Abstract
The vertebrate kidney possesses the capacity to repair damaged nephrons, and this potential is conserved regardless of the complexity of species-specific kidneys. However, many aquatic vertebrates possess the ability to not only repair existing nephrons, but also generate new nephrons after injury. Adult zebrafish have the ability to recover from acute renal injury not only by replacing lost injured epithelial cells of endogenous nephrons, but by also generating de novo nephrons. This strong regeneration potential, along with other unique characteristics such as the high degree of genetic conservation with humans, the ease of harvesting externally fertilized, transparent embryos, the accessibility to larval and adult kidneys, and the ability to perform whole organism phenotypic small molecule screens, has positioned zebrafish as a unique vertebrate model to study kidney injury. In this review, we provide an overview of the contribution of zebrafish larvae/adult studies to the understanding of renal regeneration, diseases, and therapeutic discovery.
Keywords: Acute kidney injury, Chronic kidney disease, Polycystic kidney disease, Regeneration, Small molecule screening, Zebrafish
Introduction
For more than a decade, zebrafish researchers have been developing larval and adult models of acute kidney injury (AKI). AKI is defined as a sudden loss of renal function resulting in an increase in circulating waste products [1]. AKI results from largely multifactorial insults, including but not limited to decreased renal blood flow, urinary obstruction, exposure to toxic agents, and as a consequence of sepsis, with US healthcare costs for AKI estimated at $10 billion annually [2, 3]. Each year in the United States, AKI incidents requiring dialysis affect more than 90,000 patients, while non-dialysis-dependent AKI affects more than 1.5 million patients [4–6]. AKI is also thought to be one of the risk factors for chronic kidney diseases (CKD), and there is an increased risk of end-stage renal disease in patients with severe AKI [7–12]. Despite this, no established therapies are proven to significantly prevent renal injury, accelerate the rate of renal recovery, or prevent post-injury fibrosis and chronic renal insufficiency after AKI [13]. Using the anatomically simple zebrafish kidney, researchers hope to provide insights into kidney regeneration mechanisms with the promise of developing future renal therapeutics.
The vertebrate kidney has the ability to repair damaged nephrons, which is conserved across species with varying regenerative potential. While a thorough understanding of the molecular mechanisms that govern kidney repair are still incompletely understood [14], there is a well-defined sequence of cellular events that drives functional organ recovery [15, 16]. The mammalian kidney does not likely harbor a stem cell population, but instead, the repair process is driven by dedifferentiation of surviving injured renal tubule epithelium followed by proliferation and repopulation of the denuded tubules [17–20]. This process is thought to be phenotypically and mechanistically conserved between human [20], mouse [15, 17], and larval zebrafish kidneys [21•], allowing for reagents and pathways discovered in zebrafish to be utilized for mammalian regeneration studies. In contrast to this, in adult zebrafish, an adult “stem cell” population has been identified that drives neo-nephrogenesis during both organism growth and injury events [22•]. While this mechanism does not occur in the mammalian kidney, findings from post-injury neo-nephrogenesis in zebrafish may also provide insight into pathways that promote whole nephron regeneration in the mammalian kidney after injury.
