Abstract
Gamma rhythms have been proposed to promote the feed forward or “bottom-up” flow of information from lower to higher regions in the brain during perception. On the other hand, beta rhythms have been proposed to represent feed back or “top-down” influence from higher regions to lower. The pedunculopontine nucleus (PPN) has been implicated in sleep-wake control and arousal, and is part of the reticular activating system (RAS). This review describes the properties of the cells in this nucleus. These properties are unique, and perhaps it is the particular characteristics of these cells that allow the PPN to be involved in a host of functions and disorders. The fact that all PPN neurons fire maximally at gamma band frequency regardless of electrophysiological or transmitter type, make this an unusual cell group. In other regions, for example in the cortex, cells with such a property represent only a sub-population. More importantly, the fact that this cell group’s functions are related to the capacity to generate coherent activity at a preferred natural frequency, gamma band, speaks volumes about how the PPN functions. We propose that “bottom-up” gamma band influence arises in the RAS and contributes to the build-up of the background of activity necessary for preconscious awareness and gamma activity at cortical levels.
Keywords: Arousal, beta oscillations, Ca2+ channels, gamma oscillations
Introduction
Bottom-up or feed forward brain processes depend on sensory events as stimuli activate lower brain centers and the information rises to succeeding higher centers to promote perception. Top-down or feed back processing refers to the influence imposed by higher centers on the perception of and attention to incoming stimuli. Recent studies suggest that feed forward and feed back signaling use different frequency channels, specifically gamma and beta frequencies, respectively (Bastos et al 2015). This review is concerned with questions about bottom-up processes, specifically, where does the gamma activity arise? Is the gamma band activity generated only at the level of the cortex, or does it arise from lower centers to interact with ongoing cortical activity? What mechanisms generate activity at such frequencies? Can synaptic circuits maintain such frequencies for any length of time, or are there other mechanisms involved? Which lower centers generate gamma band activity, and is it coherent with cortical gamma band activity?
Role of gamma band activity
Gamma oscillations appear to participate in sensory perception, problem solving, and memory (Eckhorn et al., 1988; Gray and Singer, 1989; Jones, 2007; Philips and Takeda, 2009; Palva et al., 2009; Voss et al., 2009), and coherence at these frequencies may occur at cortical or thalamocortical levels (Llinas et al., 1991; Singer, 1993). Indeed, synchronous gamma band activation among thalamocortical networks (Llinas et al., 2002), and in other neuronal groups is thought to contribute to the merger, or “binding”, of information originating from separate regions (Llinas and Pare, 1991). On the other hand, gamma oscillation deficits have been suggested as a pathophysiologic feature of diseases like schizophrenia and Alzheimer’s disease (Steriade and Llinas, 1988; Ribary et al., 1991; Stam et al., 2002; Uhlhass and Singer, 2010).
Gamma oscillations are thought to emerge from the dynamic interaction between intrinsic neuronal and synaptic properties of thalamocortical networks (Steriade and Llinas, 1988). That is, synaptic connections alone may not be able to maintain firing at gamma frequencies (~30–90 Hz), so that intrinsic membrane properties also appear essential to the maintenance of gamma band activity. For example, flicker fusion of visual inputs demonstrates that cortical circuits cannot “follow” individual visual stimuli presented at rates above 35 Hz or so. That is, cortical circuits appear incapable of reliably firing at gamma frequencies for any length of time. Therefore, the ability of cells with intrinsic membrane properties, coupled with synaptic interactions, is what allows the circuit as a whole to fire at a preferred frequency, and is essential to maintaining frequencies in the gamma range. The neuronal mechanisms behind such activity include the presence of inhibitory cortical interneurons with intrinsic membrane potential oscillatory activity in the gamma range (Steriade and Llinas, 1988; Llinas et al., 1991; Steriade, 1999), many of which are electrically coupled (Gibson et al., 1999), as well as of fast rhythmic bursting pyramidal neurons (Cunningham et al., 2004). At the thalamic level, thalamocortical excitatory neurons have intrinsic properties needed to generate subthreshold gamma band membrane potential oscillations (Pedroarena and Llinas, 1997).
