Abstract
Introduction
The breast cancer resistance protein (BCRP/ABCG2) is an efflux transporter in the placental barrier. By transporting chemicals from the fetal to the maternal circulation, BCRP limits fetal exposure to a range of drugs, toxicants, and endobiotics such as bile acids and hormones. The purpose of the present studies was to 1) determine whether BCRP localizes to highly-ordered, cholesterol-rich lipid raft microdomains in placenta microvillous membranes, and 2) determine the impact of cholesterol on BCRP-mediated placental transport in vitro.
Methods
BCRP expression was analyzed in lipid rafts isolated from placentas from healthy, term pregnancies and BeWo trophoblasts by density gradient ultracentrifugation. BeWo cells were also tested for their ability to efflux BCRP substrates after treatment with the cholesterol sequestrant methyl-β-cyclodextrin (MβCD, 5mM, 1 h) or the cholesterol synthesis inhibitor pravastatin (200μM, 48 h).
Results and Discussion
BCRP was found to co-localize with lipid raft proteins in detergent-resistant, lipid raft-containing fractions from placental microvillous membranes and BeWo cells. Treatment of BeWo cells with MβCD redistributed BCRP protein into higher density non-lipid raft fractions. Repletion of the cells with cholesterol restored BCRP localization to lipid raft-containing fractions. Treatment of BeWo cells with MβCD or pravastatin increased cellular retention of two BCRP substrates, the fluorescent dye Hoechst 33342 and the mycotoxin zearalenone. Repletion with cholesterol restored BCRP transporter activity. Taken together, these data demonstrate that cholesterol may play a critical role in the post-translational regulation of BCRP in placental lipid rafts.
Keywords: BCRP/ABCG2, Placenta, Lipid rafts, Cholesterol, Zearalenone, BeWo cells
Introduction
The breast cancer resistance protein (BCRP/ABCG2) is highly expressed on the apical membrane of placental syncytiotrophoblasts, serving a fetoprotective function at the interface of the maternal and fetal circulations [1, 2]. BCRP transports substrates away from the placenta and prevents the accumulation of potentially harmful xenobiotics in the fetus [3]. Substrates include commonly prescribed drugs such as the hypoglycemic agent glyburide, the chemotherapeutic drug doxorubicin and the antibiotic nitrofurantoin [4–7]. BCRP also transports estrogenic dietary chemicals that affect sexual differentiation of the fetus including the fungal toxin zearalenone [8–11]. Additionally, BCRP critically prevents cytokine-induced apoptosis and facilitates syncytial formation in placental cells [12, 13].
Few studies have offered insight into the post-translational regulation of BCRP by cholesterol and its organization in the plasma membrane. Cholesterol in the plasma membrane aggregates in structures called lipid rafts, which are ordered microdomains rich in sphingolipids and proteins [14]. Lipid raft order creates a phase-separation between its contents and the disordered phospholipid bilayer [14, 15]. Lipid rafts are critical in a number of membrane processes including signal transduction, biochemical synthesis and transport [16].
There is some evidence linking lipid raft integrity and membrane cholesterol content to BCRP function. In cells transfected to overexpress BCRP, the BCRP protein localized in lipid raft fractions after density gradient ultracentrifugation [17]. Proteomic analysis of lipid rafts in primary human T cells and mouse spermatozoa also point to the detergent resistance of the BCRP protein [18, 19]. Further, BCRP efflux activity is dependent on lipid raft integrity and cholesterol content in canine kidney cells and membrane vesicles overexpressing BCRP [17, 20, 21]. It is known that cholesterol is critical for fetal development; mothers with chronically low cholesterol are at higher risk for premature delivery and low birth weight babies [22, 23]. However, it is possible that cholesterol also plays a critical role in regulating placental BCRP and therefore influences drug transfer from the maternal to fetal circulation.
To date, the majority of studies of the BCRP transporter have investigated the localization of the protein in lipid rafts and the ability of cholesterol to regulate its activity using overexpressing cell-based systems, with little attention paid to native human tissues. Therefore, we hypothesized that BCRP localizes to lipid rafts in microvillus membranes from healthy term placentas and cultured placental cells. Further, we expected that disruption of cholesterol content in placental cells would alter BCRP function and enrichment in lipid rafts, which could potentially enhance drug transfer across the placental barrier
Materials and Methods
Chemicals
Unless stated otherwise, all chemicals were from Sigma-Aldrich (St. Louis, MO).
Cell Culture
BeWo human choriocarcinoma cells (American Type Culture Collection, Manassas, VA) were maintained in an incubator at 37°C with 5% CO2 in air in a DMEM and F12 1:1 mixture (Life Technologies, Carlsbad, CA), supplemented with 10% fetal bovine serum (Atlantic Biologicals, Miami, FL) and 1% penicillin-streptomycin (Life Technologies). This cell line recapitulates first trimester trophoblasts by secreting placental hormones and expressing transporters such as BCRP [35]. For all experiments, cells were grown to 70–80% confluence before use. For cholesterol modulation studies, cells were cultured in the presence or absence of pravastatin (PRAV, 10–200 μM) for 48 h or methyl-β-cyclodextrin (MβCD, 5 mM) for 1 h and then HBSS in the absence and presence of cholesterol-MβCD (Sigma C4951) for 30 min [16]. Time points and doses used were based on previous literature and preliminary studies indicating a reduction in cholesterol but no effect on cell viability (Supplemental Fig 1) [16]. Cells were lysed in buffer containing 20 mM Tris-HCl, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 1% Triton X-100 and a protease inhibitor cocktail (Sigma P8340).
Patient Selection and sample collection
Placentas (n = 3) were obtained with written informed consent from healthy women meeting all criteria following term delivery by scheduled Cesarean section (Supplemental Table 1). Inclusion criteria included healthy women between the ages of 18–40 years, term gestation (≥ 36 weeks), and scheduled Cesarean section without labor. Exclusion criteria included chronic medical conditions, pregnancy-induced medical conditions, maternal infection, clinical chorioamnionitis, medication use (with the exception of prenatal vitamins), maternal smoking, alcohol or drug abuse, multiple pregnancies, and known fetal chromosomal abnormalities [24]. The study was approved by the Institutional Review Boards of Robert Wood Johnson Medical School (Protocol #0220100258) and Rutgers University (Protocol #E12-024).
