Abstract
Summary
Vitamin D deficiency was common in older adults from a country with adequate sun exposure. The variables associated with this deficiency provide insight into the next steps needed to characterize older adults with this deficiency and to treat it accordingly.
Purpose
The aim of this study was to describe the prevalence of and factors associated with vitamin D deficiency among Mexican older adults.
Methods
This was a secondary analysis of the last wave of the Mexican Health and Aging Study. Vitamin D levels along with other biomarkers were obtained from a sub-sample of Mexican adults older than 60 years. Prevalence was described by sex and age group, and a multivariate analysis was performed to test the factors associated with this condition.
Results
Data from 1088 adults over the age of 60 years were analyzed. The mean serum vitamin D level was 23.1 ± 8.1 ng/mL and was significantly higher among men than women (25.6 ± 0.6 and 22.8 ± 0.5 ng/mL, respectively; p < 0.001). In total, 37.3% (n = 406) presented with vitamin D deficiency, 65% of whom were women. Low 25-(OH)-vitamin D levels were associated with female sex (OR 1.74, 95% CI 1.59–2.42), current smoking (OR 2.21, 95% CI 1.47–3.39), education (OR 1.1, 95% CI 1.06–1.13), physical activity (OR 1.74, 95% CI 1.31–2.23), and high levels of glycated hemoglobin (OR 1.16, 95% CI 1.07–1.25).
Conclusions
Vitamin D deficiency was highly prevalent in Mexican older adults and was associated with a number of factors, indicating the multifactorial causality of this deficiency.
Keywords: Vitamin D, Geriatric nutrition, Epidemiology of aging, Micronutrients
Introduction
Vitamin D deficiency has been a recent focus of clinicians and researchers because of its potential implications for global health in different age groups. In the field of aging research, vitamin D has been studied as a result of its potential role in adverse conditions, such as sarcopenia, osteoporosis, falls, fractures, and chronic disease [1].
The general prevalence of vitamin D deficiency varies widely, ranging from 7.5 to 77% [2]. This variability depends on factors such as sex, body composition, physical activity, and vitamin D intake. Regarding environmental determinants, the differences between countries are largely due to the exposure to ultraviolet B rays (UVB) [3], which is determined by the angle of the sun in the sky at a particular geographical location.
Between 70 and 95% of vitamin D levels depend on the cutaneous synthesis of cholecalciferol, a main metabolite of hepatic 7-dehydrocholesterol [4]. Aging can affect several aspects of this synthesis; for example, there is an age-dependent decrease in 7-hydrocholesterol, and this decrease lowers the concentration of epidermal cholecalciferol, resulting in an increased need for exposure to synthesize an amount similar to that obtained by younger adults [5]. Moreover, skin changes during aging, primarily wrinkling and thinning skin, have been implicated in an altered vitamin D synthesis [6]. Finally, as older adults decline, their level of physical activity decreases, which reduces their sun exposure considerably, further worsening the availability of the main components needed for vitamin D synthesis [7].
Based on these findings, it is plausible to believe that older adults in Mexico have an advantage over those living in countries with lower sun exposure, as Mexico is located at 19° 6′ N, 99° 08′ O and is thus recognized as having an appropriate level of UVB exposure [2].
Despite this assumption, there is little evidence on the prevalence of vitamin D deficiency and its associated factors in older adults living in developing countries [2], Mexico in particular. As Palacios stated, most studies have included randomly selected individuals from the general population, and thus, the elderly population has been under-represented, and sample sizes have not reached sufficient statistical power. Furthermore, differences in data could arise depending on the type of study [8].
Therefore, the aim of this study was to describe the prevalence of vitamin D deficiency in Mexican older adults and to determine the factors associated with this deficiency.
