Abstract
Paralytic Shellfish Poisoning (PSP) toxins are annually recurrent along the Massachusetts coastline (USA), which includes many small embayments and salt ponds. Among these is the Nauset Marsh System (NMS), which has a long history of PSP toxicity. Little is known, however, about the bloom dynamics of the causative organism Alexandrium fundyense within that economically and socially important system. The overall goal of this work was to characterize the distribution and dynamics of A. fundyense blooms within the NMS and adjacent coastal waters by documenting the distribution and abundance of resting cysts and vegetative cells. Cysts were found predominantly in three drowned kettle holes or salt ponds at the distal ends of the NMS – Salt Pond, Mill Pond, and Town Cove. The central region of the NMS had a much lower concentration of cysts. Two types of A. fundyense blooms were observed. One originated entirely within the estuary, seeded by cysts in the three seedbeds. These blooms developed independently of each other and of the A. fundyense population observed in adjacent coastal waters outside the NMS. The temporal development of the blooms was different in the three salt ponds, with initiation differing by as much as 30 days. These differences do not appear to reflect the initial cyst abundances in these locations, and may simply result from higher cell retention and higher nutrient concentrations in Mill Pond, the first site to bloom. Germination of cysts accounted for a small percentage of the peak cell densities in the ponds, so population size was influenced more by the factors affecting growth than by cyst abundance. Subsurface cell aggregation (surface avoidance) limited advection of the vegetative A. fundyense cells out of the salt ponds through the shallow inlet channels. Thus, the upper reaches of the NMS are at the greatest risk for PSP since the highest cyst abundances and cell concentrations were found there. After these localized blooms in the salt ponds peaked and declined, a second, late season bloom occurred within the central portions of the NMS. The timing of this second bloom relative to those within the salt ponds and the coastal circulation patterns at that time strongly suggest that those cells originated from a regional A. fundyense bloom in the Gulf of Maine, delivered to the central marsh from coastal waters outside the NMS through Nauset Inlet. These results will guide policy decisions about water quality as well as shellfish monitoring and utilization within the NMS and highlight the potential for “surgical” closures of shellfish during PSP events, leaving some areas open for harvesting while others are closed.
Keywords: paralytic shellfish poisoning, Alexandrium fundyense, dinoflagellate cysts, bloom dynamics, retention mechanism, Nauset Marsh System
1. Introduction
Harmful algal blooms (HABs) have severe economic and ecological consequences and cause serious public health problems in many coastal areas of the world (Smayda, 1990; Anderson et al., 2002; GEOHAB, 2001). In the U.S. the most serious and widespread manifestation is paralytic shellfish poisoning (PSP), a syndrome caused by human ingestion of shellfish that accumulate toxins from dinoflagellates, predominantly in the genus Alexandrium (Shumway et al., 1988; Anderson et al., 2000). In the northeastern U.S., PSP events occur annually along much of the New England coastline. Anderson (1997) described several Alexandrium1 habitats in the region that included both large-scale blooms in the coastal waters of the Gulf of Maine and small-scale blooms isolated in small embayments and salt ponds of southern New England.
The recurrent large-scale A. fundyense blooms in the Gulf of Maine have been intensively studied over many years yielding significant improvement in our understanding of their transport pathways and bloom dynamics (Franks and Anderson, 1992; Townsend et al., 2001; Anderson et al. 2005a). Several conceptual models were proposed for the region (Anderson et al., 2005b; McGillicuddy et al., 2005; Townsend et al., 2001), derived from different approaches, but with many features in common. Gulf of Maine regional blooms are thought to originate through the germination of A. fundyense resting cysts found in two major “seedbeds” – one in the Bay of Fundy and one offshore of Penobscot and Casco Bays in mid-coast Maine (Anderson et al. 2005c). Motile, vegetative cells derived from cyst germination at these locations grow and are transported within the complex Maine coastal current (MCC) system, with onshore and alongshore transport driven predominantly by the MCC flow and episodic wind forcing. These regional blooms can extend far down the coast from the original seedbeds, occasionally reaching outer Cape Cod and even the islands of Nantucket and Martha’s Vineyard, as they did during a massive regional bloom in 2005 (Anderson et al., 2005a). During that bloom, it was never clear whether high concentrations of cells observed near the Nauset Inlet along the outer Cape or the toxicity that occurred from there to the offshore islands of Martha’s Vineyard and Nantucket were from the large regional A. fundyense bloom, or instead were caused by cells produced within the Nauset Marsh System (NMS) and exported into the alongshore coastal flow to the outer Cape and offshore islands (Anderson et al., 2005a).
In contrast to the Gulf of Maine blooms in open coastal waters, the NMS is subject to recurrent, annual A. fundyense blooms that are thought to originate within the estuary (Anderson et al., 1983). The NMS supports thriving commercial and recreational shellfishing industries, and is therefore an economically and socially important system. This system is impacted by PSP toxins on a near-annual basis. Over the last several decades PSP toxicity in the NMS has worsened significantly in both intensity and duration. From 1975 to 1991, Salt Pond was closed to shellfishing roughly 50% of the time, but in the last 20 years, it has been closed 19 times (95%). Anecdotal reports also suggest that PSP toxicity events are occurring earlier and lasting longer. Toxicity generally results in the closure of shellfishing within the entire NMS for several months.
There are several explanations for these trends, including stimulation of the A. fundyense cells by eutrophication as a result of the rapid development of the watershed. Additionally, two major storms altered flushing patterns and dispersed cysts within the NMS in 1991, potentially affecting the magnitude of subsequent blooms.
A. fundyense blooms within two ponds in the NMS (Salt Pond and Mill Pond) were studied nearly 30 years ago (Anderson et al., 1983), but very little since. They are believed to be localized phenomena due to the restrictive nature of Nauset Inlet connecting to the open ocean, and the swimming behavior of the A. fundyense cells within the salt ponds (Anderson et al., 1983; Anderson and Stolzenbach, 1985). With regard to the latter, a retention mechanism for A. fundyense was described whereby vertically migrating A. fundyense cells stop swimming upwards when they reach a light level of ∼150 μE m−2 sec−1. This keeps the bulk of the population below the thin, low-salinity surface lens that is flushed out of the salt ponds within each ebb tide during daylight hours (Anderson and Stolzenbach, 1985). During the nighttime, cells aggregate in bottom waters, again avoiding advective losses with the surface layer. This retention of cells facilitates the local deposition of cysts that re-inoculate populations in the overlying waters in subsequent years – hence the point-source, self-seeding characterization of these blooms (Anderson et al., 1983).
From a management perspective, when the blooms occur and shellfish toxicity rises above quarantine levels, the entire NMS is closed for harvest. Site-specific (‘surgical’) closures of the shellfish beds have been considered, since some shellfish areas show high levels of toxicity at times, while other areas of the NMS remain below action levels. However, the lack of knowledge on the mechanism for bloom initiation and dynamics has limited the ability of resource managers to make these policy decisions.
The overall goal of this study was to characterize the distribution and dynamics of A. fundyense blooms within the NMS and adjacent coastal waters, so that information could guide policy decisions about resource monitoring and utilization. Specific objectives were 1) to determine the distribution and abundance of A. fundyense resting cysts within the NMS to establish the potential for local initiation of blooms; and 2) to determine the spatial and temporal variability of A. fundyense blooms in the salt ponds, channels, inlet, and adjacent coastal waters of the NMS as an indication of the relative importance of in situ growth within the system vs. advection from outside. Ultimately, the cyst and motile cell distributions will provide the biological input for biological/physical numerical models of the NMS similar to those already developed and used for management of the large scale Gulf of Maine blooms (e.g., McGillicuddy et al., 2005; He et al., 2008).
2. Materials and methods
2.1 Description of the study area
The NMS is a shallow estuary with extensive marshes protected from the Atlantic Ocean by a highly dynamic barrier beach with a single connection through Nauset Inlet (Fig. 1). The central estuary (hereafter termed the “central marsh”) connects to three separate drowned kettle ponds that are significantly deeper (~5–11 m maximum depth): Salt Pond (maximum depth ~9 m at station 21), Town Cove (maximum depth ~5 m at station 1), and Mill Pond (maximum depth ~11m at station 11). (Town Cove is more open at its mouth than the other two sites, but will still be referred to as a “salt pond” in this study). In these salt ponds, tidal currents are weaker and stratification due to salinity and temperature gradients is much stronger than in the shallower central marsh. Narrow, generally well-mixed shallow channels link the terminal endpoints of the system to the central estuary.
Fig. 1.
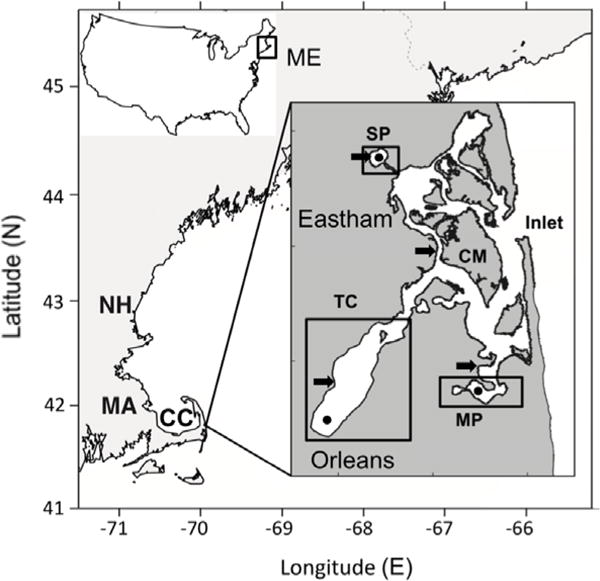
Map of the Nauset Marsh System (NMS) showing the location of the study site on outer Cape Cod (CC), Massachusetts (MA). Maps of the United States of America (US) and of the Gulf of Maine region (US northeast coast) are shown for geographical reference ME: Maine, NH: New Hampshire. The detailed insert shows the general location of the two Towns (Eastham in the northern half and Orleans in the southern half of the NMS) and the sub-systems of the NMS that were divided for analyses. MP: Mill Pond, SP: Salt Pond, TC: Town Cove, CM: Central Marsh, Inlet: the connection of the marsh with the Atlantic Ocean. The arrows indicate the locations of the weekly shellfish samples. Black dots indicate the locations of the deepest stations at Town Cove (station 1, ~ 5m), Mill Pond (station 11, ~11m) and Salt Pond (station 21, ~9m).
