Abstract
The rate at which autosomal dominant polycystic kidney disease (ADPKD) progresses to end-stage renal disease varies widely and is determined by genetic and non-genetic factors. The ability to determine the prognosis of children and young adults with ADPKD is important for the effective life-long management of the disease and to enable the efficacy of emerging therapies to be determined. Total kidney volume (TKV) reflects the sum volume of hundreds of individual cysts with potentially devastating effects on renal function. The sequential measurement of TKV has been advanced as a dynamic biomarker of disease progression, yet doubt remains among nephrologists and regulatory agencies as to its usefulness. Here, we review the mechanisms that lead to an increase in TKV in ADPKD, and examine the evidence supporting the conclusion that TKV provides a metric of disease progression that can be used to assess the efficacy of potential therapeutic regimens in children and adults with ADPKD. Moreover, we propose that TKV can be used to monitor treatment efficacy in patients with normal levels of renal function, before the pathologic processes of ADPKD cause extensive fibrosis and irreversible loss of functioning renal tissue.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary renal disorder and the fourth leading cause of chronic kidney disease (CKD) worldwide1. The disease initiates in utero and typically progresses to end-stage renal disease (ESRD) by the 6th decade of life. In the majority of patients with ADPKD, glomerular filtration rate (GFR) remains within normal limits for several decades2. Consequently, in the early stages of ADPKD, when effective therapy would have maximal long-term benefits, GFR is unhelpful for diagnosing the disease and evaluating progression. Manifestations that indicate the presence of the ADPKD, such as hypertension, flank pain, haematuria, renal lithiasis or cyst infections, can, however, begin in childhood when GFR is within the normal limits1,3.
For most nephrologists, renal cysts are the central pathogenic feature that characterizes ADPKD. The combined volume of renal cysts in ADPKD, which is reflected by an increase in the physical dimensions of the kidneys, has been used since the 1980s to forecast progression to ESRD3–5. However, despite a wealth of laboratory and clinical data consistent with the view that serial measurements of total kidney volume (TKV) can predict the future decline of renal function, the value of this information is inadequately appreciated by attending physicians and regulatory agencies. Here, we review the pathogenic mechanisms that link the progressive increase in TKV to a predictable, progressive decline in GFR, and describe how this knowledge can be used in the clinical management of patients with ADPKD and in the design of clinical trials.
The pathogenesis of cyst formation
Cysts derive from renal tubules and in rare instances from the Bowman capsule, a derivative of tubule epithelium6 (FIG. 1). The principal element in the formation of renal cysts is a sustained increase in the number of cells lining the tubule wall. In vivo evidence from animal models of ADPKD indicates that the development of renal cysts is initiated by a cell or group of cells that harbour mutations in PKD1 or PKD2 (which encode polycystin- 1 and polycystin-2, respectively)7. The divergent tubule cells enter a continuous proliferation programme in response to molecular changes that are associated with the underlying mutation. The resultant increase in the number of cells leads to expansion of the tubule wall and dilation of a tubule segment or bulging of the cyst into the renal interstitium — two processes that are possibly influenced by abnormalities in planar cell polarity. Renal tubule cells normally divide and extend tubule length. In nascent cysts, cell polarization is not uniform and dividing cells cause radial extension of the tubule wall and the formation of a cyst. Macroscopic cysts that are observed clinically by ultrasonography, CT and MRI, were once tiny tubule sprouts that expanded relentlessly. Of note, the rate of increased cell proliferation is slow in comparison to that of most malignant cancers. Unlike cancer, patients with ADPKD experience relatively low rates of exponential-like cell proliferation for many decades, enabling individual cysts to eventually reach impressive dimensions exceeding 10 cm in diameter.
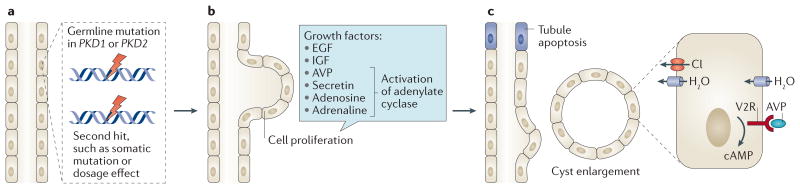
Figure 1. The formation and expansion of a tubule cyst.
a | Germline mutations in PKD1 or PKD2 do not direcly cause tubule cells to hyperproliferate. Rather, hyperproliferation and cyst formation might require a germline mutation in PKD1 or PKD2 to occur concomitantly with a somatic mutation or chemical injury. Such a ‘second hit’ would not necessarily affect a polycystin on the normal allele and could instead disrupt other related genes. A dosage threshold for polycystin might determine the timing and extent of cyst formation. b | Proliferation of tubule cells in response to growth factors results in expansion of the tubule wall, which starts to bulge into the interstitium. c | A point is reached at which the nascent cyst breaks free of the parent tubule, becoming an autonomous cyst. The separation of the cyst from the tubule might occur as a result of tubule apoptosis, which occurs via an unknown mechanism. The cyst expands and fills with fluid that is secreted into it by mechanisms involving cyclic (c)AMP-mediated chloride (Cl) influx and arginine vasopressin (AVP)-driven fluid intake. Individual cysts begin as structures with diameters of ∼0.10 mm and expand to >50 mm in advanced cases. EGF, epidermal growth factor; IGF, insulin-like growth factor; H20, water; V2R, arginine vasopressin receptor 2.
In contrast to the absorptive phenotype of healthy tubule epithelial cells, mural tubule cells in patients with ADPKD acquire a proclivity to secrete NaCl and fluid into the urine8. The combination of increased cell proliferation and fluid secretion promotes progressive enlargement of the renal cysts. Moreover, arginine vasopressin, which promotes cell proliferation and fluid secretion, is present at increased levels in patients with ADPKD and exacerbates renal cyst enlargement, enabling cysts to grow continuously for many decades9–11(FIG. 1c).
Cysts: a cause or consequence of disease
Renal cysts can form under a variety of conditions that are seemingly unrelated to polycystin mutations. Chemicals that increase cell oxidative stress induce the formation of renal cysts when administered to rodents12–14. Cysts also develop in humans with age and in patients with sclerosing glomerulopathies, arteriolar nephrosclerosis and diabetic nephropathy15. In light of evidence that renal cysts can develop independently of polycystin mutations, some investigators have suggested that cyst formation in diseases such as ADPKD begins following changes in the extracellular matrix that cause secondary changes in tubule epithelial cells16. For many years the prevailing hypothesis was that cysts do not form until a second process or ‘hit’ renders polycystin in the normal allele incapable of maintaining the normal cell phenotype17. Emerging evidence, however, suggests that a dosage threshold for polycystin determines the timing and extent of cyst formation in renal tubules18. These findings do not necessarily mean that the germ line allele bearing the poly cystin mutation cannot act alone to cause dysfunction in cell types other than those found in renal tubules. Evidence of abnormal vascular compliance19,20, changes in responses to vasodilators and vasoconstrictors21, and disturbance of osmoregulation22,23 in patients with ADPKD have raised the possibility that factors other than the anatomic distortion caused by cysts might contribute to renal dysfunction.