Methods
Design and settings
This was a cross-sectional analysis of the third (2012) wave of the Mexican Health and Aging Study (MHAS), which was a prospective panel study conducted in Mexico. The aim and design of the MHAS have been published elsewhere [9, 10]. In brief, the three waves of the MHAS (2001, 2003, and 2012) contained a representative sample of community-dwelling Mexican older adults (≥50 years old) and their respective spouses (≥18 years old). To determine the factors that influenced aging in this population, a set of questionnaires (e.g., sociodemographic, health-related, health service access, cognitive performance, and functional status) were administered to participants in face-to-face interviews. In addition, each wave included a sub-sample in which anthropometric measures and blood samples were collected.
The last wave, in 2012, assessed 18,465 participants, including 12,569 follow-up participants from 2001 on and 5896 new participants (including spouses of the selected subject, regardless of age). In this wave, anthropometrics, physical performance tests, and blood samples were additionally obtained in a sub-sample of 2089 older adults. After excluding those <60 years (n = 961), the present study evaluated 1128 participants older than 60 years old.
Measurements
Vitamin D
Between October and November 2012, biomarkers including serum 25-(OH)D levels were obtained from blood samples from a peripheral venipuncture obtained by trained personnel according to standardized protocols. Within 30 min of the venipuncture, the samples collected were centrifuged at 2500 rpm for 15 to 20 min to separate the serum, which was stored in two 2-mL tubes. Serum 25-(OH)D levels were measured with a chemiluminescent microparticle immunoassay (CMIA-Architect Abbott Laboratories. Abbott Park, IL, USA). The measurement interval of this CMIA ranged from 8 to 160 ng/mL, and the intra- and inter-assay coefficients of variation were <10%. The cutoff value for defining vitamin D deficiency was <20 ng/mL (<50 nmol/L) for serum 25-(OH)D levels, as suggested for sub-population studies by the American Task Force for vitamin D deficiency screening [11].
Independent variables
To further describe vitamin D deficiency and its related factors, variables from different domains were included. The sociodemographic characteristics included age, sex, marital status (married or not), and years of education. The health-related variables included smoking status (currently smoking), low physical activity (negative response to the following question “In the last two years, have you regularly performed exercise or other vigorous physical activity?”), self-report of six common chronic diseases (i.e., diabetes mellitus, hypertension, cardiac disease, lung disease, cancer, and stroke), geriatric conditions (i.e., falls, depression, cognitive decline, and functional dependency), anthropometry (body mass index [BMI] in kg/m2, systolic and diastolic pressure in mmHg, and waist-to-hip index), and serum biomarkers (c-reactive protein [CRP] in mg/dL, total cholesterol in mg/dL, high-density lipoprotein cholesterol [HDL-c] in mg/dL, thyroid stimulating hormone [TSH] in mIU/mL, hemoglobin in g/dL, and glycated hemoglobin percentage).
Regarding geriatric variables, falls were considered present if the older adult answered “yes” to the question “Have you fallen in the last 12 months?”. Depressive symptoms were considered present if the older adult scored ≥5 points on a previously validated five-item questionnaire for depression (a set of seven dichotomous items generated by specific questions related to mood) [12]. Subjects were considered to have cognitive decline if they failed two or more sub-tests of the brief version of the Cross-Cultural Cognitive Examination [13]. If the older adult required help for one of the eight activities of daily living listed, he or she was defined as having functional dependency. Trained study personnel performed standard anthropometric measurements, and the average of two measurements was used for this analysis. Regarding biomarkers, the same procedure for serum 25-(OH)D levels was conducted as previously described, and technical details for each of the biomarkers are available upon request or on the MHAS webpage http://www.mhasweb.org/DocumentationQuestionnaire.aspx.
Statistical analysis
Descriptive statistics were calculated to estimate the prevalence or the relative frequency of the population that had serum 25-(OH) D levels <20 ng/mL. In addition, this prevalence was described by sex and age group (60–65, 66–75, and >75 years old). A plot of the vitamin D deficiency prevalence and age stratified by sex was generated to visualize this condition across age ranges. To assess associations, a bivariate analysis including all of the previously described variables was performed; p values were obtained using t tests or chi-square tests based on the nature of the variable (continuous or categorical, respectively). Finally, a multiple logistic regression model was fit using vitamin D deficiency as the dependent variable, and all of the independent variables were simultaneously included. All analyses were performed with the statistical package software STATA 14 ® (StataCorp, 4905 Lakeway Drive, College Station, TX 77845, USA).