The mean depth of the system at Mean Low Water is <2m and the tidal range is 1–2m, so sampling was performed around high tide using shallow-draft small boats. Freshwater discharge into the NMS is dominated by groundwater from the Nauset and Monomoy lenses of the Cape Cod aquifer system (Colman and Masterson, 2007) and annual precipitation of about 100 cm yr−1. There are no riverine inputs into the NMS.
2.2 Cyst sampling
Sediment samples for cyst enumeration were collected on three days in the autumns of 2008 and 2009 (end of October and beginning of November) at 73 stations distributed throughout the NMS (Fig. 2). The same stations were sampled both years. The samples were obtained using a hand-deployed Petite Ponar Grab (Wildco, Inc). The top 0–1cm and the 1–3cm sediment layers were immediately sub-cored from the grab sample using a cutoff syringe barrel. The samples were transported and stored in dark and cold (4°C) conditions. Within 2 days from collection aliquots (5 cm3) from each sediment layer were diluted to 25ml with filtered seawater, sonicated with a Branson Sonifier 250 at a constant 40-W output for 1 min, and sieved to yield a clean 20–100 μm size fraction (Anderson et al., 2003). The samples were preserved in formalin and then exchanged into cold methanol before storage −20°C. A. fundyense cysts were counted within 3 months from processing according to standard methods for cyst identification (Anderson et al., 2003) using primuline to stain the cysts (Yamaguchi et al., 1995). Briefly, 9 ml of the sonified sediment sample were preserved by the addition of 1 ml of 10% formalin and stored at 4°C for at least 30 min. These samples were then centrifuged for 10 min with the overlying formalin removed by aspiration. The remaining pellet was suspended with 10 ml of methanol and stored at −20°C for at least 48 h. The samples were centrifuged and aspirated as before, brought up to 9 ml with Milli-Q (MQ) water plus 1 ml of primuline stain (2 mg ml−1). After staining for 30 min, the samples were centrifuged and aspirated for the final time and brought up to 5 ml with MQ water. A 1 ml aliquot was examined in a Sedgewick-Rafter slide. Green fluorescent-stained cysts were easily visualized and enumerated using a Zeiss Axioskop epifluorescence microscope at 160× with a FITC-chlorophyll long pass filter set (excitation band pass @ 450–490nm; emission long pass @ 520nm). Note that stained cysts lacking internal contents may be counted with this method. These are few in number however, as empty A.fundyense cysts are easily deformed during sonication and sieving, and most empty cysts therefore do not have the intact, elongated morphology used as a diagnostic feature for counting.
Fig. 2.
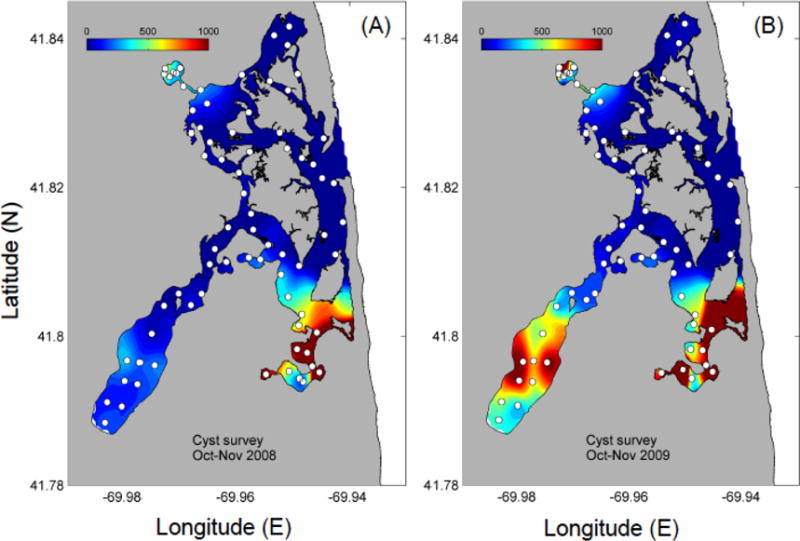
Contour maps of mean A. fundyense cyst concentrations (cysts cm−3) in: (a) 2008 and (b) 2009. White dots indicate sample sites.
2.3 Bloom sampling
High-water slack surveys were conducted at approximately weekly intervals within the NMS during the Alexandrium bloom season starting in late March and continuing until mid June 2009. The sampling happened in the morning or early afternoon depending on the timing of high tide. During the peak of the bloom in late April and early May, sampling frequency was increased to twice per week, for a total of 12 surveys. To survey at high tide, two small boats outfitted with cross-calibrated gear were required. Starting at Nauset Inlet just prior to slack-before-ebb, the boats simultaneously sampled about 30 stations total within 3 hours. This approach provided snapshots of the A. fundyense distributions at high tide, when the NMS was sufficiently deep for navigation and intrusion of coastal waters into the estuary was maximal. When conditions were unfavorable for safe operations, stations near Nauset Inlet were not sampled.
At each station, vertical profiles of salinity and temperature were obtained using two calibrated Sea-Bird SBE19 CTDs, one in each boat. Water samples for A. fundyense counts and nutrient analysis were collected with 2.5 L Niskin bottles. One sample was collected in the surface waters (< 1m) and another at 2–3m depending on the bottom depth. Only 1 sample was collected when the depth was < 2m. Three or four vertical samples were collected in the deep holes of Salt Pond and Mill Pond.
To determine if coastal Alexandrium populations were contributing to shellfish toxicity observed within the NMS, a transect off Nauset Inlet was sampled using the R/V Tioga (60ft) during 3 surveys (May 9, June 2 and June 9), a time when A. fundyense populations are common in the western Gulf of Maine and might therefore be present in the waters offshore of Cape Cod.
A. fundyense abundance was quantified using a whole cell format with an oligonucleotide probe (NA-1) that targets the North American ribotype Alexandrium catenella/tamarense/fundyense LSU rRNA (Scholin et al., 1994; Anderson et al., 2005d). In the field, 2 L of seawater were collected directly from Niskin bottles into sample-rinsed plastic bottles, concentrated by sieving onto 20-μm Nitex mesh, backwashed to a final volume of 14 ml with filtered seawater, preserved with 0.75 ml formalin (5% final), and stored on ice. Upon return to the laboratory, formalin was exchanged with cold methanol and the samples were stored at −20°C for no longer than 3 months before counting. A 7.0 ml aliquot of the sample material was filtered onto a 25 mm Cyclopore membrane (Whatman Inc., 5 μm pore size) (Scholin et al., 1997). The probing procedure was performed directly on the filters as described in Anderson et al. (2005d). For the labeling, the NA-1 probe (NA-1 Cy3; 5′ Cy3-AGTGCAACACTCCCACCA-3′) was conjugated on the 5′ end with a Cy3™ fluorochrome. The filter was semi-permanently mounted on a microscope slide, using 80% glycerol in 25× SET as a mounting medium to prevent fluorochrome degradation. Slides were stored cold (4 °C) and dark until microscopic analysis (within 1 day). Control samples containing cultured cells of A. fundyense were processed simultaneously to confirm the consistency of the staining procedure.
The entire filter (equivalent to 1 L of whole water) was enumerated for A. fundyense cells using a Zeiss Axioskop epifluorescence microscope equipped with a 10× Zeiss fluar objective (100× total magnification) coupled with a high-quality Cy3 filter set (Chroma #41032) that yielded bright orange A. fundyense target cells that could be easily discriminated from other similar autofluorescent particles (e.g., phycoerythrin-containing cells).
Nutrient samples were collected in acid-washed, sample-rinsed bottles, transported to the lab on ice and immediately frozen at −20°C. In the laboratory, each sample was analyzed within 3 months from collection for inorganic nitrate plus nitrite (hereafter termed “nitrate”), ammonium, silicate, and phosphate using a Lachat Instruments QuickChem 800 four-channel continuous flow injection system. This method is USEPA approved for nutrient analysis ranging from groundwater to the open ocean.
2.4 Toxicity data
Massachusetts Division of Marine Fisheries (MA-DMF) provided shellfish toxicity data from their routine biotoxin monitoring program. Samples were taken from mussel beds that were continuously exposed to the blooms. Within 24 hours of the start of each weekly survey, mussels were collected by shellfish officers from the Towns of Eastham and Orleans at 4 sites near the shore at 1–2m depth: Roberts Cove near Mill Pond, Salt Pond, Town Cove, and Hemenway Landing in the central marsh area in Eastham (Fig. 1).
2.5 Drifter trajectory
A single, satellite-tracked, surface drifter (1m depth) was released into the nearshore waters along the coast of Maine (near the entrance to Casco Bay) on April 9, 2009 as part of another study in that local region (Ed Laine, Bowdoin College). It moved down the coast and meandered in the Gulf of Maine before it tracked along the coast of Cape Cod in the early June period (Fig. 10). These data were kindly provided by Jim Manning of NOAA, NEFSC.