While cortical interneurons can generate membrane potential gamma oscillations through the activation of voltage-dependent, persistent sodium channels (Llinas et al., 1991), in thalamocortical neurons, the main mechanism responsible for gamma band activity involves high threshold P/Q-type voltage-gated calcium channels located in the dendrites (Pedroarena and Llinas, 1997). Moreover, the same intrinsic properties mediating gamma band oscillations are present in the thalamus of several vertebrate species, indicating considerable evolutionary conservation (Llinas and Steriade, 2006). It thus appears that at least two types of intrinsic membrane properties are essential for generating gamma band activity, sodium-dependent subthreshold oscillations and voltage-dependent high threshold calcium channels.
Voltage-gated calcium channel involvement in gamma band generation is particularly important. Indeed, calcium channels are known to play a pivotal role in determining intrinsic properties and synaptic transmission throughout the central nervous system (Katz and Miledi, 1965; Llinas and Hess, 1976; Caterall, 1988; Llinas, 1988; Llinas et al., 2007a). P/Q-type channels (also known as Cav2.1 channels) are present widely in the brain (Hillman et al., 1991; Uchitel et al., 1992; Jones, 2007; Llinas et al., 2007a). N-type calcium channels are found in the rat auditory brainstem, are restricted to the early postnatal period, and are replaced by P/Q-type channels later in development (Iwasaki and Takahashi, 1998; Westenbroek et al., 1992). Importantly, P/Q-type mutant mice have deficient gamma band activity in the EEG, abnormal sleep-wake states, ataxia, are prone to seizures (low frequency synchrony), and die by 3 weeks of age (Llinas et al., 2007b). These findings suggest that both the cortex and the thalamus may be capable of generating gamma band activity, and they do so via sub-populations of cells with sodium-dependent and/or calcium channel-dependent mechanisms. But do other regions of the brain also generate such activity?
Subcortical gamma band activity
Both the hippocampus and cerebellum have the intrinsic and synaptic properties necessary to generate gamma band oscillatory activity. Hippocampal oscillatory activity in the gamma range (30–90 Hz) has been extensively described to be functional associated with entorhinal cortex afferents (Charpak et al., 1995). Interestingly, neurons located in the entorhinal cortex can also oscillate at gamma band frequencies, suggesting a key role for such afferents in maintaining hippocampal gamma oscillations (Chrobak and Buzsaki, 1998). Recently, gamma band activity in the CA1 area was divided into high (>65 Hz) and low (~25–60 Hz) gamma frequency components that differentially couple CA1 and CA3 subfields, respectively (Colgin et al., 2009). Such differences have been proposed to “bind” CA1 high gamma oscillations with very high frequency activity from entorhinal cortex in charge of providing information about object and place recognition in rodents (Bussey et al., 1999), whereas CA1 low gamma oscillations would be locked to the slower frequencies present in the CA3 area in charge of memory storage (Colgin et al., 2009; Colgin and Moser, 2010). This suggests the use of different frequency bands for separate functions.
Similarly, a peak in gamma band power has been described in the Purkinje cell layer around the apex of the cerebellar lobule, and to a lower extent in distal white matter (Lang et al., 2006; Middleton et al., 2008). Moreover, the cerebellar activity is coherent with that of the cortex and thalamus. Cortico-cerebellar coherence at gamma frequencies is evident in monkeys during performance of a manual precision grip task (Soteropoulos and Baker, 2006), and cerebello-thalamic activity is synchronized with neocortical activity at gamma frequencies (Timofeev and Steriade, 1997). Finally, it has been proposed that both cerebellar and thalamocortical networks might oscillate at the same frequencies to enable information exchange among these brain areas (Middleton et al., 2008). It was found that gamma band activity in the motor cortex lags behind coherent activity in basal ganglia structures (Lalo et al 2008; Trottenberg et al 2006). This led to the suggestion that motor cortex gamma synchronization reflects a momentary arousal-related event for enabling the initiation of movement (Brucke et al 2012; Cheyne and Ferrari 2013; Jenkinson et al 2013). That is, structures such as the RAS and thalamus may play an early permissive role in the control of movement. Thus, there are several other regions generating gamma band activity besides the cortex and thalamus, including the hippocampus, cerebellum, basal ganglia, and importantly, the reticular activating system (RAS).