Zebrafish larvae contain a functional pronephros composed of two nephrons whose glomeruli fuse at the trunk midline. The zebrafish larval pronephric kidney is anatomically simple, while conserving the cellular and molecular complexity of higher vertebrates. Its accessibility for visualization and manipulation represents a great advantage for the study of the mechanisms involved in the pathophysiology of AKI. As with mammalian nephrons, the zebrafish pronephric tubules are segmented into different specialized regions: a neck, proximal convoluted tubule, proximal straight tubule, distal early tubule, Corpuscle of Stannius, distal late tubule, and duct [23]. The zebrafish pronephros is thought to possess a lumen by 24 h post-fertilization (hpf) and shows initial functionality by 48 hpf [24]. Development of the mesonephros, the juvenile and adult kidney, begins at 12 days post-fertilization (dpf) and remains functional the entire adult life [25]. In adult fish, the nephrogenic capacity of cells within the mesonephric field is maintained throughout life (termed neo-nephrogenesis) [22•, 25, 26], whereas in mammals the potential to generate nephrons de novo ends a few days after birth. Zebrafish mesonephric kidney nephrogenesis proceeds in an anterior to posterior manner and the kidney is divided into four morphologically distinct regions with different densities of nephrons: the anterior nephron-dense region, the medial nephron-sparse region, the medial nephron-dense region, and the posterior nephron-sparse region. Interestingly, the number of nephrons in the two medial regions is directly proportional to the body mass of the fish, indicating the existence of a mechanism that coordinates neo-nephrogenesis and renal capacity required by the fish [22•, 25]. This capacity of the zebrafish mesonephros to generate a neo-nephrogenic response provides a unique model for studying both developing nephrons and renal regeneration in an adult vertebrate organism. In this review, we provide an overview of zebrafish larvae/adult models of AKI, genetic models of chronic kidney disease (CKD), and discovery screens for compounds that enhance recovery from kidney damage.
Zebrafish Models of Acute Kidney Injury
In this section, we will review both larval and adult models of AKI. We have made a distinction between larval and adult models, since larvae do not have the capacity to generate de novo nephrons through neo-nephrogenesis. The models generated to date have focused either on translating mammalian models to zebrafish (i.e., nephrotoxins) or focused on the unique utility of the zebrafish (i.e., laser ablation, inducible transgene) (Fig. 1).
Fig. 1.
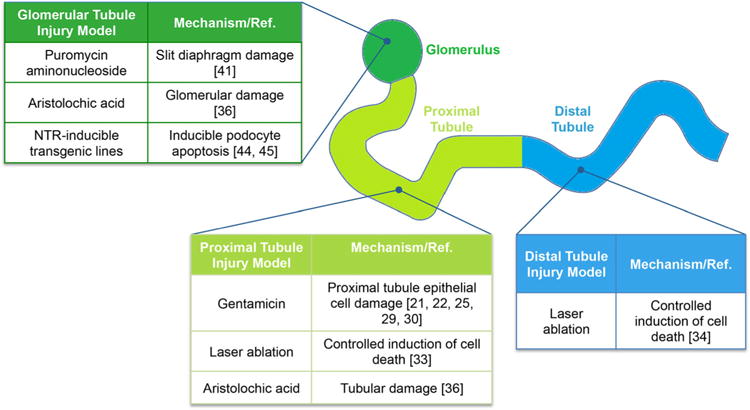
Summary of zebrafish renal injury models. The studies highlighted in the review, as indicated be reference number, are organized by location of damage in the nephron and the type of injury model
Larval Model of Gentamicin-Induced Injury
Perhaps the best-characterized model of AKI used in zebrafish is a nephrotoxic model using the aminoglycoside gentamicin. At high doses, gentamicin causes AKI as a result of proximal tubular cell damage [27, 28]. Injection of gentamicin in the circulation of 48–72 hpf zebrafish larvae results in histological changes characteristic of mammalian AKI such as flattening of the apical brush border, loss of cell polarity, and enlargement of the tubular lumen with accumulation of debris in the tubular lumen [21•, 29, 30]. The tubular obstruction as a consequence of the gentamicin-induced tubular injury results in a reduction of the pronephros’ ability to filter and eliminate fluid and subsequent development of edema [30]. Together, these findings demonstrate the promise of the zebrafish larval gentamicin AKI model as a simple model to augment mammalian AKI studies [31].