Figure 10.
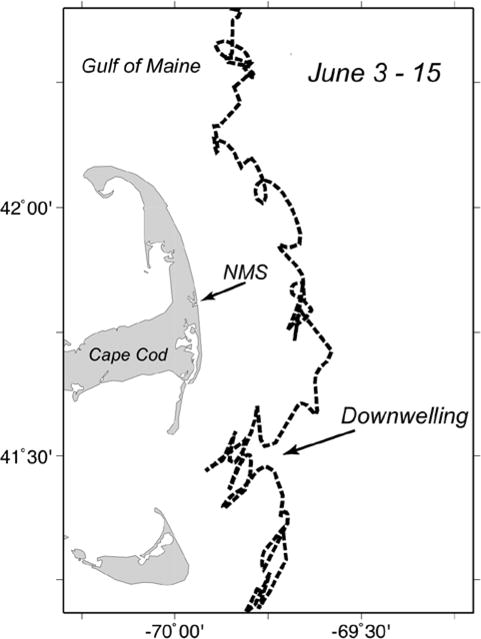
Trajectories of a drifter released in the Gulf of Maine in the spring of 2009. The drifter was released early in the spring along the Maine coast tracked with the alongshore coastal flow near Cape Cod during the period of time of our later surveys in June (7–12 June 2009). Note that the drifter turned shoreward during downwelling-favorable conditions.
3. Results
3.1 Cyst abundance and distribution
3.1.1 Autumn 2008
The highest concentrations of cysts were observed in the fine-grained, muddy sediments of Mill Pond, Salt Pond and Town Cove, the uppermost reaches of the NMS (Fig. 2a). The average cyst concentration in the top 1 cm layer in Mill Pond (1040±313 cysts cm−3) was 2.5 times and 7× higher than in Salt Pond and Town Cove, respectively (Table 1). However, the highest concentrations were not located in the deepest holes of those protected ponds, but instead were found at the margins of the ponds in depths of 1.5 to 5 m. Relatively few cysts (<60 – 80 cysts cm−3) were observed in the central marsh area. Throughout the NMS system, the mean cyst concentration was higher in the top 1 cm layer (252 cyst cm−3±29 SE) than in the underlying 1–3 cm (127 cyst cm−3±15 SE).
Table 1.
Mean cyst concentrations (cysts cm−3) and maximum number of cyst (cysts cm−3) at Mill Pond, Salt Pond, Town Cove and the Central Marsh of the NMS in the autumn of 2008 and 2009. SE: standard error.
| 2008 | Mill Pond | Salt Pond | Town Cove | Central Marsh | |
|---|---|---|---|---|---|
| Mean ± SE | Top 1 cm | 1040±313 | 418±80 | 150±31 | 81±22 |
| 1–3 cm | 415±151 | 85±31 | 199±49 | 62±22 | |
| Max | Top 1 cm | 2909 | 820 | 385 | 640 |
| 1–3 cm | 1035 | 248 | 555 | 773 | |
|
| |||||
| 2009 | Mill Pond | Salt Pond | Town Cove | Central Marsh | |
|
| |||||
| Mean ± SE | Top 1 cm | 1375±564 | 856±625 | 525±108 | 88±22 |
| 1–3 cm | 1044±546 | 739±665 | 520±126 | 120±45 | |
| Max | Top 1 cm | 4965 | 4570 | 1150 | 690 |
| 1–3 cm | 3170 | 4060 | 1190 | 980 | |
3.1.2 Autumn 2009
As in 2008, the highest concentrations of cysts were localized in Mill Pond, Salt Pond, and Town Cove with the lowest concentrations found in the central marsh area, where sediments were a mixture of silt and sand (Fig.2b; Table 1). The number of cysts observed in each of those areas was generally higher in 2009 (3.5 times for Mill Pond; 1.3× for Salt Pond, 2.1× for Town Cove and 1.1× for the central marsh area). In contrast to 2008, the mean cyst concentration was similar in both layers in 2009 (385±45 cyst cm−3, 1 cm layer; 396 cyst±57 cm−3, 1–3 cm layer). Throughout the NMS, the number of cysts in the autumn 2009 was 1.5 and 3.1 times higher than in 2008 for the 1 cm and 1–3 cm sediment layers respectively. Virtually no cysts were found near the Nauset Inlet, where the sediment was predominantly sand.
Sediment cores typically showed a top, oxygenated surface layer, below which there was anoxic black sediment. No obvious signs of bioturbation were seen, although an occasional worm was recovered during cyst processing.
3.2 Temperature, salinity and nutrients during the bloom season
The mean water temperature in the NMS increased on average from 3.6 (±0.11) °C on March 24, our first sampling day to 15.1 (±0.24) °C on June 18, the last sampling day. Temperatures were lower in the central marsh than in the three distal salt ponds (Fig. 3a), consistent with advection of water into the marsh from the coastal ocean during flood tides. Temperatures were similar within the three salt ponds through the observation period. The decrease in water temperature throughout the estuary of about 3°C recorded during the 10th survey (June 2) followed a period of upwelling winds and coastal water level setup that brought in additional cold, high salinity ocean water into the estuary. Thermal stratification was apparent in the deeper regions of the salt ponds, with near-surface temperatures up to 7°C warmer than near-bottom water (Fig. 4a–c). In the shallower regions of the central marsh, the temperature structure was vertically uniform.
Fig. 3.
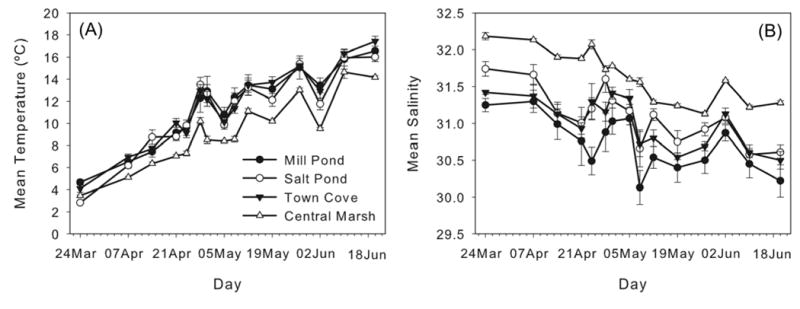
Temporal evolution of (a) mean temperature (°C) and (b) mean salinity in the NMS. Filled circle: Mill Pond; open circle: Salt Pond; filled inverted triangle: Town Cove; open triangle: central marsh. Error bars indicate standard error. X-axis labels indicate the sampling day.
Fig. 4.
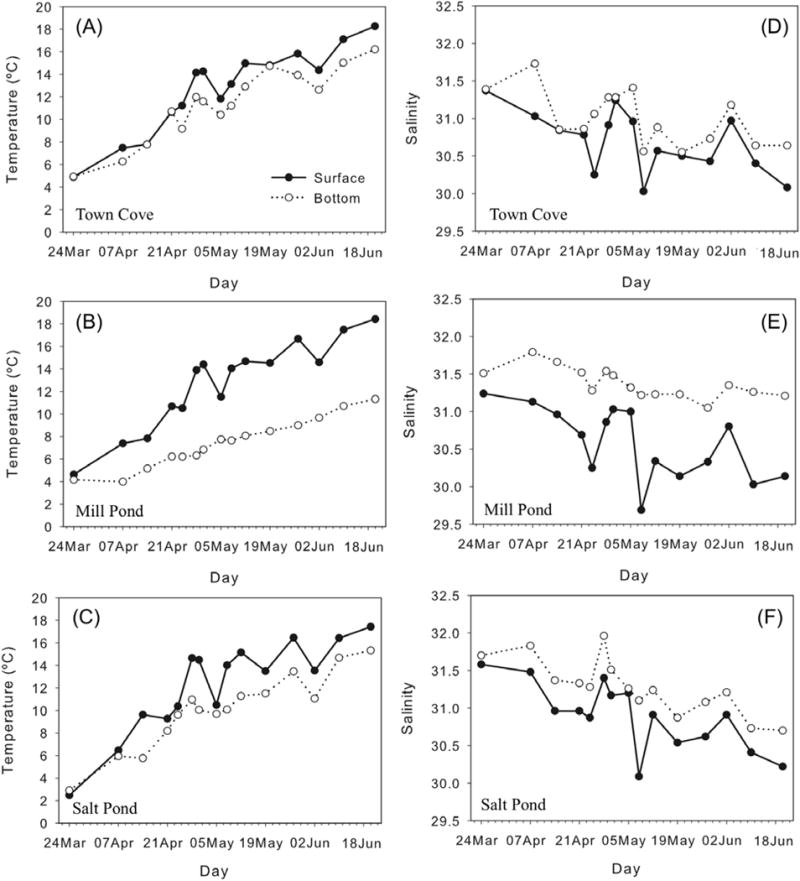
Temporal evolution of surface (filled circles) and bottom (open circles) temperature (°C) (a–c) and salinity (d–f) in the stations with highest depth at (a, d) Town Cove (station 1); (b, e) Mill Pond (station 11); and (c, f) Salt Pond (station 21). X-axis labels indicate the sampling day.
Mean salinity decreased ∼1 psu from an average of 31.9 (±0.07) psu on March 24 to 31 (±0.07) psu on June 18 (Fig. 3b). Salinity values were always higher in the central marsh than in the salt ponds, again due to advection of coastal ocean water into the marsh. Near surface salinities were slightly lower in Mill Pond than in Salt Pond or Town Cove (Fig. 3b), particularly at the station farthest from the inlet to the pond (data not shown). As with temperature, salinity variability was largely coherent among the ponds, decreasing slightly through the observations. The deep regions of the salt ponds were stratified with respect to salinity, with the strongest stratification occurring in Mill Pond (Fig. 4d–f).