Ethical issues
The MHAS was approved by the Institutional Review Boards and Ethics Committees of the University of Texas Medical Branch in the USA, the Instituto Nacional de Estadistica y Geografia (INEGI), and the Instituto Nacional de Salud Publica (INSP) in Mexico. In addition, the current analysis was registered at the Instituto Nacional de Geriatría (DI-PI-003/2016).
Results
The study included a total of 1128 adults older than 60 years. The mean age was 69.6 years (±SD 7.67 years), and the mean years of education was 4.5 years (±SD 4.3 years). Of the total sample, 51.2% were women (n = 578), and 58% (n = 654) were married, while more than half had low levels of physical activity (59.3%). The most frequently reported chronic disease was hypertension (49%), and 42.7% (n = 482) of the older adults reported recent falls (Table 1). The mean serum vitamin D level was 23.1 ng/mL (±SD 8.1 ng/mL), and 36.9% (n = 416) of the subjects had serum levels <20 ng/mL.
Table 1.
General description of the population by vitamin D deficiency status
| Total (n = 1128) | Deficient, <20 ng/mL (n = 416) | Not deficient, ≥20 ng/mL (n = 712) | p value | |
|---|---|---|---|---|
| Age, mean (SD) | 69.6 (7.67) | 70.67 (8.01) | 69.1 (7.4) | 0.001 |
| Women, n (%) | 578 (51.2) | 271 (65.1) | 307 (43.1) | <0.001 |
| Marital status, n (%) | 654 (57.9) | 212 (50.9) | 442 (62.1) | <0.001 |
| Years of education, mean (SD) | 4.5 (4.26) | 5.3 (4.5) | 4.1 (4.05) | <0.001 |
| Current smoker, n (%) | 129 (11.4) | 61 (14.6) | 68 (9.5) | 0.009 |
| Low physical activity, n (%) | 669 (59.3) | 293 (70.4) | 376 (52.8) | <0.001 |
| Diabetes mellitus, n (%) | 264 (23.4) | 123 (29.5) | 141 (19.8) | <0.001 |
| Hypertension, n (%) | 554 (49.1) | 214 (51.4) | 340 (47.7) | 0.232 |
| Cardiac disease, n (%) | 43 (3.8) | 17 (4.1) | 26 (3.6) | 0.713 |
| Lung disease, n (%) | 68 (6.04) | 33 (7.93) | 35 (4.93) | 0.041 |
| Cancer, n (%) | 31 (2.75) | 15 (3.6) | 16 (2.2) | 0.178 |
| Stroke, n (%) | 26 (2.3) | 9 (2.1) | 17 (2.3) | 0.809 |
| Falls, n (%) | 482 (42.7) | 181 (43.5) | 301 (42.2) | 0.686 |
| Depression, n (%) | 427 (37.9) | 166 (40.2) | 261 (36.7) | 0.246 |
| Cognitive decline, n (%) | 99 (8.78) | 36 (8.6) | 63 (8.8) | 0.911 |
| Functional dependency, n (%) | 203 (18) | 97 (23.3) | 106 (14.8) | <0.001 |
| BMI, mean (SD) | 28.4 (5.3) | 29.1 (5.6) | 28.1 (5.1) | 0.001 |
| Systolic pressure, mean (SD) | 143 (21.1) | 145.5 (21.6) | 141.5 (20.7) | 0.002 |
| Diastolic pressure, mean (SD) | 77.8 (11.2) | 78.4 (11.2) | 77.5 (11.1) | 0.21 |
| Waist-to-hip index, mean (SD) | 0.96 (0.07) | 0.95 (0.08) | 0.96 (0.07) | 0.037 |
| CRP, mean (SD) | 4.2 (5.79) | 4.8 (7.3) | 3.86 (4.64) | 0.008 |