Conversely, indirect evidence from studies of animals with hereditary renal cystic disorders suggests that ADPKD begins within tubule epithelial cells and affects interstitial components secondarily. Examination of cysts in their early stages of development in proximal tubules of a rat model of ADPKD revealed a structurally normal epithelium adjacent to dedifferentiated mural cells that lacked apical brush borders24 (FIG. 2a). The dedifferentiated cells expressed phosphorylated ERK, a growth-promoting kinase, and beneath them the tubule basement membranes were thickened with a widened interstitium containing scattered mono nuclear cells. By contrast, the normal epithelium that did not express phosphorylated ERK rested on tubule basement membranes and interstitial matrix that appeared normal. These findings support the view that changes caused by mutations within certain tubule cells provoke cellular dedifferentiation, aberrant synthesis of the tubule basement membrane, and secondary migration of monocytes into the interstitium.
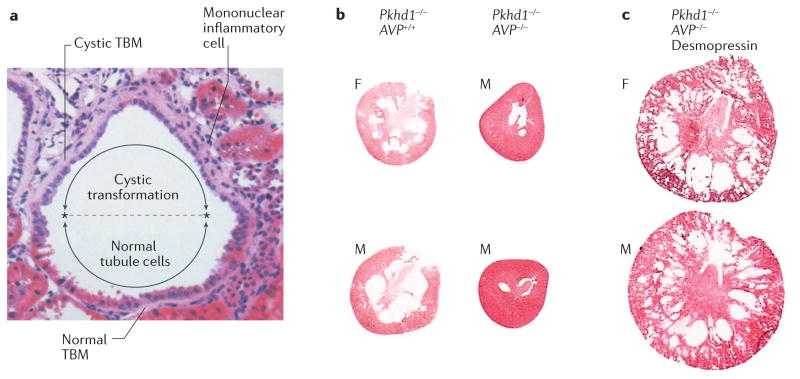
Figure 2. Features of early cyst formation.
a | Haematoxylin and eosin stain of a nascent renal cyst from a Han rat: a model of autosomal dominant polycystic kidney disease (ADPKD) caused by a missense mutation in ANKS6. The cells in the lower half of the image appear normal with brush borders typical of proximal tubules, whereas in the cystic upper half of the image the cells are flattened, with no brush borders and cramped nuclei. The extracellular matrix beneath the cystic cells is expanded and the tubule basement membrane (TBM) is thickened. Mononuclear inflammatory cells have invaded the interstitium adjacent to the cystic portion of the tubule. On microscopic analysis, a similar association between epithelial cell dedifferentiation and an abnormal underlying basement membrane has been observed in PKD1 and PKD2 murine models of ADPKD (J.J. Grantham & V.E. Torres, unpublished work). b | The first column (Pkhd1-/- AVP+/+) shows cross sections of kidneys from female (F) and male (M), 12-week-old rats, demonstrating an abundance of medullary cysts in the absence of Pkhd1 when arginine vasopressin (AVP) signalling is intact. In Pkhd1-/- AVP-/- rats (second column), which lack Pkhd1 and AVP signalling, very few cysts can be discerned. c | In Pkhd1-/- AVP-/- rats, administration of the AVP V2-receptor agonist, desmopressin, from 12–20 weeks of age results in the formation of visible cysts. Images reproduced with permission from Elsevier Ltd © Shizuko Nagao et al. Kidney Int. 63, 427–437 (2003).
More definitive evidence that cysts have a primary cellular origin is provided by a study of a rat model of autosomal recessive PKD (ARPKD), caused by mutations in Pkhd1 (REF. 10). In this model, cysts, which primarily develop in the collecting duct, are targets of arginine vasopressin V2-receptor inhibitors. Breeding of these rats with Brattleboro rats, which are unable to release vasopressin, generated double mutants that lacked circulating vasopressin. These rats exhibited very few small cysts and had normal renal function and interstitial morphology, despite the presence of homozygous Pkhd1 mutations. Administration of desmopressin at 12 weeks of age, led to the rapid development of renal cysts together with interstitial fibrosis and renal insufficiency (FIG. 2b). Moreover, renal fibrosis and declining function were only seen when cysts were present, demonstrating that mutated genes alone are insufficient to generate cysts, and that cysts are the primary offenders in this hereditary cystic disorder. This seminal study also strengthened the view that vasopressin, a normal constituent of plasma, has a central role in determining the number of cysts that form and the rate at which they grow.
Changes within the interstitial matrix in ADPKD probably occur secondary to the formation and enlargement of cysts. Cultured epithelial cells from human ADPKD cysts produce an array of chemokines and cytokines that have the potential to induce inflammation and interstitial matrix remodelling. In patients with ADPKD some of these cytokines and chemokines are excreted in the urine at levels above normal25–27. Moreover, renal cysts in animals with mutations in PKD1 orthologues also produce inflammatory chemo kines, supporting the notion that the interstitial changes observed in ADPKD are secondary to the production of inflammatory factors by renal cyst epithelial cells28,29.
Hypertension can develop in children with ADPKD even when the numbers and sizes of individual cysts are relatively modest. Renal tubules are normally 30–100 μm in diameter depending on the segment. When a cyst forms and progressively enlarges it stresses and extends adjacent capillaries, lymphatics, arterioles and venules, disturbing the renal circulation of blood and lymph. These changes can occur at microscopic levels well below the threshold of ultrasound, CT and MRI detection30,31. The relationship between hypertension and the presence of cysts has been clarified by studies in animal models. In one study hypertension and reductions in maximum urine osmolality and urine nitrate were observed only in the Pkd1-deficient mice that had renal cysts32. The investigators hypothesized that cyst expansion resulted in hypertension by reducing local blood flow and activating the intrarenal renin–angiotensin system. In another study, kidney cyst formation, along with enhanced expression of prorenin and angiotensinogen in the kidney, increased urinary levels of angiotensinogen and elevated blood pressure in mice lacking cilia (Ift88-knockouts) or polycystin-1 (Pkd1-knockouts)33. These data suggest that upregulation of the intra renal renin–angiotensin system is directly linked to the absence of cilia or polycystin-1 and contributes to the development of hypertension in PKD33.
Mechanisms of renal cyst enlargement
The rate at which a kidney enlarges in a patient with ADPKD depends on the number of cysts, the rates at which individual cysts expand, and the effects of intrinsic and extrinsic growth factors — TKV (the sum volume of the two kidneys) is a composite indicator of all of these variables.
Renal cyst formation
The formation of a renal cyst seems to be a stochastic event, which remains poorly understood. Cysts that form in utero can expand at rates many times faster than cysts that develop post-partum34. In some patients, in utero cyst formation and growth is so exuberant that at birth they are misdiagnosed as having ARPKD or bilateral Wilms tumours. In the vast majority of patients, however, in utero cyst formation is modest and patients are born without evidence of ADPKD, with renal cysts becoming evident only in the 2nd or 3rd decades of life. Patients with ADPKD associated with mutations in PKD2 form fewer cysts than those with mutations in PKD135. Genetic and acquired factors that can promote cyst formation, including excessive body size, increased dietary intake of salt, protein and acid precursors, and inflammatory chemokines, are the subject of intense study7,36–38.
Determinants of the rate of cyst growth
Each cyst in a polycystic kidney seems to function autonomously39. The expansion of individual cysts causes a concomitant increase in TKV, with the growth rates of individual cysts culminating in an exponential increase in the rate of total kidney growth. Mathematical models indicate that an exponential rate of kidney growth requires the growth rates of individual cysts to remain relatively constant. The TKV of each patient will therefore increase at a rate determined by the cyst number and the combined rates of growth of individual cysts. By this formulation, the oldest and largest cysts will have a more commanding effect on the change in TKV than will younger and smaller cysts.