Waking and REM sleep
During waking and rapid eye movement (REM) sleep, the EEG shows low amplitude, high frequency activity at beta/gamma frequencies (~20–30/30–90 Hz) (Buzsaki and Draguhn, 2004). The pedunculopontine nucleus (PPN) is most active during waking and REM sleep (Garcia-Rill 2015). The PPN is the arm of the RAS that modulates ascending projections through the thalamus (modulating arousal) and descending projections through the pons and medulla (modulating posture and locomotion) (Garcia-Rill 2015), and is composed of different populations of cholinergic, glutamatergic, and GABAergic neurons (Wang and Morales, 2009). Extracellular recordings of PPN neurons in vivo identified six categories of thalamic projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital wave generation (Steriade et al., 1990). Some of these neurons had low rates of spontaneous firing (<10 Hz), but most had high rates of tonic firing in the beta/gamma range (20–80 Hz). It has been shown that PPN neurons exhibit beta/gamma frequencies in vivo during active waking and REM sleep, but not during slow wave sleep (Sakai, et al., 1990; Steriade, et al., 1990; Kayama, et al., 1992; Datta and Siwek, 2002; Datta, et al., 2009; Boucetta, et al., 2014). Similarly, the presence of gamma band activity has been confirmed in the cortical EEG of the cat in vivo when the animal is active (Steriade, et al., 1990 Steriade, et al., 1991); and in the region of the PPN in humans during stepping, but not at rest (Fraix, et al., 2013). A recent study showed that PPN neurons fired at low frequencies ~10 Hz at rest, but the same neurons increased firing to gamma band frequencies when the animal woke up, or when the animal began walking on a treadmill (Goetz et al 2016). That is, the same cells were involved in both arousal and motor control. Thus, there is ample evidence for gamma band activity during active waking and movement in the PPN in vitro, in vivo, and across species, including man.
Mechanism behind PPN gamma activity
A number of recent publications have described the mechanisms behind gamma band activity in the PPN (Garcia-Rill et al 2013, 2014, 2015, 2016; Kezunovic et al., 2011; Urbano et al 2014), and will not be reiterated. Briefly, these oscillations are mediated by voltage-dependent, high threshold N- and P/Q-type calcium channels that are present in every PPN neuron, regardless of cell or transmitter type. These channels are distributed along the dendrites of PPN cells (Hyde et al 2013a). Presumably, afferent input traveling through “specific” sensory pathways diverges to activate “non-specific” reticular pathways to activate PPN dendrites. However, gamma band activity during waking has different mechanisms than gamma band activity during REM sleep. Injections of glutamate into the PPN increased waking and REM sleep (Datta et al., 2001a), while injections of the glutamatergic receptor agonist N-methyl-D-aspartic acid (NMDA) increased only waking (Datta et al., 2001b), and injections of the glutamatergic receptor agonist kainic acid (KA) increased only REM sleep (Datta, 2002). Intracellularly, protein kinase C (PKC), which modulates KA receptors, enhances N-type channel activity and has no effect on P/Q-type channel function (Stea et al. 1995), but CaMKII, which modulates NMDA receptors, was shown to modulate P/Q-type channel function (Jiang et al. 2008).
That is, the two calcium channel subtypes are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. Moreover, there are three cell types in the PPN, those bearing only N-type calcium channels, those with both N- and P/Q-type, and those with only P/Q-type calcium channels (Luster et al 2014, 2015). The implications from all of these results is that, a) there is a “waking” pathway mediated by CaMKII and P/Q-type channels and a “REM sleep” pathway mediated by cAMP/PK and N-type channels, and b) different PPN cells fire during waking (those with N+P/Q and only P/Q-type) vs REM sleep (those with N+P/Q and only N-type).