Upon collection, placenta samples for lipid raft analysis were snap frozen and stored at −80°C until use. Samples for immunohistochemistry were stored in PAXgene Tissue Containers containing PAXgene tissue stabilizer (Qiagen, Germantown, MD).
Assays for cholesterol, protein and cell viability and growth inhibition
All colorimetric and fluorescent assays were performed using a SpectraMax M3 Multimode Microplate Reader and analyzed with SpectraMax SoftMax Pro 6.3 software (Molecular Devices, Sunnyvale, CA). For analysis of cholesterol in cell lysates and lipid raft fractions, an Amplex Red based detection kit was used according the instructions provided by Sigma-Aldrich. Samples were compared to a standard curve generated from human low density lipoprotein prepared in either cell lysis buffer (20 mM tris-HCl, 150 mM NaCl, 5 mM EDTA, pH = 7.4) or lipid raft extraction buffer (see below). Protein content of cell lysates and lipid raft fractions was quantified using a Detergent Compatible analysis kit (BioRad, Hercules, CA) based on the Lowry method [25].
Viability was measured as a function of the ability of the cells to convert resazurin to fluorescent resorufin as previously described [26]. To assess growth, cell number quantified using a Beckman Coulter Z1 Particle Counter (Indianapolis, IN). At all concentrations used, pravastatin had no effect on either cell growth or viability (Supplemental Fig. 1).
Subcellular Fractionation
Ultracentrifugation and density gradient methods were employed to obtain total cell membranes from BeWo cells, brush border membranes from human term placenta, and lipid rafts using a Beckman L7-55 ultra-centrifuge (Beckman Coulter, Brea, CA) [27–29].
Membrane Isolation from BeWo Cells
Plasma membranes were collected from BeWo cells using a Percoll-based ultra-centrifugation method as previously described [27]. All ultra-centrifugation steps for membrane isolation were performed using a Type 40.1 Ti rotor (Beckman Coulter, Indianapolis, IN).
Placental Brush Border Membrane Extraction
Crude brush border MVM extracts were prepared from human term placentas using an MgCl2-based centrifugation method as previously described [28]. Pure MVM were prepared by subjecting the crude MVM to the protocol a second time. All ultra-centrifugation steps for brush border membrane extractions were performed using a Type 60 Ti rotor (Beckman Coulter). Whole homogenates, nuclear fractions, and crude and pure MVM were analyzed for markers of apical and endothelial membranes, cytoplasm, nuclei and mitochondria using techniques in Western blotting.
Lipid Raft Extraction
Lipid raft fractions were prepared using a method adapted from a prior report [29]. Brush border membrane extracts and BeWo plasma membranes were incubated in TNE buffer (25 mM Tris HCl, 150 mM NaCl, 5 mM EDTA), supplemented with 1% Lubrol or 1% Triton X-100, respectively, on ice for 1 h. The mixtures were then mixed with OptiPrep (60% iodixanol) to a final concentration of 40% iodixanol in a final volume of 3 mL. This was added to the bottom of an open-top ultra-centrifuge tube and overlayed with 6 mL of 30% iodixanol and then 2 mL of 5% iodixanol. Samples were centrifuged at 260,000 g for 4 h at 4°C using an SW41 swinging bucket rotor (Beckman Coulter). Detergent-resistant lipid rafts migrated up the tube and concentrated at the 30–5% interface. Ten 1.4 mL fractions were collected from each tube with a pipette, starting from the least dense fractions at the top of the tube.
Western Blotting
Unless otherwise stated, all Western blotting was performed using equipment from BioRad as previously described [30]. Primary antibodies were used to detect BCRP (BXP-53, 1:5000; Enzo Life Sciences, Farmingdale, NY), β-actin (ab8227, 1:2000, Abcam, Cambridge, MA), transferrin receptor 1 (TFR-1, ab108985, 1:5000 Abcam), placental alkaline phosphatase (PLAP, ab133602, 1:10,000, Abcam), multidrug resistance-associated protein 1 (MRP1, ab3368, 1:2000, Abcam), cluster of differentiation 34 (CD34, ab81289 1:10,000, Abcam), histone H2A (25785, 1:1000, Cell Signaling, Danvers, MA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, G8795, 1:1000, Sigma-Aldrich). HRP-linked secondary antibodies (anti-rabbit, anti-rat or anti-mouse, 1:2000 Sigma-Aldrich) were used to detect primary antibodies. After the addition of a Luminata Forte Western HRP substrate (Millipore, Billerica, MA), chemiluminescent protein-antibody complexes were visualized using a Fluorchem Imager (ProteinSimple, Santa Clara, CA). Semi-quantitative analysis of bands of the blots was performed using AlphaView Software (ProteinSimple).
Flow Cytometry
Phycoerthyrin-labeled anti-BCRP antibody (5D3) (R&D Systems, Minneapolis, MN) was used to detect BCRP expression in BeWo cells according to the manufacturer’s protocol. Phycoerthyrin-labeled IgG antibody was used as a negative control. Cells were washed three times and then resuspended in 2% paraformaldehyde/PBS for flow cytometric analysis using a Gallios/FC500 Cytometer (Beckman Coulter, Indianapolis, IN) (excitation wavelength, 488 nm; emission wavelength, 575 nm).
Transporter Function
The transport activity of BCRP in BeWo cells was measured by analyzing the cellular retention of two BCRP transporter substrates, Hoescht 33342 and zearalenone, an estrogenic mycotoxin [9, 31].
Hoescht 33342 Transport
After experimental treatments, BeWo cells were detached from the culture plates with trypsin, washed, resuspended in medium, and added to a 96-well round bottom plate at a density of 200,000 cells/well in a final volume of 200 μL. Plates were centrifuged (500 g, 5 minutes, 5°C) and the medium removed. Cells were then resuspended in 200 μL of growth medium containing Hoechst 33342 (5 μM). After 30 min at 37°C in the presence or absence of the BCRP-specific inhibitor, Ko143 (1 μM), cells centrifuged, washed and re-suspended in substrate-free growth medium with or without Ko143. After 1 h at 37°C, the cells were washed and resuspended in 50 μL ice cold PBS for analysis. Quantification of intracellular fluorescence was performed using a Cellometer Vision automated cell counter (Nexcelom Bioscience, Lawrence, MA) fitted with a VB-450-302 filter (excitation/emission = 375/450 nm). The total number of cells analyzed for each sample ranged from 200 to 2000 and were normalized for cell size.