The rates at which individual cysts grow within a poly cystic kidney are determined by intrinsic and extrinsic factors, both of which remain relatively constant throughout the life of the patient. The location of the specific mutation within the length of PKD1 or PKD2 does not seem to determine the rate of cyst formation or the intrinsic rate of cyst growth40,41. Truncating mutations in PKD1, however, cause more severe renal cystic disease than missense mutations41. As mentioned above, mutations in PKD2 lead to fewer cysts than mutations in PKD1, although, the combined rates of individual cyst growth are not appreciably different with either mutation. As patients with mutations in PKD2 have fewer cysts, they have smaller kidneys and reach ESRD nearly two decades later than those with mutations in PKD1 — a fact that favours the view that cysts cause the renal insufficiency that is eventually experienced by most patients.
Extrinsic factors that affect the rate of cyst and kidney growth in ADPKD have also been identified. Rapid cyst growth in utero could be caused, to some extent, by the high concentrations of growth factors that attend fetal development. Post-partum, cyst and kidney growth can be modified by growth factors, including epidermal growth factor42 and insulin-like growth factor43, and by activators of adenylyl cyclase, such as arginine vasopressin, secretin, adenosine and adrenaline8. Of these, vasopressin has a powerful effect on the growth of cysts derived from the distal tubules and collecting ducts of patients9. High water intake can suppress the release of arginine vasopressin. Water intake varies widely between patients, however, and in the vast majority of those with ADPKD, plasma levels of vasopressin fluctuate within the antidiuretic range and exert a tonic growth- promoting effect on cysts that bear vasopressinV2 receptors.
Pathological consequences of renal cysts
As mentioned earlier, renal cysts secrete cytokines and chemokines as they expand, which lead to inflammation and fibrosis in the adjacent interstitium. Cysts can also obstruct urine flow in the affected tubule and in adjacent renal tubules, which causes apoptosis of the upstream tubule segments and separation of the glomeruli from the proximal convoluted tubule (resulting in atubular glomeruli)44. Cysts that develop in the distal tubule and collecting duct segments can impede urine flow in the nephrons that drain into them, which can result in an astounding loss of viable upstream tubule structures in portions of the kidney in which cysts have not formed (FIG. 3). The pathological consequences resulting from obstruction by renal cysts might modify the rate of kidney enlargement and contribute to the reduction in GFR. Our observations of >100 sets of kidneys from patients with ADPKD and ESRD indicate that renal volume can cease to increase or can even decrease in advanced cases of ADPKD (J.J. Grantham & V. Torres, unpublished work). We hypothesize that such declines in renal volume result from the progression of robust fibrosis leading to contraction of the parenchyma, leaving the cysts and residual surviving nephrons encased within thick bands of scar tissue.
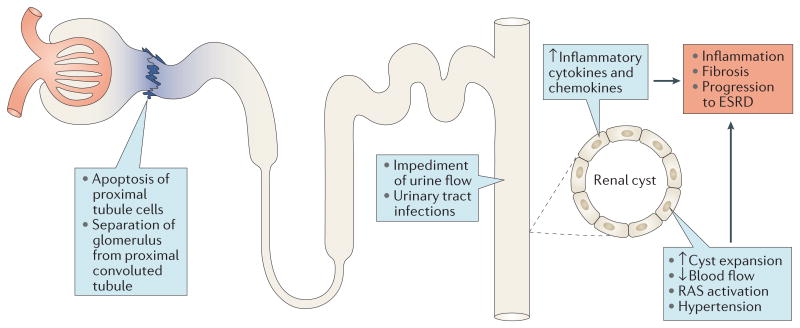
Figure 3. Potential influence of collecting duct cyst formation on upstream tubule segments and renal function.
The formation of a renal cyst reduces or stops flow in the collecting duct of origin and, through compression, in adjacent normal tubules, capillaries, venules and arterioles. These effects lead to activation of the renin–angiotensin–system (RAS), which results in hypertension. The reduced flow of urine in the upstream nephrons that drain into a collecting duct cyst leads to apoptosis of proximal tubule cells at the junction with the glomerulus, resulting in the formation of atubular glomeruli and disconnected downstream nephron segments that undergo extensive apoptosis. The degenerating tubules and expanding cysts release inflammatory cytokines and chemokines that contribute to fibrosis and progression to end-stage renal disease (ESRD).
TKV as a measure of ADPKD progression
Similar to other chronic progressive kidney disorders, ADPKD progression is typically assessed by changes in serum creatinine concentration or estimated GFR (eGFR). Consequently, patients diagnosed with ADPKD in adulthood are often told that their disease was most likely inactive during their first two to three decades of life, as serum creatinine levels are typically within normal limits during this time. We now know that irreversible structural changes appear early in childhood in patients with ADPKD and progress over their lifetime, despite apparently normal renal function during the first 20–30 years. This paradox might relate to the capacity of the kidney to compensate for the loss of functioning nephrons by increasing the rate of single nephron filtration in surviving nephrons45. The effects of compensation are most readily observed in living kidney donors, in whom the GFR of the residual kidney is only 22% lower than the GFR of two kidneys in age-matched controls46. Evidence suggests that similar compensatory adjustment occurs in the surviving nephrons of patients with slowly progressive renal diseases. The compensatory maintenance of GFR obfuscates the damage that is being done by the primary disease process47. Given the wide variance of GFR within and between individuals, measurement of GFR is an insensitive way to monitor the early, irreversible renal changes that occur in all slowly progressive renal disorders. Alternative biomarkers of disease progression, such as urinary albumin excretion for glomerular diseases, have been suggested, but as yet have not won widespread support from regulatory agencies48. In ADPKD, a reduction in renal blood flow seems to precede the decline in GFR, but the use of MRI to measure changes in renal blood flow requires further validation49.
An increase in TKV is widely accepted to be the dominant feature of ADPKD progression. TKV can be estimated from an equation that utilizes radiologic measurements of renal length, width and thickness. By contrast, CT scans and MRI permit accurate and reproducible volumetric reconstructions from the sum of individual slices of each kidney and can demonstrate changes in TKV over intervals as short as 6 months. In 2000, two research groups independently used CT to evaluate TKV as a surrogate marker of disease progression in longitudinal pilot studies of patients with ADPKD50,51. Preliminary evidence from these studies indicated that TKV increased from year to year, raising the possibility that the rate of TKV growth, which reflects the enlargement of cysts, might serve as a sensitive metric of disease progression. These results have been repeatedly confirmed. Fifteen longitudinal studies in hundreds of patients with ADPKD, ranging from 4 to 77 years of age now provide incontrovertible evidence that TKV increases annually in ADPKD (TABLE 1; FIG. 4). The mean rate of kidney enlargement ranges from 3.4% to 12.6% per year in patients receiving standard of care, although rates of <0.5% per year and >20% per year have been recorded in individual patients50,51. To our knowledge, no studies have attempted to explain the wide differences in kidney growth rates in these disparate cohorts.