Ascending Projections
The main ascending output of the PPN is to the intralaminar thalamus (ILT), specifically, the parafascicular nucleus (Pf). The ILT and Pf receive projections from the cholinergic PPN nuclei with both symmetrical and asymmetrical terminals (Capozzo et al. 2003; Erro et al. 1999; Kha et al. 2000; Kobayashi and Nakamura 2003). In turn, Pf neurons send widespread projections to the cortex, striatum, subthalamic nucleus, and substantia nigra (Herrero et al. 2002; Van der Werf et al. 2002). The Pf is thought to be involved in maintaining consciousness and selective attention in primates (Minamimoto and Kimura 2002; Raeva 2006). We found that all Pf cells recorded manifested P/Q-type calcium channels and fired maximally at gamma band frequency (Kezunovic et al 2012). Moreover, these channels were distributed along the dendrites of the neurons, just as in PPN (Hyde et al 2013b).
These findings provided novel insights into the function of the Pf, demonstrating that it generates gamma band oscillatory activity in the presence of sufficient excitation from the PPN. We suggest that, rather than participating in the temporal binding of sensory events, gamma band activity generated in the PPN and relayed to the Pf may help stabilize coherence related to arousal, providing a stable activation state during waking, and relay such activation to the cortex, which thus participates in “non-specific” thalamocortical processing. Most of our thoughts and actions are driven by preconscious processes. We speculate that continuous sensory input will induce gamma band activity in the PPN that is relayed to the Pf to participate in the processes of preconscious awareness, and provide the essential stream of information for the formulation of many of our actions (Garcia-Rill et al 2013, 2014, 2015, 2016; Urbano et al 2014). Figure 1 shows a wiring diagram of the projections and mechanisms described.
Figure 1. Wiring diagram and mechanisms behind bottom-up gamma band activity.
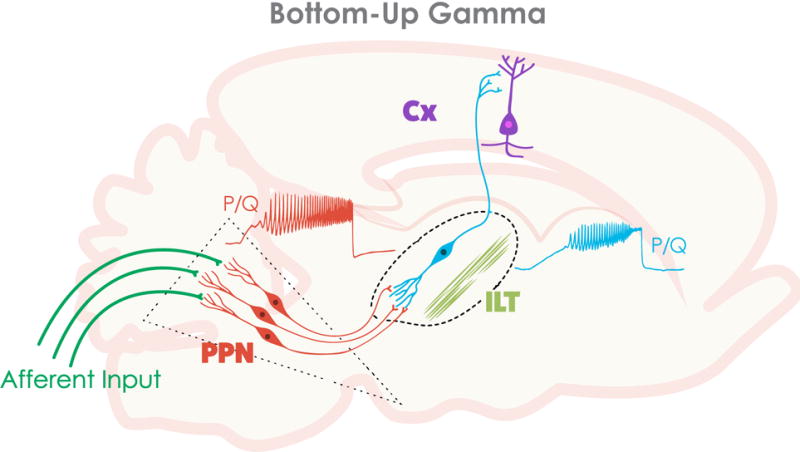
Afferent input (green lines) that originates from collateral activation of the RAS by sensory systems activates the dendrites of PPN neurons (red) in the posterior midbrain. The presence of inputs to dendritic P/Q- and N-type calcium channels set off oscillations at gamma band that influence firing frequency. The output of the PPN ascends to the intralaminar thalamus (ILT), especially the parafascicular nucleus, activating its dendrites to oscillate at gamma frequency via high threshold calcium channels (blue). These cells in turn project to the cortex, particularly to upper cortical layers where the non-specific thalamic inputs terminate, to activate cortical neurons (purple). Once cortical, hippocampal, basal ganglia, and cerebellar cells are activated, the generation and maintenance of gamma band activity in the brain can more easily be maintained through synaptic AND intrinsic membrane properties.
