Breast cancer burden is high in low-resource countries. From 1980 to 2010, new breast cancer cases increased by more than 50% worldwide.1 Disease burden increased even more rapidly in low- and middle-income countries (LMICs), where more than half of breast cancer cases now occur. Moreover, breast cancer disproportionately affects young women in LMICs, such that 23% of new breast cancer cases occur among women age 15 to 49 years in LMICs versus 10% in high-income countries.1
Breast cancer mortality is also higher in LMICs compared with high-income countries, and reasons for this are multifactorial. One contributing factor is a lack of breast cancer awareness and early detection in LMICs. For example, more than 90% of women with newly diagnosed breast cancer in the United States have locoregional disease, whereas more than half of women with newly diagnosed breast cancers in LMICs have stage III or IV disease.2,3 According to an analysis from the 2003 World Health Survey, only 2.2% of women age 40 to 69 years in LMICs had received any breast cancer screening.4 In addition to insufficient early detection, other factors contributing to delayed diagnosis include poverty, cultural and religious beliefs, misconceptions about the disease, and fear of mastectomy.5 Women's autonomy in health care decision making may also be limited in some cultures.5
The WHO, along with many national cancer control programs, recommends population-based screening mammography for detection of early-stage breast cancer in high-income countries, even though there continues to be honest and sometimes heated debate regarding this recommendation.6–8 It is worthwhile to consider the possible benefits versus harms of breast cancer screening in LMICs, which have received far less attention. In this commentary, we discuss breast cancer screening and early detection in LMICs with a particular focus on Malawi. We highlight areas of uncertainty and suggest pragmatic strategies for moving forward in light of current evidence gaps.
Health care systems in LMICs may face strong incentives and pressure to adopt health care interventions such as screening mammography that are well established in high-resource settings, with implicit assumptions that benefits demonstrated in more developed countries will generalize to less developed countries. Such assumptions are inherently problematic and unrealistic in settings of severe resource scarcity. For example, there are compelling reasons to believe that breast cancer screening would perform differently in LMICs than in high-income countries. Factors that could reduce efficacy of breast cancer screening in LMICs include a younger population with lower breast cancer incidence, shorter life expectancy, more prevalent competing causes of death, and higher prevalence of biologically aggressive subtypes for which patient outcomes are less likely to be affected by screening. Conversely, breast cancer screening could have greater impact in LMICs if it increases breast cancer awareness and early detection of symptomatic disease. For example, there may be more diffuse effects than would be expected in resource-rich settings where strong health care systems and higher levels of awareness narrow the scope of breast cancer screening principally to detection of asymptomatic disease. Indeed, for weak health care systems, it is plausible that effects beyond breast cancer may be realized and may extend to cancer more generally or to women's health. Investments in HIV programs have similarly had far-reaching effects beyond providing antiretroviral therapy, and antiretroviral therapy clinics are now established vehicles for effective delivery of many other essential health services. In Malawi, commonly piggybacked health services in HIV clinics now include cervical cancer screening, Kaposi sarcoma treatment, nutritional supplementation, and reproductive health and mother-child wellness initiatives, all of which seek to maximize impacts from initial investments for HIV.
Despite recent controversies about screening mammography in high-income countries and a scarcity of high-quality data for this approach in LMICs, it is often assumed that wherever mammography is available, it must benefit women. This may be the case, even when screening is available only in the private sector without clearly defined eligibility guidelines, quality control measures, or follow-up procedures.9 Examples of this exist in Malawi, where a major intersection in Lilongwe (the capital) features a billboard advertising screening mammography in a private clinic promoted by a famous young Malawian breast-cancer survivor. However, the cost of a screening mammogram in Lilongwe is approximately US$90 in a country with an annual gross domestic product per capita of US$253.10 Moreover, screening is often directly marketed to and used for women who can pay for it, without clear eligibility criteria accounting for age, comorbidities, or projected life expectancy. In Lilongwe, mammography sponsors have distributed coupons for discounted screening mammography at public breast cancer awareness events to unselected audiences of women primarily in their 20s and 30s. Benefits of screening mammography have not been clearly demonstrated for average-risk women in these age groups anywhere in the world, nor is it recommended for them in consensus guidelines.