Table 1. TKV measurements by CT and MRI in clinical studies.
| Reference | Mode of TKV measurement | Age range (no of patients) | TKV range (ml) | Longitudinal study (Yes/No) | Annual TKV growth rate (%) | Drug used in trial |
|---|---|---|---|---|---|---|
| Lee et al. (1981)75 | CT | Adult (10) | 406–4,950 | No | NA | NA |
| Thomsen et al. (1981)76 | CT | 16–66 (43) | 700–≥4,000 | No | NA | NA |
| King et al. (2000)50 | MRI | 21–50 (9) | 569–1,985 | Yes | 4.3 | NA |
| Sise et al. (2000)51 | CT | 25–46 (10) | 386–1,933 | Yes | 9.6 | NA |
| Ruggenti et al. (2005)77 | CT | 35–58 (12) | 2,435* | Yes | 5.9 | Octreotide |
| Grantham et al. (2006)52 | MRI | 15–46 (241) | 276–3,877 | Yes | 5.3 | NA |
| Antiga et al. (2006)78 | CT | 33–58 (13) | 1,469–4,295 | Yes | 6.3 | NA |
| Serra et al. (2010)62 | MRI | 18–40 (100) | 558–1,753 | Yes | ∼10.3 | Sirolimus |
| Walz et al. (2010)61 | MRI | 44* (433) | 308–7,681 | Yes | ∼12.6 | Everolimus |
| Cadnapaphornchai et al. (2011)79 | MRI | 4–21 (77) | ∼160–∼1,750‡ | Yes | 7.4 | NA |
| Tokiwa, et al. (2011)80 | MRI | 21–72 (73) | 405–9,652 | Yes | 3.4 | NA |
| Higashihara, et al. (2012)81 | MRI | 45* (86) | 1,839* | Yes | 5.6 | NA |
| Torres, et al. (2013)63 | MRI | 18–50 (1445) | 1,705/1,668§ | Yes | 5.5 | Tolvaptan |
| Caroli et al. (2013)66 | MRI | 37* (75) | 1,557/2,161§ | Yes | 6.6 | Octreotide |
| Chen et al. (2014)82 | MRI | 4–77 (532) | 1,265* | Yes | 5.7 | NA |
| Schrier et al. (2014)65 | MRI | 15–49 (558) | 1,164–1,264‖ | Yes | 6.6 | ACE inhibitor or ARB |
| Cadnapaphornchai et al. (2014)68 | MRI | 8–22 (110) | 571/572§ | Yes | 10.3 | Pravastatin |
Average at baseline.
Estimated from graphs.
Averages for control/experimental groups.
Averages of four groups at baseline.
ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CT, computed tomography; MRI, magnetic resonance imaging; NA, not applicable; TKV, total kidney volume.
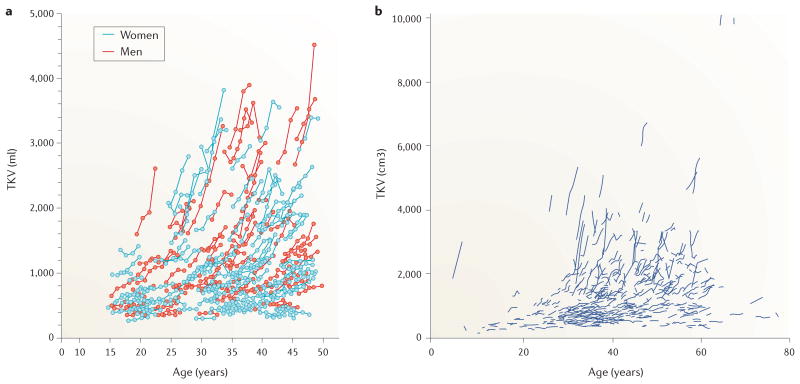
Figure 4. Relationship between age and total kidney volume (TKV) in patients with autosomal dominant polycystic kidney disease.
The relationships between age and TKV are illustrated for individual patients from two large longitudinal studies in a | the USA53 and b | in China82. An exponential-like pattern in TKV increase was observed in both studies. Considerable heterogeneity exists in the rates of TKV increase, ranging from <0.5% per year to >20% per year. The mean rate of TKV growth was 5.3% per year in participants from the US study and 5.7% per year in participants from the Chinese study. Part a | reproduced with permission from Massachusetts Medical Society © Jared J. Grantham et al. N. Engl. J. Med. 354, 2122–2130 (2006). Part b | reproduced with permission from PLOS © Dongping Chen. et al. PLoS ONE 9, e92232 (2014), which is licensed under a Creative Commons Attribution 4.0 International Licence. To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease longitudinal study of 241 patients with ADPKD and baseline eGFR >70 ml/min, explored the hypothesis that TKV is a sensitive indicator of disease progression52,53. After 12 years, during which GFR was measured by iothalamate clearance and renal MRI scans were obtained at 1, 2, 3, 6, 8, 10, and 12 years post-baseline35,39,52,54, several important facts emerged: cysts account for the sustained increase in kidney volume; each kidney of a pair enlarges at approximately the same rate with few exceptions; TKV progresses as an exponential-like process from birth to late adulthood; each patient has a signature rate of kidney growth; in relatively young patients GFR can remain within normal limits for several decades despite the presence of enlarging kidneys; GFR decline becomes detectable at variable ages and the subsequent rate of decline is inversely related to the rate of increase in TKV; kidneys are smaller and GFR decline is slower in patients with PKD2 mutations than in those with PKD1 mutations, owing to the formation of fewer cysts; TKV at baseline predicts the development of stage 3 CKD (as assessed using KDOQI criteria) within 8 years. The progressive increase in TKV with age has been confirmed in young children and in adults in the USA and elsewhere (TABLE 1). These findings support the conclusion that the sequential measurement of TKV provides a quantifiable index of disease progression. TKV can also serve as an indicator to evaluate the efficacy of candidate therapeutic agents that target cyst formation and growth.
Researchers who performed an analysis of 590 patients with ADPKD proposed classification of the disease into typical (class 1) ADPKD, which accounted for 95% of the kidneys analysed, or atypical (class 2) ADPKD on the basis of pre-specified imaging findings. Stratification of class 1 patients according to five height-adjusted rates of TKV growth in relation to age, revealed an increased frequency of ESRD after 10 years in patients with higher rates of TKV growth at baseline. The age at which ESRD was diagnosed was also lower in these patients, affirming the power of TKV to predict prognosis55. Of note, the measurement of TKV by segmentation of CT or MRI scans might be impractical in some countries. In circumstances in which CT and MRI are not available, determination of TKV by ellipsoid estimations or measurement of renal length using ultrasonography might be informative for the purpose of stratifying the risk of progression in patients with ADPKD55,56.
Despite the available evidence, the use of TKV as a measure of ADPKD severity or progression has met with substantial resistance. In response to a request from the FDA, the Polycystic Kidney Disease Outcomes Consortium (PKDOC) gathered retrospective and prospective TKV data from 2,355 patients with ADPKD to chart the decline in eGFR as a function of baseline TKV57. Patients with ‘preserved’ renal function (eGFR ≥50 ml/min/1.73 m2) or ‘reduced’ renal function (eGFR <50 ml/min/1.73 m2) and a TKV of >1 l had a greater risk of developing ESRD than those with a TKV of <1 l. Thus, a large weight of evidence indicates that TKV has a predominant role in determining the extent to which GFR can decline in patients with ADPKD. As a result of the PKDOC findings, the FDA and the European Medicines Agency have accepted TKV as a prognostic biomarker to select participants with ADPKD for clinical trials. This acceptance falls short, however, of approving the use of TKV as a surrogate end point. As changes in TKV occur as a direct consequence of the pathogenic processes of cyst growth in ADPKD, we think that TKV fulfils the criteria for a surrogate end point that is reasonably likely to predict clinical benefit; the FDA requires that in order to be approved, a therapeutic agent must have a beneficial effect on such an end point58. Our position is that TKV could be used as a surrogate primary end point in clinical trials provided that other biomarkers such as eGFR, urinary albumin excretion48, urine monocyte chemotactic protein-1 (REF. 25), and tubular biomarkers of toxicity such as kidney injury molecule-1 (REF. 59), and lipocalin60 do not raise concerns of nephrotoxicity of the agent being tested.
