2004;519(1–3):153–165. [Google Scholar]
- Sun XX, Lee YJ, Choi JK, Kim EK. Synergistic effect of sophorolipid and loess combination in harmful algal blooms mitigation. Mar Pollut Bull. 2004;48(9–10):863–872. doi: 10.1016/j.marpolbul.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Yasumoto T, Seino N, Murakami Y, Murata M. Toxins produced by benthic dinoflagellates. Biol Bull. 1987;172(1):128–131. [Google Scholar]
- Yu ZM, Zou JZ, Ma XN. Application of clays to removal of red tide organisms: II. Coagulation of different species of red tide organisms with montmorillonite and effect of clay pretreatment. Chin J Oceanol Limnol. 1994;12(4):316–324. [Google Scholar]
- Zeng D, Wu J, Kennedy JF. Application of a chitosan flocculant to water treatment. Carbohydr Polym. 2008;71(1):135–139. [Google Scholar]
- Zhang ZG, Luan ZK, Zhao Y, Cui JH, Chen ZY, Li YZ. Breakage and regrowth of flocs coagulation with polyaluminum chloride (PACl) Environ Sci. 2007;28(2):346–351. [PubMed] [Google Scholar]
- Zou H, Pan G, Chen H. Effects of ionic strength on the flocculation and removal of cynaobacterial cells of Microcystis aeruginosa by clays. Environ Sci. 2005;26:148–151. [PubMed] [Google Scholar]
- Zou H, Pan G, Chen H, Yuan X. Removal of cyanobacterial blooms in Taihu Lake using local soils II. Effective removal of Microcystis aeruginosa using local soils and sediments modified by chitosan. Environ Pollut. 2006;141(2):201–205. doi: 10.1016/j.envpol.2005.08.042. [DOI] [PubMed] [Google Scholar]