Adult Model of Gentamicin-Induced Injury
While larval zebrafish AKI models have considerable advantages such as the simplicity of the kidney, and ease to manipulate and visualize the organism, the adult zebrafish mesonephric kidney, with its potent regenerative capacity, represents a relevant model to understanding differing repair potentials when compared to the limited regeneration potential of mammalian metanephric kidneys [19, 22•, 25, 26, 32]. Using gentamicin-induced injury, the zebrafish mesonephros has been shown to undergo de novo formation of nephrons (neo-nephrogenesis) [22•, 25]. Utilizing this model, two independent groups used the transgenic zebrafish lines, lhx1a:EGFP [22•] and wt1b:GFP [25], to follow GFP expression in cellular aggregates within the nephrogenic field and characterize the regenerative response following gentamicin-induced injury. Using the lhx1a:EGFP line, one study found populations of lhx1a+ single cells, cellular aggregates, and renal vesicle-like bodies, which are believed to be nephron progenitors and early stage nephrons [22•]. In agreement with the lhx1a:EGFP studies, another group using the wt1b:GFP line identified wt1b+ cellular aggregates form 72 to 96 h following gentamicin treatment that could give rise to new nephrons [25]. In serial transplantation assays, using purified lhx1a+ tubular aggregates of 10–30 cells, it was discovered that these cells can form new functional nephrons in recipient adult zebrafish, and able to maintain a self-renewal capacity [22•]. Further characterization of this renal “stem-cell” population in an adult vertebrate, including defining the cellular niche, will provide a solid foundation for understanding the differences between potent renal regeneration (zebrafish) and a limited renal regeneration potential (mammals).
Laser Ablation-Induced Injury
In an attempt to overcome some of the limitations of gentamicin-induced damage, mainly bilateral (complete) damage at undefined time points after delivery, researchers have developed a laser ablation model in the larval zebrafish kidney [33]. Injury is driven by the induction of cell death in a small area of the renal field with a low degree of larval lethality, thus allowing for a longer timeframe to study the injury than allowed by gentamicin-driven injury n[33]. The increased spatial and temporal specificity of the damage and the fact that varying regions of the kidney can be targeted allows for the study of repair processes in different segments of the tubule. Using controlled laser ablation to target the distal tubule in a larval transgenic zebrafish expressing GFP in the kidney tubule, it was demonstrated that the repair process is driven by cell migration and this process is independent of cell proliferation [34]. Results from this work showed that after unilateral epithelial injury proliferation is confined in the injured kidney to cells adjacent to the wound, supporting the idea that the initial signals inducing repair are intrinsic to the injured tubule itself. The main limitations of this model are the uncertain relevance of the physiological characteristics of laser-driven damage to mammalian AKI and the relatively small field of damage induced. However, this model still represents a useful tool to answer questions that require a highly controlled degree of injury.
Aristolochic Acid-Induced Injury
Aristolochic acid (AA) is a nephrotoxic compound found in the birthwort (Aristolochiaceae) plant family and was used in the past for treatment of arthritis and wound healing [35]. To date, in zebrafish, the effect of AA-induced nephrotoxicity has only been evaluated in developing embryos by soaking of the embryos at 24 hpf in 10 ppm of AA for 5–7 h, and found to induce tubular and glomerular damage [36]. Although tubular and interstitial damage is associated with AA treatment in mammals, glomerular damage has not been reported in mice and humans with AA nephropathy [37, 38]. While this model has a high appeal because zebrafish larvae could simply be soaked in a chemical toxin, more work needs to be done to determine if this approach actually leads to classic AKI hallmarks.
Puromycin Aminonucleoside (PAN)-Induced Injury
The glomeruli are responsible for the filtration of low-molecular-weight plasma waste products into the urine, while retaining large macromolecules. The glomerular filtration barrier (GFB) is a complex structure containing endothelial, mesangial, and podocyte cells. Perturbation of the GFB integrity, and in particular damage to the podocytes, results in leakage of blood plasma proteins into the urine, a hallmark of most glomerular diseases termed proteinuria [39, 40]. Progressive proteinuria frequently leads to permanent glomerular damage and renal failure. Therefore, there is a need to develop models that can serve to understand GFB function under normal and damage conditions. The glomerular toxin Puromycin aminonucleoside (PAN) has been used to study podocyte injury in zebrafish embryos. When injected into the circulation of 2.5 dpf larvae, PAN toxicity leads to effacement of podocyte foot processes and development of edema [41]. Similar changes in glomerular function have also been observed in rats treated with PAN, validating the use of the zebrafish pronephros to study glomerular injury [42].