Using TKV to monitor treatment efficacy
Advanced ADPKD is associated with a number of ultrastructural changes in the kidneys involving extensive apoptosis and replacement of viable renal parenchyma by thick fibrotic bands. Nephrons in the mammalian kidney do not regenerate following renal injury, and reversal of established cysts and associated fibrosis, or restoration of renal function, in patients or models of ADPKD has not been demonstrated. In this light it is important to emphasize that effective treatment of ADPKD will likely be reflected by a slowing of disease progression rather than through restoration of renal structure and function.
Several clinical trials over the past few years have used sequential TKV measurements together with eGFR as indicators of the response to treatment (TABLE 1). In randomized controlled clinical trials, the mTOR inhibitors everolimus61 and rapamycin62 failed to consistently change the rate of TKV increase and had no effect on eGFR in patients with ADPKD. The 3-year TEMPO 3:4 trial, which included 1,445 patients with ADPKD and preserved eGFR levels, found that the vasopressin V2-receptor antagonist, tolvaptan, slowed the annual rate of TKV increase by 49% and reduced the rate of renal function decline by 31% (as measured by the slope of the reciprocal of serum creatinine concentration (1/Scr))63. A sub-analysis of the TEMPO 3:4 trial showed that tolvaptan also reduced the urinary excretion of albumin48 and monocyte chemotactic protein-1 (REF. 64).
The HALT-PKD trial assessed the effect of blood-pressure lowering using angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin II-receptor blockers on the rate of TKV growth in 558 patients with hypertension and ADPKD65. The rate of TKV growth was slowed and the rate of eGFR decline marginally diminished in participants assigned to a low blood- pressure target (95/60 mmHg to 110/75 mmHg) compared with those assigned to a standard blood-pressure target (120/70 mm Hg to 130/80 mm Hg). The reduction in the rate of TKV growth in the low blood-pressure group was independent of the antihypertensive used.
The long-acting somatostatin analogue, octreotide, which inhibits the production of cAMP, was assessed in the 3-year ALADIN trial66, in which 75 participants with ADPKD were randomly assigned to receive either octreotide or a placebo. At 1 year and at 3 years, the percent increase in TKV was lower in patients who received octreotide than in those who received placebo, whereas the overall rate of GFR decline tended to be slower in the octreotide group than in the placebo group but did not reach statistical significance. The results of a larger randomized controlled clinical trial assessing the efficacy of another long-acting somatostatin analogue, lanreotide, to halt disease progression in 18–60 year-old patients with ADPKD and stage 3 CKD will become available later this year67.
In a 3-year pilot study, pravastatin, which inhibits vasopressin-mediated MAP kinase stimulation of cyst growth, slowed TKV growth in children with ADPKD. eGFR levels remained within the normal range throughout the trial68. Additional studies over longer periods of treatment are needed to determine if statins also reduce the rate of GFR decline.
In the light of current knowledge, we think that an inverse, sustained association between TKV and GFR in ADPKD is indisputable. A host of preclinical studies in animals with renal cystic disease have identified drugs that, when administered early in the disease, slow the rates of TKV increase and GFR decline, so extend lifespan69. By contrast, the extent to which the increase in TKV causes the decline in GFR in patients with ADPKD has not been clearly resolved by the clinical trials discussed above70. Trials that were designed to test the hypothesis that the increase in TKV is responsible for the decline in GFR in ADKPD, have yielded mixed results. The most convincing therapeutic effect was found for tolvaptan in the TEMPO 3:4 trial63. A post hoc analysis of the trial data revealed that in patients with stage 1, stage 2 and stage 3 CKD at baseline, tolvaptan treatment reduced the rate of TKV growth by 1.99%, 3.12%, and 2.61% per year, and reduced annual eGFR decline by 0.40 ml/min/1.73 m2, 1.13 ml/min/1.73 m2, and 1.66 ml/min/1.73 m2, respectively71. In the HALT-PKD trial, intensive compared to standard blood- pressure control significantly slowed kidney growth without a significant overall effect on the rate of eGFR decline. Nevertheless, the more intensive blood-pressure lowering strategy did slow the rate of decline in GFR after the first 4 months of treatment65. Furthermore, a post hoc analysis of the HALT-PKD trial showed more pronounced and significant effects of intensive blood-pressure control among patients who had rapidly progressive disease55,72. These patients not only showed improvements in the rate of TKV increase and eGFR decline after the first 4 months of treatment, but also in the overall rate of eGFR decline. These results suggest that the beneficial effects of a drug on eGFR might be more clearly demonstrated in patients with rapid disease progression in whom the decline in GFR has started than in patients with slowly progressive disease and preserved GFR.
Regulatory agencies must show that a drug has a substantial beneficial effect on GFR before it can be approved to treat ADPKD. This mandate presents investigators and clinicians who want to treat ADPKD before GFR declines with a conundrum — if patients with slowly progressive disease and normal GFR values are included in clinical trials the effect size of a drug will be diluted. Consequently, in our opinion, study populations for ADPKD clinical trials should be enriched with patients who have rapidly progressive disease and stage 2 or early stage 3 CKD in order to enable a robust effect on the rate of eGFR decline to be demonstrated.
The annual rate of TKV growth is approximately 5% per year52,54,62,63,65,66 (TABLE 1). This growth is promoted to a large extent by vasopressin, which stimulates mural cell proliferation and fluid secretion into the cysts. Tolvptan inhibits both cell proliferation and fluid secretion, whereas rapamycin, everolimus, and pravastatin target only cell proliferation. The use of agents with dual action, such as tolvaptan, and trial durations longer than 1–2 years might enable the confirmation of beneficial effects in future studies.
We think that change in TKV indicates disease progression at an earlier stage and with greater precision than change in eGFR. Moreover, enlarging kidneys frequently cause discomfort and physical dysfigurement long before they fail to function adequately73. Delaying kidney growth targets the major physical abnormality in ADPKD as well as the disruption of microscopic renal functional units that causes renal insufficiency.
Predicting TKV and GFR
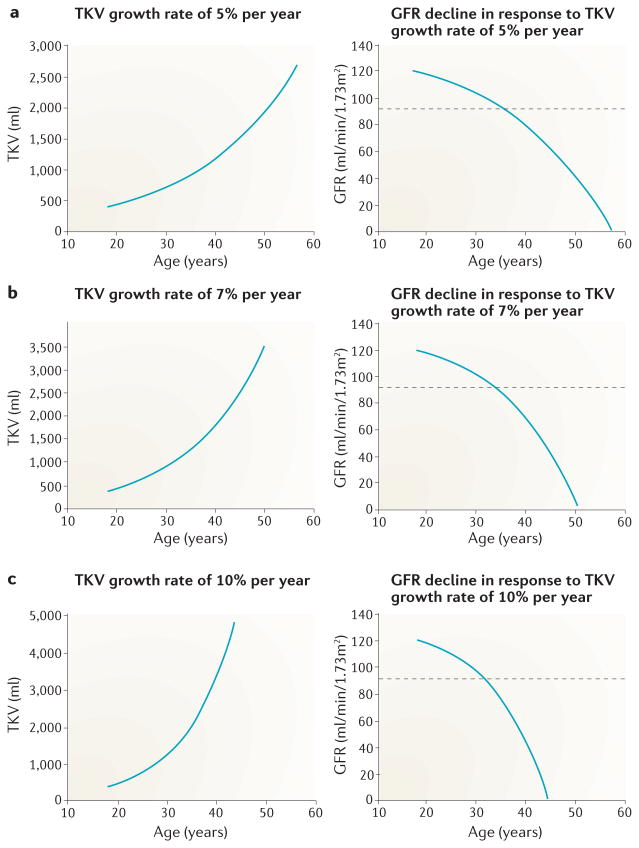
Assuming that the annual rate of TKV growth within individual patients is fairly stable, the degree to which their kidneys will enlarge in the future can be predicted. In this model, the annual rate of TKV growth increases exponentially with age (FIG. 5). Assuming that TKV is structurally linked to glomerular filtration, GFR in this model would exponentially decrease with advancing age — the inverse of the annual rate of TKV growth. GFR values might decline early in the course of disease but do not fall53 below the normal limit of 90 ml/min/1.73 m2 for two to three decades, after which GFR decline seems to accelerate.
