Abstract
Schizophrenia (SZ) is a severe mental disorder with unknown etiology and elusive neuropathological and neurobiological features have been a focus of many theoretical hypotheses and empirical studies. Current genetic and neurobiology information relevant to SZ implicates neuronal developmental and synaptic plasticity abnormalities, and neurotransmitter, microglial and oligodendrocytes dysfunction. Several recent theories have highlighted the neurovascular unit as a potential contributor to the pathophysiology of SZ. We explored the biological plausibility of a link between SZ and the neurovascular system by examining insights gained from genetic, neuroimaging and postmortem studies, which include gene expression and neuropathology analyses. We also reviewed information from animal models of cerebral angiogenesis in order to understand better the complex interplay between angiogenic and neurotrophic factors in development, vascular endothelium/blood brain barrier remodeling and maintenance, all of which contribute to sustaining adequate regional blood flow and safeguarding normal brain function. Microvascular and hemodynamics alterations in SZ highlight the importance of further research and reveal the neurovascular unit as a potential therapeutic target in SZ.
Keywords: Schizophrenia, angiogenesis, gene expression, neurovascular unit, endothelial cells
1. Background: Role of angiogenesis during neurodevelopment and CNS function
Schizophrenia (SZ) is a disabling psychiatric disorder that affects multiple brain functions, impairs real-world functioning and is only partially responsive to pharmacological treatments.
Although multiple pathological processes in the neurobiology of SZ have been identified to date, including neurotransmitter system dysfunctions (e.g., dopaminergic systems and the glutamatergic system), myelin, immune-response and infectious origins (Arion et al., 2007; Hakak et al., 2001; Harrison and Weinberger, 2004; Lewis et al., 1999; Meltzer, 1987; Wolf et al., 1993), none have accounted for heterogeneous array of clinical symptoms consistently, making pharmacological intervention difficult and only partially efficacious.
Vascular involvement in the pathophysiology of SZ (Bleuler, 1911) has gained some salience in recent years to explain and unify physiologic abnormalities seen in SZ (Hanson and Gottesman, 2005; Moises et al., 2015; Schmidt-Kastner et al., 2012). These hypotheses place impairment of the brain microvascular system as the central mechanism in the pathophysiology of SZ.
A fundamental physiological process in development and maintenance of the microvascular system is angiogenesis. Angiogenesis is the process of formation of new capillaries from existing vessels and it is the pivotal event in neovascularization during embryogenesis and throughout the life span. Angiogenesis is also engaged in response to brain injury, brain function demands and it is responsible for coupling the capillary endothelium with astroglial cells and neurons.
During brain development neurovascular coupling mechanisms assure simultaneous generation of neuroblasts and blood vessels. The primary perineuronal vascular network that surrounds the ventral neural tube is established very early during embryogenesis, in mice this occurs at approximately E7.5–E8.5 days (Risau, 1997). Vascularization of the neuroepithelium occurs via sprouting angiogenic endothelial progenitor cells from the pial surface to the periventricular areas by E11 and generates a vascular plexus (Risau, 1994). Formation of the blood brain barrier (BBB) is likely to begin at this time. Tracer-injection studies in embryos demonstrate permeability of brain endothelial cells in the first few days of vessel formation, but it becomes tightly restricted by E15.5 (Ben-Zvi et al., 2014) suggesting that the BBB has already formed. Diverse angiogenic factors including PDGF, IGF-1, TGFB, GDNF, BDNF, bFGF etc. secreted by neuroblasts, most notably VEGF are required to induce angiogenesis (Hellbach et al., 2014; Raab et al., 2004; Virgintino et al., 2003). Accumulated evidence from animal models also reveals that a preexisting vasculature is a necessary physical substrate for oligodendrocyte (OLG) precursor cells migration during early brain and spinal cord development. Interestingly, OLG precursor cells migration in vivo was disrupted in mice with defective vascular architecture, but was normal in mice lacking pericytes (Tsai et al., 2016). Thus, endothelial-OLG precursor cells interactions appeared to be critical for coordination of migration and differentiation of these precursors into OLG. Astrocytes migration, however, is different from OLG precursors during early development. Astrocytes progenitors migrate following radial glia without secondary tangential migration and, therefore, occupy restricted spatial domains related to their developmental site of origin (Tsai et al., 2012).
As brain circuitry matures during adolescence and attains adult levels of myelination-dependent saltatory action potential conduction, adequate access to blood supply and oxygen tension become central factors in the processes of the formation and compaction of myelin segments. Hypoxia inducible factors (HIF1/2a) stabilization in OLG progenitors as a result of oxygen strain inhibits their differentiation into OLGs. Remarkably, these undifferentiated OLG progenitors possess paracrine activity that induces robust postnatal angiogenesis in vivo and directly stimulate endothelial cell proliferation in vitro (Yuen et al., 2014). Given the strong evidence for the involvement of OLGs and myelin abnormalities in the pathophysiology of SZ (reviewed in (Haroutunian et al., 2014)), the functional impact of oligodendroglial by endothelial cell (EC) interactions may be fundamental to the pathophysiology of SZ and may explain, at least partially, the observed gene expression changes specific to OLG and ECs in SZ.
This review aims to assess the accumulated evidence for the involvement and role(s) of the brain microvasculature in SZ and answer the following questions: (1) is there evidence for regional brain hemodynamics changes in SZ? (2) What are the morphological abnormalities in BBB/microvascular structure in the brains of individuals with SZ? (3) Does microvascular/BBB gene expression signature associated with SZ show changes in angiogenesis/vascular remodeling and offer support for an anomalous BBB in SZ? (4) Could the genes involved in angiogenesis and associated with it signaling pathways be potential risk factors in SZ from the genomic and transcriptomic perspective? (5) Do animal models aimed at revealing the mechanism of cerebral angiogenesis demonstrate cognitive and socioemotional deficit similar to those observed in individuals with SZ?
2. Regional brain hemodynamic changes associated with SZ clinical symptoms
That the neurovascular system is involved in SZ has been repeatedly documented by neuroimaging studies. Extensive reviews of structural and functional neuroimaging in SZ (Buchsbaum and Hazlett, 1998; Lopes et al., 2015), including discordant monozygotic twins (Weinberger et al., 1992), have demonstrated that abnormalities in brain hemodynamics are generally consistent with historical concept of hypofrontality in individuals with SZ (Berman et al., 1986) and reflect functional deficit of frontal cortex (Davidson and Heinrichs, 2003; Taylor, 1996; Weinberger et al., 1986). Frontal hypoperfusion has been consistently documented in clinical cases involving first episode, neuroleptic-naïve (Andreasen et al., 1997; Catafau et al., 1994; Erkwoh et al., 1997; Rubin et al., 1994) and untreated individuals with chronic SZ (Kim et al., 2000) suggesting that this characteristic is independent of the length of disease, its treatment and duration. Other regions, where hypoperfusion in SZ has been extensively documented, include middle and anterior cingulate, temporal and parietal regions (Ojeda et al., 2002; Schultz et al., 2002; Yucel et al., 2002) and thalamus (Clark et al., 2001). More recently, MRI based investigation of alterations in the volume of small arterial (pial) and arteriolar vessels in brains of individuals with SZ suggested that microvascular abnormalities maybe more widespread across the entire brain (Hua et al., 2016). Reduced CBF has been also associated with psychotic symptoms. For example, it has been shown that reduced CBF was associated with greater severity of negative symptoms in frontal, cingulate and superior temporal cortices (Pinkham et al., 2011). In contrast, positive symptoms have been associated with increased CBF in temporal, cingulate and superior frontal gyri (McGuire et al., 1993; Pinkham et al., 2011) and in subfields of hippocampus (Schobel et al., 2013). Although most neuroimaging studies have made the underlying assumption that the SZ hypofunction in CBF reflects neuronal hypometabolism, a more direct association of SZ with cerebrovascular circulation and function is equally plausible.
The evolutionary increase of cortical neuronal complexity, massive expansion of the cortical surface and cell number in humans depends on higher blood supply due to oxygen and nutrients requirements. Not surprisingly cerebral cortical vascularization is dramatically enhanced in humans relative to monkeys and cats (Lauwers et al., 2008). Moreover comparison of newborn and adult cerebral cortical depth in non-human primates using 3D-imaging has shown that relative cortical vascular volume nearly doubles in adults compared to newborns and that this increase occurred predominantly at the capillary level (Risser et al., 2009). Increase in capillary volume is sustained by the lengthening of pre-existing segments and by the formation of new segments, while the contribution of capillary diameter is minor and only marginal at the perforating vessel level. These findings indicate that structural adaptations of the cerebral vascular system induce multiple changes that include: vessel density and segment length, a decrease in inter-capillary distances and, to a lesser extent, vessel diameter increase. Therefore, postnatal cortical maturation typically described in terms of synaptogenesis, gliogenesis and interconnectivity is accompanied, and perhaps preceded, by an intensive remodeling of microvascular architecture.
Moreover, recent studies in mice using combination of high-throughput histology and computation models also suggest that the known changes in cortical blood flow (CBF) induced by changes in the neurotransmitter microenvironment are controlled at the level of microvessels (Blinder et al., 2013), likely through constriction or stiffening of contractile proteins in pericytes. The restricted lateral perfusion of cerebral cortical tissue is an additional distinctive parameter of brain hemodynamics. Although the microvasculature forms interconnected loops with a topology that follow organization of cortical columns, blood flow sourced by penetrating arterioles is effectively drained by penetrating venules limiting lateral perfusion. Experimental results for pathological condition such as local ischemia are also consistent with restricted lateral perfusion within the cerebral cortex, which prevents blood in neighboring penetrating vessels from entering the area previously sourced by the occluded vessel (Blinder et al., 2013), thus limiting damaging impact on neural cells proximal to supplying impaired vessel and sparing those in neighboring columns. Therefore, it is anticipated that reduced blood perfusion documented in individuals with SZ and possibility of associated with it, mild hypoxia will be restricted to cortical columns with reduced CBF.
Astrocytes, which encase by their end-feet, almost the entire parenchymal arterioles and capillaries also play a pivotal role in regulating CBF. By contrast, the processes from neurons are rarely in direct contact with brain blood vessels (Koehler et al., 2009). Astrocytes sensing synaptic activity and the release of calcium and major neurotransmitters, such as glutamate, may additionally influence CBF by modulating the vascular smooth muscle tone around larger vessels, such as arterioles. Spill over glutamate elicits NMDA receptor signaling that engages the calcium syncytium by raising intracellular Ca2+ concentration in astrocytic end-feet modulating vascular tone via several pathways. The first involves the vasodilator-nitric oxide release by neurons, which activates smooth muscle guanylate cyclase. This activation results in a rise in cGMP levels causing dilatation of vessels. The second pathway involves phospholipase A2 metabolism in both neurons and astrocytes, which results in prostaglandins release, which acts on vessels to dilate and induces arachidonic acid metabolism into 20-hydroxyeicosatetraeonic acid in smooth muscle, which constricts vessels (Attwell et al., 2010). A role of increased Ca2+- sensitive K+ current in astrocyte ends-foot on vasodilation and CBF has also been demonstrated (reviewed by (Koehler et al., 2009)). Given the accumulated evidence of regional abnormalities of amino acidergic (glutamatergic and GABAergic) and monoaminergic (dopaminergic and serotonergic) neurotransmission in SZ (Bunney and Bunney, 2000; Davis et al., 1991; Meyer-Lindenberg et al., 2002; Otsuki and Akiyama, 1997) and region-specific effect of ketamine (NMDA receptor antagonist) on CBF (Tamminga, 1999; Tamminga et al., 1995), the contribution of disturbed neuronal circuits to regional hemodynamic changes associated with clinical symptoms and cognitive performance in SZ appeared to be critical. Thus, there is ample evidence for strong interactions between the microvasculature and neurobiological processes known to be involved in SZ. On the one hand, the microvasculature influences the development and maintenance of brain circuitry, while at the same time changes in neurotransmitter and related systems influence CBF and potentially regulate and alter microvascular architecture dynamically.