Inducible Transgenic Lines for AKI
Generation of transgenic lines is a widely use method in the zebrafish field for understanding the function of genes involved in kidney development and regeneration. Given the density of the metanephric kidney, in vivo imaging of glomerular structures in mammalian systems is technically difficult. Taking advantage of the transparency of zebrafish embryos and larvae allows for imaging of podocytes in the zebrafish glomerulus. For example, a transgenic zebrafish line expressing GFP under the control of the podocin promoter was generated to visualize podocytes for dissection and electron microscopy analysis [43]. Using this transgenic line in a crb2b morphant with compromised glomerular function that leads to pericardial edema and formation of pronephric cysts, there was a loss of podocin:GFP expression, validating the potential use of this transgenic line for the in vivo, real-time study of glomerular injuries [43].
A limitation of disrupting glomerular function in zebrafish embryonic kidneys is that it leads to death within a few days. In order to model human diseases that lead to disruption of the glomerular filtration barrier, inducible transgenic zebrafish lines have been generated [25, 44, 45•]. These lines were generated using the bacterial nitroreductase (NTR) gene under the control of the podocin promoter, such that NTR expression is limited to the podocytes of both pronephric and mesonephric kidneys. Glomerular injury can be induced by treatment in either larvae or adult zebrafish with the pro-drug metronidazole (Mtz), as this is converted by NTR into a DNA cross-linking agent that induces cell death [46, 47]. Using podocin:NTR-GFP transgenic larvae subjected to Mtz treatment, pericardial edema and podocyte depletion as suggested by reduction in GFP expression were observed [44]. Close examination of the glomerulus in Mtz-treated zebrafish also revealed the presence of podocyte foot process effacement, a phenotype resembling nephrotic syndrome in humans. Interestingly, glomerular barrier functionality and foot process structures were recovered within a week after washout of Mtz. A group of proliferating cells in the glomeruli was detected during the recovery period suggesting the presence of a resident progenitor cell population [44]. A complimentary study used a similar transgenic line podocin:NTR-mCherry to investigate the mechanism of podocyte damage and regeneration in adult zebrafish [45•]. Similar to the observations in larvae, Mtz treatment of adult zebrafish leads to severe edema and decreased fluorescent reporter intensity, as well as an impaired GFB and podocyte foot process effacement [45•]. Interestingly, employing the wt1b:GFP transgenic line that was previously used as a marker for kidney regeneration studies, it was demonstrated that wt1b expression is reinitiated in cells on the wall of Bowman’s capsule after Mtz-mediated podocyte injury [25, 45•]. The recent description in a mammalian system of a parietal epithelial cell population in Bowman’s capsule that can act as podocyte progenitors positions zebrafish as a great alternative model to investigate the identity of these cells and their mechanism to repair glomerular damage [48, 49].
Fluorescent Conjugates to Delineate Injury
Small dextran conjugates delivered to the kidney via the circulation can be endocytosed by the proximal tubule, enabling specific labeling of the renal epithelial tubular cells in zebrafish [24, 30]. However, using such dextran conjugates in high-throughput small molecule or genetic screens is not feasible, since individual injection of the conjugates into larval zebrafish is required. To overcome this limitation, researchers have developed a small fluorescent molecule as an alternative tool for visualization of the proximal tubule after injury [50]. The compound, named PT-Yellow, specifically labels the proximal tubule of zebrafish embryos soaked for 20 min. It is absorbed into the blood, filtered by the glomerulus, and endocytosed by the proximal tubular epithelial cells. Additionally, injection of PT-Yellow into adult zebrafish results in labeling of the mesonephric proximal tubules and a significant loss of fluorescence follows gentamicin-induced injury [50]. These observations indicate that PT-Yellow may be useful for studying normal and altered proximal tubule function in the zebrafish.