Figure 5. Hypothetical inverse relationship between total kidney volume (TKV) and glomerular filtration rate (GFR) in patients with autsomal dominant polycystic kidney disease (ADPKD).
Increasing rates of TKV growth are shown by exponential-like curves. The corresponding GFR curves are based on the assumption that, in the absence of modifying factors, GFR decline is linked to the progressive increase in TKV in patients with ADPKD. At the age of 18 years, TKV is assumed to be 400 ml53 and GFR 120 ml/min/1.73 m2. GFR in each succeeding year is determined from the equation GFR = (GFRt-1) – (TKVt/400) where t is age and t-1 is age minus 1 year. GFR decline is determined by the fractional increase in TKV (TKVt/400). The model illustrates the initial decades during which GFR remains within normal limits shown by values above the dashed horizontal line depicting the lower limit of chronic kidney disease stage 1; the escalating rate of GFR decline over time; and the inverse relationship between GFR decline and the formation and expansion of cysts as measured by TKV. a | For a TKV increase of 5% per year, GFR reaches 0.0 ml/min/1.73 m2 at 57 years of age. b | For a TKV increase of 7% per year GFR reaches 0.0 ml/min/1.73 m2 at 50 years of age. c | For a TKV increase of 10% per year GFR reaches 0.0 ml/min/1.73 m2 at 43 years of age.
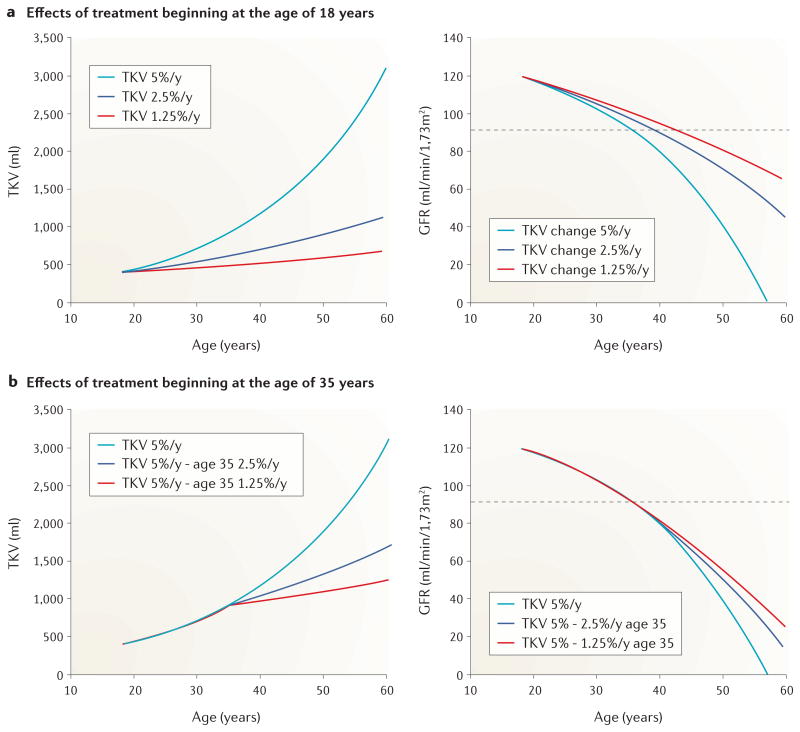
We hypothesize that the rate of GFR decline could be reduced markedly if patients with ADPKD receive treatment during the early stages of the disease (FIG. 6). We predict that early treatment has the potential to delay the progression of renal dysfunction by more than a decade. When therapy is started at 18 years of age, the reduction in the rate of TKV growth is predicted to shift from 5% to 2.5% or 1.25% per year; a change that yields a vastly different effect on GFR decline than that induced by starting therapy later in life. Starting treatment at 35 years of age, for example, would be expected to produce more modest benefit.
Figure 6. Hypothetical effect of starting therapy for autosomal dominant polycystic kidney disease (ADPKD) at 18 years or 35 years of age.
These graphs, which are based on the assumption that decline in glomerular filtration rate (GFR) in ADPKD is linked to the progressive increase in total kidney volume (TKV), show the potential effect of treatment on the annual rate of TKV growth. GFR values above the dashed horizontal line fall within chronic kidney disease stage 1. The baseline rate of 5% increase in TKV per year can be reduced to 2.5% or 1.25% per year regardless of whether treatment is started at 18 years or 35 years of age. a | If treatment is started at 18 years of age, a patient with a TKV growth rate of 5% per year will experience a decline in GFR to 0.0 ml/min/1.73 m2 at 57 years of age; a patient with a TKV growth rate of 2.5% per year will reach a GFR of 0.0 ml/min/1.73 m2 at around 70 years of age; a patient with a TKV growth rate of 1.25% per year will reach a GFR of 0.0 ml/min/1.73 m2 at >70 years of age. b | If treatment is initiated at 35 years of age, reductions in TKV from baseline and in the rate of GFR decline are more modest than if treatment is started at 18 years of age. A TKV growth rate reduction to 2.5% per year at the age of 35 years will lead to a GFR decline to 0.0 ml/min/1.73 m2 at 65 years of age, whereas a TKV growth rate reduction to 1.25% per year will lead to a GFR decline to 0.0 ml/min/1.73 m2 at 70 years of age. The model illustrates that reductions in the rate of TKV growth might be associated with substantial preservation of renal function if treatment is initiated relatively early in life, and that beginning treatment by 35 years of age could provide a modest, but worthwhile preservation of renal function.
The hypothesized relationships between TKV growth and GFR decline presented above should not be taken literally; rather, they are an idealized view of the potential quantitative relationship between TKV growth and GFR decline. Large absolute changes in TKV will be greater than absolute changes in GFR; however, relative changes in TKV will likely be associated with a relative change in GFR. The TEMPO 3:4 trial reported a 49% reduction in the rate of TKV growth and a 31% reduction in the slope of 1/Scr in the tolvaptan group63. Using the CKD-EPI equation to estimate GFR, the rate of yearly eGFR decline was 1.0 ml/min/1.73 m2 less than in the tolvaptan group tham om the placebo group. Although statistically significant this effect was viewed by critics as being of minor clinical relevance74. According to our model, initiation of therapy at 35 years of age could be sufficient to reduce the rate of TKV growth by 50%, which could potentially extend useful renal function by approximately 8 years (FIG. 6). We believe that the models described above will help physicians to generate reasonable expectations for prolonging the renal function of patients with ADPKD.