Zearalenone Transport
For zearalenone transport studies, BeWo cells, grown and treated in 6 well culture dishes, were loaded with zearalenone (10 μM) in HBSS in the presence or absence of Ko143 (1 μM). After 1 h at 37°C, cells were washed with HBSS, and then incubated in zearalenone-free HBSS in the absence and presence of Ko143. After 30 min at 37°C, cells were washed, collected in lysis buffer and stored at −80°C. Quantification of intracellular ZLN was performed using an ELISA kit (Abnova, Taipei City, Taiwan), and standards were prepared in lysis buffer [9].
Immunohistochemistry
For immunohistochemistry, tissue was embedded in paraffin and 5 μm thick sections prepared. After deparaffinization, tissue sections were quenched in 2% H2O2 (10 min, room temperature). Tissue sections were then blocked with an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) followed by 5% serum corresponding to the source of the primary antibody. After 2 h at room temperature, tissues sections were incubated with primary antibodies to BCRP (BXP-21, 1:100; abcam), TFR-1 (ab108985, 1:200 Abcam), PLAP (ab133602, 1:1000, Abcam), or CD34 (ab81289 1:10,000, Abcam). After 16 h at 4°C, tissue sections were washed and incubated with biotinylated secondary antibodies for 60 min at room temperature (Vector Laboratories). Tissue sections were then stained using a 3,3′-diaminobenzidine peroxidase substrate kit (Vector Laboratories). After counterstaining with hematoxylin, tissue sections were dehydrated and imaged by light microscopy on a Olympus BX51 microscope (Waltham, MA) fitted with a ProgRes C14+ camera (Jenoptik, Jena, Germany). Negative controls for each secondary antibody are provided (Supplemental Fig 2).
Statistical analysis
Data were presented as mean ± SE and analyzed using Graphpad Prism 5.0 software (Graphpad Software Inc., La Jolla, CA). Data were normally distributed, and two-way analysis of variance post-test or one-way analysis of variance with Bonferroni post-test was used to determine significance, which was set at p < 0.05.
Results
Localization of the BCRP transporter in human term placentas
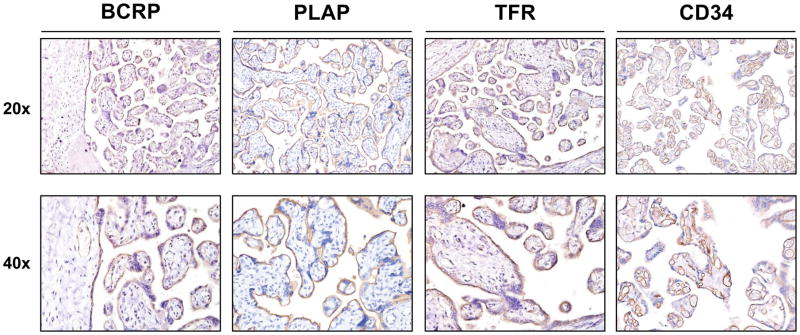
BCRP protein was highly expressed on syncytiotrophoblasts and fetal endothelial cells (Fig. 1). Placental alkaline phosphatase (PLAP) and transferrin receptor (TFR), markers for raft and non-raft regions of cell membranes, respectively, were also expressed in syncytiotrophoblasts. TFR was also present on fetal endothelial cells while CD34, a marker for endothelial membranes, was localized only in the fetal endothelium (Fig. 1).
Fig 1. Localization of the BCRP transporter and plasma membrane markers in human term placentas.
Healthy, term placentas were fixed in PAXgene tissue stabilizer and then subjected to routine tissue processing and paraffin embedding. Sections (5 μm) were prepared and stained with antibodies against BCRP, PLAP, TFR or CD34 as indicated in the Materials and Methods. Antibody binding was visualized using a Vectastain DAB kit (brown staining) and counterstained with hematoxylin (blue staining).
Localization of the BCRP transporter to lipid raft-enriched fractions in human term placentas
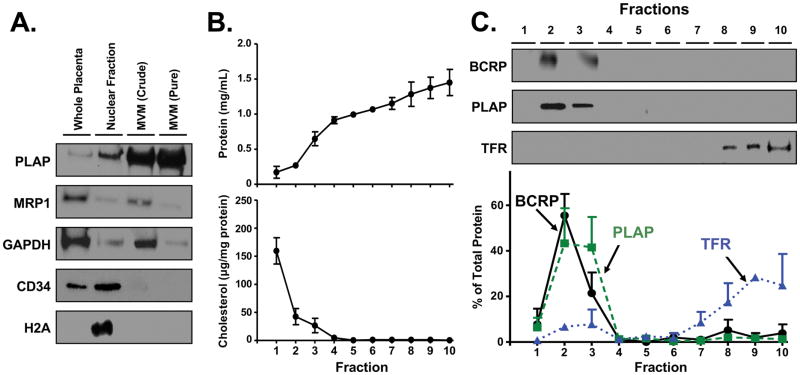
MVM was extracted and purified from healthy term placentas and tested for contamination using Western blotting (Fig. 2A). PLAP, also a marker for MVM, was highly enriched in both crude and purified MVM fractions of the tissue when compared to whole placenta homogenates. MRP1, a marker of syncytiotrophoblast basolateral membranes, was present in whole homogenates and crude MVM, but little or no expression of this protein was evident in purified fractions. Similar results were obtained for GAPDH, a marker of cytoplasmic contamination. CD34 was present only in whole placenta homogenates and nuclear fractions. Low levels of CD34 remained in crude MVM preparations but were not detected following further purification. H2A, a nuclear protein marker, was found only in nuclear fractions. These data confirmed that pure MVM were prepared.
Fig 2. Localization of the BCRP transporter in lipid raft-enriched fractions in human term placentas.
A. Western blot of placental MVM. Western blotting was performed on MVM isolated from human term placentas using ultracentrifugation. B. Protein and cholesterol analysis of fractions from placental MVM ultracentrifugation. Placenta MVMs were subjected to density gradient ultracentrifugation in order to isolate detergent-resistant lipid rafts. Ten 1.4 mL fractions were collected and analyzed for protein or cholesterol content as described in the Materials and Methods. Data are presented as mean ± SE (n = 3 placentas). C. Western blot of fractions from placental MVM ultracentrifugation. Western blots from 1 representative placenta are shown. Data are presented as mean ± SE (n = 3 placentas).