Age is one of the main variables that need to be taken into account when assessing CBF changes in SZ patients. In general, there is consensus that CBF declines as a function of age. While negative correlations between age and CBF have been observed in the cingulate, frontal and parietal regions, it appears that individuals with SZ may experience unique and additional, age-independent, CBF impairments in frontal and parietal areas that are likely related to the expression of negative symptoms in late life (Schultz et al., 2002). In addition to age, antipsychotic treatment is a common variable that may have strong impact on regional CBF in SZ patients. The documented outcomes of antipsychotics treatment on CBF in SZ patients show conflicting results ranging from substantial to minor, or no efficacy of medication. Normalizing effect of antipsychotic treatment on regional CBF has been more evident in patients treated with atypical antipsychotics and show that increases in regional blood perfusion are positively correlated with cognitive performance improvement (Ertugrul et al., 2009; Lindenmayer et al., 2007). Examination of brains from antipsychotic-treated monkeys have shown that while the neuronal cell density has a tendency to increase in the parietal cortex of antipsychotic-exposed monkeys, glial cell densities and EC’s densities remain unchanged (Konopaske et al., 2007). One possible outcome of antipsychotic-treatment is that increase of neuronal density may stabilize abnormal regional neurotransmission in SZ resulting in positive effects on local CBF modulated by contractile mechanisms in pericytes (Blinder et al., 2013). This evidence of antipsychotic-induced normalization, or partial normalization of CBF, suggests that hypoperfusion and reduced CBF may be more profound in untreated persons with SZ and suggest that hypoperfusion and reduced CBF may be components of the core pathophysiology of SZ.
3. Microvascular/ blood brain barrier abnormalities in SZ: evidence from postmortem studies
Given strong evidence for hypoperfusion in many cortical regions in SZ it is surprising that evidence of structural abnormalities in microvasculature are rare and inconsistent. This may be explained, at least in part, by differences in the brain regions studied, by methodological differences, and by the complexity of surveying the microvasculature in cerebral cortical tissue quantitatively. Early reports suggested abnormalities in the microvasculature in SZ, such as reduction of retinal vascular bed during progressive course of SZ (Cotton et al., 1940) and generally simplified angio-architecture with reduced arborization of the brain vessels in the orbito-frontal cortex of paranoid hallucinatory SZ individuals (Senitz and Winkelmann, 1991). Wide-spread small vessel disease in the cerebral white matter with small foci of demyelination has been shown in patients with SZ psychosis (Bruton et al., 1994). Morphometric analysis of electron microscopy images from the superficial layers (layers I-II) of prefrontal and visual cortices of individuals with SZ showed the presence of ultrastructural abnormalities of capillaries including thickening deformation of basal lamina and cytoplasmic vacuolation of endothelial cells, although the area of capillaries and their diameter did not show significant differences between SZ and controls (Uranova et al., 2010). Consistent with results in frontal cortex, mean capillary diameter was not significantly different in layers III and V of the anterior cingulate cortex between elderly antipsychotic-free SZ patients and age matching controls, irrespective of evident cortical thickness reduction in both layers in SZ (Sinka et al., 2012). Lack of differences in mean capillary length densities was also demonstrated in prefrontal and anterior cingulate cortices between control and SZ patients, although mean cortical volume and thickness was significantly reduced in SZ patients (Kreczmanski et al., 2005; Schmitz et al., 2005). Similar results were obtained in several subcortical regions including caudate, putamen, nucleus accumbens, mediodorsal thalamic nucleus and amygdala using several parameters of microvascular integrity (total capillary length, mean capillary density and length per neuron) (Kreczmanski et al., 2009). Noninvasive approach to assess cerebral vascular abnormalities in SZ by retinal imaging, since retinal and cerebral microvessels are structurally and functionally homologous, suggested that wider venules in SZ patients may represent a proxy marker of familial vulnerability to psychosis symptoms (Meier et al., 2013).
It is assumed that shear stress caused by blood flow is critical for vascular endothelial cell survival and continued vessel patency. Therefore, reduction of CBF in frontal cortex in SZ may directly impact shear stress and subsequently survival outcome of the vascular endothelium. Endothelial cell death is typically followed by collapse of the capillary walls, leaving only remnants of the extracellular matrix in the form of a thin string. Thus, the lack of gross pathological lesions of the microvasculature in SZ and the absence of thinning, reduced tortuosity, or string vessels in the cerebral cortex and subcortical structures, which are prominent features in Alzheimer’s disease (Bailey et al., 2004; Hunter et al., 2012; Richard et al., 2010) arguing against lingering microvascular degeneration in SZ.
Given the strong association with cognitive and adaptive functioning decline at the onset of SZ, similar to that observed in normal aging, early-stage schizophrenia and normal aging might share common underpinnings (Tang et al., 2009). Studies of age-related changes in the cerebral microvasculature of cognitively normal individuals have led to varying conclusions. While some human studies have shown that various structural parameters in the cerebral cortical microvasculature network remain stable during aging (Hunziker et al., 1979; Meier-Ruge et al., 1980; Meier-Ruge et al., 1992; Schulz-Dazzi, 1986), others demonstrated significant decrease in density of vessels supplying the cerebral cortex and hippocampus compared to young controls accompanied by the increased diameter of the remaining vessels (Bell and Ball, 1981). Age-dependent increase in intercapillary distance, indicating reduction of vessel density, and increased diameter of vessels were also demonstrated in cortical regions and hippocampi of old rats compare to their adult counterparts (Amenta et al., 1995; Sonntag et al., 1997). Age related shrinkage of cortical thickness consequent to partial atrophy may have significant impact on morphometric parameters characterizing cerebral microvasculature in normal aging and could be the basis for reported inconsistencies.
The lack of unequivocal support for reduced cerebral capillary networks in SZ and normal aging suggests that non-morphometric parameters may be more informative to the understanding of the mechanisms contributing to regional hypoperfusion seen in SZ. Ultrastructural evaluation of BBB structural integrity and its associated permeability is one of these approaches.
The perivascular environment is highly susceptible to changes in ECs/BBB integrity and can therefore be used to gauge microvascular disturbances in SZ. Most notable abnormalities documented in the pericapillary environment in SZ include swelling of astrocytic end-feet and apoptotic pericapillary OLGs (Uranova et al., 2010). Assessment of the deeper layers (layer V) of the frontal cortex has indicated significant reduction of pericapillary OLGs in SZ (Vostrikov et al., 2008).
Based on the inflammatory-vascular theory of SZ (Hanson and Gottesman, 2005), brains of individuals with SZ are likely to display features of reactive astrogliosis and microglial infiltrations, due to concurrent inflammation associated microvascular/BBB damage and persistent hypoxia during the course of disease. Other studies have also suggested that activation of microglial cells maybe more relevant to the pathophysiology of psychosis (Bernstein et al., 2009; Steiner et al., 2008) and reflective of heterogenic pathophysiology of paranoid vs. residual SZ.
Overall, the data on neuroinflammation and astrogliosis in SZ (Arnold et al., 1996; Arnold et al., 1998; Bernstein et al., 2009; Casanova et al., 1990; Damadzic et al., 2001; Falke et al., 2000; Radewicz et al., 2000; Wierzba-Bobrowicz et al., 2004) are contradictory and inconclusive, which may be attributable to small sample sizes, and the inclusion of non-psychiatric controls, including elderly controls, without adequate assessment of common and uncommon neuropathologic lesions. (Schnieder and Dwork, 2011). Additionally, possible anti-inflammatory effects of antipsychotics on microglial activation (Kato et al., 2011) should be taken into account.
Stereological cell counting methods may shed additional light on protracted effect of vascular/BBB integrity impairment in SZ. Region specific glial cell loss (all types) was demonstrated in anterior cingulate cortex in SZ (Stark et al., 2004). Glial cell losses include significant reduction of GFAP-positive astrocytes restricted to layer V of the dorsolateral prefrontal cortex (Rajkowska et al., 2002) and astrocytes adjacent to blood vessels in gyral and underlying white matter (Webster et al., 2001; Webster et al., 2005), which is consistent with reported reduction of astrocytic markers in deep cortical layer of anterior cingulate cortex in SZ (Katsel et al., 2011; Steffek et al., 2008). In contrast, evaluation of several subcortical regions including internal capsule, putamen and thalamic nuclei in SZ showed increased expression of several astrocyte-specific markers (Barley et al., 2009), however, the contribution of potential confounding factors such as age, dementia and neuroleptic exposure (Arnold et al., 1996) cannot be ruled out.
4. Microvascular/blood brain barrier transcriptome signature associated with SZ
Reports of microarray-based genome-wide gene expression studies in SZ performed on crude brain tissue homogenates have not pinpointed abnormalities in the cerebral vasculature transcriptome. It has been argued (Harris et al., 2008) that the brain vascular endothelium volume represents only 1 – 3% of the brain volume (depending on different estimates (Lauwers et al., 2008)) crude brain tissue homogenates and thus, it is unlikely to contribute to overall transcriptional alteration in the disease state considerably. However, recent stereological analyses suggest that the numbers of vascular ECs and neurons maybe much closer to each other than previously thought. The estimated ratio of ECs to neurons in the human amygdala, a brain region associated with SZ, is estimated to be near 1:1 (Garcia-Amado and Prensa, 2012). In non-human primates parietal cortex, the ECs to neurons ratio is 1:3, where ECs constitute near 15% of total cells (Konopaske et al., 2007). Thus, while there are inconsistencies in reported cell-type counts in deferent brain regions, it is, nevertheless, irrefutable that EC-microvasculature is a noteworthy component of the total brain tissue transcriptome. A recent RNA-seq based approach on acutely purified different brain cells populations (Zhang et al., 2014) identified EC/BBB -specific markers and has provided tools for the interrogation of EC/BBB-specific genes in total brain transcriptome analyses.
We examined 1306 genes identified in the transcriptome of purified mouse cerebral microvascular ECs (pericyte-depleted and enriched in comparison to peripheral ECs) (Daneman et al., 2010) in a whole-tissue block homogenate-based microarray dataset from 15 cerebrocortical regions and the hippocampus of individuals with SZ (Katsel et al., 2005a; Katsel et al., 2005b). This subset of genes, therefore, represents transcripts specifically enriched in ECs and the BBB and identified BBB forming molecules and pathways related to angiogenesis (Daneman et al., 2010). Six hundred and fifty-seven of EC/BBB -specific transcripts appeared to be significantly changed in the 15 brain cortical regions and the hippocampus of individuals with SZ using microarray analysis tools, as previously described (Katsel et al., 2008; Katsel et al., 2009). Three hundred and eleven of these genes corresponded to a subset uniquely enriched genes in ECs (between 2 - 750 fold) compare to the other subsets of transcripts from acutely purified CNS cell types, including neurons, astrocytes, myelinating oligodendrocytes, oligodendrocyte progenitors and microglia (Zhang et al., 2014). Near 24% (~300) of EC/BBB -specific transcripts (Daneman et al., 2010) were differentially expressed in the brain regions examined in persons with SZ relative to controls.