Nutrient concentrations were high in the three salt pond sites compared to the low concentrations in the central marsh (Fig. 5). In particular, Mill Pond was higher than the other parts of the NMS (P < 0.005, t-test for unpaired samples) due mainly to the high concentrations recorded at the bottom of the deepest station (station 11) (see Fig. 5). Silicate had the highest concentrations, followed by ammonium, phosphate and nitrate. Ammonium and nitrate concentrations generally decreased through the observation period (Fig. 5). Ammonium concentrations were greatest in the deep holes of Mill Pond and Salt Pond, with particularly high concentrations in the bottom waters of Mill Pond (mean value 59.9±12μmol L−1). Nitrate concentrations were typically lower than ammonium (mean values <3 μmol L−1 in Mill Pond and <1.7 μmol L−1 in Salt Pond), but high nitrate concentrations were associated with lower salinity regions in the upper reaches of the ponds, particularly in upper Mill Pond where nitrate concentration exceeded 7 μmol L−1 at times.
Fig. 5.
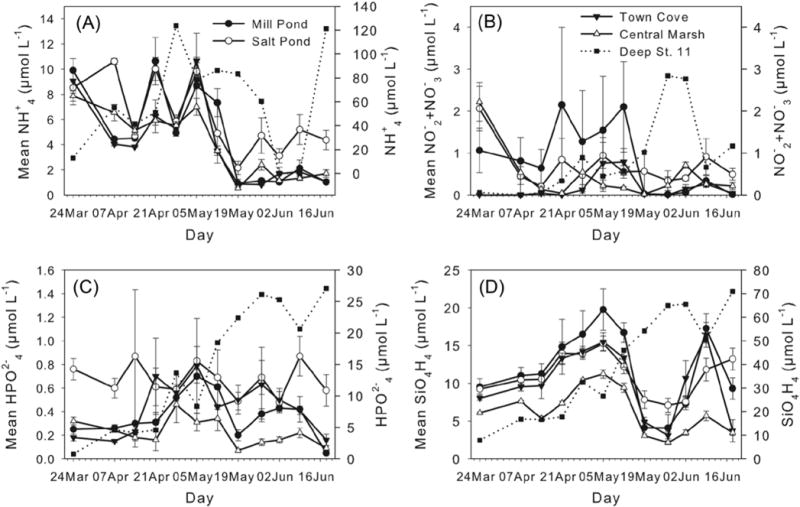
Temporal evolution of mean nutrient concentrations: (a) Mean NH+4 (μmol L−1), (b) mean NO−2 +NO−3 (μmol L−1), (c) mean HPO2−4 (μmol L−1) and (d) mean SiO4H4 (μmol L−1). Filled circle: Mill Pond; open circle: Salt Pond; filled inverted triangle: Town Cove; open triangle: central marsh. Filled squares and dashed line and right axis value indicate the bottom nutrient concentrations at the depth hole in Mill Pond (station 11). Error bars indicate standard error. X-axis labels indicate the sampling day.
3.3 Alexandrium fundyense cells and shellfish toxicity
Alexandrium fundyense mean concentration in Mill Pond was already over 100 cells L−1 at the beginning of the sampling period (24 March 2009). At that time, virtually no cells were observed in Salt Pond, Town Cove, or the central marsh (Fig. 6a, 7). A. fundyense bloomed as early as mid April in Mill Pond (~ 1,000 cells L−1), the area of highest cyst abundance within the NMS (Fig. 2a). An increase of one order of magnitude in the number of cells (from ~ 2,900 to ~ 29,000 cells L−1) was recorded two-weeks later on April 28 (Fig. 6ab). The maximum concentrations (~ 37,000 cells L−1) were observed on May 8 (Fig. 6a). Subsequently, cell abundance remained high (~ 14,500 cells L−1) through mid-May, but decreased dramatically (from 14,400 to 27 cells L−1) at the end of that month (from May 19 to May 27) (Fig. 6a, 7). Thereafter, the number of cells remained <100 cells L−1 until the end of the sampling period (June 18).
Fig. 6.
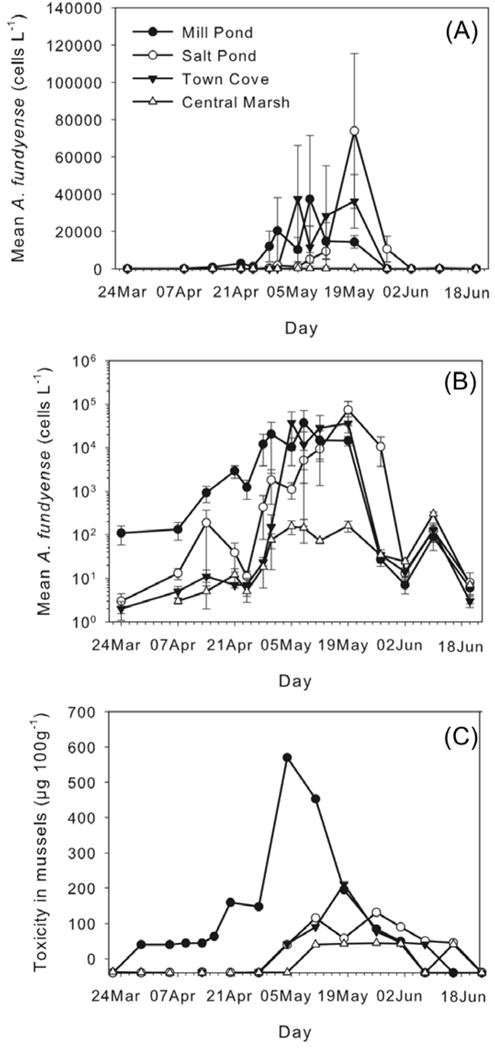
Temporal evolution of mean A. fundyense cell concentrations (cells L−1): (a) linear scale and (b) logarithmic scale. (c) Temporal evolution of toxicity in mussels (μg 100g−1), data recorded by MA- DMF. Filled circle: Mill Pond; open circle: Salt Pond; Filled inverted triangle: Town Cove; open triangle: central marsh. Error bars in (a) and (b) indicate standard error. X-axis labels indicate the sampling day.
Fig. 7.
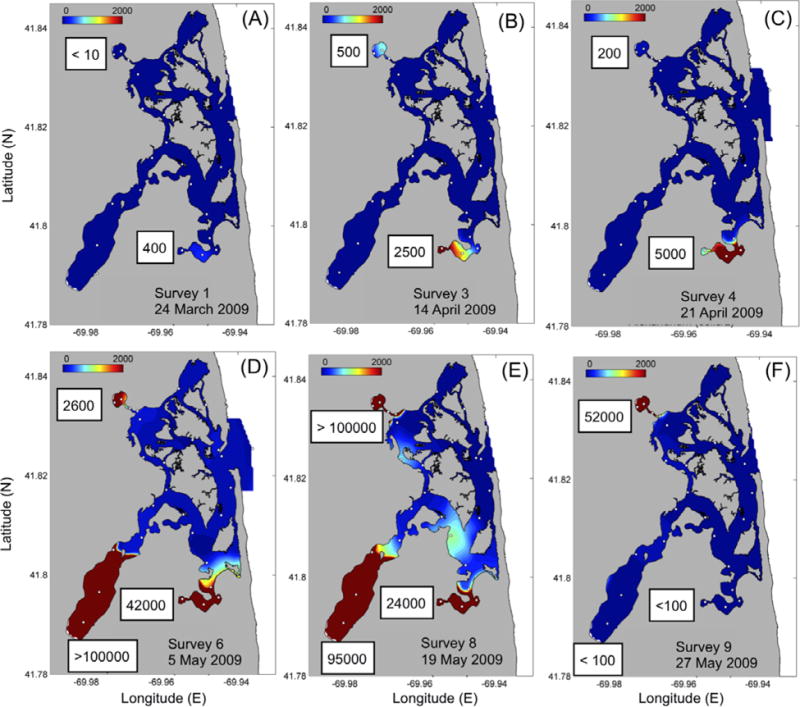
Distribution of A. fundyense cell concentrations (cells L−1) during (a) March 24, (b) April 14 (c) April 21, (d) May 5, (6) May 19 and (f) May 27. Maximum number of cells for Mill Pond, Town Cove and Salt Pond indicated in the white squares. White dots indicate sample sites.
Development of the blooms within Town Cove and Salt Pond was delayed nearly a month relative to that in Mill Pond (Fig. 6a, 7). In Town Cove the bloom peaked (~37,000 cells L−1) at the beginning of May (May 5) and in Salt Pond the peak (~74,000 cells L−1) was recorded later that month (May 19). As was the case in Mill Pond, after May 19 the cells concentrations decreased dramatically in Town Cove. A week later, Salt Pond followed as cell concentrations dropped to <100 cells L−1 (Fig. 6a).
Cell concentrations within the central marsh remained relatively low (~100 cells L−1) throughout the entire study (Fig. 6a, 7), although on June 9 the number of A. fundyense cells in the central marsh waters (~300 cells L−1) was greater than in any of the ponds (Fig. 6b).
In general, shellfish toxicity in the salt ponds followed the same pattern observed in cell abundance for those areas. Generally, the highest shellfish toxicities at the monitoring sites were recorded 1–2 weeks after the highest cell concentrations in the three salt ponds. The central marsh did not show any clear relationship between toxicity and cell concentrations. Mill Pond reached its highest toxicity on May 5 (Fig. 6c). It also had the longest interval of detectable toxicity (from March 29 to June 1 – approximately two months). Town Cove and Salt Pond became detectably toxic later (May 4) and remained so for less time (ending June 14 and June 21 respectively – an interval of 5 to 6 weeks). The highest toxicity levels in these two salt ponds were 3–4 times lower than in Mill Pond. Toxicity in the central marsh was always below or near detection limits (~ 40 μg 100 g−1) and was detectable at low values for 4 successive weeks (May 12 to June 2). After the toxicity decreased below detection limits on June 8, it increased to just over 40 μg 100 g−1 on June 16 (Fig. 6c), consistent with the time when the highest number of A. fundyense cells were observed in the central marsh (June 9) (Fig. 6b).