Detergent-based density gradient ultracentrifugation was then performed on pure MVM to isolate lipid raft, and non-lipid raft fractions. Ten fractions were collected starting from the least dense fractions at the top of the gradient and analyzed for overall protein and cholesterol content. Lower density fractions at the top (fractions 1–3) typically represent detergent-resistant raft microdomains, while higher density fractions at the bottom (fractions 7–10) typically represent detergent-soluble cell components, including non-raft membranes [reviewed in 27]. Protein and cholesterol analysis of lipid raft fractions showed that only 11.7% of total protein was localized to fractions 1–3 while 96.0% of cholesterol (normalized to protein) was localized to the same fractions (Fig. 2B).
Western blotting of the density gradient fractions showed that approximately 84.8% of total PLAP protein content was found in fractions 2 and 3 while 84.3% of total TFR protein was localized in the bottom 4 fractions (7–10) (Fig. 2C). Similar to PLAP, 76.9% of total BCRP protein was found in lipid raft-enriched fractions 2 and 3.
Localization of the BCRP transporter following cholesterol depletion of BeWo cells
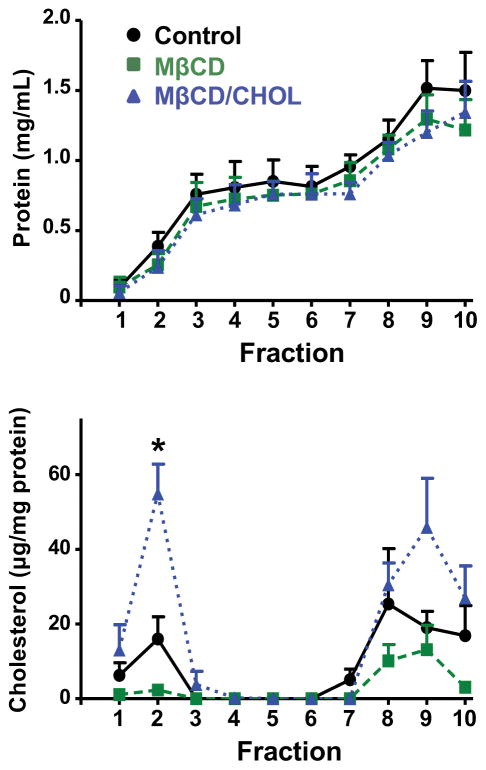
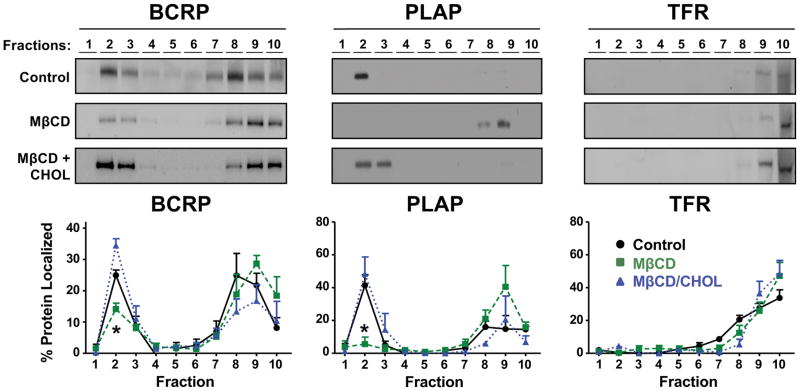
In order to characterize density gradient fractions collected from BeWo cells, we first assessed total protein and cholesterol. Following centrifugation, 14.0% of total protein was localized to lipid raft fractions (fractions 1–3), while 25.1% of cholesterol (normalized to protein) was localized to the same fractions (Fig. 3). We next analyzed BeWo cells for expression of BCRP in lipid rafts. Analysis of the gradient fractions by Western blotting showed that 46.3% of total PLAP was found in fractions 2 and 3, while 90.3% of total TFR was found in fractions 7–10 (Fig. 4). BCRP co-localized with PLAP in lipid rafts, with 33.6% of total BCRP contained in fractions 2 and 3.
Fig 3. Protein and cholesterol content of density gradient fractions from BeWo cells following ultracentrifugation.
Plasma membranes collected from BeWo cells treated with HBSS, MβCD (5 mM, 1 h), or MβCD (5 mM, 1 h) and then MβCD-cholesterol (5 mM, 30 min) were subjected to density gradient ultracentrifugation in order to isolate detergent-resistant lipid rafts. Ten 1.4 mL fractions were collected and analyzed for protein or cholesterol content as described in the Materials and Methods. Data are presented as mean ± SE (n = 3 independent experiments).
Fig 4. Localization of the BCRP transporter following cholesterol depletion of BeWo cells.
Western blots were performed using membrane fractions collected from BeWo cells following treatment with vehicle (Control), 5 mM methyl-β-cyclodextrin (MβCD) for 1 h, or 5 mM MβCD for 1 h and then 5 mM cholesterol-loaded MβCD for 30 min (MβCD + CHOL). Western blots show BCRP along with lipid raft (PLAP) and non-lipid raft (TFR) markers in fractions obtained from 1 representative experiment. Enrichment of proteins in different fractions was semi-quantified using densitometry of Western blots from 3 independent experiments. Data are presented as mean ± SE *p < 0.05 compared to control within each fraction.
To determine the extent to which cholesterol influences the localization of BCRP in lipid rafts, BeWo cells were treated with the cholesterol sequestrant MβCD. MβCD treatment reduced the content of BCRP and PLAP in lipid raft fractions by 51.4% and 92.6%, respectively (Fig. 4). This redistribution of BCRP and PLAP was reversed when cells were replenished with cholesterol after MβCD treatment. Greater than 90% of TFR content was localized to the higher density fractions and MβCD treatment did not affect its distribution. Protein analysis showed that BeWo lipid rafts (fractions 1–3) accounted for 13.3% and 12.2% of total protein in MβCD and cholesterol replenished treatment groups, respectively (Fig. 3). By comparison, cholesterol analysis showed that fractions 1–3 accounted for 11.4%, and 40.9% of total cholesterol in MβCD and cholesterol replenished treatment groups, respectively (Fig. 3).