| Total cholesterol, mean (SD) | 198 (45.3) | 201.5 (48.7) | 195.9 (43.1) | 0.046 |
| HDL-c, mean (SD) | 41.1 (10.5) | 41.8 (11.4) | 40.6 (9.9) | 0.066 |
| TSH, mean (SD) | 2.85 (5.2) | 3.4 (7.25) | 2.52 (3.59) | 0.006 |
| Hemoglobin, mean (SD) | 14.8 (2.12) | 14.8 (1.99) | 14.8 (2.1) | 0.927 |
| Glycated hemoglobin | 0.39 (0.48) | 0.46 (0.49) | 0.34 (0.47) | 0.002 |
As shown in Table 1, vitamin D-deficient subjects were older (70.67 vs. 69.1 years, p < 0.001), were more often current smokers (14.6 vs. 9.5%, p < 0.05), had a higher mean BMI (29.1 vs. 28.1 kg/m2, p < 0.001), had a higher prevalence of diabetes (29.5 vs. 19.8%, p < 0.001), more frequently reported low activity levels (70.4 vs. 52.8%, p < 0.001), and were more dependent (23.3 vs. 14.8%, p < 0.001). In addition, some biomarkers also significantly differed between older adults with vitamin D deficiency and those without vitamin D deficiency, including systolic pressure (145.5 vs. 141.5 mmHg, p = 0.002) and CRP level (4.8 vs. 3.86 mg/dL, p = 0.008) (Table 1).
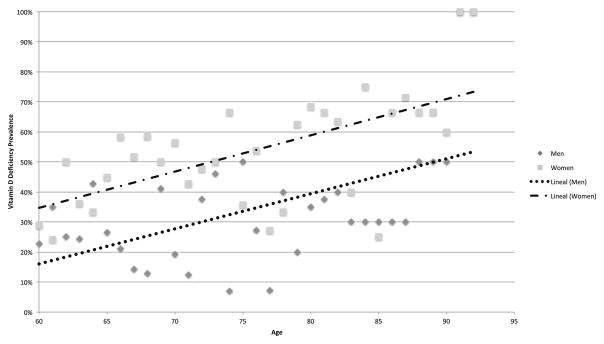
Table 2 shows the results of the stratified analysis by sex and age group. Vitamin D deficiency for the total sample significantly increased with age; however, after stratification by sex, this relationship was only present among women. In addition, vitamin D deficiency was consistently higher among women than men (Fig. 1).
Table 2.
Prevalence of vitamin D deficiency according to sex and age group
| Total (n = 416) | 60–65 years (n = 146) | 66–75 years | >75 years (n = 104) | p value | |
|---|---|---|---|---|---|
| Men, n (%) | 145 (26.3) | 68 (27.9) | 43 (22.1) | 34 (30.1) | 0.255 |
| Women, n (%) | 271 (46.8) | 78 (36.7) | 123 (52.1) | 70 (54.2) | 0.001 |
| Total, n (%) | 416 (36.9) | 146 (32.1) | 166 (38.5) | 104 (42.9) | 0.029 |
Fig. 1.
Prevalence of vitamin D deficiency by age category and sex
In the multivariate analysis (Table 3), the multiple logistic regression model (R2 = 0.115, p < 0.001) showed that vitamin D deficiency was independently associated with age (odds ratio [OR] 1.02, 95% confidence interval [CI] 1.01–1.05; p < 0.001), female sex (OR 1.97, 95% CI 1.59–2.42; p < 0.001), years of education (OR 1.1, 95% CI 1.06–1.13; p < 0.001), current smoking (OR 2.21, 95% CI 1.47–3.39; p < 0.001), low physical activity (OR 1.74, 95% CI 1.31–2.23; p < 0.001), BMI (OR 1.03, 95% CI 1.01–1.06; p < 0.004), systolic pressure (OR 1.02, 95% CI 1.01–1.03; p = 0.045), CRP level (OR 1.02, 95% CI 1.01–1.05; p = 0.04), TSH level (OR 1.03, 95% CI 1.01–1.07; p = 0.013), and glycated hemoglobin level (OR 1.16, 95%CI 1.07–1.25; p < 0.001).