Top differentially expressed in SZ genes that were distinctively enriched in EC/BBB are shown in Table 1 (p-val sorted). Core analyses for the pathways and networks that were represented in differentially expressed genes in SZ were performed with Ingenuity Pathway Analysis (IPA) and Metacore. Angiogenesis was among the top biological function with majority of genes exhibiting downregulation in SZ (p-val=3.65E-34; activation Z-score= −1.77). The other prominently downregulated EC/BBB-related functions in SZ include migration and proliferation of cells (p-val=1.8E-38; activation Z-score= −2.73), while functional categories representing apoptosis tended to be upregulated (p-val=3.7E-40; activation Z-score= 2.28). Pathway analysis performed by both IPA and Metacore platforms (Tables 2 and 3) indicate enrichment for well-established angiogenesis-related pathways, such as WNT-, VEGF-, IGF1-, oncostatin M-, angiopoietin-, ephrin receptor- signaling, in which the majority of pathway-associated genes demonstrated downregulation in the analyzed brain regions from individuals with SZ. These findings are in agreement with the overall transcription pattern of laser capture microdissected cerebral vascular ECs from the prefrontal cortex of individuals with SZ in which majority of pathway and biological processes enriched in ECs were directionally associated with downregulation suggesting functional impairment of cerebral vasculature and BBB (Harris et al., 2008), and further supported by dysregulated VEGF signaling in dorsolateral prefrontal cortex (Fulzele and Pillai, 2009) and thalamus (Chu et al., 2009) in SZ. Interestingly, study of systemic blood factors in antipsychotic-naïve young relatives at familial high risk for psychosis and first-episode SZ patients showed that an anti-angiogenic factor - soluble fms-like tyrosine kinase (sFLT1) was significantly increased in the plasma of familial high risk for SZ individuals compared to controls (Lizano et al., 2016). Upregulation of sFLT1, the factor that binds and reduces circulation of pro-angiogenic factors, including VEGF, and blunts their beneficial effect on vascular endothelium homeostasis, suggest that the vascular dysfunction may presage the onset of psychosis in young relatives of persons at familiar high risk for SZ.
Table 1.
Differentially expressed EC/microvascular genes in SZ across 15 cortical regions (p-Val sorted) (as described in (Katsel et al., 2005a)).
| Symbol | Entrez Gene Name | Affymetrix | p-value (corr)* |
Fold change SZ* | t-scores* | Fold enriched in EC† |
|---|---|---|---|---|---|---|
| ICAM2 only EC | intercellular adhesion molecule 2 | 213620_s_at | 1.09E-10 | −1.48 | −7.98 | 730.3 |
| SPARC | secreted protein, acidic, cysteine-rich (osteonectin) | 212667_at | 7.55E-09 | −1.77 | −7.51 | 34.1 |
| CEBPD | CCAAT/enhancer binding protein delta | 203973_s_at | 6.09E-08 | −1.65 | −7.46 | 4.1 |
| CLDN5 | claudin 5 | 204482_at | 1.30E-07 | −1.38 | −7.01 | 676.9 |
| IFITM1 | interferon induced transmembrane protein 1 | 201601_x_at | 6.53E-07 | −1.61 | −7.37 | 50.2 |
| CDH5 | cadherin 5 | 204677_at | 6.84E-07 | −1.30 | −5.69 | 306.7 |
| IL13RA1 | interleukin 13 receptor, alpha 1 | 201887_at | 1.22E-06 | −1.35 | −5.23 | 3.2 |
| IL6ST | interleukin 6 signal transducer (GP130, oncostatin M receptor) | 212195_at | 1.22E-06 | −1.53 | −6.19 | 18.7 |
| IFITM2 | interferon induced transmembrane protein 2 | 201315_x_at | 1.23E-06 | −1.59 | −6.81 | 4.4 |
| EMP1 | epithelial membrane protein 1 | 201324_at | 1.37E-06 | −1.81 | −6.31 | 6.4 |
| STOM | stomatin | 201061_s_at | 1.66E-06 | −1.38 | −5.36 | 5.8 |
| PECAM1 | platelet/endothelial cell adhesion molecule 1 | 208982_at | 1.88E-06 | −1.38 | −5.34 | 291.1 |
| MAFF | v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog F | 36711_at | 3.88E-06 | −1.64 | −7.02 | 4.1 |
| ELTD1 | EGF, latrophilin and seven transmembrane domain 1 | 219134_at | 4.65E-06 | −1.15 | −3.75 | 584.4 |
| SDPR | serum deprivation response | 222717_at | 6.26E-06 | −1.13 | −3.65 | 69.4 |
| ANGPTL4 | angiopoietin-like 4 | 223333_s_at | 6.39E-06 | −1.25 | −4.22 | 7.2 |
| ADAMTS1 | ADAM metallopeptidase with thrombospondin type 1 | 222162_s_at | 2.06E-05 | −1.39 | −6.21 | 5.2 |
| LAMC1 | laminin, gamma 1 | 200771_at | 3.11E-05 | −1.21 | −3.01 | 7.9 |
| VWF | von Willebrand factor | 202112_at | 3.36E-05 | −1.39 | −4.65 | 43.1 |
| ANGPT2 | angiopoietin 2 | 205572_at | 5.80E-05 | −1.24 | −3.98 | 183.5 |
| FLT1 | fms-related tyrosine kinase 1, VEGF receptor 1 | 226498_at | 7.63E-05 | −1.36 | −4.57 | 411.2 |
| FN1 | fibronectin 1 | 212464_s_at | 1.01E-04 | −1.27 | −4.29 | 137.1 |
| MSN | moesin | 200600_at | 1.65E-04 | −1.39 | −4.62 | 23.4 |
| EDN1 | endothelin 1 | 222802_at | 1.72E-03 | −1.17 | −3.21 | 106.8 |
| COL4A1 | collagen, type IV, alpha 1 | 211980_at | 2.41E-04 | −1.36 | −5.44 | 44.7 |
| TAGLN | transgelin | 205547_s_at | 3.77E-03 | −1.92 | −3.19 | 3.1 |
| SLC2A1 | solute carrier family 2 (facilitated glucose transporter), member 1 | 201250_s_at | 4.65E-03 | −1.13 | −0.98 | 35.5 |
| TIE1 | tyrosine kinase with immunoglobulin-like and EGF-like domains 1 | 204468_s_at | 8.81E-03 | −1.20 | −3.91 | 388.2 |
| COL4A2 | collagen, type IV, alpha 2 | 211964_at | 9.64E-03 | −1.32 | −2.42 | 52.7 |
| ADM | adrenomedullin | 202912_at | 1.11E-02 | −1.33 | −2.05 | 31.2 |
| EPAS1 | endothelial PAS domain protein 1 | 200878_at | 1.51E-02 | −1.15 | −1.73 | 5.1 |
p-Val (corr) Benjamini-Hochberg corrected (Benjamini and Hochberg, 1995), one-way ANOVA; Fold change in SZ compare to controls, shown for hippocampus; t-scores, contrast analysis 15 brain regions (all p<0.001).
Fold enriched in EC compare to neurons, astrocytes, OPCs, oligodendrocytes (excluding microglia), based on an RNA-Seq transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex (Zhang et al., 2014) (http://web.stanford.edu/group/barres_lab/brain_rnaseq.html).
Table 2.
Ingenuity Canonical Pathways enriched in vascular endothelium differentially expressed genes in SZ.
| Pathways | p-val | z-score | Total genes | In Data (genes) |
|---|---|---|---|---|
| Interferon Signaling | 3.72E-11 | −3.05 | 36 | 13 |
| Tight Junction Signaling | 1.57E-09 | −2 | 140 | 21 |
| Integrin Signaling | 3.81E-07 | −1.53 | 191 | 23 |
| Cardiac Hypertrophy Signaling | 2.17E-07 | −0.43 | 223 | 24 |
| Ephrin Receptor Signaling | 5.34E-06 | −1.29 | 163 | 18 |
| Axonal Guidance Signaling | 1.46E-05 | NA | 442 | 31 |
| VEGF Signaling | 3.55E-05 | −1.90 | 92 | 12 |
| IGF1 Signaling | 7.48E-05 | −1 | 91 | 11 |
| Oncostatin M Signaling | 8.28E-05 | −2.45 | 28 | 6 |
| Wnt/PCP pathway | 8.49E-04 | −0.38 | 63 | 8 |
| Angiopoietin Signaling | 1.63E-03 | −1.41 | 66 | 8 |
Table 3.
Metacore process networks enriched in vascular endothelium differentially expressed genes in SZ.
| Process Networks | p-val | Total genes | In Data (genes) |
|---|---|---|---|
| WNT signaling | 7.27E-07 | 177 | 32 |
| Regulation of angiogenesis | 8.02E-07 | 222 | 36 |
| Cell junctions | 1.65E-06 | 162 | 29 |
| Integrin-mediated cell-matrix adhesion | 5.86E-06 | 214 | 33 |
| Regulation of epithelial-to-mesenchymal transition | 1.55E-05 | 225 | 33 |
| Actin filaments | 1.85E-05 | 176 | 28 |
| Anti-Apoptosis mediated by external signals via MAPK and JAK/STAT | 2.25E-05 | 179 | 28 |
| Wnt, beta-catenin, Notch, VEGF, IP3 and integrin signaling | 7.93E-05 | 151 | 24 |
4.1 Receptor tyrosine kinase family
Notably, IGF1-, VEGF-, ephrin receptors belong to the receptor tyrosine kinase family, which are known to trigger multiple signaling cascades, including PI3K-AKT-mTOR and MAPK signaling. Both cascades have been shown to be dysregulated in thalamic nuclei from individuals with SZ, but not from individuals with bipolar disorder or major depression (Chu et al., 2009). Detected abnormalities are corroborated by earlier reports of reduced AKT-GSK3b (Emamian et al., 2004; Kozlovsky et al., 2001, 2002; Zhao et al., 2006) and insulin receptor content and autophosphorylation levels (Zhao et al., 2006), suggesting that aberrant insulin receptor signaling in SZ may be linked to reduced glucose metabolism in cerebral cortical regions of the brains of SZ patients (Buchsbaum et al., 2007). Growth factors, PDGFβ and TGFβ are involved in the recruitment of pericytes and maturation of the BBB (reviewed by (Blanchette and Daneman, 2015)), signifying the involvement of receptor tyrosine kinase signaling pathway in the development and maintenance of the cerebrovascular system.
4.2 Proangiogenic signals
Differentially expressed in SZ EC’s angiopoietins (ANGPT2 and ANGPTL4, Table 1) are the signaling molecules secreted in the neurovascular unit and involved in maintenance of the vascular plexus and BBB integrity. Proangiogenic signal, VEGF-A secreted by astrocytes, also promotes endothelial sprouting from existing vasculature by stimulating motility, filopodia extension and proliferation, and, together with Notch signaling, controls whether specific endothelial cells become lead tip cells, or trailing stalk cells(Eilken and Adams, 2010). In contrast to elevated circulating blood VEGF levels in patients with SZ (Pillai et al., 2015), reduced cerebral VEGF gene expression was observed in the current study and reported in the prefrontal cortex of individuals with SZ (Fulzele and Pillai, 2009).