Effects of cholesterol on BCRP transport activity
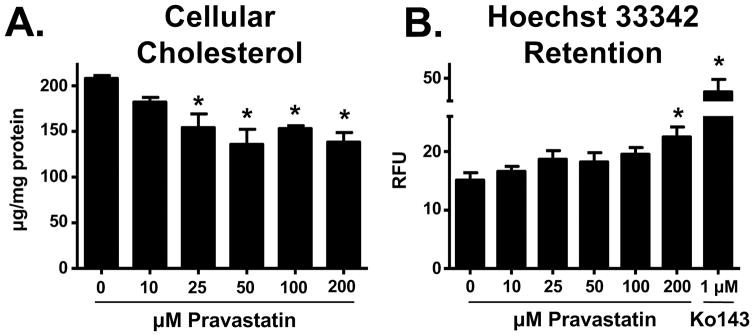
Initially, we characterized BCRP transport activity in BeWo cells using the fluorescent substrate, Hoechst 33342 (Fig. 5B). Ko143, a specific functional inhibitor of BCRP [32], increased the cellular accumulation of Hoechst 33342 by 172%. To determine whether reduced cholesterol content affected BCRP function, cells were treated with pravastatin and assessed for cholesterol content and Hoechst 33342 retention. Pravastatin treatment (48 h) caused a concentration-dependent decrease in total cellular cholesterol (Fig. 5A), and this resulted in an increase in the retention of Hoechst 33342 up to 47% (Fig. 5B).
Fig 5. Relationship between cholesterol content and BCRP substrate retention in BeWo cells.
BeWo cells were treated with vehicle or pravastatin (10 – 200 μM) for 48 h. A. Effects of pravastatin on cholesterol content. Cholesterol was measured in cell lysates using the Amplex Red assay. B. Effects of pravastatin on BCRP efflux. Efflux transporter activity was indirectly measured by the ability of cells to retain the BCRP substrate Hoechst 33342. The BCRP substrate was quantified in relative fluorescence units (RFU) using a Nexcelom Cellometer (n = 3 independent experiments).*p<0.05 relative to control. Data are presented as mean ± SE.
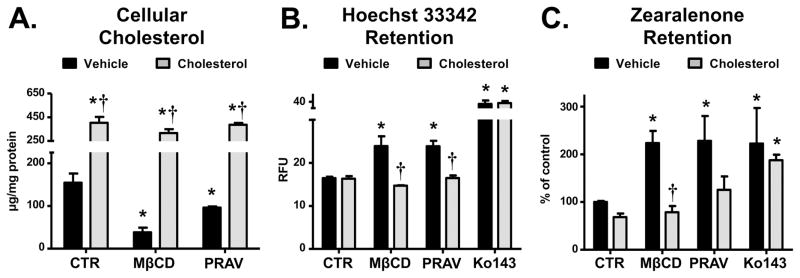
To determine if repletion with cholesterol would restore BCRP efflux activity, BeWo cells were treated in a separate set of experiments with MβCD (5 mM, 1 h) or pravastatin (200 μM, 48 h) and then exogenous cholesterol (5 mM MβCD-cholesterol, 30 min). The retention of Hoecsht 33342 and the estrogenic mycotoxin zearalenone were then measured. Treatment of BeWo cells with MβCD and pravastatin reduced cellular cholesterol content by 75.3% and 37.8%, respectively (Fig. 6A), which increased Hoechst 33342 accumulation by 20% (Fig. 6B) and zearalenone retention by 125% (Fig. 6C) for both treatments. BeWo cells under the same treatment conditions were analyzed for loading of Hoechst 33342 or Rhodamine 123, a fluorescent chemical that is not transported by BCRP (Supplemental Fig. 3). Neither MβCD nor pravastatin showed significant differences in the loading of dyes compared to control. Repletion of cholesterol in MβCD and pravastatin-treated cells restored cellular cholesterol, which lowered the cellular retention of Hoechst 33342 and zearalenone to levels that were not significantly different than control cells (Fig. 6A–C). Ko143 increased the cellular accumulation of both Hoechst 33342 (Fig. 6B) and zearalenone (Fig. 6C), and, as expected, exogenous cholesterol did not reverse the inhibitory effects of Ko143.
Fig 6. Effects of modulating cholesterol on BCRP transporter activity in BeWo cells.
Cholesterol content in BeWo cells was modulated by treatment with MβCD (1 h, 5 mM) or pravastatin (PRAV, 48 h, 200 μM). Cholesterol repletion groups were treated with cholesterol-loaded MβCD (30 min, 5 mM) immediately before the experiment. A. Effects of MβCD and pravastatin on BeWo cell cholesterol content. Cells were treated with MβCD or pravastatin, lysates prepared and cholesterol content measured using an Amplex Red assay (n = 3 samples). B. Effects of modulating BeWo cell cholesterol on BCRP efflux transporter activity using Hoechst 33342 retention. Efflux transporter activity was measured by the ability of cells to retain the BCRP substrate Hoechst 33342. The BCRP substrate was quantified in relative fluorescence units (RFU) using a Nexcelom Cellometer and expressed as mean ± SD (n = 3 independent experiments). C. Effect of modulating BeWo cell cholesterol on BCRP efflux transporter activity using zearalenone retention. Efflux transporter activity was measured by the ability of the cells to retain the BCRP substrate zearalenone. Black bars represent cells where cholesterol was not restored. Grey bars represent cells where cholesterol was restored. Data are presented as mean ± SE (n = 4–6). Asterisks (*) represent statistically significant differences (p < 0.05) compared to control cells where cholesterol was not restored. Daggers (†) represent statistically significant differences (p < 0.05) compared to cells with the same treatment (CTR, MβCD or PRAV) where cholesterol was not restored.
Effects of lowering cholesterol on BeWo cell expression of BCRP
To determine whether the effects of lowering cholesterol on BCRP efflux activity were due to changes in BCRP expression, cells treated with MβCD (5 mM, 1 h) or pravastatin (200 μM, 48 h) were analyzed by Western blotting and flow cytometry. Treatment of BeWo cells with the cholesterol inhibitors did not alter total BCRP expression in the cells (Supplemental Fig. 4A) or its insertion in the plasma membrane (Supplemental Fig. 4B).