In addition, LMICs often lack the necessary infrastructure to ensure high-quality mammography and subsequent follow-up care.11 Operating a mammography unit continuously requires a consistent supply of electricity and x-ray films, as well as engineers, technicians, and radiologists, all of which may be lacking in many LMICs. Four mammography units were donated to Malawi in 2012, one to each tertiary referral hospital, with the intent to provide the first publicly available mammography services in the country, but these units have yet to become operational.12 Mammography screening programs have also been estimated to cost US$16,000 to US$37,000 per life saved, which exceeds per capita health care budgets in many LMICs by a significant margin.9,13
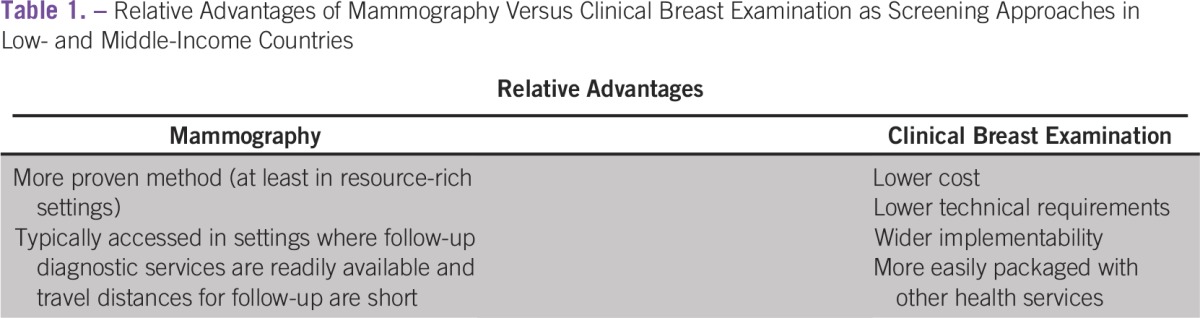
International guidelines recommend clinical breast examination (CBE) as a preferred approach to screening in settings in which mammography screening is not available.5,14 Even in high-resource settings, there is some evidence that annual CBE might be as effective as screening mammography in lowering breast cancer mortality.15-18 Relative advantages for mammography versus CBE with respect to implementation are detailed in Table 1.
Table 1.
Relative Advantages of Mammography Versus Clinical Breast Examination as Screening Approaches in Low- and Middle-Income Countries
In LMICs, two clinical trials in Egypt found that CBE conducted by physicians was effective and cost-effective in rural and urban areas.19,20 In Malaysia, training rural nurses to perform CBE resulted in significant breast cancer downstaging (77% v 37% late-stage diagnoses).21 In an ongoing cluster-randomized trial in India, CBE performed by female community health care workers detected more early-stage (I to IIA) cancers (18.8 v 8.1 per 100,000 women) in intervention versus control villages; no differences were observed for stage IIB and higher-stage cancers.22 A cross-sectional study in Nepal comparing CBE performed by female community health care workers with examinations by surgeons reported interobserver agreement of 64% for lump detection, with 70% sensitivity and 95% specificity.23 Moreover, modeling studies have suggested that CBE is cost-effective in low-resource settings.24,25
In addition to health care workers, lay volunteers can also be trained to perform CBE. A study in rural Sudan screened approximately 10,000 women age 18 years or older by using this approach. Seventeen of those screened had carcinoma in situ or breast cancer, including eight with carcinoma in situ and four with early-stage breast cancer. In control villages, only four women self-referred for breast symptoms, three of whom had advanced-stage breast cancer.26 In Tanzania, laypersons in villages were trained to provide screening for a variety of cancers by using basic history and physical examination. After 3 years, breast cancer downstaging was one of the most significant results of the program, evidenced by a 74% increase in stage I to II breast tumors.27
In LMICs where health care systems are significantly weakened by limited resources and human capacity, it is worth emphasizing that anticipated impacts of widespread breast cancer screening would not be limited to detecting asymptomatic disease. For example, in Malawi, 47% of women with pathologically confirmed breast cancer at the tertiary referral hospital in Lilongwe had symptom durations greater than 12 months,28 and only 44% of randomly selected women from rural and urban areas in the Lilongwe district were aware of breast cancer as a disease.29 Therefore, if CBE were effectively scaled up throughout Malawi in a manner that engages communities with effective downstream referral, anticipated benefits might be large with respect to improved cancer awareness and earlier identification of unaddressed, prevalent, symptomatic disease. In addition, there may be collateral effects on other public health problems apart from breast cancer, including promotion of healthier lifestyles among women as well as increased cancer awareness and destigmatization. These off-target effects of breast cancer screening are no less important simply because they are harder to define and measure than the number of early-stage breast cancers diagnosed.