Genetic Models of Kidney Disease
Since zebrafish are amenable to genetic modification, strength is to identify mutants that model the etiology of different types of kidney disease. As part of a large-scale mutagenesis screen, numerous recessive mutations affecting the development of the zebrafish pronephric kidney were identified and characterized [24, 51]. Since the screens were performed to uncover mutants that compromised kidney function based upon renal cyst development at 2 dpf, these mutants were found to be more akin to CKD, and affected architecture and function of glomeruli and terminal differentiation of tubular epithelial cells [24]. A second screen, using insertional mutagenesis with a pseudo-typed retrovirus as the mutagen, also assessed cystic kidneys. This study recovered twelve different mutant loci and two of the genes identified, vhnf1 and pkd2, were previously shown to be responsible for cystic kidney diseases in humans [52–54]. Six of the loci were found to encode components of intraflagellar transport complex B, a multi-protein complex essential for cilia function, biogenesis, maintenance, and signaling [54–56], findings consistent with a role of cilia in polycystic kidney disease (PKD) pathogenesis [57]. PKD, a common CKD, is characterized by the formation of multiple epithelium-lined renal cysts, renal enlargement, and abnormal tubule development [58]. Given the identification of multiple homologs of human PKD genes and the observation of characteristic phenotypes associated with the formation of pronephric kidney cyst, the kidney of the zebrafish larvae serves as a good model for the discovery and study of PKD-associated genes [54, 59–61]. There is now evidence in mammalian systems pointing to primary cilia defects in the pathogenesis of PKD [62–66]. The role of cilia function in response to injury was investigated in a model of pronephric and mesonephric obstruction in zebrafish embryos and adult, respectively. The findings from this study suggest that expression of foxj1a, a transcription factor required for cilia formation, is a primary response to injury and is needed to maintain enhanced cilia function in injured epithelial cells. Interestingly, foxj1a expression is also increased in uninjured zebrafish cystic mutants representing a putative novel therapeutic target for PKD [60].
Small Molecule Screening Assays
Phenotypic in vivo high-throughput screening (HTS) has become a widely used drug discovery method [67–70]. Since traditional HTS use has not increased the number of newly marketed drugs [71], it has been proposed that HTS screens which better recapitulate in vivo events are needed to improve the discovery of new drug candidates [70, 72, 73]. To comprehend pathophysiological processes, the interactions between compounds, pathways, cells, tissues, and organs must be understood; whole-animal models have been shown to be more informative in this regard than in vitro and ex vivo models [74]. The concept is supported by a review of FDA-approved new molecular entities over a decade, which showed that the majority of first-in-class agents were discovered in phenotypic, not target-based screens [75]. The zebrafish is uniquely positioned for chemical screens. Due to its small size and high fecundity, it is compatible with multi-well plate formats used in large-scale, phenotype-based screens. Because small molecule examination occurs in a physiologically relevant context, zebrafish screens select for compounds with favorable physiochemical properties. Despite using limited numbers of compounds, zebrafish screens have yielded valuable compounds for cardiac [76–79], vascular [80], cancer [81], and CNS biology [82, 83], among others. The best example, from a therapeutic standpoint, is a prostaglandin E2 analog, which accelerates hematopoietic stem cell engraftment in the bone marrow and has been moved into Phase 1b clinical trials within 2 years of the initial screen of 2500 compounds [84].