Discussion
Because of BCRP’s enrichment in the placenta, and its ability to transport toxicants from the fetus to the maternal circulation, this transporter provides a critical fetoprotective function [33]. Our studies demonstrate that BCRP is localized to lipid rafts in syncytiotrophoblasts and BeWo cells. This is based on findings that BCRP can be isolated in detergent-resistant, cholesterol-rich cell membranes after density gradient centrifugation where it co-localizes with PLAP, a well-characterized lipid raft marker [34]. In BeWo cells, but not MVM, BCRP is also localized in non-lipid raft fractions. BeWo cells are derived from a human choriocarcinoma [35], and differences in the trafficking of BCRP between these cells and primary human tissue may be due to the transformed phenotype. It has also been proposed that some proteins present in lipid raft fractions are simply not as intimately packed within lipid rafts and are susceptible to Triton digestion, which was used only for lipid raft isolation in BeWo cells [36]. Further, it is common that even well-established lipid raft proteins, like caveolin-1, display a bimodal distribution in gradient centrifugation experiments under certain conditions and localize to both raft and nonraft fractions [37–39]. It has also been proposed that some fraction of a particular protein can be bound to certain fatty acids that drive the association with lipid rafts [36]. This post-translational modification could, in theory, result in two distinct populations of the same protein: one that associates with rafts and one that does not. Further studies will be needed to determine what drives the bimodal distribution of BCRP, but it is clear that cholesterol plays a functional role in the distribution of BCRP in placental cells.
Our studies measuring efflux transport activity of BCRP used two substrates, Hoechst 33342 and zearalenone. Hoechst 33342 is a fluorescent substrate often used for measuring BCRP efflux activity [31], while zearalenone is an estrogenic mycotoxin produced by fungi that occur naturally on cereal crops. We previously identified zearalenone as a novel substrate for BCRP [9]. In utero exposure to zearalenone has been shown to cause developmental toxicities including feminization, mammary epithelial proliferation and precocious puberty [40]. The fact that BCRP can efflux zearalenone may make it a critical protein in protecting the fetus against toxicity by this environmental contaminant.
Maternal cholesterol is vital to the development of the fetus. For instance, low maternal serum cholesterol has been noted as a cause of both preterm delivery and lower birth weight [41]. As indicated above, cholesterol is also crucial to the integrity of lipid rafts, which have been implicated as mediators of a number of key metabolic processes needed for fetal development including transmembrane trafficking of nutrients. For example, lipid raft integrity is important for the maternal-to-fetal transport of folate, a cofactor important in the development of the nervous system [42]. In this regard, maternal exposure to fumonisin B1, which is known to disrupt lipid raft integrity in the placenta, has the potential to inhibit receptor-mediated folate transport to the fetus resulting in toxicity [43]. While it is known that cholesterol and lipid rafts are important in pregnancy, understanding how they modulate efflux transporters such as BCRP will be critical to understanding mechanisms by which the fetus is protected from exposure to xenobiotics. Without affecting BCRP protein expression, both pravastatin and MβCD inhibited most but not all BCRP efflux transport activity by modulating levels of cellular cholesterol, a process that disrupts lipid rafts [44]. Further, unlike other statins, pravastatin is not a direct inhibitor of BCRP at the concentrations used [45]. These data indicate that cholesterol is important in regulating BCRP efflux activity. However, the precise mechanism is not clear. Although lipid rafts may be critical for maximal BCRP activity (see further below), it is also possible that cholesterol modulates BCRP directly. For example, BCRP has been shown to have a cholesterol-binding motif and this may directly influence the change in conformation of BCRP to an active state [20]. Additionally, it has been postulated that cholesterol aids in the interaction of small, hydrophobic substrates with BCRP, by “filling in” an unoccupied space in the active site [reviewed in 46, 47]. This may, in part, explain why efflux of zearalenone, a small, hydrophobic compound, was more susceptible to the effects of cholesterol reduction than Hoechst 33342, a larger, hydrophilic compound.
Lipid rafts are more ordered than the surrounding phospholipid bilayer and can therefore promote protein-protein interactions. For example, lipid rafts have been reported to enhance the dimerization of glial-cell-derived neurotrophic factor and the high-affinity IgE receptor [16]. Efflux of xenobiotics by BCRP is dependent on its dimerization [48]. Lipid rafts may help to bring BCRP monomers in proximity to one another, while stabilizing a conformation of BCRP that exposes critical cysteine residues needed for dimerization [16, 49]. At the present time, the precise role of raft and non-raft BCRP in BeWo cells in mediating the Hoechst 33342 and zearalenone efflux is not known. It is possible that reducing cellular cholesterol affects both raft and non-raft BCRP, but more studies are needed to clarify this possibility.
Supplementary Material
Cells were treated with increasing concentrations of pravastatin (10 – 250 μM). After 48 h, viability was determined using the Alamar Blue assay and cell growth by counting cells using a Coulter Counter. Data are presented as mean ± SE (n = 4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to vehicle control.
Healthy, term placentas were fixed in PAXgene tissue stabilizer and then subjected to routine tissue processing and paraffin embedding. Sections (5 μm) were prepared and stained as indicated in the Materials and Methods without primary antibody. Anti-rabbit (left), anti-mouse (center), and anti-rat (right) secondary antibodies were used. Antibody binding was visualized using a Vectastain DAB kit (brown staining) and counterstained with hematoxylin (blue staining).
BeWo cells were treated with vehicle (control) and MβCD (1 h, 5 mM) or pravastatin (48 h, 200 μM) to lower cellular cholesterol. Cells were then treated with the BCRP substrate (Hoechst 33342) or the non-BCRP substrate (rhodamine) for 30 min and cellular fluorescence quantified using a Nexcelom Cellometer. Data are presented as mean relative fluorescence units ± SE (n = 3).
BeWo cells were treated with vehicle (control), pravastatin (48 h, 200 μM) or MβCD (1 h, 5 mM). A. Western blot analysis of pravastatin and MβCD treated BeWo cells. Cell lysates were prepared and analyzed by Western blotting for expression of BCRP (72 kDa). Expression was normalized to the loading control β-actin (42 kDa) and presented as mean ± SE (n = 3). B. Flow cytometric analysis of pravastatin and MβCD treated BeWo cells. Treated cells were incubated with phycoerthyrin-labeled anti-BCRP antibody (5D3) or IgG control.