Classical cancer screening paradigms and messaging must be adapted to the LMIC context. The HIV implementation science field now champions pragmatic scale-up of proven multicomponent interventions to maximize population-level outcomes in LMICs. Similar approaches may be attractive for cancer screening as well. We are currently conducting a pilot breast cancer education and CBE screening intervention in Lilongwe among women attending diverse health clinics. The major objectives are to assess uptake and feasibility of packaging CBE with other health services, performance characteristics of CBE performed by trained lay breast health promoters, and completion rates for referrals among women with detected abnormalities. These preliminary data will help inform wider scale-up of breast cancer awareness and screening efforts throughout Malawi.
Even as the screening mammography debate evolves in resource-rich settings, mammography is being actively promoted and implemented in many resource-limited countries in the world, including Malawi. We believe there is agreement within the global health community that high breast cancer burden and mortality in LMICs require an urgent response, but competing health needs and local realities require that available resources be optimally used to provide the best value for populations overall. This may be particularly true, given that several breast cancer screening approaches are available that can be packaged together in varying combinations. We believe more evidence is needed to guide large-scale breast cancer screening approaches in LMICs under varying socioeconomic and cultural conditions, and we emphasize that although CBE has been shown to result in cancer downstaging in LMIC settings, effects on breast cancer–specific mortality remain unclear. Limited cancer diagnosis, treatment, and registration throughout LMICs also limit the impact of screening interventions as well as metrics for their evaluation and must be simultaneously strengthened. We eagerly await results of ongoing studies, including our own work, to define optimal approaches in Malawi, with the expectation that successful strategies here may be quite different from those in other LMIC settings.
Footnotes
Supported by Grants No. K01TW009488, R21CA180815, and U54CA190152 from the National Institutes of Health (S.G.), Grant No. 5R25TW009340 from the National Institutes of Health Fogarty International Center (L.G.), and by the State Department Fulbright Scholar Program.
AUTHOR CONTRIBUTIONS
Financial support: Satish Gopal
Administrative support: Satish Gopal
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Lily A. Gutnik
No relationship to disclose
Beatrice Matanje-Mwagomba
No relationship to disclose
Vanessa Msosa
No relationship to disclose
Suzgo Mzumara
No relationship to disclose
Blandina Khondowe
Employment: Center for Medical Diagnostics, Lilongwe
Agnes Moses
No relationship to disclose
Racquel E. Kohler
No relationship to disclose
Lisa A. Carey
Research Funding: GlaxoSmithKline, Genentech/Roche
Clara N. Lee
No relationship to disclose
Satish Gopal
No relationship to disclose
REFERENCES
- 1.Institute for Health Metrics and Evaluation (IHME) The Challenge Ahead: Progress and Setbacks in Breast and Cervical Cancer. Seattle, WA: IHME; 2011. [Google Scholar]
- 2.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–318. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 4.Akinyemiju TF. Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: Analysis of the World Health Survey. PLoS One. 2012;7:e48834. doi: 10.1371/journal.pone.0048834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yip CH, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: Early detection resource allocation. Cancer. 2008;113:2244–2256. doi: 10.1002/cncr.23842. [DOI] [PubMed] [Google Scholar]
- 6.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311:1327–1335. doi: 10.1001/jama.2014.1398. [DOI] [PubMed] [Google Scholar]
- 7.Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: Systematic review of incidence trends. BMJ. 2009;339:b2587. doi: 10.1136/bmj.b2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med. 2014;174:448–454. doi: 10.1001/jamainternmed.2013.13635. [DOI] [PubMed] [Google Scholar]
- 9.Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10:89–92. [PMC free article] [PubMed] [Google Scholar]
- 10.The World Bank. GDP per capita (current US$): Malawi. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD/countries/1W?display=default.