Table 3.
Multiple logistic regression model. Only significant variables with vitamin D deficiency as the dependent variable are presented
| OR (95% CI) | p value | |
|---|---|---|
| Age | 1.02 (1.01–1.05) | <0.001 |
| Women | 1.97 (1.59–2.42) | <0.001 |
| Years of education | 1.1 (1.06–1.13) | <0.001 |
| Current smoker | 2.21 (1.47–3.39) | <0.001 |
| Low physical activity | 1.74 (1.31–2.23) | <0.001 |
| BMI | 1.03 (1.01–1.06) | 0.004 |
| Systolic pressure | 1.02 (1.01–1.03) | 0.045 |
| CRP | 1.02 (1.01–1.05) | 0.04 |
| TSH | 1.03 (1.01–1.07) | 0.013 |
| Glycated hemoglobin | 1.16 (1.07–1.25) | <0.001 |
R2 = 0.1151, p < 0.001, for the model
Discussion
Our main findings show that vitamin D deficiency (25OHD <20 ng/mL) is common in Mexican older adults, with a prevalence of 37%. Additionally, the other factors associated with this deficiency—both unique to our population and previously described in other older adult groups—included sex (female), current smoking, educational level, physical activity, and high levels of glycated hemoglobin.
Flores et al. found a lower prevalence of vitamin D deficiency after analyzing the results of the National Health and Nutrition Survey 2006 (ENSANUT 2006) to estimate vitamin D concentrations in all age groups [14]. According to these authors, 13% of Mexican older adults (>60 years) presented vitamin D deficiency; however, the sample size was small (n = 158) and not representative of older adults.
Our findings are similar to those reported for Australia, a country that is considered to have sufficient sun exposure during the year in almost all of its territories due to its geographical location (27° 00′ S, 133° 00′ E). According to this previous report, the prevalence of vitamin D deficiency in Australia was 31%; the lowest levels of 25-(OH)D were observed among participants living in the south (>35° S) as a result of the lower sun exposure [15].
Other reports from countries with different localizations show a wide range in the prevalence of vitamin D deficiency in older adults. A recent study by Klenk et al. that evaluated a group of community-dwelling individuals living in Germany reported a higher prevalence of vitamin D deficiency (79%) than that observed in our study [3]. In the USA, another country with insufficient sun exposure for at least 1 month a year as well as with a high percentage of African-Americans, who are reported to have one of the highest rates of vitamin D deficiency worldwide, the prevalence of vitamin D deficiency was 42% [16].
The heterogeneity in prevalence could occur as the result of differences in the cutoff values used to define vitamin D deficiency, with studies that use higher cutoff values tending to report higher prevalence. In our study, the cutoff value proposed by the U.S. Preventive Services Task Force (<20 ng/mL of serum 25-(OH) D) [11, 17] was used, and as comparisons were made with previous studies that used the same value, the differences in the reported prevalence can be attributed to variables other than the cutoff points.
Despite having sufficient sun exposure, the prevalence of vitamin D deficiency in Mexico was high and could be explained by other associated factors. In fact, Portela et al. [18–20] reported that the prevalence of vitamin D deficiency is linked with factors such as sex, age, genetics, diet, adiposity, and comorbidities.