Conclusions
As described in this Review, the sequential measurement of TKV provides a metric of disease progression that can be used to assess the efficacy of potential therapeutic regimens in children and adults with ADPKD. TKV is inherently linked to the pathogenesis of disease, justifying the use of TKV as a measure of disease progression. Importantly, it is reasonable to suggest that TKV can be used to monitor treatment efficacy in patients with normal eGFR levels before the progression of pathologic processes cause extensive fibrosis and irreversible loss of functioning renal tissue, leading to measure able declines in renal function. The association of reductions in the rates of kidney growth with reductions in GFR decline, as shown in the TEMPO trial, will need to be demonstrated in additional trials to strengthen the rationale for accepting TKV as a surrogate end point in clinical trials for ADPKD. As the possibility exists that an effect on kidney growth might not always be associated with protection of renal function, we propose that TKV should be considered an appropriate surrogate end point when a strong mechanistic argument exists to support the potential benefit of an interventional drug and when concerns regarding nephrotoxicity can be eliminated using a pre-specified panel of functional and chemical biomarkers.
The marketing of tolvaptan for the treatment of ADPKD has been approved in Japan, Canada, Europe, Switzerland and South Korea based largely on data from the TEMPO trial that used TKV as a primary end point. In the USA, a second trial (REPRISE) is examining the effect of tolvaptan on eGFR change, but not on TKV, in patients with CKD stage 2 to early stage 4. A robust outcome would resolve doubt about the effect size of the drug on eGFR decline and possibly move TKV closer to being accepted as a surrogate end point. Until an end point is available that can be used early in the disease process, pharmaceutical companies will be reluctant to invest in the evaluation of drugs targeting early events in cyst formation and growth — we believe that TKV stands an excellent chance of becoming that early surrogate end point.
Key points.
In autosomal dominant polycystic kidney disease (ADPKD), renal cyst formation begins in utero and continues throughout life
Renal cysts originate in tubules and contribute to the development of renal insufficiency
Individual renal cysts progressively expand at a constant rate that can differ widely from the growth rate of neighbouring cysts
Cysts disrupt the renal ultrastructure in the early stages of ADPKD and cause renin-dependent hypertension, albuminuria, interstitial inflammation, fibrosis, and the destruction of functioning nephrons.
Evidence indicates that the annual rate of kidney growth in ADPKD is inversely linked to the decline in glomerular filtration rate so can be used to predict future decline in glomerular function
Longitudinal studies in thousands of patients have provided evidence to validate the use of total kidney volume as a prognostic marker and as a potential indicator of treatment efficacy in ADPKD
Planar cell polarity.
Planar polarization refers to the spatial orientation of a group of cells, whereby the cells and their structures are orientated within the same plane.
Desmopressin.
A synthetic and highly specific vasopressin V2 agonist.
Acknowledgments
Competing interests: J.J.G and V.E.T have consulted for and received research grants from Otsuka Pharmaceutical Development and Commercialization, Inc.
Footnotes
Author contributions: J.J.G and V.E.T contributed equally to researching data for the article, discussion of the content, and revising or editing the manuscript before submission.
References
- 1.Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 2.Franz KA, Reubi FC. Rate of functional deterioration in polycystic kidney disease. Kidney Int. 1983;23:526–529. doi: 10.1038/ki.1983.51. [DOI] [PubMed] [Google Scholar]
- 3.Dalgaard OZ. Bilateral polycystic disease of the kidneys; a follow-up of 284 patients and their families. Dan Med Bull. 1957;4:128–133. [PubMed] [Google Scholar]
- 4.Levine E, Grantham JJ. The role of computed tomography in the evaluation of adult polycystic kidney disease. Am J Kidney Dis. 1981;1:99–105. doi: 10.1016/s0272-6386(81)80036-4. [DOI] [PubMed] [Google Scholar]
- 5.Gabow PA, Ikle DW, Holmes JH. Polycystic kidney disease: prospective analysis of nonazotemic patients and family members. Ann Intern Med. 1984;101:238–247. doi: 10.7326/0003-4819-101-2-238. [DOI] [PubMed] [Google Scholar]
- 6.Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987;31:1145–1152. doi: 10.1038/ki.1987.121. [DOI] [PubMed] [Google Scholar]
- 7.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta. 2011;1812:1291–1300. doi: 10.1016/j.bbadis.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:102–108. doi: 10.1681/ASN.2007060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer E, et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:361–368. doi: 10.2215/CJN.04560510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner KD, Jr, Evan AP. Renal cystic disease induced by diphenylthiazole. Kidney Int. 1983;24:43–52. doi: 10.1038/ki.1983.124. [DOI] [PubMed] [Google Scholar]
- 13.Gardner KD, Jr, Evan AP, Reed WP. Accelerated renal cyst development in deconditioned germ-free rats. Kidney Int. 1986;29:1116–1123. doi: 10.1038/ki.1986.116. [DOI] [PubMed] [Google Scholar]
- 14.Gardner KD, Jr, et al. Endotoxin provocation of experimental renal cystic disease. Kidney Int. 1987;32:329–334. doi: 10.1038/ki.1987.213. [DOI] [PubMed] [Google Scholar]
- 15.Grantham JJ. Acquired cystic kidney disease. Kidney Int. 1991;40:143–152. doi: 10.1038/ki.1991.192. [DOI] [PubMed] [Google Scholar]
- 16.Carone FA, Kanwar Y. Tubular cell and matrix changes in renal cystic disease. Contrib Nephrol. 1993;101:1–6. doi: 10.1159/000422098. [DOI] [PubMed] [Google Scholar]
- 17.Torres VE, Harris PC. Polycystic kidney disease in 2011: connecting the dots toward a polycystic kidney disease therapy. Nat Rev Nephrol. 2012;8:66–68. doi: 10.1038/nrneph.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int. 2015;88:699–710. doi: 10.1038/ki.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr Hypertens Rev. 2013;9:2–11. doi: 10.2174/1573402111309010002. [DOI] [PubMed] [Google Scholar]
- 20.Nowak KL, Farmer H, Cadnapaphornchai MA, Gitomer B, Chonchol M. Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw013. http://dx.doi.org/10.1093/ndt/gfw013. [DOI] [PMC free article] [PubMed]
- 21.Merta M, et al. Role of endothelin and nitric oxide in the pathogenesis of arterial hypertension in autosomal dominant polycystic kidney disease. Physiol Res. 2003;52:433–437. [PubMed] [Google Scholar]
- 22.Devuyst O, Torres VE. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:459–470. doi: 10.1097/MNH.0b013e3283621510. [DOI] [PubMed] [Google Scholar]
- 23.Bichet DG. A defect in vasopressin secretion in autosomal dominant polycystic kidney disease. Kidney Int. 2012;82:1051–1053. doi: 10.1038/ki.2012.271. [DOI] [PubMed] [Google Scholar]
- 24.Nagao S, et al. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;63:427–437. doi: 10.1046/j.1523-1755.2003.00755.x. [DOI] [PubMed] [Google Scholar]
- 25.Zheng D, et al. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2003;14:2588–2595. doi: 10.1097/01.asn.0000088720.61783.19. [DOI] [PubMed] [Google Scholar]
- 26.Meijer E, et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am J Kidney Dis. 2010;56:883–895. doi: 10.1053/j.ajkd.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Azurmendi PJ, et al. Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol Dial ransplant. 2009;24:2458–2463. doi: 10.1093/ndt/gfp136. [DOI] [PubMed] [Google Scholar]
- 28.Karihaloo A, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2011;22:1809–1814. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swenson-Fields KI, et al. Macrophages promote polycystic kidney disease progression. Kidney Int. 2013;83:855–864. doi: 10.1038/ki.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74:1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeman T, et al. Blood pressure and renal function in autosomal dominant polycystic kidney disease. Pediatr Nephrol. 1997;11:592–596. doi: 10.1007/s004670050343. [DOI] [PubMed] [Google Scholar]