- 11.Corbex M, Burton R, Sancho-Garnier H. Breast cancer early detection methods for low and middle income countries, a review of the evidence. Breast. 2012;21:428–434. doi: 10.1016/j.breast.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Kaminjolo S. India donates cancer detecting equipment to Malawi. The Nation, February 17, 2004. [Google Scholar]
- 13.Harford JB. Breast-cancer early detection in low-income and middle-income countries: Do what you can versus one size fits all. Lancet Oncol. 2011;12:306–312. doi: 10.1016/S1470-2045(10)70273-4. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) WHO position paper on mammography screening. Geneva, Switzerland: WHO; 2014. [PubMed] [Google Scholar]
- 15.Miller AB, Wall C, Baines CJ, et al. Twenty-five-year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: Randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher SW. ACP Journal Club: Annual mammography screening did not reduce long-term breast cancer mortality in women 40 to 59 years of age. Ann Intern Med. 2014;160:JC7. doi: 10.7326/0003-4819-160-10-201405200-02007. [DOI] [PubMed] [Google Scholar]
- 17.Jatoi I. Screening clinical breast examination. Surg Clin North Am. 2003;83:789–801. doi: 10.1016/S0039-6109(03)00028-8. [DOI] [PubMed] [Google Scholar]
- 18.Anderson BO. Breast cancer: Thinking globally. Science. 2014;343:1403. doi: 10.1126/science.1253344. [DOI] [PubMed] [Google Scholar]
- 19.Boulos S, Gadallah M, Neguib S, et al. Breast screening in the emerging world: High prevalence of breast cancer in Cairo. Breast. 2005;14:340–346. doi: 10.1016/j.breast.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Denewer A, Hussein O, Farouk O, et al. Cost-effectiveness of clinical breast assessment-based screening in rural Egypt. World J Surg. 2010;34:2204–2210. doi: 10.1007/s00268-010-0620-3. [DOI] [PubMed] [Google Scholar]
- 21.Devi BC, Tang TS, Corbex M. Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: A pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol. 2007;18:1172–1176. doi: 10.1093/annonc/mdm105. [DOI] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical breast examination: Preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103:1476–1480. doi: 10.1093/jnci/djr304. [DOI] [PubMed] [Google Scholar]
- 23.Hyoju SK, Agrawal CS, Pokhrel PK, et al. Transfer of clinical breast examination skills to female community health volunteers in Nepal. Asian Pac J Cancer Prev. 2011;12:3353–3356. [PubMed] [Google Scholar]
- 24.Duffy SW, Tabar L, Vitak B, et al. Tumor size and breast cancer detection: What might be the effect of a less sensitive screening tool than mammography? Breast J. 2006;12:S91–S95. doi: 10.1111/j.1075-122X.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 25.Okonkwo QL, Draisma G, der Kinderen A, et al. Breast cancer screening policies in developing countries: A cost-effectiveness analysis for India. J Natl Cancer Inst. 2008;100:1290–1300. doi: 10.1093/jnci/djn292. [DOI] [PubMed] [Google Scholar]
- 26.Abuidris DO, Elsheikh A, Ali M, et al. Breast-cancer screening with trained volunteers in a rural area of Sudan: A pilot study. Lancet Oncol. 2013;14:363–370. doi: 10.1016/S1470-2045(12)70583-1. [DOI] [PubMed] [Google Scholar]
- 27.Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. Int J Cancer. 2015;136:2875–2876. doi: 10.1002/ijc.29348. [DOI] [PubMed] [Google Scholar]
- 28.Kohler RE, Moses A, Krysiak R, et al. Pathologically confirmed breast cancer in Malawi: A descriptive study—Clinical profile of breast cancer. Malawi Med J. 2015;27:10–12. doi: 10.4314/mmj.v27i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohler RE. Breast cancer in Malawi [doctoral thesis] University of North Carolina, Chapel Hill: NC; 2015. [Google Scholar]