Our results, as well as those from Oliveri et al. [21], Portela [18], and Linnebur [22], demonstrate a strong correlation between female sex and vitamin D deficiency. These results could be explained by the variations in hormone secretion between the sexes, an association that merits exploration in future research. With respect to age, as shown by Choi et al. [23] and Daly et al. [15], lower levels of vitamin D were observed in older people in our sample, as expected [24]. Regarding education, low levels of education were surprisingly related to better vitamin D status; this finding is consistent with Forrest et al. Our hypothesis concerning this finding is that less educated people may have more sun exposure as a result of performing activities related to agriculture and livestock; however, these findings should be further studied in future research.
In our study, vitamin D deficiency occurred among older people with low physical activity, functional dependency, and comorbidities such as diabetes. These associations have been previously reported [25–27] and can be explained by the “reverse causality” phenomenon described by Thacher [1]. This phenomenon suggests that ill people are less likely to participate in more outdoor activities and consequently have less sun exposure than those in better health conditions. Additionally, as vitamin D3 (cholecalciferol) is stored in adipose tissue and a vitamin D reserve is generated during summer, older adults who are not exposed to sunlight during this season are at a higher risk of having vitamin D deficiency.
In this sense, it is conceivable that a higher BMI would be correlated with a better vitamin D status; nonetheless, our results as well as those by Linnebur [22] have demonstrated that serum 25(OH)D levels are lower among elderly individuals with a higher BMI than among those with a normal BMI (19.0 vs. 26.2 ng/mL). This relationship could result from the sedentary lifestyle that characterizes individuals with a higher BMI, as they may practice less outdoor activities and thus have reduced sun exposure. As Bell et al. reported, in people with obesity, vitamin D is sequestered in fat tissue, reducing its release into the circulation and therefore its bioavailability [28].
Although causal mechanisms were not studied in this work, it is reasonable to suggest that vitamin D deficiency is part of a complex homeostatic imbalance that is characterized by the presence of multimorbidity and other physical impairments and an increased risk of frailty, disability, and dependence; in turn, these factors promote a deficient nutritional status, which, in addition to a low sun exposure, contributes to permanent vitamin deficiency.
This study on the prevalence of vitamin D deficiency and its associated factors provides an overview of the health conditions of older adults in Mexico. The findings thus support the design of strategies and interventions based on a population’s specific characteristics and needs to ensure that better results in the clinical setting and in public health can be achieved. Based on our results, interventions that focus on modifiable determinants of vitamin D deficiency, such as those promoting adequate sun exposure as well as a healthy diet and physical activity, need to be addressed to reduce the prevalence of vitamin D deficiency in this population.
Some limitations should be considered to interpret our results in context. This study used a cross-sectional design, which limited the possibility of interpreting the causality of the associations. In addition, some variables that were not available in our dataset could have been associated with vitamin D deficiency (such as place of residence, nutritional factors, and other hormones). In particular, vitamin D levels show well-described seasonal variations, and data on these changes were not available in our study, indicating the need for further studies on this topic [29].
Another possible limitation of our study is the use of self-report questionnaires, which may have introduced recall bias. However, different epidemiological studies that have aimed to characterize large populations have used self-report, as it is a methodology that has demonstrated effectiveness in achieving results and reducing time and costs [30].
An important strength of our study is that it is the first population-based study to report on the prevalence of vitamin D deficiency and its determinants in older Mexican adults and is one of the few studies in Latin America that has focused on this population group [2, 31]; furthermore, the quality of the method used to determine the levels of vitamin D as well as the sample size diminished the variability in our results.
These findings present opportunities for new research in the field, as there is little evidence on the determinants and mechanisms of vitamin D deficiency in the older Mexican population. In this respect, we consider it important for future research, especially studies aiming to explain causality, to measure the percentage of fat mass rather than BMI, parathyroid hormone levels, environmental and lifestyle factors that account for more substantial variations in serum 25OHD levels [32], characteristics of solar exposure, levels of pollution, types of clothes, and race and ethnicity to better determine the role of these components in vitamin D reserve.
Acknowledgments
Funding
The 2012 version of the Mexican Health and Aging Study was supported in part by the National Institutes of Health/National Institute on Aging (R01AG018016, R Wong, PI). This manuscript was supported by the Instituto Nacional de Geriatría grant number DI-C2016-MC-004.