Acknowledgments
Funding
This work was supported by the National Institutes of Environmental Health Sciences [Grants ES020522, ES005022, ES007148], a component of the National Institutes of Health.
References
- 1.Hahnova-Cygalova L, Ceckova M, Staud F. Fetoprotective activity of breast cancer resistance protein (BCRP, ABCG2): expression and function throughout pregnancy. Drug metabolism reviews. 2011;43(1):53–68. doi: 10.3109/03602532.2010.512293. [DOI] [PubMed] [Google Scholar]
- 2.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer research. 2001;61(8):3458–64. [PubMed] [Google Scholar]
- 3.Jani M, Ambrus C, Magnan R, Jakab KT, Beery E, Zolnerciks JK, Krajcsi P. Structure and function of BCRP, a broad specificity transporter of xenobiotics and endobiotics. Archives of toxicology. 2014;88(6):1205–48. doi: 10.1007/s00204-014-1224-8. [DOI] [PubMed] [Google Scholar]
- 4.Bircsak KM, Gupta V, Yuen PY, Gorczyca L, Weinberger BI, Vetrano AM, Aleksunes LM. Genetic and Dietary Regulation of Glyburide Efflux by the Human Placental Breast Cancer Resistance Protein Transporter. The Journal of pharmacology and experimental therapeutics. 2016;357(1):103–13. doi: 10.1124/jpet.115.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang H, Unadkat JD, Mao Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab Dispos. 2007;35(12):2154–8. doi: 10.1124/dmd.107.018044. [DOI] [PubMed] [Google Scholar]
- 6.Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(13):2943–50. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 7.Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285:111–117. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 8.Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72:967–975. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- 9.Xiao J, Wang Q, Bircsak KM, Wen X, Aleksunes LM. Screening of Environmental Chemicals Identifies Zearalenone as a Novel Substrate of the Placental BCRP/Transporter. Toxicology research. 2015;4(3):695–706. doi: 10.1039/C4TX00147H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilakivi-Clarke L, Cho E, Clarke R. Maternal genistein exposure mimics the effects of estrogen on mammary gland development in female mouse offspring. Oncology reports. 1998;5(3):609–16. doi: 10.3892/or.5.3.609. [DOI] [PubMed] [Google Scholar]
- 11.Jefferson WN, Williams CJ. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reproductive toxicology. 2011;31(3):272–9. doi: 10.1016/j.reprotox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evseenko DA, Paxton JW, Keelan JA. The xenobiotic transporter ABCG2 plays a novel role in differentiation of trophoblast-like BeWo cells. Placenta. 2007;28(Suppl A):S116–20. doi: 10.1016/j.placenta.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(13):3592–605. doi: 10.1096/fj.07-8688com. [DOI] [PubMed] [Google Scholar]
- 14.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harbor perspectives in biology. 2011;3(10):a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucero HA, Robbins PW. Lipid rafts-protein association and the regulation of protein activity. Archives of biochemistry and biophysics. 2004;426(2):208–24. doi: 10.1016/j.abb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Simons K, Toomre D. Lipid rafts and signal transduction. Nature reviews Molecular cell biology. 2000;1(1):31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 17.Storch CH, Ehehalt R, Haefeli WE, Weiss J. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. The Journal of pharmacology and experimental therapeutics. 2007;323(1):257–64. doi: 10.1124/jpet.107.122994. [DOI] [PubMed] [Google Scholar]
- 18.Nixon B, Bielanowicz A, McLaughlin EA, Tanphaichitr N, Ensslin MA, Aitken RJ. Composition and significance of detergent resistant membranes in mouse spermatozoa. Journal of cellular physiology. 2009;218(1):122–34. doi: 10.1002/jcp.21575. [DOI] [PubMed] [Google Scholar]
- 19.Lin SL, Chien CW, Han CL, Chen ES, Kao SH, Chen YJ, Liao F. Temporal proteomics profiling of lipid rafts in CCR6-activated T cells reveals the integration of actin cytoskeleton dynamics. J Proteome Res. 2010;9(1):283–97. doi: 10.1021/pr9006156. [DOI] [PubMed] [Google Scholar]
- 20.Hegedus C, Telbisz A, Hegedus T, Sarkadi B, Ozvegy-Laczka C. Lipid regulation of the ABCB1 and ABCG2 multidrug transporters. Advances in cancer research. 2015;125:97–137. doi: 10.1016/bs.acr.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Telbisz A, Muller M, Ozvegy-Laczka C, Homolya L, Szente L, Varadi A, Sarkadi B. Membrane cholesterol selectively modulates the activity of the human ABCG2 multidrug transporter. Biochimica et biophysica acta. 2007;1768(11):2698–713. doi: 10.1016/j.bbamem.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Bream EN, Leppellere CR, Cooper ME, Dagle JM, Merrill DC, Christensen K, Simhan HN, Fong CT, Hallman M, Muglia LJ, Marazita ML, Murray JC. Candidate gene linkage approach to identify DNA variants that predispose to preterm birth. Pediatric research. 2013;73(2):135–41. doi: 10.1038/pr.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta obstetricia et gynecologica Scandinavica. 2012;91(6):726–35. doi: 10.1111/j.1600-0412.2012.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memon N, Bircsak KM, Archer F, Gibson CJ, Ohman-Strickland P, Weinberger BI, Parast MM, Vetrano AM, Aleksunes LM. Regional expression of the BCRP/ABCG2 transporter in term human placentas. Reproductive toxicology. 2014;43:72–7. doi: 10.1016/j.reprotox.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 26.Fields RD, Lancaster MV. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. American biotechnology laboratory. 1993;11(4):48–50. [PubMed] [Google Scholar]