3.4 Vertical distributions of Alexandrium fundyense cells
The vertical structure of cell and nutrient concentration from the deepest stations in Mill Pond (station 11, ~11 m depth) and Salt Pond (station 21, ~9 m depth) are shown for samples that corresponded with the peak of the bloom in each pond (Fig 8). In both cases, A. fundyense cell concentrations revealed subsurface maxima, below the surface layer but above the nutrient rich bottom waters. A mid-water column maximum in cell concentration was a common feature through most of the surveys. An exception was the first survey in Mill Pond at the start of bloom, when the maximum cell concentrations were found in near-bottom waters.
Fig. 8.
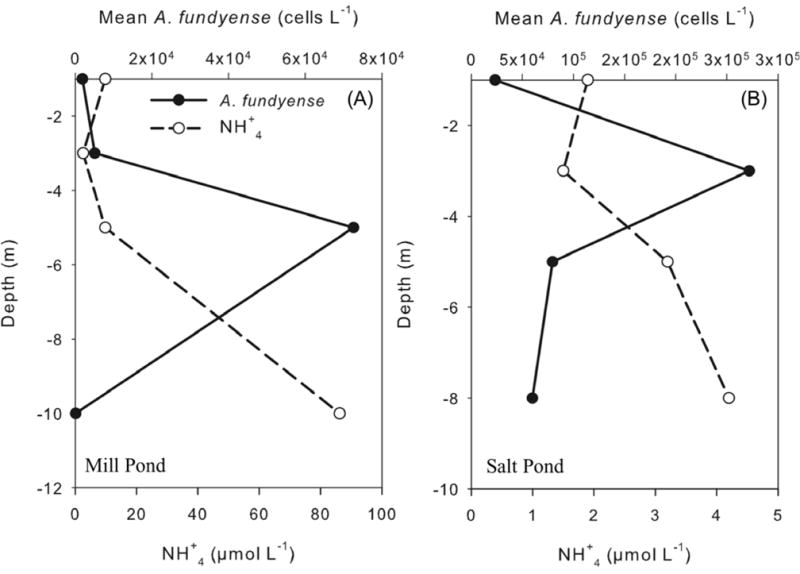
Vertical distributions of mean A. fundyense cell concentration (cells L−1) and mean NH+4 (μmol L−1) concentrations in (a) the deepest station (station11) at Mill Pond on May 12 and (b) the deepest station (station 21) at Salt Pond on May 19.
3.5 Alexandrium fundyense cells in the central marsh
During the period of relatively high cell concentrations in the central marsh, a sequence of survey snapshots is shown for June 2, 9, and 18 (Fig. 9). On June 2 (Fig. 9a), there was a significant decrease in the number of cells throughout the NMS (Fig. 6ab), coinciding with a decrease in temperature and an increase in salinity in the marsh (Fig. 3). One week later (Fig. 9b), the cells were present throughout the central marsh, with low concentrations appearing in the salt ponds. The origin of the increase in cell concentrations in the marsh is suggested by the drifter track, which indicates transport of surface water along the outer Cape and toward the coast due to a downwelling event at that time (Fig. 10). A. fundyense cells were present in coastal waters near Nauset Inlet (Fig. 9), presumably from the large-scale Gulf of Maine A. fundyense bloom that was occurring at that time (D. M. Anderson, unpub. data).
Fig. 9.
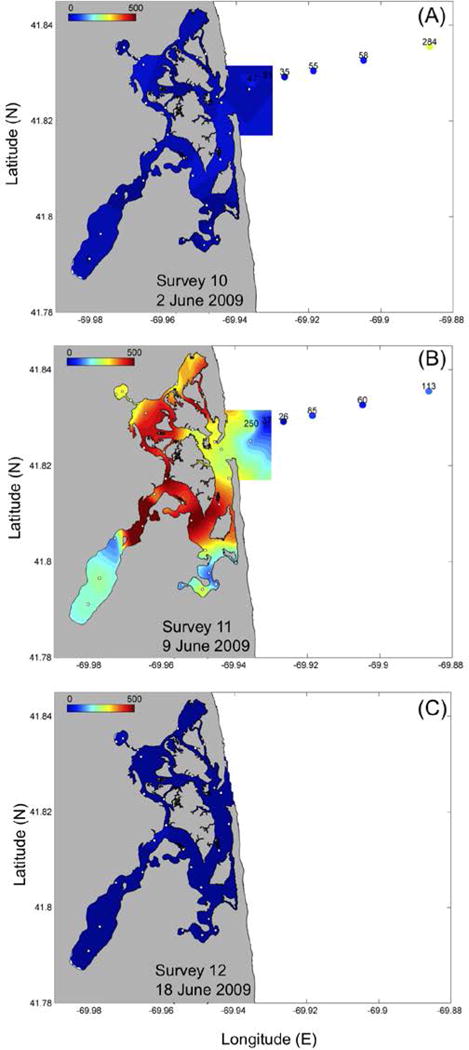
Distribution of A. fundyense cell concentrations (cells L−1) during (a) June 2, (b) June 9 and (c) June 18. Note the change of scale from 0–2000 in Fig.7 to 0–500 cells L−1. White dots indicate sample sites.
4. Discussion
This is the first study describing the dynamics of A. fundyense blooms and toxicity in the entire NMS, as previous investigations were restricted to the individual salt ponds (e.g., Anderson et al., 1983; Anderson and Stolzenbach, 1985). This is also the first description of A. fundyense cyst distributions in the area (Fig. 2, Table 1). In the observations presented to this point and in the discussion below, we identify a number of features and mechanisms that underlie the annually recurrent blooms in the system. In this section we synthesize the important findings of the study, including 1) the accumulation of cysts predominantly in three salt ponds at the distal ends of the estuary; 2) the direct link between these cyst seedbeds and the initiation sites of early-season A. fundyense blooms; 3) the importance of factors regulating growth and cell accumulation as the major determinants of A. fundyense population size in the salt ponds, rather than cyst abundance; 4) the growth and retention of A. fundyense populations within the salt ponds despite significant tidal exchange with the central marsh; 5) highly localized toxicity consistent with the cyst and cell distributions; 6) limited advection of A. fundyense cells from the NMS to adjacent coastal waters; and conversely, 7) the introduction of A. fundyense cells into the NMS from Gulf of Maine bloom populations in coastal waters near the Nauset Inlet, leading to a late season, short-lived bloom in the estuary. These results identify features and mechanisms that help to explain localized blooms of A. fundyense and other cyst-forming HAB species in many regions of the world (e.g., Anglés et al., 2010; Estrada et al., 2010; Hattenrath et al. 2010). The results also have significant management implications for the NMS, as issues such as stimulation of blooms by anthropogenic nutrients supplied via groundwater or biogeochemical processes, or the possibility of site-specific shellfish closures can now be considered.
4.1 Bloom initiation and development
Two types of blooms were observed within the NMS. One type was initiated by cyst germination in the three salt ponds, leading to three localized blooms that largely remained within those salt ponds, each with a duration between 5 and 8 weeks, with limited dispersal of cells into the central marsh area. A second, late season bloom also occurred within the NMS after the salt pond blooms had ended. That episode was short lived (~1 week in duration) and was caused by cells from a widespread coastal bloom of A. fundyense that were carried to the shore and through the Nauset Inlet by downwelling-favorable winds and tidal currents.
Looking at the first of these blooms in more detail, the results of our surveys unequivocally demonstrate that the early-season 2009 A. fundyense blooms in the NMS are initiated from germination of cysts in bottom sediments in three distinct seedbeds. The spatial match between the appearance of the first vegetative A. fundyense cells (Figs. 6ab, 7) and the highest cyst concentrations (Fig. 2a), both restricted to the salt ponds, and the lack of cells in the central marsh where cysts were scarce, confirm this cyst-based local origin. The absence of significant numbers of cells in the central marsh throughout the bloom season (Figs. 6ab, 7) further suggests that the transport of A. fundyense cells from the Gulf of Maine through Nauset Inlet (Anderson et al., 2005a) is not a valid initiation mechanism for the early-season blooms, although, as discussed below, such intrusions do happen later in the season and can lead to a second wave of toxicity.
The relatively low concentrations of A. fundyense cells in the central marsh in 2009 (Figs. 6ab, 9) also indicates that export of cells from the NMS to adjacent coastal waters is likely to be minimal, as cells have to pass through that central area to exit via Nauset Inlet. This issue arose during the 2005 A. fundyense bloom in the region (Anderson et al., 2005a), when large numbers of cells were observed just outside Nauset Inlet and toxicity occurred both within the central marsh and from the inlet to the southern extreme of Cape Cod (Monomoy Island), extending offshore to the islands of Martha’s Vineyard and Nantucket. The origin of the cells responsible for that part of the regional toxicity was in question. It now seems probable that those cells and the very high toxicities within the NMS and adjacent coastal toxicity were from regional Gulf of Maine A. fundyense populations advecting along the outer Cape, and not from a pulse of “locally-grown” cells exported from the NMS. Nevertheless, it will be informative to conduct genetic analysis of the A. fundyense populations within the NMS to ascertain the relative abundance of local strains versus those introduced from afar, particularly since the latter have much higher intrinsic toxicities (Maranda et al., 1985; Anderson et al., 1994).