Footnotes
Compliance with ethical standards
Conflict of interest
None.
List of names for PubMed
Carrillo-Vega MF
García-Peña C
Gutiérrez-Robledo LM
Pérez-Zepeda MU
References
- 1.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arabi A, El Rassi R, El-Hajj Fuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat Rev Endocrinol. 2010;6:550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- 3.Klenk J, Rapp K, Denkinger MD, Nagel G, Nikolaus T, Peter R, Koenig W, Bohm BO, Rothenbacher D. Seasonality of vitamin D status in older people in Southern Germany: implications for assessment. Age Ageing. 2013;42:404–408. doi: 10.1093/ageing/aft042. [DOI] [PubMed] [Google Scholar]
- 4.Atli T, Gullu S, Uysal AR, Erdogan G. The prevalence of vitamin D deficiency and effects of ultraviolet light on vitamin D levels in elderly Turkish population. Arch Gerontol Geriatr. 2005;40:53–60. doi: 10.1016/j.archger.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Reis JP, von Muhlen D, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124:e371–e379. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durvasula S, Gies P, Mason RS, Chen JS, Henderson S, Seibel MJ, Sambrook PN, March LM, Lord SR, Kok C, Macara M, Parmenter TR, Cameron ID. Vitamin D response of older people in residential aged care to sunlight-derived ultraviolet radiation. Arch Osteoporos. 2014;9:197. doi: 10.1007/s11657-014-0197-9. [DOI] [PubMed] [Google Scholar]
- 7.Scott D, Blizzard L, Fell J, Ding C, Winzenberg T, Jones G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol. 2010;73:581–587. doi: 10.1111/j.1365-2265.2010.03858.x. [DOI] [PubMed] [Google Scholar]
- 8.Palacios C, González L. La deficiencia de vitamina D es un problema global de salud pública. An Venez Nutr. 2014;27:57–72. [Google Scholar]
- 9.Wong R, Michaels-Obregon A, Palloni A. Cohort profile: the Mexican Health and Aging Study (MHAS) Int J Epidemiol. 2015 doi: 10.1093/ije/dyu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong R, Michaels-Obregon A, Palloni A, Gutierrez-Robledo LM, Gonzalez-Gonzalez C, Lopez-Ortega M, Tellez-Rojo MM, Mendoza-Alvarado LR. Progression of aging in Mexico: the Mexican Health and Aging Study (MHAS) 2012. Salud Publica Mex. 2015;57(Suppl 1):S79–S89. doi: 10.21149/spm.v57s1.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeFevre ML Force USPST. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:133–140. doi: 10.7326/M14-2450. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Navarro SG, Fuentes-Cantu A, Avila-Funes JA, Garcia-Mayo EJ. Validity and reliability of the screening questionnaire for geriatric depression used in the Mexican Health and Age Study. Salud Publica Mex. 2007;49:256–262. doi: 10.1590/s0036-36342007000400005. [DOI] [PubMed] [Google Scholar]
- 13.Mejia-Arango S, Gutierrez LM. Prevalence and incidence rates of dementia and cognitive impairment no dementia in the Mexican population: data from the Mexican Health and Aging Study. J Aging Health. 2011;23:1050–1074. doi: 10.1177/0898264311421199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores M, Sánchez-Romero LM, Macías N, Lozada A, Díaz E, Barquera S. INdSP, editor. Resultados de la ENSANUT 2006. Secretaría de Salud; Cuernavaca, México: 2011. Concentraciones séricas de vitamina D en niños, adolescentes y adultos mexicanos (Mexico) [Google Scholar]
- 15.Daly RM, Gagnon C, ZX L, Magliano DJ, Dunstan DW, Sikaris KA, Zimmet PZ, Ebeling PR, Shaw JE. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol. 2012;77:26–35. doi: 10.1111/j.1365-2265.2011.04320.x. [DOI] [PubMed] [Google Scholar]