As discussed in the genetic screen section, compromised kidney function in the zebrafish embryo and larvae is associated with pericardial edema, axis curvature, and, in the case of cystic kidney diseases, also a defective left–right asymmetry of the body plan [24, 54]. Together, these phenotypic characteristics have enabled the use of the zebrafish embryo for a chemical screen of compounds that ameliorate cysts in a model of PKD. The chemical modifier screen was done using pkd2 mutant zebrafish larvae that develop cystic kidneys by treating with a collection of compounds and using a computer algorithm to quantify the body axis curvature [59]. This research identified a role of the HDAC inhibitor, VPA, for the reduction of axis curvature and kidney cyst formation in zebrafish and lessening the progression of cyst formation in a mouse model of PKD [59]. Another study performed screening for compounds capable of increasing the size of the kidney field through proliferation-based mechanisms [85]. The screen identified a compound, PTBA, which was able to induce an increased proliferation of renal progenitor cells with persistent expansion of the kidney field [85]. Follow-up studies demonstrated that in a zebrafish larvae AKI model, a PTBA analog (m4PTB) enhanced functional renal recovery [21•]. In addition, m4PTB enhances renal recovery and reduces renal fibrosis after IR-AKI in mice by promoting cell cycle progression of the surviving regenerating renal tubular epithelial cells [21•]. Further investigation of m4PTB revealed that the compound also had activity in the aristolochic acid-induced mouse model [86]. Importantly, the cellular mechanism by which m4PTB ameliorates recovery from AKI, namely increased proliferation of dedifferentiated progenitor cells, is preserved among the two species and models [21•, 86], and mirrors that seen in human clinical specimens [20]. Validating the use of the zebrafish system as a novel, innovative model to identify agents that ameliorate recovery from kidney damage.
Conclusion
The insights gained over the past decade into the applicability of zebrafish models of kidney injury and disease to mammalian models of renal damage have provided valuable groundwork for utilizing the zebrafish as a therapeutic discovery model. While several valid models of AKI and CKD have been developed in zebrafish, and potential therapeutic compounds have been identified in zebrafish screens that have been successfully translated to mammalian models, more work needs to be done to determine if these hits will effective in the clinic. An important first step is to elucidate whether the molecular mechanisms found during zebrafish AKI are ultimately conserved in human injury and disease events. Such studies would help to provide an early evaluation step in the discovery process to predict whether a compound should be moved forward for development.
Acknowledgments
The laboratory of Neil Hukriede was supported by the National Institutes of Health Grants 2R01 DK069403, 1RC4 DK090770, 2R01 HD053287, and 1P30DK079307. The laboratory of Maria Cecilia Cirio was supported by the Agencia Nacional de Promoción Científica y Tecnológica de Argentina, FONCyT, PICT 2013. The laboratory of Mark de Caestecker was supported by National Institutes of Health Grants 1R01 HL093057 and 1RC4DK090770.
Footnotes
This article is part of the Topical Collection on Zebrafish as a Model for Pathobiology.
Compliance with Ethics and Guidelines
Conflict of Interest: Maria Cecilia Cirio and Neil Hukriede have no conflicts of interest. Mark de Caestecker is a consultant for Nephrogenix.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334(22):1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nature clinical practice. Nephrology. 2006;2(7):364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 5.Lameire NH, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 6.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 7.Bucaloiu ID, et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81(5):477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 8.Chawla LS, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2012;79(12):1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011;7(4):209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wald R, et al. Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125(6):585–593. doi: 10.1016/j.amjmed.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Wu VC, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011;80(11):1222–1230. doi: 10.1038/ki.2011.259. [DOI] [PubMed] [Google Scholar]
- 13.Molitoris BA, et al. Designing clinical trials in acute kidney injury. Clin J Am Soc Nephrol. 2012;7(5):842–843. doi: 10.2215/CJN.12801211. [DOI] [PubMed] [Google Scholar]
- 14.Davidson AJ. Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatr Nephrol. 2011;26(9):1435–1443. doi: 10.1007/s00467-011-1795-z. [DOI] [PubMed] [Google Scholar]
- 15.Bacallao R, Fine LG. Molecular events in the organization of renal tubular epithelium: from nephrogenesis to regeneration. Am J Physiol. 1989;257(6 Pt 2):F913–F924. doi: 10.1152/ajprenal.1989.257.6.F913. [DOI] [PubMed] [Google Scholar]