4.3 Notch signaling
While early genetic studies linked polymorphisms in genes involved in the Notch pathway to SZ (Passos et al., 2006; Schmidt-Kastner et al., 2006; Tiwari et al., 2010), very few hypothesis-free gene expression changes in the Notch pathway have been reported in SZ. However, our hypothesis-driven analyses based on expression patterns of EC/BBB-related transcripts showed that consistent with earlier findings NOTCH −3, −4 (Sinkus et al., 2013) and JAG1(Kerns et al., 2010) were affected in the brain regions of the persons with SZ. NOTCH4 and JAG1 are distinctly enriched in vascular ECs, while NOTCH3 and HES5, the downstream NOTCH signaling effector, that are significantly affected in SZ (Kerns et al., 2010) are highly expressed in astrocytes compare to other neural cell-types (Zhang et al., 2014) suggesting cross-talk between perivascular astrocytes and the vascular endothelium.
Motility and filopodia extension of endothelial tip cells share many structural and functional features with axonal growth cones. While significantly affected in SZ (Table 2; Axonal guidance signaling) along with ephrin receptor signaling, semaphoring / plexin, netrin and slit / roundabout receptors appeared to be guidance cues in endothelial sprouting and possibly coordinate the formation of growing vessels and neural cells. These pathways show a tendency to be downregulated in analyzed brain regions in individuals with SZ (Table 2). While changes in the expression of genes involved in axonal guidance signaling prefrontal cortex (Arion et al., 2010) and cerebellum (Eastwood et al., 2003) in SZ have been ascribed, predominantly, to neural function, including synaptic plasticity and neural progenitors migration during development, the above analysis suggests that guidance and maturation of the microvasculature is another plausible feature of these guidance signaling systems.
4.4 WNT/β-catenin signaling
Recent evidence suggests that a unique brain-specific angiogenic mechanism integrates a WNT/β-catenin signaling pathway that is elicited by WNT ligands released by neuroblasts (Daneman et al., 2009). In addition, activation of WNT signaling initiates maturation of the BBB (reviewed by (Blanchette and Daneman, 2015)). Genetic disruption of WNT signaling leads to severe CNS angiogenesis defects (reduction in vessel number, loss of capillary beds, and the formation of hemorrhagic vascular malformations) with loss of BBB integrity, indicating that both processes are tightly linked (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008). Earlier studies indicated that WNT-1 immunoreactivity is increased, while β-catenin is reduced in the CA3 and CA4 pyramidal cell layers of the hippocampus (Cotter et al., 1998; Miyaoka et al., 1999) and is associated with low protein levels of GSK3B in prefrontal cortex from individuals with SZ (Kozlovsky et al., 2000). However, other studies have shown conflicting results; the initial reduction of GSK3B protein levels in prefrontal cortex of SZ (Beasley et al., 2001) was not confirmed in the same region using samples from a different SZ cohort (Beasley et al., 2002). These differences may be due to vagaries of crude brain homogenate studies since a recent evaluation of WNT signaling using a cell-type specific approach showed elevated mRNA levels of WNT7B and frizzled-1, while downstream mediators of WNT signaling, DAAM1 and LEF1 were decreased in LCM isolated parvalbumin-positive neurons from individuals with SZ (Pietersen et al., 2014). An RNA- seq pilot study of human induced pluripotent stem cell derived into forebrain neural progenitor cells from patients with SZ, additionally, show perturbation in canonical WNT signaling related to SZ (Brennand et al., 2011; Topol et al., 2015). These findings suggested that disturbed Wnt signaling in SZ is likely more common and not limited to only neuronal phenotypes. Moreover, a recent genetic study found polymorphisms in the CTNNB1 and WNT7B genes to be associated with SZ (Levchenko et al., 2015).
WNT signals are transduced by at least two distinct pathways: the canonical WNT/β-catenin pathway and the β-catenin independent diverse non-canonical WNT pathways (Wnt/PCP and Wnt/Ca2+). During neovascularization, angiogenesis can be envisioned as the coordinated growth and migration of EC sheets towards an angiogenic signal. ECs polarization is likely responsible for the pattern formation, which further organized into the complex vascular networks. Planar cell polarity signaling (WNT/PCP, Table 2) is a central mechanism, which impacts extracellular polarity cues and has important implications for vascular remodeling and in coordinated assembly of ECs into vascular structures (Cirone et al., 2008). WNT/PCP signaling is regulated at multiple levels: downstream, or upstream of the dishevelled homolog, DVL. For example, one of the WNT/PCP signaling branches involves the asymmetrical localization of frizzled by the protocadherin CELSR receptor and VANGL protein by recruiting PRICKLE, a putative nuclear translocation receptor to the plasma membrane, which subsequently binds DVL and inhibits PCP signaling. Both, vascular endothelium enriched VANGL1 and PRICKLE1 genes (Zhang et al., 2014) were among three hundred eleven differentially expressed genes of the EC/BBB ensemble (please see above) and showed upregulation in the 15 cortical regions studied in SZ (Tables 2 and 3; Wnt/PCP signaling). Negative effect on WNT signaling in SZ further supported by the upregulation of secreted frizzled-related proteins, SFRP2 and, particularly SFRP4, which is a potent angiogenesis inhibitor (Muley et al., 2010), in neural progenitor cells derived from SZ patients (Topol et al., 2015).
4.5 Endothelial basement membrane
Ultrastructural analysis of capillaries in prefrontal and visual cortices in SZ showed thickening and deformation of the basal lamina (Uranova et al., 2010). Collagen IV is a type of collagen found primarily in the basal lamina, a structural component of the basement membrane and known for its role in angiogenesis and differentiation of EC. Six genes, COL4A1-6 are known to encode type IV collagen subunits. At least two of them, COL4A1-2 showed significant decreases in the cingulate, temporal and parietal cortices in SZ (Table 1) and downregulated COL4A1 mRNA levels were shown in LCM isolated ECs from the prefrontal cortex of individuals with SZ (Harris et al., 2008). Two other essential components of vascular basement membrane are laminin and SPARC (osteonectin) (both shown in Table 1). SPARC is one of the most downregulated EC-specific genes in SZ and it is additionally involved in pericytes recruitment to the microvessels, vascular remodeling, modulation of the mitogenic activity of VEGF on ECs, and reduces the association of VEGF with its cell-surface receptors (Kupprion et al., 1998).
4.6 Vascular inflammatory responses
Identified biological process networks related to adhesion, transport and vascular remodeling shown in Table 3 are integral processes in angiogenesis and the maintenance of the vascular endothelium. Gene expression changes associated with these processes are indicative of functional impairment of cerebral vasculature and BBB integrity (Harris et al., 2008). A recent vascular transcriptome study showed that the inflammatory response is an intrinsic component in VEGF elicited signaling in human ECs (Schweighofer et al., 2009). Specifically, VEGF and IL1 treatment of human umbilical vein EC elicits expression changes in genes involved in the regulation of vascular permeability, EC lineage specialization, epithelial to mesenchymal transition, which is characteristic to vascular remodeling, and genes, previously implicated in axon guidance (Schweighofer et al., 2009). Once again, these observations highlight a parallelism between axon growth guidance and vascular sprouting.
Transcriptome data from postmortem brain studies in SZ suggest “hypo-inflammatory” signature associated with SZ supported by two independent microarray studies (Harris et al., 2008; Katsel et al., 2005b; Roussos et al., 2012), which were confirmed in large coexpression dataset from seven independent transcriptome studies of SZ (Mistry et al., 2013). Overall, decrease clustering and reduced number of genes participating in immune-response modules suggests functional dysregulation of brain immune related processes in SZ. Reports of expression patterns of immune response loci genetically associated with SZ represented by the major histocompatibility complex genes (Kano et al., 2011; Morgan et al., 2016; Sinkus et al., 2013), which may be influenced by prolong smoking are generally agree that the upregulation pattern of MHC genes, which is normally associated with infection and systemic inflammation fails to occur in individuals with SZ.
Recently, increased expression of structural variants of complement component 4 (Sekar et al., 2016), which is part of classical complement cascade activation have been linked to excessive complement activity in the development of SZ and associated with reduced synaptic densities in SZ brains. Role of the complement system is also increasingly recognized as contributor to the brain endothelial activation (Wu et al., 2016). Moreover, the critical contribution of microglia to the repair of damaged BBB, which involves mechanisms elicited by purinergic receptor signaling, has been demonstrated recently (Lou et al., 2016). Additional factors, including age, duration of illness, cognitive and functional deterioration, regional susceptibility, can argue for (Arion et al., 2007; Najjar and Pearlman, 2015; Narayan et al., 2008; Saetre et al., 2007; Shao and Vawter, 2008), or against inflammation in SZ (Arnold et al., 1998). Despite these discordant observations, whether perivascular inflammation influences angiogenesis and the integrity of the neurovascular unit remains an important question.
4.7 HIF1A signaling
During embryonic and early postnatal development increasing demands for oxygen, supply of nutrients and low levels of oxygenation are prerequisites for angiogenesis and formation of mature vascular plexus in the brain. This process could be disrupted by fetal hypoxia, which have all been proposed as a common environmental risk factor for SZ (Hanson and Gottesman, 2005; Schmidt-Kastner et al., 2006; Tsuang, 2000). Hypoxia-inducible factor 1 alpha (HIF1A) and vascular-specific endothelial PAS domain protein 1 (EPAS1, HIF2A) are among the main transcription factors that mediate the effect of hypoxia on the expression of genes involved in angiogenesis (Tian et al., 1997). Activated HIF1A/EPAS1 initiates transcription of multiple target paracrine signaling genes involved in angiogenesis, vascular remodeling and tone regulation. As we mentioned above, the majority of genes involved in these processes appeared to be downregulated in analyzed cortical regions from individuals with SZ compare to controls (Tables 1–3). In agreement with the directionality of changes in angiogenesis/vascular remodeling in analyzed brain regions from individuals with SZ, EC-specific EPAS1 (Table 1) and HIF1A (not shown) transcription factors showed modest but significant reduction of mRNA levels in the same regions in SZ.
A concise summary of the plausible mechanisms of postnatal angiogenesis and their effects in the brains of healthy and SZ individuals are shown in Figure One.
Figure 1.
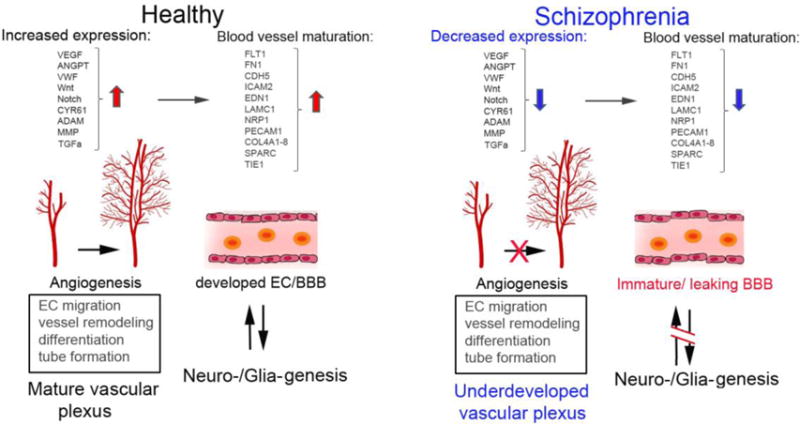
Plausible mechanisms (including participating genes) that take place in postnatal angiogenesis and their general effects in healthy and schizophrenia brains.