A key feature of bloom initiation in the NMS is the difference in bloom timing in the salt ponds. Cells of A. fundyense were first observed in Mill Pond, where the population increased several weeks to a month before similar concentrations were observed in Salt Pond (Figs. 6ab, 7). This temporal pattern of bloom initiation and development was also seen in shellfish toxicity (Fig. 6c). PSP toxin was first detected in shellfish from Mill Pond, approximately three weeks earlier than in Salt Pond. Cysts were roughly 2.5 times more abundant in Mill Pond than in Salt Pond (Table 1), which would seem to lead to a larger inoculum from the sediments and thus earlier bloom development, but the volume of Mill Pond at mean sea level is 2.7 times larger (480,000 vs 180,000 m3), so initial cell concentrations should have been roughly similar. With mean cyst abundances of 1,040 and 418 cysts cm−3 in Mill and Salt Ponds respectively, volumes as given above, and bottom surface areas of 180,000 and 100,000 m2, if all the cysts in the top 1 cm of sediment germinated at once, the vegetative cell concentration in the ponds would be 3,500 and 2,300 cells L−1 respectively. However, not all cysts germinate at once, or even in a season. If only 20% of the cysts in that 1 cm surface sediment layer germinate in a 6–8 week season, as is suggested by ongoing germination flux experiments in the ponds (E. Vahtera, unpub. data), and, for simplification purposes, if the germination rate were constant through time, we can calculate a germination flux rate of ~0.4% day−1. A week of germination would lead to an inoculum with cell concentrations of ~70 −100 cells L−1, in the ponds, roughly equivalent to what was observed in the early stages of the blooms. In subsequent weeks, the same germination flux would be provided, but those cells would be greatly outnumbered by the dividing cells in the water column. With an estimated inoculum of 700 and 460 cells L−1 from the top 1 cm of Mill Pond and Salt Pond respectively (20% of the total possible inoculum from that layer), the germination inoculum over a season would be between 1.8 and 0.6% of the peak cell densities in the two salt ponds. In these systems, population size is therefore not regulated by the abundance of cysts in bottom sediments, but rather by the factors affecting cell growth and retention, although an encystment inoculum is necessary to start the bloom process.
Differences in the timing of bloom initiation among the salt ponds could relate to nutrient concentrations. Nitrate and ammonium were higher in Mill Pond than in Salt Pond throughout the bloom season, with nitrate on average 1.4 times greater in Mill Pond than in Salt Pond (Fig. 5ab). The difference likely arises from difference in nearby residential development, as houses with septic tanks and leaching fields surround Mill Pond, whereas the Cape Cod National Seashore, established in 1961, prevented development around Salt Pond. Mill Pond also had lower surface salinities and was more stratified than the other ponds (Figs. 4), likely contributing reduced vertical exchange, lower dissolved oxygen concentrations, and higher ammonium concentrations in the bottom waters. Cell concentrations in the deepest part of Mill Pond decreased below the pycnocline (Fig. 8), where the highest ammonium concentrations were measured (Fig. 5a) and the water samples had a sulphurous odor consistent with low dissolved oxygen concentrations (also, Howes et al., 2003). The mean ammonium concentrations in the bottom waters of Mill Pond late in the season (~80μM) were high, but A. fundyense does grow well at those concentrations (Anderson et al., 1984). The cells in Mill Pond thus seem to achieve a fine balance among nutrients, oxygen, and light. They avoid the anoxic bottom waters, but also avoid the surface layer, and in doing so, are able to access high nutrient concentrations in the lower layer yet maintain favorable light conditions.
An additional potential factor in timing of bloom initiation among the ponds is the influence of temperature on cyst germination rates (Anderson, 1980; Rengefors and Anderson, 1998; Itakura and Yamaguchi, 2001; Kim et al., 2002) and population growth rates (Watras et al., 1982; Anderson et al., 1983; Stock et al., 2005). Although the temperatures were largely similar in the salt ponds (Fig. 3a), the upper reaches of Mill Pond were fresher and had somewhat higher temperatures than similar parts of Salt Pond and Town Cove (p<0.005, t-test for unpaired samples). The relationship between A. fundyense growth rate and temperature is quite steep at low temperatures (Watras et al., 1982), so small changes in temperature can lead to fairly large differences in germination and growth rate.
4.2 Population development and retention
The bloom development inside the ponds and the lack of transport into the central marsh waters (Fig. 7) suggest the existence of a retention mechanism for A. fundyense cells. Anderson and Stolzenbach (1985) documented a flushing mechanism for Salt Pond in which salty, inflowing waters sank to the pond bottom, displacing and mixing with more brackish waters linked to groundwater intrusions, and the surface layer was preferentially advected from the pond during the ebb tide. The A. fundyense population concentrated in subsurface layers at or below the pycnocline, presumably to avoid the high light levels of the surface waters. Consequently, few cells were found in the surface layer that exited the pond during the ebb, leading to significant retention of the bloom in the pond despite the tidal exchange. The input of fresh groundwater to the ponds creates stratification that reduces vertical exchange between the surface and bottom layers, and a shallow sill at the pond inlet presents a physical barrier to horizontal advection of bottom waters.
The observations in 2009 were consistent with the previous observations in Salt Pond (Anderson and Stolzenbach, 1985) in that subsurface maxima in cell concentrations were found regularly at or below the pycnocline (e.g., Fig. 8). The three salt ponds in NMS have similar retentive bathymetry, with deep holes separated from the central marsh by relatively shallow sills. Recent results from a 3d numerical model of NMS suggest that residence times in the bottom waters of the ponds are on the order of 3 to 5 days, compared with about 1 day for the central marsh (D. Ralston, unpublished data). Previous results from a depth-averaged model of NMS also found that the residence times of the ponds were significantly longer than for the central marsh, albeit with shorter residence times overall due to differences in model resolution (Aubrey et al., 1997). The importance of retention mechanisms on dinoflagellate bloom development has been described for several species in other ecosystems (Seliger et al., 1970; Heany and Talling, 1980; Tyler and Selinger, 1978; 1981; Crespo et al., 2006).
4.3 Bloom termination
The A. fundyense population grew by asexual reproduction until sexuality was induced, leading to gamete fusion, planozygote formation, and eventual cyst deposition. Numerous planozygotes were observed in the salt ponds populations in the late stages of these blooms, and cyst abundance after the blooms, in autumn 2009, was more than 2.5 times greater than in autumn 2008 (Fig. 2, Table 1), so encystment was likely a factor in bloom termination. This was also observed by Anderson et al. (1983) who quantified the large planozygotes as a fraction of the A. fundyense populations in these salt ponds. The bloom declines in 2009 coincided with a general decrease in nutrient concentration throughout the marsh on May 19 (Fig. 5). Nutrient stress has been proposed as a factor leading to cyst formation in culture (Von Stosch, 1973; Pfiester, 1976; Watanabe et al., 1982; Anderson and Lindquist, 1985), but it remains unclear whether this is the only mechanism leading to encystment of A. fundyense field populations. Recently, Figueroa et al. (2011) reported that salinity and temperature are also important factors regulating A. minutum planozygote and cyst formation. Anderson et al. (1983) observed that planozygotes appeared approximately six divisions after the first vegetative cells were seen in Salt and Mill Ponds. If such a mechanism was present in our study, it could explain the earlier detection of planozygotes (April 28) and bloom termination in Mill Pond compared to Salt Pond (first planozygotes on May 19).
Grazing was not quantified in this study, but in previous investigations in Cape Cod salt ponds, Turner and Anderson (1983) found that copepods and planktonic polychaete larvae (Polydora spp.) grazed non-selectively on toxic A. fundyense. The impact of copepod grazing on the bloom was minimal, but due to their greater abundance, grazing impact of polychaete larvae was substantial. Estimates of grazing loss ranged from 3–16% day−1 in the early stages of the bloom to 100% day−1 at peak population density, thus inflicting rapid and significant losses on the bloom population. Watras et al. (1985) estimated grazing and A. fundyense growth rates in Salt Pond, providing evidence that rates of grazing (from Polydora spp. and the tintinnid ciliate Favella sp.) often exceeded growth rates. These studies showed that mortality due to zooplankton grazing had the potential to suppress bloom development when grazers were abundant. Grazing is likely a key regulatory mechanism of A. fundyense in Cape Cod embayments, although this study did not directly quantify its impact on the bloom dynamics in 2009. We observed, but did not quantify, both tintinnids and copepods while counting the A. fundyense cells in the samples collected from the NMS, highlighting the possibility of grazing regulation.
Another significant loss factor in the 2009 bloom was parasitism. Data collected on Amoebophrya parasite prevalence documented host infection rates in Salt Pond as high as 90% (M. Sengco, unpub. data; Brosnahan, 2010). Abrupt declines in A. fundyense host abundance were observed following peaks in parasite prevalence. Similar impacts from parasites have been observed for other dinoflagellate blooms (e.g., Park et al., 2004; Chambouvet et al., 2008).