- 27.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(22):10104–8. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez V, Henriquez M, Llanos P, Riquelme G. Isolation and purification of human placental plasma membranes from normal and pre-eclamptic pregnancies. a comparative study. Placenta. 2004;25(5):422–37. doi: 10.1016/j.placenta.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Bayyareddy K, Zhu X, Orlando R, Adang MJ. Proteome Analysis of Cry4Ba Toxin-Interacting Aedes aegypti Lipid Rafts using geLC-MS/MS. Journal of proteome research. 2012;11(12):5843–5855. doi: 10.1021/pr3006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng R, Po I, Mishin V, Black AT, Heck DE, Laskin DL, Sinko PJ, Gerecke DR, Gordon MK, Laskin JD. The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with UVB light and nitrogen mustard. Toxicology and applied pharmacology. 2013;272(2):345–355. doi: 10.1016/j.taap.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bircsak KM, Gibson CJ, Robey RW, Aleksunes LM. Assessment of drug transporter function using fluorescent cell imaging. Curr Protoc Toxicol. 2013;57(Unit 23.6) doi: 10.1002/0471140856.tx2306s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Molecular cancer therapeutics. 2002;1(6):417–25. [PubMed] [Google Scholar]
- 33.Mao Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharmaceutical research. 2008;25(6):1244–55. doi: 10.1007/s11095-008-9537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saslowsky DE, Lawrence J, Ren X, Brown DA, Henderson RM, Edwardson JM. Placental alkaline phosphatase is efficiently targeted to rafts in supported lipid bilayers. The Journal of biological chemistry. 2002;277(30):26966–70. doi: 10.1074/jbc.M204669200. [DOI] [PubMed] [Google Scholar]
- 35.Pattillo RA, Gey GO. The Establishment of a Cell Line of Human Hormone-synthesizing Trophoblastic Cells in Vitro. Cancer research. 1968;28(7):1231–1236. [PubMed] [Google Scholar]
- 36.Schuck S, et al. Resistance of cell membranes to different detergents. Proc Natl Acad Sci U S A. 2003;100(10):5795–800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98(10):5619–24. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, et al. Quantifying raft proteins in neonatal mouse brain by ‘tube-gel’ protein digestion label-free shotgun proteomics. Proteome Sci. 2007;5:17. doi: 10.1186/1477-5956-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radeva G, Sharom FJ. Isolation and characterization of lipid rafts with different properties from RBL-2H3 (rat basophilic leukaemia) cells. Biochem J. 2004;380(Pt 1):219–30. doi: 10.1042/BJ20031348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belli P, Bellaton C, Durand J, Balleydier S, Milhau N, Mure M, Mornex JF, Benahmed M, Le Jan C. Fetal and neonatal exposure to the mycotoxin zearalenone induces phenotypic alterations in adult rat mammary gland. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2010;48(10):2818–26. doi: 10.1016/j.fct.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, Muenke M. Adverse Birth Outcome Among Mothers With Low Serum Cholesterol. Pediatrics. 2007;120(4):723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 42.Gelineau-van Waes J, Starr L, Maddox J, Aleman F, Voss KA, Wilberding J, Riley RT. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model, Birth defects research. Part A. Clinical and molecular teratology. 2005;73(7):487–97. doi: 10.1002/bdra.20148. [DOI] [PubMed] [Google Scholar]
- 43.Stevens VL, Tang J. Fumonisin B1-induced sphingolipid depletion inhibits vitamin uptake via the glycosylphosphatidylinositol-anchored folate receptor. The Journal of biological chemistry. 1997;272(29):18020–5. doi: 10.1074/jbc.272.29.18020. [DOI] [PubMed] [Google Scholar]
- 44.Hartel S, Diehl HA, Ojeda F. Methyl-beta-cyclodextrins and liposomes as water-soluble carriers for cholesterol incorporation into membranes and its evaluation by a microenzymatic fluorescence assay and membrane fluidity-sensitive dyes. Analytical biochemistry. 1998;258(2):277–84. doi: 10.1006/abio.1998.2594. [DOI] [PubMed] [Google Scholar]
- 45.Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005;68:800–807. doi: 10.1124/mol.105.014019. [DOI] [PubMed] [Google Scholar]
- 46.Telbisz A, Hegedus C, Varadi A, Sarkadi B, Ozvegy-Laczka C. Regulation of the function of the human ABCG2 multidrug transporter by cholesterol and bile acids: effects of mutations in potential substrate and steroid binding sites. Drug metabolism and disposition: the biological fate of chemicals. 2014;42(4):575–85. doi: 10.1124/dmd.113.055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polgar O, Robey RW, Morisaki K, Dean M, Michejda C, Sauna ZE, Ambudkar SV, Tarasova N, Bates SE. Mutational analysis of ABCG2: role of the GXXXG motif. Biochemistry. 2004;43:9448–9456. doi: 10.1021/bi0497953. [DOI] [PubMed] [Google Scholar]
- 48.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annual review of pharmacology and toxicology. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 49.Kage K, Fujita T, Sugimoto Y. Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2. Cancer science. 2005;96(12):866–72. doi: 10.1111/j.1349-7006.2005.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells were treated with increasing concentrations of pravastatin (10 – 250 μM). After 48 h, viability was determined using the Alamar Blue assay and cell growth by counting cells using a Coulter Counter. Data are presented as mean ± SE (n = 4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to vehicle control.
Healthy, term placentas were fixed in PAXgene tissue stabilizer and then subjected to routine tissue processing and paraffin embedding. Sections (5 μm) were prepared and stained as indicated in the Materials and Methods without primary antibody. Anti-rabbit (left), anti-mouse (center), and anti-rat (right) secondary antibodies were used. Antibody binding was visualized using a Vectastain DAB kit (brown staining) and counterstained with hematoxylin (blue staining).
BeWo cells were treated with vehicle (control) and MβCD (1 h, 5 mM) or pravastatin (48 h, 200 μM) to lower cellular cholesterol. Cells were then treated with the BCRP substrate (Hoechst 33342) or the non-BCRP substrate (rhodamine) for 30 min and cellular fluorescence quantified using a Nexcelom Cellometer. Data are presented as mean relative fluorescence units ± SE (n = 3).
BeWo cells were treated with vehicle (control), pravastatin (48 h, 200 μM) or MβCD (1 h, 5 mM). A. Western blot analysis of pravastatin and MβCD treated BeWo cells. Cell lysates were prepared and analyzed by Western blotting for expression of BCRP (72 kDa). Expression was normalized to the loading control β-actin (42 kDa) and presented as mean ± SE (n = 3). B. Flow cytometric analysis of pravastatin and MβCD treated BeWo cells. Treated cells were incubated with phycoerthyrin-labeled anti-BCRP antibody (5D3) or IgG control.