Active angiogenic mechanisms aiming development of adequate vascular supply take place during early development (neurogenesis) and after birth, during myelination and synaptic plasticity, the processes that entail extraordinary metabolic demands. Growth factors, signaling angiogenic pathways and tissue remodeling proteins are released from neural cells, leading to the building of well-developed vascular plexus that maintain healthy brain function. In schizophrenia reduced gene expression of angiogenesis signals and remodeling proteins suggests reduced vessel sprouting/microvascular length, leading to an overall reduced blood flow and oxygenation of brain tissue. At the cellular level, the ECs begin to reduce the expression of growth factor receptors affecting the survival capacity of vascular endothelium and formation of endothelial junction in BBB. Many of these factors combine may create conditions for permeable/underdeveloped BBB, which result in spill over systemic plasma proteins into the brain tissue affecting wide-range of neural processes, including myelination, formation perineuronal nets-ECM and ultimately neuronal connectivity.
The analysis undertaken in this review does not include transcriptional changes specific to astrocytes, which play a pivotal role in angiogenesis, maintenance of endothelial junctions in BBB, transport across BBB and dynamic regulation of cerebral circulation (reviewed in (Koehler et al., 2009)). Astrocytes- secreted angiotensinogen, a precursor for angiotensin II - a powerful vasoconstrictor of arterioles and larger vessels (Yang et al., 1999) can also regulates permeability of BBB. As has been shown before, angiotensin II binding to type 1 angiotensin receptors expressed by ECs of BBB elicits downstream signaling that restricts passage of molecular tracers across human BBB (Wosik et al., 2007). Both, astrocytes and ECs secrete extracellular matrix (ECM) proteins to generate and maintain the basement membranes of vascular endothelium. The composition of the ECM is altered at the sites of BBB damage and the basement membrane disruption. For example, it has been shown that activated astrocytes at the sites of BBB damage by the leaked fibrinogen increase deposition of the chondroitin sulfate proteoglycan -neurocan and induced glial scar formation (Schachtrup et al., 2010). In SZ, strong increases in the chondroitin sulfate proteoglycan -positive glial cells were detected in the deep amygdala nuclei and entorhinal cortex compare to the normal controls, but not in bipolar disorder subjects (Pantazopoulos et al., 2010) demonstrating increased production of ECM proteins as an distinctive aspect of the pathophysiology of SZ.
In this review we have not discussed pericytes transcriptional changes relevant to SZ because many of the pericytes genes overlap with ECs (Daneman et al., 2010) making it difficult to draw distinctions from transcription-based data sets derived from mixed cell populations.
5. Evidence for vascular component association with SZ from the existing genetic data
SZ is a disorder with estimated heritability ranging from 64 to 90% (Lichtenstein et al., 2009; O’Donovan et al., 2003; Sullivan et al., 2003). SZ is thought to be a highly polygenic disorder where each genetic variant has a very small risk effect. Recently, it has been proposed that the genetic risk for SZ may operate at the pathway level (Sullivan, 2012) and hypothesize that multiple polygenetic variations alter a more limited set of biological pathways in SZ. Intra- and inter-cellular pathways related to energy supply to neurons and their protection from ischemia have been implicated by pathway enrichment analyses (Moises et al., 2015). More specifically, Moises et al. established a candidate pathway related to energy supply to synapses and protection from ischemia based on genes discovered in family association studies. The enrichment of that pathway with SZ-associated variants was confirmed using the PGC-SCZ genome-wide association study (GWAS) results, the largest GWAS analysis, conducted by the Psychiatric Genomics Consortium-Schizophrenia Workgroup. PGC-SZ GWAS comprises a sample set of 36,989 SZ cases and 113,075 controls and identified 108 common variants that show statistical associations with SZ (Schizophrenia Working Group of the Psychiatric Genomics, 2014). In addition, this interpretation of functional genomics data was supported by the significant overrepresentation of the genes involved in post-ischemic repair in postmortem gene expression studies. The constructed functional genomics data candidate pathways affected in SZ suggested strong representation of genes associated with PI3K/AKT signaling pathway, which exert an influence on vasoconstriction via reuptake of serotonin, dopamine and norepinephrine and vasodilation by modulating nitric oxide production. Taken together these data suggest that SZ may be a mild adult vascular-ischemic disorder (Moises et al., 2015). To further explore this hypothesis, we tested whether SZ-candidate genes, defined as genes that localize within PGC-SZ associated regions, that are also EC/BBB -specific markers (as described in previous section) show transcriptional changes in SZ. By leveraging the microarray dataset from 15 brain cortical regions and the hippocampus of individuals with SZ (Katsel et al., 2005a; Katsel et al., 2005b), we identified 11 out of 18 genes that were dysregulated in SZ (Table 4). This set of 18 genes are significantly enriched for differential expressed genes (Chi square = 7.43, df = 1, p = 0.0064) when compared to the overall significant differential expressed gene probes (11,600 out of 44,928 gene probes), indicating a significant dysregulation of gene expression for SZ-candidate genes that participate in EC/BBB related functions.
Table 4.
Gene expression changes for identified vascular endothelium specific GWAS SZ genes.
| Symbol | Gene Name | Affymetrix | Entrez | t-score |
|---|---|---|---|---|
| MANA2A | mannosidase, alpha, class 2A, member 1 | 226538_at | 4124 | −6.33 |
| NCK1 | NCK adaptor protein 1 | 211063_s_at | 4690 | −3.94 |
| CTNND1 | catenin delta 1 | 208862_s_at | 1500 | −3.12 |
| SLC39A8 | solute carrier family 39 (zinc transporter), member 8 | 219869_s_at | 64116 | −3.08 |
| NFATC3 | nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 3 | 210555_s_at | 4775 | −2.87 |
| STAG1 | stromal antigen 1 | 202294_at | 10274 | −2.66 |
| BAG5 | BCL2 associated athanogene 5 | 202985_s_at | 9529 | −2.18 |
| DPP4 | dipeptidyl-peptidase 4 (CD26, adenosine deaminase complexing protein 2) | 203717_at | 1803 | −2.16 |
| STAB1 | stabilin 1 | 38487_at | 23166 | −1.81 |
| SRPK2 | SRSF protein kinase 2 | 203181_x_at | 6733 | 3.06 |
| DGKZ | diacylglycerol kinase zeta | 207556_s_at | 8525 | 4.99 |
| RRAS | related RAS viral (r-ras) oncogene homolog | 212647_at | 6237 | ns |
| PSMB10 | proteasome subunit beta 10 | 202659_at | 5699 | ns |
| STAT6 | signal transducer and activator of transcription 6, IL-4 induced | 201331_s_at | 6778 | ns |
| GATAD2A | GATA zinc finger domain containing 2A | 238324_at | 54815 | ns |
| EGR1 | early growth response 1 | 201694_s_at | 1958 | ns |
| PPP1R13B | protein phosphatase 1 regulatory subunit 13B | 216347_s_at | 23368 | ns |
| VSIG2 | V-set and immunoglobulin domain containing 2 | 228232_s_at | 23584 | ns |
ns - non significant
6. Neurobehavioral sequelae of mutations of the endothelial cells/microvascular genes
Few studies have evaluated neurobehavioral consequences of manipulation of EC genes. Here, we will review the pertinent reports and will propose the future directions to advance the field.
6.1 SPARC, secreted protein acidic and cysteine rich
Campolongo and colleagues(Campolongo et al., 2012) assessed the behavioral abnormalities in adult mice with constitutive deletion of the Sparc gene (the association of Sparc with EC was discussed above and it is one of the most downregulated EC-specific genes in SZ). In an open field test, both Sparc+/− and Sparc−/− mice showed no significant changes in horizontal locomotor activity; however, mice of both genotypes exhibited more anxiety-related responses. Sparc−/− mice demonstrated decreased time spent and distance travelled in the center of open field, and mice of both genotypes displayed less rearing events. In the light dark box test, both Sparc+/− and Sparc−/− mice spent less time in the lit compartment of the box. No effects of deletion were seen in elevated plus maze. Taken together, these behavioral changes suggest elevated anxiety-related behaviors in Sparc mutants. Somewhat unexpectedly, increased anxiety of Sparc knockout mice was associated with decreased depression-like behaviors. Both Sparc mutant mice showed less immobility in forced swim test that is widely used to evaluate efficacy of anti-depressants rather than depression-related behaviors(Nestler and Hyman, 2010). More physiologically relevant behavioral tests will be needed to confirm this phenotype. In addition, the authors evaluated the effects of deletion on the response to spatial novelty and to a novel object in the novel object recognition and novel object exploration tests. Compared to wild-type (WT) mice, Sparc−/− mice demonstrated less exploration of novel object without alteration in general exploratory activity. In order to uncover the underlying mechanisms, neuronal activity and adult neurogenesis in the dentate gyrus (DG) were assessed. Compared to WT mice, Sparc−/− mice showed higher levels of neuronal activity assessed by footshock-induced c-Fos expression in the DG and decreased cell proliferation but not maturation or survival in the subgranular zone of the DG. While Sparc is most abundantly expressed in EC, recent studies from the Barres lab have also demonstrated that astrocytes secrete SPARC to regulate synaptogenesis and glutamate receptor levels(Clarke and Barres, 2013). As this model does not include cell type-specific deletion, it is impossible to link the behavioral changes and brain pathology in Sparc−/− mice to altered functions of Sparc in EC.
6.2 CLDN5, claudin 5
Claudin 5 is a tight junction protein and has a key role in the formation of paracellular pores that function in mediating selective ion permeability at the BBB (Anderson, 2001; Nitta et al., 2003). Campbell and colleagues (Campbell et al., 2012) demonstrated that administration of short interfering RNA against Cldn5 significantly improved neurological severity scores in mice with focal oedema. These effects were observed as early as 8 h post-injury and lasted for 1 week. The treatment led to decreased cell death in the CA1 region of the hippocampus and improved performance in the T-maze. Kanoski et all (Kanoski et al., 2010) studied the effects of a high-energy diet on hippocampal function and BBB integrity in the rat. Consumption of the high-energy diet impaired the hippocampus-dependent but not hippocampus-independent discrimination task in the T-maze. Deficient hippocampal memory was associated with decreased mRNA expression of tight junction proteins, including Claudin-5 and −12, in the choroid plexus and the BBB. Consistent with impairment of hippocampus-dependent memory, an increased permeability of BBB was observed in the hippocampus, but not in the striatum and prefrontal cortex in rats exposed to high-energy diet. De Senna and associates (de Senna et al., 2015) assessed the effects of treadmill training on cognitive and motor behavior in diabetic rats, and associated expression of BBB proteins in the hippocampus and striatum, including Cldn5 and aquaporin 4. Diabetes decreased Cldn5 expression in the hippocampus and striatum and AQP4 in the hippocampus. Exercise restored levels of Cldn5 in the striatum, but not in the hippocampus of diabetic rats. Reduced expression of aquaporin 4 was not influenced by exercise. The data suggest exercise can improve memory retention and enhance motor skills, possibly by restoring expression of the key BBB factors. Although attributing the effects of these genetic and environmental manipulations to EC/BBB disturbances alone is not warranted, the findings are consistent with hypotheses that link the behavioral consequences of down-regulation of Sparc and claudin 5 to EC/BBB and to behaviors reminiscent of aspects of SZ.