- 16.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 17.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 18.Bussolati B, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166(2):545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2(3):284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Loverre A, et al. Increase of proliferating renal progenitor cells in acute tubular necrosis underlying delayed graft function. Transplantation. 2008;85(8):1112–1119. doi: 10.1097/TP.0b013e31816a8891. [DOI] [PubMed] [Google Scholar]
- 21•.Cianciolo Cosentino C, et al. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24(6):943–953. doi: 10.1681/ASN.2012111055. This publication validates the strategy that discoveries made using a zebrafish embryonic screening model are directly translatable to mammalian models of AKI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Diep CQ, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470(7332):95–100. doi: 10.1038/nature09669. This publication identifies a stem/progenitor cell in the adult zebrafish that is important for driving neo-nephrogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingert RA, et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3(10):1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond IA, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125(23):4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- 25.Zhou W, et al. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol. 2010;299(5):F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimschuessel R. A fish model of renal regeneration and development. ILAR J. 2001;42(4):285–291. doi: 10.1093/ilar.42.4.285. [DOI] [PubMed] [Google Scholar]
- 27.Karasawa T, et al. Calreticulin binds to gentamicin and reduces drug-induced ototoxicity. Toxicol Sci. 2011;124(2):378–387. doi: 10.1093/toxsci/kfr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Novoa JM, et al. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 29.Cianciolo Cosentino C, et al. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp. 2010 doi: 10.3791/2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hentschel DM, et al. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288(5):F923–F929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 31.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310(2):379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson CS, Holzemer NF, Wingert RA. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp. 2011;54 doi: 10.3791/2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmyre A, et al. Collective epithelial migration drives kidney repair after acute injury. PLoS One. 2014;9(7):e101304. doi: 10.1371/journal.pone.0101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 2002;17(4):265–277. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]
- 36.Ding YJ, Chen YH. Developmental nephrotoxicity of aristolochic acid in a zebrafish model. Toxicol Appl Pharmacol. 2012;261(1):59–65. doi: 10.1016/j.taap.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Sato N, et al. Acute nephrotoxicity of aristolochic acids in mice. J Pharm Pharmacol. 2004;56(2):221–229. doi: 10.1211/0022357023051. [DOI] [PubMed] [Google Scholar]
- 38.Vanherweghem JL, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341(8842):387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 39.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354(13):1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 40.Tryggvason K, Wartiovaara J. How does the kidney filter plasma? Physiology (Bethesda) 2005;20:96–101. doi: 10.1152/physiol.00045.2004. [DOI] [PubMed] [Google Scholar]
- 41.Hentschel DM, et al. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol. 2007;293(5):F1746–F1750. doi: 10.1152/ajprenal.00009.2007. [DOI] [PubMed] [Google Scholar]
- 42.Ryan GB, Rodewald R, Karnovsky MJ. An ultrastructural study of the glomerular slit diaphragm in aminonucleoside nephrosis. Lab Invest. 1975;33(5):461–468. [PubMed] [Google Scholar]
- 43.He B, et al. Podocin-green fluorescence protein allows visualization and functional analysis of podocytes. J Am Soc Nephrol. 2011;22(6):1019–1023. doi: 10.1681/ASN.2010121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, et al. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013;83(6):1193–1200. doi: 10.1038/ki.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23(6):1039–1047. doi: 10.1681/ASN.2011080776. This publication describes the generation of a zebrafish transgenic model for the study of glomerular pathogenesis and podocyte regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3(6):948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pisharath H, Parsons MJ. Nitroreductase-mediated cell ablation in transgenic zebrafish embryos. Methods Mol Biol. 2009;546:133–143. doi: 10.1007/978-1-60327-977-2_9. [DOI] [PubMed] [Google Scholar]
- 48.Appel D, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20(2):333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grouls S, et al. Lineage specification of parietal epithelial cells requires beta-catenin/Wnt signaling. J Am Soc Nephrol. 2012;23(1):63–72. doi: 10.1681/ASN.2010121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sander V, et al. The small molecule probe PT-Yellow labels the renal proximal tubules in zebrafish. Chem Commun (Camb) 2015;51(2):395–398. doi: 10.1039/c4cc08075k. [DOI] [PubMed] [Google Scholar]