A short-term increase in the number of A. fundyense cells was observed in the central marsh, and to a lesser extent, in the salt ponds, after the blooms declined in those ponds (Figs. 6b, 9). This occurred after a decrease in temperature and an increase in salinity (Fig. 3ab) in the marsh, suggesting an input of coastal water to the marsh. The presence of A. fundyense cells in the central marsh waters at that time (Figs. 6b, 9) as well as the drifter track that originated from the Maine coast in (Fig. 10) suggest that the cells in this second bloom originated outside the marsh, likely from the regional Gulf of Maine bloom that was ongoing at the time (D.M. Anderson, unpub. data). Although this possibility has to be examined through the genetic analysis of samples collected in the marsh and in offshore waters, it appears that the driving force was a downwelling-favorable wind pattern that carried cells to shore from the coastal bloom, allowing them to enter the marsh. This type of downwelling-driven delivery of cells has been described before for this area (Keafer et al., 2005) and for other ecosystems (Crespo et al., 2006; 2007). This second bloom was short-lived, disappearing one week after it was initially observed (Fig. 9), and consistent with the short residence times of the central marsh compared with the retentive nature of the salt ponds. The observations indicate a temporal mismatch between the blooms in the NMS and those in the Gulf of Maine. The latter tend to occur in late May and June in Massachusetts coastal waters (e.g., Anderson et al., 2005a), at the time the NMS salt pond blooms are ending. Nevertheless, the documentation of this bloom delivery mechanism has important implications for the NMS, as it suggests that regulatory officials need to remain alert for sudden toxicity increases after the salt pond blooms have declined. The 2009 Gulf of Maine bloom was relatively small in Massachusetts coastal waters. In years with larger blooms in the western Gulf of Maine (e.g., 2005), the input of cells into the NMS might be much larger, and the resulting toxicity threat larger as well.
4.4 Shellfish toxicity
A general relationship between cell abundance and shellfish toxicity was apparent during the early phases of the blooms (Figs. 6), with peak toxicity lagging peak cell abundance in the salt ponds by 1–2 weeks. This may reflect the nature of the sampling schedule, as shellfish and plankton samples were taken on approximately the same day every week, but is also consistent with the idea that shellfish integrate water quality conditions over many tidal cycles. The toxicity measured in shellfish reflects integrated cell abundance during the week prior to sampling. Similarly, the relationship between cell abundance and shellfish toxicity late in the bloom depends on toxin retention and depuration rates by the shellfish. The shellfish sampling stations are typically located near the shore in relatively shallow water (1–2 m). In NMS observations, typically the highest cell concentrations were not near the surface but instead below the surface near the pycnocline (3–5 m). The mismatch between shellfish sampling depth and maximum cell concentration can have important management implications since the toxicity recorded at monitoring stations may not reflect the real dimensions of the bloom.
While the seasonal progression of cell concentrations and shellfish toxicity suggested a qualitative link, direct quantitative relationships were not strong. For example, maximum toxicities in Salt Pond and Town Cove were less than in Mill Pond (Fig. 6c), despite relatively similar peak cell densities (Fig. 6ab). The best correlations were found comparing shellfish toxicities with cell concentrations at nearby stations lagged by about 1 week. Very roughly, cell concentrations of about 100 cells L−1 occurred about 1 week before the first toxicity data that exceeded the bioassay detection threshold (40 μg 100 g−1), and cell concentrations of 500 to 1000 cells L−1 preceded the first detection of toxicity exceeding the regulatory threshold (80 μg 100 g−1). The correlations between cell concentration and toxicity varied between the embayments, perhaps depending on the position of the shellfish sampling location relative to the depths of high cell concentration in each pond. Variability in toxin composition and content in natural populations of A. fundyense could also be a factor. Toxin variability has been reported in the Gulf of Maine (Maranda et al., 1985; Anderson et al., 1994; Etheridge and Roesler, 2005) where a north-to-south trend of decreasing Alexandrium toxicity has been described and is the result of northern isolates containing more potent toxin congeners (Anderson et al., 1994). Cape Cod lies at the southern end of this range, and generally has A. fundyense strains with low toxin content, but there has not been a study of toxin composition differences among NMS populations, nor was that measured here.
Overview
This study adds significantly to our knowledge of the dynamics of A. fundyense blooms in the NMS, and contributes to our understanding of the dynamics of cyst-forming dinoflagellates and HAB species in estuaries and restricted embayments worldwide. Rarely is the evidence as strong as we have documented here linking cyst seedbeds or accumulation sites with bloom initiation and localized blooms. Having three salt ponds with localized cyst accumulations (Fig. 2) followed by blooms that begin and remain localized at those sites (Fig. 7) provides unequivocal evidence for the cyst-based origin of the early-season NMS blooms. The salt ponds are highly retentive of cells compared with the central marsh, particularly below the pycnocline where stratification limits vertical mixing and the sill elevation presents a physical barrier to tidal exchange. The observations were generally consistent with prior findings that cell retention is related to the avoidance of the surface layer by A. fundyense cells (Anderson and Stolzenbach, 1985). The salt ponds are thus ideal incubators for these annually recurrent point source blooms. Given this high degree of bloom localization, shellfish monitoring could be enhanced within the ponds using mussels bags located at the mid-depth layers (~3 to 5 m below the surface) to provide early warning of the impending localized blooms. At the same time that the three salt ponds are closed due to major blooms, it may be possible to keep the central marsh area open for shellfishing due to the significantly lower cell concentrations and toxicity. This type of site-specific or surgical closure could help to diminish the negative impacts of A. fundyense blooms on recreational and commercial shellfishing in the area. However, the sudden, late-season introduction of A. fundyense cells into the NMS via Nauset Inlet demonstrates that monitoring and vigilance are necessary, especially during the widespread, coastal blooms that occur in the Gulf of Maine.
Acknowledgments
We especially thank Mike Conner and staff from the Town of Eastham and staff from the Town of Orleans for small boat operations and collection of shellfish. Mike Hickey/Terry O’Neill from the Massachusetts Division of Marine Fisheries (MA DMF) for providing the shellfish toxicity data, Megan Tyrell for her support in permitting the work within the Cape Cod National Seashore (CACO), Jim Manning (National Oceanograhic and Atmospheric Administration Northeast Fisheries Center) for providing the drifter data, and the crew of the R/V Tioga. We also thank the staff and students from the Anderson Laboratory in WHOI who assisted with the collection and analysis of many cell and cyst samples, especially to K. Norton, J. Selwyn, N. Ishimaru, and B. Conroy. This work was supported by NOAA Grant NA06OAR4170021, NPS Grant H238015504 and by the Woods Hole Center for Oceans and Human Health through NSF Grants OCE-0911031 and OCE-0430724 and NIEHS Grant 1P50-ES01274201. B.G.C. was supported by a Xunta de Galicia Ángeles Alvariño fellowship and the Stanley W. Watson Chair for Excellence in Oceanography under a Postdoctoral program at the Woods Hole Oceanographic Institution.
Footnotes
Both A. tamarense and A. fundyense occur in the Gulf of Maine region. We consider these to be varieties of the same species (Anderson et al., 1994; Brosnahan et al., 2010). Neither antibody nor oligonucleotide probes can distinguish between them, and only detailed analysis of the thecal plates on individual cells can provide this resolution. This is not practical for field samples. Accordingly, for this study, the name A. fundyense is used to refer to both forms.
References
- Anderson DM. The effects of temperature conditioning on the development and germination of Gonyaulax tamarensis (Dinophyceae) hypnozygotes. J Phycol. 1980;16:166–172. [Google Scholar]
- Anderson DM, Chisholm SW, Watras CJ. The importante of life cycle events in the population dynamics of Gonyaulax tamarensis. Mar Biol. 1983;76:179–183. [Google Scholar]
- Anderson DM, Kulis DM, Binder BJ. Sexuality and cyst formation in the dinoflagellate Gonyaulax tamarensis: Cyst yield in batch cultures. J Phycol. 1984;20:418–425. [Google Scholar]
- Anderson DM, Stolzenbach KD. Selective retention of two dinoflagellates in a well-mixed estuarine embayment: the importante of diel vertical migration and surface avoidance. Mar Ecol Progr Ser. 1985;25:39–50. [Google Scholar]
- Anderson DM, Lindquist NL. Time-course measurements of phosphorus depletion and cyst formation in the dinoflagellate Gonyaulax tamarensis Lebour. J Exp Mar Biol Ecol. 1985;86:1–13. [Google Scholar]
- Anderson DM, Kulis DM, Doucette GJ, Gallager JC, Balech E. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeast United States and Canada as determined by morphology, bioluminiscence, toxin compostion and mating compatibility. Mar Biol. 1994;120:467–478. [Google Scholar]