6.3 FLT1, fms-related tyrosine kinase 1 (VEGF receptor 1)
As a receptor for VEGFA, VEGFB and PGF, this tyrosine-protein kinase plays a critical role in the development of vasculature during embryogenesis and regulates endothelial cell proliferation and survival in adulthood (Seetharam et al., 1995; Sela et al., 2008). An indirect indication of the possible role of FLT1 in behavior comes from two studies that assessed effects of enriched environment, or spatial training in the Morris water maze test on expression of VEGF in the hippocampus or over-expression of VEGF in this brain region (Cao et al., 2004; During and Cao, 2006). Both enriched environment and training increased hippocampal expression of VEGF. Consistent with this effect, over-expression of VEGF in the hippocampus increased neurogenesis and improved cognition in adult animals. In contrast, placental growth factor, which signals through Flt1, decreased neurogenesis and learning, but increased endothelial cell proliferation. New animal models of this receptor are needed to directly evaluate its role in possible neurobehavioral abnormalities related to symptoms of SZ.
6.4 FN1, Fibronectin 1
Fibronectin is a major element of the ECM in the brain and is involved in the molecular mechanisms of neuronal development, cell adhesion and migration (Humphries et al., 2015; Schwarzbauer and DeSimone, 2011). Alternative splicing at three conserved genomic regions gives rise to multiple FN polypeptides (Kornblihtt et al., 1996), which play specific roles in FN dimer secretion and adhesion. In order to assess the role of the FN’s alternative exons splicing in vivo, Chauhan and associates generated two constitutive mouse models (Chauhan et al., 2005) devoid of extra domain A (EDA) exon regulated splicing in the FN gene that constitutively include (EDA+/+), or exclude the same exon (EDA−/−). EDA−/− mice exhibited decreased motor-coordination skills on accelerated rotarod and rearing activity in open field, while EDA+/+ mice showed diminished ambulation in the open field test. No group differences were found in anxiety- or depression-related behaviors. Although the data implicate the FN1 gene in the brain functions, limited brain and behavioral evaluation provides little if any insight into a possible link of FN1 to SZ in humans. As with all other genes being reviewed here, the biological mechanisms whereby abnormal expression of FN1 may be responsible for aspects of SZ remain poorly understood and await the development of relevant experimental models to link this gene to the disorder.
To conclude, our brief overview of the available models clearly demonstrated how little is known regarding the relationship of the dysregulated EC/BBB genes to behavioral outcomes relevant to schizophrenia; however, the studies mentioned above provide evidence to suggest that further exploration of these relationships could provide valuable insights. The major roadblock in the field is scarcity of animal models with conditional deletion or over-expression of the specific EC factors in a time, region-, and cell type-specific manner. The composition of BBB is very complex (Blanchette and Daneman, 2015). Sorting out convergent and divergent contributions of the different components of BBB will be necessary to understand their specific roles in relation to neurobehavioral consequences. Combining new genetic, imaging and circuitry approaches will help address these difficult questions.
7. Perspective: Vascular remodeling and hypoxia signaling as risk factors in SZ
Active angiogenic mechanisms elicited by growth factors and tissue remodeling proteins released by neuronal and glial cells promote vascularization and distribution of adequate blood supply during neuro- and glia- genesis and provide for a well-developed and plastic vascular plexus that maintains healthy brain function throughout the lifespan.
While morphometric data on area of coverage, total length, mean density and diameters of cerebral vascular endothelium in individuals with SZ are sparse, conflicting and inconclusive, the cerebral vascular transcriptome changes and functional genomics data reinforced by the ultrastructural abnormalities of capillaries and pericapillary cellular environment and regional CBF deficit are supportive of cortical vascular/BBB dysfunction in SZ. The evaluation of the vascular component of the multiregional cortical transcriptome described above, additionally, supports a hypothesis of a defective cerebral vasculature in SZ characterized by aberrant transcriptional changes within the multiple pathways involved in the maintenance and activation of angiogenesis, such as receptor tyrosine kinase-, Notch-, WNT- and hypoxia-signaling; genes associated with structural integrity of vascular basal membrane and ECM/BBB. The hypothesis is strengthened further by independent gene-set studies of SZ-associated variants and confirmation of previous analyses (Moises et al., 2015; Schmidt-Kastner et al., 2012) showing strong representation of vascular specific markers among SZ candidate genes.
Defective cerebral angiogenesis linked to an immature vascular plexus may create conditions influencing a wide-range of neural processes, including BBB permeability, myelination, formation of perineuronal nets-ECM and neuronal connectivity. This hypothesis can be tested in multiple ways, including (i) pharmacological intervention targeting vascular endothelium remodeling, similar to the one initiated by Ehrenreich, et al (Ehrenreich et al., 2007) using recombinant human erythropoietin, which improves ischemic tissue recovery by stimulating angiogenesis (Adelibieke et al., 2013; Chai et al., 2016; Mengozzi et al., 2012), and showed a significant improvement in cognitive function even in chronic SZ patients; (ii) development of sensitive neuroimaging in live subjects such as delayed contrast extravasation (Israeli et al., 2010) and molecular techniques for BBB integrity assessment in postmortem brain tissue. (iii) Animal models of defective angiogenesis and cerebral hypoperfusion can unveil the consequences of immature vascular plexus, permeable BBB and mild ischemia on neurobiology and behavioral traits, testing plausible mechanisms and potential treatments. Inarguably, examination of gene-environment interaction between SZ susceptibility genes and angiogenesis will shed light on the effect of prenatal or perinatal hypoxia, like it has been initiated in mice haploinsufficient for reelin (Howell and Pillai, 2014).
Vulnerability of OLGs and related myelin deficits, neuronal mitochondrial dysfunction and impaired energy metabolism, synaptic response to both negative and positive symptoms of SZ, are all features that can stem from persistent mild cerebral ischemia (Moises et al., 2015), including oxygenation and perfusion compromises caused by cerebral microvascular dysfunction. No single factor such as angiogenesis or vascular inflammation is likely to be responsible for the molecular changes, including repair capacity in vascular endothelium, and behavioral manifestations seen in SZ, however, the evidence reviewed here suggests that multiple events affecting coupling in the neurovascular unit are likely to play a significant role in the etiopathology of SZ. The pathways and mechanisms highlighted in this review provide a rational, evidence-based, basis for hypotheses testing in future research.
HIGHLIGHTS.
Angiogenic processes guide development and maintenance cerebral vascular endothelium, which guarantees adequate regional blood flow and safeguard normal brain function.
Genetic, neuroimaging, postmortem gene expression and morphological studies implicate cerebral microvasculature as a potential contributor to the pathophysiology of schizophrenia.
Microvascular gene expression signature show changes in angiogenesis/vascular remodeling and offer support for an anomalous blood brain barrier function in schizophrenia.
Neurobehavioral consequences and limitations of manipulating the mechanisms of cerebral angiogenesis in animal models relevant to schizophrenia are discussed.
Acknowledgments
This manuscript was supported by NIH grant MH097997 to PK and MP (sub-award) and Veterans Administration MIRECC to VH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adelibieke Y, Shimizu H, Saito S, Mironova R, Niwa T. Indoxyl sulfate counteracts endothelial effects of erythropoietin through suppression of Akt phosphorylation. Circ J. 2013;77:1326–1336. doi: 10.1253/circj.cj-12-0884. [DOI] [PubMed] [Google Scholar]
- Amenta F, Ferrante F, Mancini M, Sabbatini M, Vega JA, Zaccheo D. Effect of long-term treatment with the dihydropyridine-type calcium channel blocker darodipine (PY 108-068) on the cerebral capillary network in aged rats. Mech Ageing Dev. 1995;78:27–37. doi: 10.1016/0047-6374(94)01513-l. [DOI] [PubMed] [Google Scholar]
- Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci. 2001;16:126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Arion D, Horvath S, Lewis DA, Mirnics K. Infragranular gene expression disturbances in the prefrontal cortex in schizophrenia: signature of altered neural development? Neurobiol Dis. 2010;37:738–746. doi: 10.1016/j.nbd.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Trojanowski JQ, Moberg PJ, Gur RE. Glial fibrillary acidic protein-immunoreactive astrocytosis in elderly patients with schizophrenia and dementia. Acta Neuropathol (Berl) 1996;91:269–277. doi: 10.1007/s004010050425. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C. Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry. 1998;55:225–232. doi: 10.1001/archpsyc.55.3.225. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res. 2004;26:573–578. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Beasley C, Cotter D, Everall I. An investigation of the Wnt-signalling pathway in the prefrontal cortex in schizophrenia, bipolar disorder and major depressive disorder. Schizophr Res. 2002;58:63–67. doi: 10.1016/s0920-9964(01)00376-0. [DOI] [PubMed] [Google Scholar]
- Beasley C, Cotter D, Khan N, Pollard C, Sheppard P, Varndell I, Lovestone S, Anderton B, Everall I. Glycogen synthase kinase-3beta immunoreactivity is reduced in the prefrontal cortex in schizophrenia. Neurosci Lett. 2001;302:117–120. doi: 10.1016/s0304-3940(01)01688-3. [DOI] [PubMed] [Google Scholar]
- Bell MA, Ball MJ. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol. 1981;53:299–318. doi: 10.1007/BF00690372. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman K, Zec R, Weinberger D. Physiologic Dysfunction of Dorsolateral Prefrontal Cortex in Schizophrenia. Arch Gen Psychiatry. 1986;43:126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Steiner J, Bogerts B. Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert Rev Neurother. 2009;9:1059–1071. doi: 10.1586/ern.09.59. [DOI] [PubMed] [Google Scholar]
- Blanchette M, Daneman R. Formation and maintenance of the BBB. Mech Dev. 2015;138(Pt 1):8–16. doi: 10.1016/j.mod.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias, Reprinted 1950. International Univ Press; New York: 1911. [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Glboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011 doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton CJ, Stevens JR, Frith CD. Epilepsy, psychosis, and schizophrenia: clinical and neuropathologic correlations. Neurology. 1994;44:34–42. doi: 10.1212/wnl.44.1.34. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, Hof PR. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. Am J Psychiatry. 2007;164:1072–1081. doi: 10.1176/ajp.2007.164.7.1072. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Hazlett EA. Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull. 1998;24:343–364. doi: 10.1093/oxfordjournals.schbul.a033331. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Campbell M, Hanrahan F, Gobbo OL, Kelly ME, Kiang AS, Humphries MM, Nguyen AT, Ozaki E, Keaney J, Blau CW, Kerskens CM, Cahalan SD, Callanan JJ, Wallace E, Grant GA, Doherty CP, Humphries P. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat Commun. 2012;3:849. doi: 10.1038/ncomms1852. [DOI] [PubMed] [Google Scholar]