- 32.Fonseca JM, et al. Renal cyst growth is the main determinant for hypertension and concentrating deficit in Pkd1-deficient mice. Kidney Int. 2014;85:1137–1150. doi: 10.1038/ki.2013.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saigusa T, et al. Activation of the intrarenal reninangiotensin-system in murine polycystic kidney disease. Physiol Rep. 2015;3:e12405. doi: 10.14814/phy2.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol. 2010;5:889–896. doi: 10.2215/CJN.00550110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PC, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2006;17:3013–3019. doi: 10.1681/ASN.2006080835. [DOI] [PubMed] [Google Scholar]
- 36.Helal I, et al. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2013;28:380–385. doi: 10.1093/ndt/gfs417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klawitter J, et al. Bioactive lipid mediators in polycystic kidney disease. J Lipid Res. 2013;55:1139–1149. doi: 10.1194/jlr.P042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kistler AD, et al. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS ONE. 2013;8:e53016. doi: 10.1371/journal.pone.0053016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grantham JJ, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–116. doi: 10.1038/sj.ki.5002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heyer CM, et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:2872–2884. doi: 10.1681/ASN.2015050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang YH, et al. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;27:1861–1868. doi: 10.1681/ASN.2015060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harskamp LR, et al. Urinary EGF receptor ligand excretion in patients with autosomal dominant polycystic kidney disease and response to tolvaptan. Clin J Am Soc Nephrol. 2015;10:1749–1756. doi: 10.2215/CJN.09941014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Zhang Y, Yuan L, Fu L, Mei C. Rosiglitazone inhibits insulin-like growth factor1-induced polycystic kidney disease cell growth and p70S6 kinase activation. Mol Med Rep. 2013;8:861–864. doi: 10.3892/mmr.2013.1588. [DOI] [PubMed] [Google Scholar]
- 44.Galarreta CI, et al. Tubular obstruction leads to progressive proximal tubular injury and atubular glomeruli in polycystic kidney disease. Am J Pathol. 2014;184:1957–1966. doi: 10.1016/j.ajpath.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayslett JP, Kashgarian M, Epstein FH. Functional correlates of compensatory renal hypertrophy. J Clin Invest. 1968;47:774–799. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahim HN, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bricker NS. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N Engl J Med. 1972;286:1093–1099. doi: 10.1056/NEJM197205182862009. [DOI] [PubMed] [Google Scholar]
- 48.Gansevoort RT, et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 trial. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv422. http://dx.doi.org/10.1093/ndt/gfv422. [DOI] [PMC free article] [PubMed]
- 49.Torres VE, et al. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2007;2:112–120. doi: 10.2215/CJN.00910306. [DOI] [PubMed] [Google Scholar]
- 50.King BF, et al. Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:1505–1511. doi: 10.1681/ASN.V1181505. [DOI] [PubMed] [Google Scholar]
- 51.Sise C, et al. Volumetric determination of progression in autosomal dominant polycystic kidney disease by computed tomography. Kidney Int. 2000;58:2492–2501. doi: 10.1046/j.1523-1755.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 52.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–157. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
- 53.Grantham JJ, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 54.Chapman AB, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irazabal MV, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhutani H, et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88:146–151. doi: 10.1038/ki.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrone RD, et al. Qualification of total kidney volume as a prognostic biomarker for use in clinical trials evaluating patients with polycystic kidney disease. Am J Kidney Dis. 2014;63:B119. [Google Scholar]
- 58.U.S. Department of Health & Human Services, Food & Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics. 2014 http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf.
- 59.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 60.Trachtman H, et al. Urinary neutrophil gelatinase-associated lipocalcin in D + HUS: a novel marker of renal injury. Pediatr Nephrol. 2006;21:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 61.Walz G, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 62.Serra AL, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 63.Torres VE, Gansevoort RT, Czerwiec FS. Tolvaptan in autosomal dominant polycystic kidney disease. N Engl J Med. 2013;368:1259. doi: 10.1056/NEJMc1300762. [DOI] [PubMed] [Google Scholar]
- 64.Grantham JJ, et al. Tolvaptan suppresses monocyte chemotactic protein-1 in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw060. http://dx.doi.org/10.1093/ndt/gfw060. [DOI] [PMC free article] [PubMed]
- 65.Schrier RW, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caroli A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382:1485–1495. doi: 10.1016/S0140-6736(13)61407-5. [DOI] [PubMed] [Google Scholar]
- 67.Meijer E, et al. Rationale and design of the DIPAK 1 study: a randomized controlled clinical trial assessing the efficacy of lanreotide to Halt disease progression in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2014;63:446–455. doi: 10.1053/j.ajkd.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cadnapaphornchai MA, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014;9:889–896. doi: 10.2215/CJN.08350813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torres VE, Harris PC. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J Intern Med. 2007;261:17–31. doi: 10.1111/j.1365-2796.2006.01743.x. [DOI] [PubMed] [Google Scholar]
- 70.Ong AC, Devuyst O, Knebelmann B, Walz G, ERA-EDTA Working Group for Inherited Kidney Diseases Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385:1993–2002. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 71.Torres VE, et al. Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: results from the TEMPO 3:4 trial. Clin J Am Soc Nephrol. 2016;11:803–811. doi: 10.2215/CJN.06300615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irazabal MV, et al. Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the HALT-PKD clinical trial. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw294. http://dx.doi.org/10.1093/ndt/gfw294. [DOI] [PMC free article] [PubMed]
- 73.Grantham JJ. Rationale for early treatment of polycystic kidney disease. Pediatr Nephrol. 2015;30:1053–1062. doi: 10.1007/s00467-014-2882-8. [DOI] [PubMed] [Google Scholar]
- 74.Cardiovascular and Renal Drugs Advisory Committee Tolvaptan: slowing progression of Autosomal Dominant Polycystic Kidney Disease (ADPKD) 2013 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM364583.pdf.
- 75.Lee KR, Grantham JJ, Cook PN. Volume estimation from computed tomography for polycystic kidney disease. Automedica. 1981;4:75–79. [Google Scholar]
- 76.Thomsen HS, Madsen JK, Thaysen JH, Damgaard-Petersen K. Volume of polycystic kidneys during reduction of renal function. Urol Radiol. 1981;3:85–89. doi: 10.1007/BF02927815. [DOI] [PubMed] [Google Scholar]
- 77.Ruggenenti P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–216. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 78.Antiga L, et al. Computed tomography evaluation of autosomal dominant polycystic kidney disease progression: a progress report. Clin J Am Soc Nephrol. 2006;1:754–760. doi: 10.2215/CJN.02251205. [DOI] [PubMed] [Google Scholar]
- 79.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW. Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol. 2011;6:369–376. doi: 10.2215/CJN.03780410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tokiwa S, Muto S, China T, Horie S. The relationship between renal volume and renal function in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2011;15:539–545. doi: 10.1007/s10157-011-0428-y. [DOI] [PubMed] [Google Scholar]
- 81.Higashihara E, et al. Renal disease progression in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2012;16:622–628. doi: 10.1007/s10157-012-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen D, et al. Clinical characteristics and disease predictors of a large Chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS ONE. 2014;9:e92232. doi: 10.1371/journal.pone.0092232. [DOI] [PMC free article] [PubMed] [Google Scholar]