- 16.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Portela ML, Mónico A, Barahona A, Dupraz H, Sol Gonzales-Chaves MM, Zeni SN. Comparative 25-OH-vitamin D level in institutionalized women older than 65 years from two cities in Spain and Argentina having a similar solar radiation index. Nutrition. 2010;26:283–289. doi: 10.1016/j.nut.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Mateo-Pascual C, Julián-Viñals R, Alarcón-Alarcón T, Castell-Alcalá MV, Iturzaeta-Sánchez JM, Otero-Piume A. Vitamin D deficiency in a cohort over 65 years: prevalence and association with sociodemographic and health factors. Rev Esp Geriatr Gerontol. 2014;49:210–216. doi: 10.1016/j.regg.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 20.McCarroll K, Beirne A, Casey M, McNulty H, Ward M, Hoey L, Molloy A, Laird E, Healy M, Strain JJ, Cunningham C. Determinants of 25-hydroxyvitamin D in older Irish adults. Age Ageing. 2015;44:847–853. doi: 10.1093/ageing/afv090. [DOI] [PubMed] [Google Scholar]
- 21.Oliveri B, Plantalech L, Bagur A, Wittich AC, Rovai G, Pusiol E, López Giovanelli J, Ponce G, Nieva A, Chaperón A, Ladizesky M, Somoza J, Casco C, Zeni S, Parisi MS, Mautalen CA. High prevalence of vitamin D insufficiency in healthy elderly people living at home in Argentina. Eur J Clin Nutr. 2004;58:337–342. doi: 10.1038/sj.ejcn.1601786. [DOI] [PubMed] [Google Scholar]
- 22.Linnebur SA, Vondracek SF, Vande Griend JP, Ruscin JM, McDermott MT. Prevalence of vitamin D insufficiency in elderly ambulatory outpatients in Denver, Colorado. Am J Geriatr Pharmacother. 2007;5:1–8. doi: 10.1016/j.amjopharm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Choi EY. 25(OH)D status and demographic and lifestyle determinants of 25(OH)D among Korean adults. Asia Pac J Clin Nutr. 2012;21:526–535. [PubMed] [Google Scholar]
- 24.Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185–191. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm-Leen ER, Hall YN, Deboer IH, Chertow GM. Vitamin D deficiency and frailty in older Americans. J Intern Med. 2010;268:171–180. doi: 10.1111/j.1365-2796.2010.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semba RD, Garrett E, Johnson BA, Guralnik JM, Fried LP. Vitamin D deficiency among older women with and without disability. Am J Clin Nutr. 2000;72:1529–1534. doi: 10.1093/ajcn/72.6.1529. [DOI] [PubMed] [Google Scholar]
- 27.Hirani V, Cumming RG, Le Couteur DG, Naganathan V, Blyth F, Handelsman DJ, Waite LM, Seibel MJ. Low levels of 25-hydroxy vitamin D and active 1,25-dihydroxyvitamin D independently associated with type 2 diabetes mellitus in older Australian men: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2014;62:1741–1747. doi: 10.1111/jgs.12975. [DOI] [PubMed] [Google Scholar]
- 28.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D—endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu HJ, Kwon MJ, Woo HY, Park H. Analysis of 25-hydroxyvitamin D status according to age, gender, and seasonal variation. J Clin Lab Anal. 2016;30:905–911. doi: 10.1002/jcla.21955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunes DP, Duarte YA, Santos JL, Lebrao ML. Screening for frailty in older adults using a self-reported instrument. Rev Saude Publica. 2015;49:2. doi: 10.1590/S0034-8910.2015049005516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 32.Fuleihan GE, Bouillon R, Clarke B, Chakhtoura M, Cooper C, McClung M, Singh RJ. Serum 25-hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res. 2015;30:1119–1133. doi: 10.1002/jbmr.2536. [DOI] [PubMed] [Google Scholar]