- Campolongo M, Benedetti L, Podhajcer OL, Pitossi F, Depino AM. Hippocampal SPARC regulates depression-related behavior. Genes Brain Behav. 2012;11:966–976. doi: 10.1111/j.1601-183X.2012.00848.x. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Stevens JR, Kleinman JE. Astrocytosis in the molecular layer of the dentate gyrus: a study in Alzheimer’s disease and schizophrenia. Psychiatry Res. 1990;35:149–166. doi: 10.1016/0925-4927(90)90017-z. [DOI] [PubMed] [Google Scholar]
- Catafau AM, Parellada E, Lomena FJ, Bernardo M, Pavia J, Ros D, Setoain J, Gonzalez-Monclus E. Prefrontal and temporal blood flow in schizophrenia: resting and activation technetium-99m-HMPAO SPECT patterns in young neuroleptic-naive patients with acute disease. J Nucl Med. 1994;35:935–941. [PubMed] [Google Scholar]
- Chai HT, Yip HK, Sun CK, Hsu SY, Leu S. AG490 suppresses EPO-mediated activation of JAK2-STAT but enhances blood flow recovery in rats with critical limb ischemia. J Inflamm (Lond) 2016;13:18. doi: 10.1186/s12950-016-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan AK, Moretti FA, Iaconcig A, Baralle FE, Muro AF. Impaired motor coordination in mice lacking the EDA exon of the fibronectin gene. Behav Brain Res. 2005;161:31–38. doi: 10.1016/j.bbr.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Chu TT, Liu Y, Kemether E. Thalamic transcriptome screening in three psychiatric states. J Hum Genet. 2009;54:665–675. doi: 10.1038/jhg.2009.93. [DOI] [PubMed] [Google Scholar]
- Cirone P, Lin S, Griesbach HL, Zhang Y, Slusarski DC, Crews CM. A role for planar cell polarity signaling in angiogenesis. Angiogenesis. 2008;11:347–360. doi: 10.1007/s10456-008-9116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Kopala L, Li DK, Hurwitz T. Regional cerebral glucose metabolism in never-medicated patients with schizophrenia. Can J Psychiatry. 2001;46:340–345. doi: 10.1177/070674370104600405. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Kerwin R, al-Sarraji S, Brion JP, Chadwich A, Lovestone S, Anderton B, Everall I. Abnormalities of Wnt signalling in schizophrenia–evidence for neurodevelopmental abnormality. Neuroreport. 1998;9:1379–1383. doi: 10.1097/00001756-199805110-00024. [DOI] [PubMed] [Google Scholar]
- Cotton JM, Lewis NDC, Egenhofer AW. Vascular bed of the retina in mental disease. Arch Neur Psych. 1940;43:891–900. [Google Scholar]
- Damadzic R, Bigelow LB, Krimer LS, Goldenson DA, Saunders RC, Kleinman JE, Herman MM. A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: absence of significant astrocytosis. Brain Res Bull. 2001;55:611–618. doi: 10.1016/s0361-9230(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. 2003;122:69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- de Senna PN, Xavier LL, Bagatini PB, Saur L, Galland F, Zanotto C, Bernardi C, Nardin P, Goncalves CA, Achaval M. Physical training improves non-spatial memory, locomotor skills and the blood brain barrier in diabetic rats. Brain Res. 2015;1618:75–82. doi: 10.1016/j.brainres.2015.05.026. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–155. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, Heinz G, Erdag S, Jahn H, Degner D, Ritzen M, Mohr A, Wagner M, Schneider U, Bohn M, Huber M, Czernik A, Pollmacher T, Maier W, Siren AL, Klosterkotter J, Falkai P, Ruther E, Aldenhoff JB, Krampe H. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206–220. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Erkwoh R, Sabri O, Steinmeyer EM, Bull U, Sass H. Psychopathological and SPECT findings in never-treated schizophrenia. Acta Psychiatr Scand. 1997;96:51–57. doi: 10.1111/j.1600-0447.1997.tb09904.x. [DOI] [PubMed] [Google Scholar]
- Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, Senturk S, Erbas B, Cila A, Ulug B. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res. 2009;174:121–129. doi: 10.1016/j.pscychresns.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res. 2000;93:103–110. doi: 10.1016/s0165-1781(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Fulzele S, Pillai A. Decreased VEGF mRNA expression in the dorsolateral prefrontal cortex of schizophrenia subjects. Schizophr Res. 2009;115:372–373. doi: 10.1016/j.schres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Amado M, Prensa L. Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One. 2012;7:e38692. doi: 10.1371/journal.pone.0038692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005;6:7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856–1877. doi: 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- Harris LW, Wayland M, Lan M, Ryan M, Giger T, Lockstone H, Wuethrich I, Mimmack M, Wang L, Kotter M, Craddock R, Bahn S. The cerebral microvasculature in schizophrenia: a laser capture microdissection study. PLoS One. 2008;3:e3964. doi: 10.1371/journal.pone.0003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2004;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hellbach N, Weise SC, Vezzali R, Wahane SD, Heidrich S, Roidl D, Pruszak J, Esser JS, Vogel T. Neural deletion of Tgfbr2 impairs angiogenesis through an altered secretome. Hum Mol Genet. 2014;23:6177–6190. doi: 10.1093/hmg/ddu338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KR, Pillai A. Effects of prenatal hypoxia on schizophrenia-related phenotypes in heterozygous reeler mice: a gene x environment interaction study. Eur Neuropsychopharmacol. 2014;24:1324–1336. doi: 10.1016/j.euroneuro.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Brandt AS, Lee S, Blair NI, Wu Y, Lui S, Patel J, Faria AV, Lim IA, Unschuld PG, Pekar JJ, van Zijl PC, Ross CA, Margolis RL. Abnormal Grey Matter Arteriolar Cerebral Blood Volume in Schizophrenia Measured With 3D Inflow-Based Vascular-Space-Occupancy MRI at 7T. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Paul NR, Humphries MJ, Morgan MR. Emerging properties of adhesion complexes: what are they and what do they do? Trends Cell Biol. 2015;25:388–397. doi: 10.1016/j.tcb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Hunter JM, Kwan J, Malek-Ahmadi M, Maarouf CL, Kokjohn TA, Belden C, Sabbagh MN, Beach TG, Roher AE. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS One. 2012;7:e36893. doi: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker O, Abdel’Al S, Schulz U. The aging human cerebral cortex: a stereological characterization of changes in the capillary net. J Gerontol. 1979;34:345–350. doi: 10.1093/geronj/34.3.345. [DOI] [PubMed] [Google Scholar]
- Israeli D, Tanne D, Daniels D, Last D, Shneor R, Guez D, Landau E, Roth Y, Ocherashvilli A, Bakon M, Hoffman C, Weinberg A, Volk T, Mardor Y. The application of MRI for depiction of subtle blood brain barrier disruption in stroke. Int J Biol Sci. 2010;7:1–8. doi: 10.7150/ijbs.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano S, Nwulia E, Niwa M, Chen Y, Sawa A, Cascella N. Altered MHC class I expression in dorsolateral prefrontal cortex of nonsmoker patients with schizophrenia. Neurosci Res. 2011;71:289–293. doi: 10.1016/j.neures.2011.07.1818. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato TA, Monji A, Mizoguchi Y, Hashioka S, Horikawa H, Seki Y, Kasai M, Utsumi H, Kanba S. Anti-Inflammatory properties of antipsychotics via microglia modulations: are antipsychotics a ‘fire extinguisher’ in the brain of schizophrenia? Mini Rev Med Chem. 2011;11:565–574. doi: 10.2174/138955711795906941. [DOI] [PubMed] [Google Scholar]
- Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology. 2011;36:1171–1177. doi: 10.1038/npp.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005a;77:241–252. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res. 2005b;79:157–173. doi: 10.1016/j.schres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, Haroutunian V. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. 2008;33:2993–3009. doi: 10.1038/npp.2008.19. [DOI] [PubMed] [Google Scholar]
- Katsel P, Tan W, Haroutunian V. Gain in brain immunity in the oldest-old differentiates cognitively normal from demented individuals. PLoS One. 2009;4:e7642. doi: 10.1371/journal.pone.0007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns DC, Vong GS, Barley K, Dracheva S, Katsel P, Casaccia P, Haroutunian V, Byne W. Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res. 2010;120:150–158. doi: 10.1016/j.schres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Mohamed S, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:542–548. doi: 10.1176/appi.ajp.157.4.542. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32:1216–1223. doi: 10.1038/sj.npp.1301233. [DOI] [PubMed] [Google Scholar]
- Kornblihtt AR, Pesce CG, Alonso CR, Cramer P, Srebrow A, Werbajh S, Muro AF. The fibronectin gene as a model for splicing and transcription studies. FASEB J. 1996;10:248–257. doi: 10.1096/fasebj.10.2.8641558. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3beta immunoreactivity in postmortem frontal cortex of schizophrenic patients. Am J Psychiatry. 2000;157:831–833. doi: 10.1176/appi.ajp.157.5.831. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophr Res. 2001;52:101–105. doi: 10.1016/s0920-9964(00)00174-2. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol. 2002;12:13–25. doi: 10.1016/s0924-977x(01)00131-6. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HW, Hof PR, Schmitz C. Microvessel length density, total length, and length per neuron in five subcortical regions in schizophrenia. Acta Neuropathol. 2009;117:409–421. doi: 10.1007/s00401-009-0482-7. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Schmidt-Kastner R, Heinsen H, Steinbusch HWM, Hof PR, Schmitz C. Stereological studies of capillary length density in the frontal cortex of schizophrenics. Acta Neuropathol. 2005;109:510–518. doi: 10.1007/s00401-005-1003-y. [DOI] [PubMed] [Google Scholar]
- Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- Lauwers F, Cassot F, Lauwers-Cances V, Puwanarajah P, Duvernoy H. Morphometry of the human cerebral cortex microcirculation: general characteristics and space-related profiles. Neuroimage. 2008;39:936–948. doi: 10.1016/j.neuroimage.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Levchenko A, Davtian S, Freylichman O, Zagrivnaya M, Kostareva A, Malashichev Y. Beta-catenin in schizophrenia: Possibly deleterious novel mutation. Psychiatry Res. 2015;228:843–848. doi: 10.1016/j.psychres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP, Khan A, Iskander A, Abad MT, Parker B. A randomized controlled trial of olanzapine versus haloperidol in the treatment of primary negative symptoms and neurocognitive deficits in schizophrenia. J Clin Psychiatry. 2007;68:368–379. doi: 10.4088/jcp.v68n0303. [DOI] [PubMed] [Google Scholar]
- Lizano PL, Keshavan MS, Tandon N, Mathew IT, Mothi SS, Montrose DM, Yao JK. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: A preliminary study. Schizophr Res. 2016;170:115–122. doi: 10.1016/j.schres.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes R, Soares R, Coelho R, Figueiredo-Braga M. Angiogenesis in the pathophysiology of schizophrenia - a comprehensive review and a conceptual hypothesis. Life Sci. 2015;128:79–93. doi: 10.1016/j.lfs.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A. 2016;113:1074–1079. doi: 10.1073/pnas.1520398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Shah GM, Murray RM. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-s. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Hunziker O, Schulz U, Tobler HJ, Schweizer A. Stereological changes in the capillary network and nerve cells of the aging human brain. Mech Ageing Dev. 1980;14:233–243. doi: 10.1016/0047-6374(80)90123-2. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Ann N Y Acad Sci. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Meier MH, Shalev I, Moffitt TE, Kapur S, Keefe RS, Wong TY, Belsky DW, Harrington H, Hogan S, Houts R, Caspi A, Poulton R. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiatry. 2013;170:1451–1459. doi: 10.1176/appi.ajp.2013.13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY. Biological studies in schizophrenia. Schizophr Bull. 1987;13:77–111. doi: 10.1093/schbul/13.1.77. [DOI] [PubMed] [Google Scholar]