- Anderson DM. Bloom dynamics of toxic Alexandrium species in the northeastern United States Limnol. Oceanogr. 1997;42:1009–1022. [Google Scholar]
- Anderson DM, Hoagland P, Kaoru Y, White AW. Estimated annual impacts from harmful algal blooms (HABs) in the United States. Woods Hole Oceanographic Inst Tech Rept, WHOI-2000-11 2000 [Google Scholar]
- Anderson DM, Gilbert PM, Burkholder JM. Harmful algal blooms and eutrophication: nutrient sources, compositions and consequences. Estuaries. 2002;25:704–726. [Google Scholar]
- Anderson DM, Fukuyo Y, Matsuoka K. Cyst methodologies. In: Hallegraeff GM, Anderson DM, Cembella AD, editors. Manual on Harmful Marine Microalgae, Monographs on Oceanographic Methodology, 11. UNESCO; 2003. pp. 165–190. [Google Scholar]
- Anderson DM, Keafer BA, McGillicuddy DJ, Mickelson MJ, Keay KE, Libby PS, Manning JP, Mayo CA, Whittaker DK, Hickey JM, He R, Lynch DR, Smith KW. Initial observations of the 2005 Alexandrium fundyense bloom in southern New England: General patterns and mechanisms. Deep-Sea Res II. 2005a;52(19–21):2856–2876. [Google Scholar]
- Anderson DM, Keafer BA, Geyer WR, Signell RP, Loder TC. Toxic Alexandrium blooms in the western Gulf of Maine: the plume advection hipothesis revisited. Limnol Oceanogr. 2005b;50(1):328–345. [Google Scholar]
- Anderson DM, Stock CA, Keafer BA, Bronzino Nelson A, Thompson B, McGillicuddy DJ, Keller M, Matrai PA, Martin J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep-Sea Res II. 2005c;52(19–21):2522–2542. [Google Scholar]
- Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, Scholin CA. Identification and enumeration of Alexandrium spp. From the Gulf of Maine using molecular probes. Deep-Sea Res II. 2005d;52(19–21):2467–2490. [Google Scholar]
- Anglés S, Jordi A, Gracés E, Basterretxea G, Palanques A. Alexandrium minutum resting cyst distribution in a confined site. Deep-Sea Res II. 2010;57:210–221. [Google Scholar]
- Aubrey DG, Voulgaris G, Spencer WD, O’Malley SP. Tidal circulation and Flushing characteristics of the Nauset marsh system. Woods Hole Oceanographic Inst Tech Rept, WHOI-97-1l 1997 [Google Scholar]
- Brosnahan M. Ph D thesis. Massachusetts Institute of Technology and the Woods Hole Oceanographic Institution Joint Program; 2010. Life cycle studies of the red tide dinoflagellate species complex Alexandrium tamarense. [Google Scholar]
- Chambouvet A, Morin P, Dominique M, Guillou L. Control of toxic marine dinoflagellate blooms by serial parasitic killers. Science. 2008;322(5905):1254–1257. doi: 10.1126/science.1164387. [DOI] [PubMed] [Google Scholar]
- Colman JA, Masterson JP. Monitoring ground water quality in coastal ecosystems. United Stated Geological Survey open file report 2007-1149 2007 [Google Scholar]
- Crespo BG, Figueiras FG, Porras P, Teixeira IG. Downwelling and dominance of autochthonous dinoflagellates in the NW Iberian margin: the example of the Ría de Vigo. Harmful Algae. 2006;5:770–781. [Google Scholar]
- Crespo BG, Figueiras FG, Groom S. Role of across-shelf currents in the dynamics of harmful dinoflagellate blooms in the northwestern Iberian upwelling. Limnol Oceanogr. 2007;52:2668–2678. [Google Scholar]
- Estrada M, Solé J, Anglés S, Garcés E. The role of resting cysts in Alexandrium minutum population dynamics. Deep-Sea Res II. 2010;57:308–321. [Google Scholar]
- Etheridge SM, Roesler CS. Effects of temperature, irradiance, and salinity on photosynthesis, growth rate, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep-Sea Res II. 2005;52(19–21):2491–2500. [Google Scholar]
- Figueroa RI, Vázquez JA, Massanet A, Murado MA, Bravo I. Interactive effects of salinity and temperature on planozygote and cyst formation of Alexandrium minutum (Dinophyceae) in culture. J Phycol. 2011;47:13–24. doi: 10.1111/j.1529-8817.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- Franks PJS, Anderson DM. Toxic phytoplankton blooms in the Southwestern Gulf of Maine: Testing hypotheses of physical control using historical data. Mar Biol. 1992;112:165–174. [Google Scholar]
- Glibert P, Pitcher G, editors. GEOHAB. Global ecology and oceanography of harmful algal blooms, science plan. SCOR and IOC; Baltimore and Paris: 2001. [Google Scholar]
- Hattenrath TK, Anderson DM, Gobler CJ. The influence of anthropogenic nitrogen loading and meteorological conditions on the dynamics and toxicity of Alexandrium fundyense blooms in a New York (USA) Estuary. Harmful Algae. 2010;9:402–412. [Google Scholar]
- He R, McGillicuddy DJ, Keafer B, Anderson D. Gulf of Maine Harmful Algal Bloom in Summer 2005: part 2. J Geophys Res. 2008;113:C07040. doi: 10.1029/2007JC004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heany SI, Talling JF. Dynamic aspects of dinoflagellate distribution patterns in a small productive lake. J Ecol. 1980;68:75–94. [Google Scholar]
- Howes BL, Ramsey JS, Kelley SW, Côté JM. Water quality and habitat health of the embayment systems of Orlean, MA. Progress report from the 2001 field season 2003 [Google Scholar]
- Itakura S, Yamaguchi M. Germination characteristics of naturally occurring cysts of Alexandrium tamarense (Dinophyceae) in Hiroshima Bay, Inland Sea of Japan. Phycologia. 2001;40:263–267. [Google Scholar]
- Keafer BA, Churchill JH, Anderson DM. Blooms of the toxic dinoflagellate Alexandrium fundyense in the Casco Bay region of the western Gulf of Maine: advection from offshore source populations and interactions with the Kennebec River plume. Deep-Sea Res II. 2005;52(19–21):2631–2655. [Google Scholar]
- Kim YO, Park MH, Han MS. Role of cyst germination in the bloom initiation of Alexandrium tamarense (Dinophyceae) in Masan Bay, Korea. Aquat Microb Ecol. 2002;29:279–286. [Google Scholar]
- Maranda L, Anderson DM, Shimizu Y. Comparison of toxicity between populations of Gonyaulax tamarensis of Eastern North American waters. Estuar Coast, and Shelf Sci. 1985;21:401–410. [Google Scholar]
- McGillicuddy DJ, Jr, Anderson DM, Lynch DR, Townsend DW. Mechanisms regulating large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: results from a physical-biological model. Deep-Sea Res II. 2005;52(19–21):2698–2714. [Google Scholar]
- Park MG, Yih W, Coats W. Parasites and phytoplankton, with special emphasys on dinoflagellate infections. J Eukaryot Microbiol. 2004;51(2):145–155. doi: 10.1111/j.1550-7408.2004.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Pfiester LA. Sexual reproduction of Peridinium willei (Dinophyceae) J Phycol. 1976;12:234–238. [Google Scholar]
- Rengefors K, Anderson DM. Environmental and endogenous regulation of cyst germination in two freshwater dinoflagellates. J Phycol. 1998;34(4):568–577. [Google Scholar]
- Seliger HH, Carpenter JH, Loftus M, McElroy WD. Mechanisms for the accumulation of high concentrations of dinoflagellates in a bioluminescent bay. Limnol Oceanog. 1970;15:234–245. [Google Scholar]
- Scholin CA, Herzog M, Sogin M, Anderson DM. Identification of Group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae) species. II. Sequence analysis of a fragment of the large-subunit ribosomal RNA gene. J Phycol. 1994;30:999–1011. [Google Scholar]
- Scholin CA, Miller P, Buck K, Chavez F, Harris P, Haydock P, Howard J, Cangelosi G. Detection and quantification of Pseudo-nitzschia australis in cultured and natural populations using LSU rRNA-targeted probes. Limnol Oceanogr. 1997;42(5):1265–1272. [Google Scholar]
- Shumway SE, Sherman-Caswell S, Hurst JW. Paralytic shellfish poisoning in Maine: monitoring a monster. J Shellfish res. 1988;7(4):643–652. [Google Scholar]
- Smayda TJ. Novel and nuisance phytoplankton blooms in the sea: evidence for a global epidemic. In: Graneli E, Sundström B, Edler L, Anderson DM, editors. Toxic marine phytoplankton. Elsevier; New York: 1990. pp. 29–40. [Google Scholar]
- Stock CA, McGillicuddy DJ, Solow AR, Anderson DM. Evaluating hypotheses for the initiation and development of Alexandrium fundyense blooms in the western Gulf of Maine using a coupled physical-biological model. Deep-Sea Res II. 2005;52(19–21):2715–2744. [Google Scholar]
- Townsend DW, Pettigrew NR, Thomas AC. Offshore blooms of the red tide dinoflagellate Alexandrium sp In the Guld of Maine. Cont Shelf Res. 2001;21:347–369. [Google Scholar]
- Turner JT, Anderson DM. Zooplankton grazing during dinoflagellate blooms in Cape Cod embayment, with observations of predation upon tintinnids by copepods. Marine Ecology. 1983;4:359–374. [Google Scholar]
- Tyler MA, Seliger HH. Annual subsurface transport of a red tide dinoflagellate to its bloom area: water circulation patterns and organism distributions in the Chesapeake Bay. Limnol Oceanogr. 1978;23:227–246. [Google Scholar]
- Tyler MA, Seliger HH. Selection for a red tide organismo: physiological responses to the physical environment. Limnol Oceanogr. 1981;26:310–324. [Google Scholar]
- Von Stosch HA. Observation on vegetative reproductionand sexual cycles of two freshwater dinoflagellate, Gymnodinium pseudopalustre and Woloszynzkia apiculate sp. nov. Br Phycol J. 1973;8:105–134. [Google Scholar]
- Watanabe MM, Watanabe M, Fukuyo Y. Encystment and excystment of red tide flagellates. I. Induction of encystment of Scrippsiella trochoidea. Nat (Japan) Inst Environ Stud Res Rep No. 30: Eutrophication and red tides in the coastal amrine environment. 1982:43–52. [Google Scholar]
- Watras CJ, Chisholm SW, Anderson DM. Regulationof growth in an estuarine clone of Gonyaulax tamarensis Lebour: salinty–dependent temperature responses. J Exp Mar Biol Ecol. 1982;62:25–37. [Google Scholar]
- Watras CJ, Garcon VC, Olson RJ, Chisholm SW, Anderson DM. Th effect of zooplankton grazing on estuarine blooms of the toxic dinoflagellate Gonyaulax tamarensis. J Plankton Res. 1985;7:891–908. [Google Scholar]
- Yamaguchi M, Itakura S, Imai I, Ishida Y. A rapid and precise technique for enumeration of restinga cysts of Alexandrium sp (Dinophyceae) in natural sediments. Phycologia. 1995;34:207–214. [Google Scholar]