- 51.Driever W, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- 52.Bingham C, et al. Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001;68(1):219–224. doi: 10.1086/316945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mochizuki T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272(5266):1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 54.Sun Z, et al. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131(16):4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 55.Kishimoto N, et al. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell. 2008;14(6):954–961. doi: 10.1016/j.devcel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Sun Z. Qilin is essential for cilia assembly and normal kidney development in zebrafish. PLoS One. 2011;6(11):e27365. doi: 10.1371/journal.pone.0027365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 58.Smith LA, et al. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol. 2006;17(10):2821–2831. doi: 10.1681/ASN.2006020136. [DOI] [PubMed] [Google Scholar]
- 59.Cao Y, et al. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci USA. 2009;106(51):21819–21824. doi: 10.1073/pnas.0911987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hellman NE, et al. The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch. Proc Natl Acad Sci USA. 2010;107(43):18499–18504. doi: 10.1073/pnas.1005998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang L, et al. A possible zebrafish model of polycystic kidney disease: knockdown of wnt5a causes cysts in zebrafish kidneys. J Vis Exp. 2014;94:e52156–e52156. doi: 10.3791/52156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20(1):23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin F, et al. Kidney-specific inactivation of the KIF3A sub-unit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100(9):5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15(1):105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 65.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18(5):1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 66.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13(10):2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 67.Lansbury PT., Jr Back to the future: the ‘old-fashioned’ way to new medications for neurodegeneration. Nat Med. 2004;10(Suppl):S51–S57. doi: 10.1038/nrn1435. [DOI] [PubMed] [Google Scholar]
- 68.Lee JA, et al. Modern phenotypic drug discovery is a viable, neoclassic pharma strategy. J Med Chem. 2012;55(10):4527–4538. doi: 10.1021/jm201649s. [DOI] [PubMed] [Google Scholar]
- 69.Murphey RD, et al. A chemical genetic screen for cell cycle inhibitors in zebrafish embryos. Chem Biol Drug Des. 2006;68(4):213–219. doi: 10.1111/j.1747-0285.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 70.Sams-Dodd F. Target-based drug discovery: is something wrong? Drug Discov Today. 2005;10(2):139–146. doi: 10.1016/S1359-6446(04)03316-1. [DOI] [PubMed] [Google Scholar]
- 71.Lawrence S. Drug output slows in 2006. Nat Biotechnol. 2007;25(10):1073. [Google Scholar]
- 72.Kamb A. Opinion: what’s wrong with our cancer models? Nat Rev Drug Discov. 2005;4(2):161–165. doi: 10.1038/nrd1635. [DOI] [PubMed] [Google Scholar]
- 73.Prior M, et al. Back to the future with phenotypic screening. ACS Chem Neurosci. 2014;5(7):503–513. doi: 10.1021/cn500051h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deo RC, MacRae CA. The zebrafish: scalable in vivo modeling for systems biology. Wiley Interdiscip Rev Syst Biol Med. 2011;3(3):335–346. doi: 10.1002/wsbm.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10(7):507–519. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 76.Peal DS, et al. Novel chemical suppressors of long QT syndrome identified by an in vivo functional screen. Circulation. 2011;123(1):23–30. doi: 10.1161/CIRCULATIONAHA.110.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burns CG, et al. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1(5):263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- 78.Peterson RT, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22(5):595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 79.Milan DJ, et al. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107(10):1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 80.Hao J, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5(2):245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White RM, et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature. 2011;471(7339):518–522. doi: 10.1038/nature09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kokel D, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6(3):231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rihel J, et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327(5963):348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Groh ED, et al. Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol. 2010;21(5):794–802. doi: 10.1681/ASN.2009080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Novitskaya T, et al. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am J Physiol Renal Physiol. 2014;306(5):F496–F504. doi: 10.1152/ajprenal.00534.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