- Mengozzi M, Cervellini I, Villa P, Erbayraktar Z, Gokmen N, Yilmaz O, Erbayraktar S, Manohasandra M, Van Hummelen P, Vandenabeele P, Chernajovsky Y, Annenkov A, Ghezzi P. Erythropoietin-induced changes in brain gene expression reveal induction of synaptic plasticity genes in experimental stroke. Proc Natl Acad Sci U S A. 2012;109:9617–9622. doi: 10.1073/pnas.1200554109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Mistry M, Gillis J, Pavlidis P. Meta-analysis of gene coexpression networks in the post-mortem prefrontal cortex of patients with schizophrenia and unaffected controls. BMC Neurosci. 2013;14:105. doi: 10.1186/1471-2202-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res. 1999;38:1–6. doi: 10.1016/s0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- Moises HW, Wollschlager D, Binder H. Functional genomics indicate that schizophrenia may be an adult vascular-ischemic disorder. Transl Psychiatry. 2015;5:e616. doi: 10.1038/tp.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan LZ, Rollins B, Sequeira A, Byerley W, DeLisi LE, Schatzberg AF, Barchas JD, Myers RM, Watson SJ, Akil H, Bunney WE, Vawter MP. Microarrays (Basel) 2016. Quantitative Trait Locus and Brain Expression of HLA-DPA1 Offers Evidence of Shared Immune Alterations in Psychiatric Disorders; p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muley A, Majumder S, Kolluru GK, Parkinson S, Viola H, Hool L, Arfuso F, Ganss R, Dharmarajan A, Chatterjee S. Secreted frizzled-related protein 4: an angiogenesis inhibitor. Am J Pathol. 2010;176:1505–1516. doi: 10.2353/ajpath.2010.090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161:102–112. doi: 10.1016/j.schres.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008;1239:235–248. doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan MC, Williams NM, Owen MJ. Recent advances in the genetics of schizophrenia. Hum Mol Genet. 2003;12(2):125–133. doi: 10.1093/hmg/ddg302. [DOI] [PubMed] [Google Scholar]
- Ojeda N, Ortuno F, Arbizu J, Lopez P, Marti-Climent JM, Penuelas I, Cervera-Enguix S. Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp. 2002;17:116–130. doi: 10.1002/hbm.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki S, Akiyama K. Neurochemical studies of schizophrenia in Japan. Psychiatry Clin Neurosci. 1997;51:347–356. doi: 10.1111/j.1440-1819.1997.tb02598.x. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos GS, Gattaz WF, Tavares H, Kieling C, Timm S, Wang AG, Berg RH, Werge T, Dias-Neto E. Analysis of coding-polymorphisms in NOTCH-related genes reveals NUMBL poly-glutamine repeat to be associated with schizophrenia in Brazilian and Danish subjects. Schizophr Res. 2006;88:275–282. doi: 10.1016/j.schres.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Pietersen CY, Mauney SA, Kim SS, Passeri E, Lim MP, Rooney RJ, Goldstein JM, Petreyshen TL, Seidman LJ, Shenton ME, McCarley RW, Sonntag KC, Woo TU. Molecular profiles of parvalbumin-immunoreactive neurons in the superior temporal cortex in schizophrenia. J Neurogenet. 2014;28:70–85. doi: 10.3109/01677063.2013.878339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Howell KR, Ahmed AO, Weinberg D, Allen KM, Bruggemann J, Lenroot R, Liu D, Galletly C, Weickert CS, Weickert TW. Association of serum VEGF levels with prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.96. [DOI] [PubMed] [Google Scholar]
- Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R, Gur R. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 2011;194:64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 2002;57:127–138. doi: 10.1016/s0920-9964(02)00339-0. [DOI] [PubMed] [Google Scholar]
- Richard E, van Gool WA, Hoozemans JJ, van Haastert ES, Eikelenboom P, Rozemuller AJ, van de Berg WD. Morphometric changes in the cortical microvascular network in Alzheimer’s disease. J Alzheimers Dis. 2010;22:811–818. doi: 10.3233/JAD-2010-100849. [DOI] [PubMed] [Google Scholar]
- Risau W. Molecular biology of blood-brain barrier ontogenesis and function. Acta Neurochir Suppl (Wien) 1994;60:109–112. doi: 10.1007/978-3-7091-9334-1_28. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Risser L, Plouraboue F, Cloetens P, Fonta C. A 3D-investigation shows that angiogenesis in primate cerebral cortex mainly occurs at capillary level. Int J Dev Neurosci. 2009;27:185–196. doi: 10.1016/j.ijdevneu.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Roussos P, Katsel P, Davis KL, Siever LJ, Haroutunian V. A system-level transcriptomic analysis of schizophrenia using postmortem brain tissue samples. Arch Gen Psychiatry. 2012;69:1205–1213. doi: 10.1001/archgenpsychiatry.2012.704. [DOI] [PubMed] [Google Scholar]
- Rubin P, Holm S, Madsen PL, Friberg L, Videbech P, Andersen HS, Bendsen BB, Stromso N, Larsen JK, Lassen NA. Regional cerebral blood flow distribution in newly diagnosed schizophrenia and schizophreniform disorder. Psychiatry Res. 1994;53:57–75. doi: 10.1016/0165-1781(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP. An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry. 2012;17:1194–1205. doi: 10.1038/mp.2011.183. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Steinbusch WM, Schmitz C. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84:253–271. doi: 10.1016/j.schres.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Kreczmanski P, Schmidt-Kastner R, Heinsen H, Hof PR. No alterations in capillary length density in the prefrontal cortex of schizophrenics. Schizophr Bull. 2005;31:261–261. doi: 10.1007/s00401-005-1003-y. [DOI] [PubMed] [Google Scholar]
- Schnieder TP, Dwork AJ. Searching for Neuropathology: Gliosis in Schizophrenia. Biol Psychiatry. 2011;69:134–139. doi: 10.1016/j.biopsych.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, O’Leary DS, Boles Ponto LL, Arndt S, Magnotta V, Watkins GL, Hichwa RD, Andreasen NC. Age and regional cerebral blood flow in schizophrenia: age effects in anterior cingulate, frontal, and parietal cortex. J Neuropsychiatry Clin Neurosci. 2002;14:19–24. doi: 10.1176/jnp.14.1.19. [DOI] [PubMed] [Google Scholar]
- Schulz-Dazzi U. [Quantitative analysis of capillary and neuron images under normal conditions and in dementia] Biull Eksp Biol Med. 1986;101:102–105. [PubMed] [Google Scholar]
- Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer B, Testori J, Sturtzel C, Sattler S, Mayer H, Wagner O, Bilban M, Hofer E. The VEGF-induced transcriptional response comprises gene clusters at the crossroad of angiogenesis and inflammation. Thromb Haemost. 2009;102:544–554. doi: 10.1160/TH08-12-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Schizophrenia Working Group of the Psychiatric Genomics, C. Daly MJ, Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component 4. Nature. 2016 doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, Keshet E. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–1574. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]
- Senitz D, Winkelmann E. [Neuronal structure abnormality in the orbito-frontal cortex of schizophrenics] J Hirnforsch. 1991;32:149–158. [PubMed] [Google Scholar]
- Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol Psychiatry. 2008;64:89–97. doi: 10.1016/j.biopsych.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka L, Kovari E, Santos M, Herrmann FR, Gold G, Hof PR, Bouras C, Giannakopoulos P. Microvascular changes in late-life schizophrenia and mood disorders: stereological assessment of capillary diameters in anterior cingulate cortex. Neuropathol Appl Neurobiol. 2012;38:696–709. doi: 10.1111/j.1365-2990.2012.01263.x. [DOI] [PubMed] [Google Scholar]
- Sinkus ML, Adams CE, Logel J, Freedman R, Leonard S. Expression of immune genes on chromosome 6p21.3–22.1 in schizophrenia. Brain Behav Immun. 2013;32:51–62. doi: 10.1016/j.bbi.2013.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B. Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry. 2004;161:882–888. doi: 10.1176/appi.ajp.161.5.882. [DOI] [PubMed] [Google Scholar]
- Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res. 2008;103:71–82. doi: 10.1016/j.schres.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bernstein HG, Bielau H, Farkas N, Winter J, Dobrowolny H, Brisch R, Gos T, Mawrin C, Myint AM, Bogerts B. S100B-immunopositive glia is elevated in paranoid as compared to residual schizophrenia: a morphometric study. J Psychiatr Res. 2008;42:868–876. doi: 10.1016/j.jpsychires.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. Puzzling over schizophrenia: schizophrenia as a pathway disease. Nat Med. 2012;18:210–211. doi: 10.1038/nm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Glutamatergic aspects of schizophrenia. Br J Psychiatry Suppl. 1999:12–15. [PubMed] [Google Scholar]
- Tamminga CA, Holcomb HH, Gao XM, Lahti AC. Glutamate pharmacology and the treatment of schizophrenia: current status and future directions. Int Clin Psychopharmacol. 1995;10(Suppl 3):29–37. 29–37. [PubMed] [Google Scholar]
- Tang B, Chang WL, Lanigan CM, Dean B, Sutcliffe JG, Thomas EA. Normal human aging and early-stage schizophrenia share common molecular profiles. Aging Cell. 2009;8:339–342. doi: 10.1111/j.1474-9726.2009.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF. Cerebral blood flow activation and functional lesions in schizophrenia. Schizophr Res. 1996;19:129–140. doi: 10.1016/0920-9964(95)00000-3. [DOI] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Zai CC, Muller DJ, Kennedy JL. Genetics in schizophrenia: where are we and what next? Dialogues Clin Neurosci. 2010;12:289–303. doi: 10.31887/DCNS.2010.12.3/atiwari. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol A, Zhu S, Tran N, Simone A, Fang G, Brennand KJ. Altered WNT Signaling in Human Induced Pluripotent Stem Cell Neural Progenitor Cells Derived from Four Schizophrenia Patients. Biol Psychiatry. 2015;78:e29–34. doi: 10.1016/j.biopsych.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, Fancy SP. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351:379–384. doi: 10.1126/science.aad3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry. 2010;11:567–578. doi: 10.3109/15622970903414188. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Errede M, Robertson D, Girolamo F, Masciandaro A, Bertossi M. VEGF expression is developmentally regulated during human brain angiogenesis. Histochem Cell Biol. 2003;119:227–232. doi: 10.1007/s00418-003-0510-y. [DOI] [PubMed] [Google Scholar]
- Vostrikov V, Orlovskaya D, Uranova N. Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. World J Biol Psychiatry. 2008;9:34–42. doi: 10.1080/15622970701210247. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun. 2001;15:388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- Webster MJ, O’Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience. 2005;133:453–461. doi: 10.1016/j.neuroscience.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Kosno-Kruszewska E, Lechowicz W, Pasennik E, Schmidt-Sidor B. Degeneration of microglial cells in frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol. 2004;42:157–165. [PubMed] [Google Scholar]
- Wolf SS, Hyde TM, Weinberger DR. Neurobiology of schizophrenia. Curr Opin Neurol Neurosurg. 1993;6:86–92. [PubMed] [Google Scholar]
- Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci. 2007;27:9032–9042. doi: 10.1523/JNEUROSCI.2088-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zou Q, Ding X, Shi D, Zhu X, Hu W, Liu L, Zhou H. Complement component C3a plays a critical role in endothelial activation and leukocyte recruitment into the brain. J Neuroinflammation. 2016;13:23. doi: 10.1186/s12974-016-0485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Gray TS, Sigmund CD, Cassell MD. The angiotensinogen gene is expressed in both astrocytes and neurons in murine central nervous system. Brain Res. 1999;817:123–131. doi: 10.1016/s0006-8993(98)01236-0. [DOI] [PubMed] [Google Scholar]
- Yucel M, Pantelis C, Stuart GW, Wood SJ, Maruff P, Velakoulis D, Pipingas A, Crowe SF, Tochon-Danguy HJ, Egan GF. Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry. 2002;159:251–254. doi: 10.1176/appi.ajp.159.2.251. [DOI] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr Res. 2006;84:1–14. doi: 10.1016/j.schres.2006.02.009. [DOI] [PubMed] [Google Scholar]