Bottom-up gamma
The original description of the RAS specifically suggested that it participates in tonic or continuous arousal (Moruzzi and Magoun, 1949), and lesions of this region were found to eliminate tonic arousal (Watson et al., 1974). This raises the question of how a circuit can maintain such rapid, recurrent activation. Expecting a circuit of 5 or 10 synapses to reliably relay 20–60 Hz cycling without failing is unrealistic. Without the intrinsic properties afforded by rapidly oscillating channels, such as those described recently for the PPN and Pf, beta/gamma band activity could not be maintained. The combination of channels capable of fast oscillations and of circuitry that involves activating these channels probably are both required for the maintenance of gamma band activity (Garcia-Rill 2015; Garcia-Rill et al 2013, 2014, 2015, 2016; Llinas, 1988; Llinas et al., 1991; Kezunovic et al., 2011). The PPN and Pf, in which every cell manifests gamma band activity, then becomes a gamma-making machine. We speculate that it is the continued activation of the RAS during waking that allows the maintenance of the background of gamma activity necessary to support the state capable of reliably assessing the world around us on a continuous basis- preconscious awareness.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P20 GM103425 and P30 GM110702 to Dr. Garcia-Rill.
References
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, et al. Visual areas exert feedforward and feedback through distinct frequency channels. Neuron. 2015;85:390–40. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, gabaergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucke C, Huebl J, Kempf F, Krauss JK, Yarrow, et al. Pallidal gamma activity is correlated to movement amplitude in patients with dystonia. Clin Neurophysiol. 2008;119(S1):49. doi: 10.1016/j.clinph.2008.01.106. [DOI] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Capozzo A, Florio T, Cellini R, Moriconi U, Scarnati E. The pedunculopontine nucleus projection to the parafascicular nucleus of the thalamus: an electrophysiological investigation in the rat. J Neural Transm. 2003;110:733–747. doi: 10.1007/s00702-003-0820-1. [DOI] [PubMed] [Google Scholar]
- Caterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1988;24:307–323. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Charpak S, Paré D, Llinás RR. The entorhinal cortex entrains fast CA1 hippocampal oscillations in the anaesthetized guinea-pig: role of the monosynaptic component of the perforant path. Eur J Neurosci. 1995;7:1548–1557. doi: 10.1111/j.1460-9568.1995.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Cheyne G, Ferrari P. MEG studies of motor cortex gamma oscillations: evidence for a gamma “fingerprint” in the brain? Frontiers Human Neurosci. 2013;7:#575. doi: 10.3389/fnhum.2013.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010;25:319–329. doi: 10.1152/physiol.00021. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, et al. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Nat Acad Sci USA. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainite receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental nmda receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2002;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer J Physiol Reg Integ Comp Physiol. 2001;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. Doi: none. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neurosci. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, et al. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. Doi: none. [DOI] [PubMed] [Google Scholar]
- Erro E, Lanciego JL, Gimenez-Amaya JM. Relationships between thalamostriatal neurons and pedunculopontine projections to the thalamus: a neuroanatomical tract-tracing study in the rat. Exp Brain Res. 1999;127:162–170. doi: 10.1007/s002210050786. Doi: none. [DOI] [PubMed] [Google Scholar]
- Fraix V, Bastin J, David O, Goetz L, Ferraye M, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLOS ONE. 2013;8:e83919. doi: 10.1371/journal.pone.0083919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E. Waking and the Reticular Activating System. New York: Academic Press; 2015. p. 330. [DOI] [Google Scholar]
- Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, et al. Gamma band activity in the RAS-intracellular mechanisms. Exp Brain Res. 2014;232:1509–1522. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, et al. Implications of gamma band activity in the pedunculopontine nucleus. J Neural Transm. 2015;123:655–665. doi: 10.1007/s00702-015-1485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, D’Onofrio S, Luster B, Mahaffey S, Urbano FJ, et al. The 10 Hz Frequency: a fulcrum for transitional brain states. Translat Brain Rhyth. 2016;1:7–13. doi: 10.15761/TBR.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, et al. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J Neural Transm. 2016;123:667–678. doi: 10.1007/s00702-016-1577-7. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Nat Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;8:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, et al. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci USA. 1991;88:7076–7080. doi: 10.1073/pnas.88.16.7076. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JR, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013a;115:1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Visualization of fast calcium oscillations in the parafascicular nucleus. J Eur Physiol (Pflug Arch) 2013b;465:1327–1340. doi: 10.1007/s00424-013-1264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J Physiol (Lond) 1998;509:419–442. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Kuhn AA, Brown P. Gamma oscillations in the human basal ganglia. Exp Neurol. 2013;245:72–76. doi: 10.1016/j.expneurol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, et al. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Nat Acad Sci USA. 2008;105:341–346. doi: 10.1073/pnas.0710213105. Doi: 10.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Calcium channels in higher-level brain function. Proc Nat Acad Sci USA. 2007;14:17903–17904. doi: 10.1073/pnas.0709509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kha HT, Finkelstein DI, Pow DV, Lawrence AJ, Horne MK. Study of projections from the entopeduncular nucleus to the thalamus of the rat. J Comp Neurol. 2000;426:366–377. doi: 10.1002/1096-9861(20001023)426:3<366::AID-CNE2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The effect of calcium on acetylcholine release from motor nerve terminals. Proc R Soc Lond B Biol Sci. 1965;161:483–495. doi: 10.1098/rspb.1965.0017. Doi: none. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-J. [DOI] [PubMed] [Google Scholar]
- Kezunovic N, Hyde J, Simon C, Urbano FJ, Garcia-Rill E. Gamma band activity in the developing parafascicular nucleus (Pf) J Neurophysiol. 2012;107:772–784. doi: 10.1152/jn.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, et al. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Nakamura Y. Synaptic organization of the rat parafascicular nucleus, with special reference to its afferents from the superior colliculus and the pedunculopontine tegmental nucleus. Brain Res. 2003;980:80–91. doi: 10.1016/S0006-8993(03)02921-4. [DOI] [PubMed] [Google Scholar]
- Lalo E, Thobois S, Sharott A, Polo G, Mertens P, et al. Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci. 2008;28:3008–3016. doi: 10.1523/JNEUROSCI.5295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinás RR. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J Physiol (Lond) 2006;571:101–120. doi: 10.1113/jphysiol.2005.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Choi S, Urbano FJ, Shin HS. Gamma band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci USA. 2007a;104:17819–17824. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Nat Acad Sci USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci USA. 1976;73:2520–2523. doi: 10.1073/pnas.73.7.2520. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Paré D. Of dreaming and wakefulness. Neurosci. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-Y. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Soonwook C, Urbano FJ, Hee-Sup S. γ-Band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Nat Acad Sci USA. 2007b;104:17819–17824. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E. High-Threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep. 2015;3:e12431. doi: 10.14814/phy2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster B, Urbano FJ, Garcia-Rill E. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep. 2016:e12787. doi: 10.14814/phy2.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, et al. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58:763–774. doi: 10.1016/j.neuron.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamimoto T, Kimura M. Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophysiol. 2002;87:3090–3101. doi: 10.1152/jn.2002.87.6.3090. Doi: none. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol. 1949;1:455–473. Doi: none. [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage. 2009;49:3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Pedroarena C, Llinás RR. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci USA. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. Doi: nonbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int J Psychophysiol. 2009;73:350–354. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Raeva SN. The role of the parafascicular complex (CM-Pf) of the human thalamus in the neuronal mechanisms of selective attention. Neurosci Behav Physiol. 2006;36:287–295. doi: 10.1007/s11055-006-0015-y. [DOI] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-C. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in informtion processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Baker SN. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol. 2006;95:1194–1206. doi: 10.1152/jn.00935.2005. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, et al. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. Doi: none. [DOI] [PubMed] [Google Scholar]
- Stea A, Soomg TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Steriade M. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Lydic R, Baghdoyan HA, editors. Handbook of Behavioral State Control Cellular and molecular mechanisms. CRC Press; New York: 1999. pp. 327–347. [DOI] [Google Scholar]
- Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. Doi: none. [DOI] [PubMed] [Google Scholar]
- Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Fast (mainly 30–100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. J Physiol. 1997;504:153–168. doi: 10.1111/j.1469-7793.1997.153bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottenberg T, Fogelson N, Kuhn AA, Kivi A, Kupsch A, et al. Subthalamic gamma activity in patients with Parkinson’s disease. Exp Neurol. 2006;200:56–65. doi: 10.1016/j.expneurol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Uchitel OD, Protti DA, Sanchez V, Cherkesey BD, Sugimori M, et al. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and trnsmitter release in mammalian synapses. Proc Natl Acad Sci USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dial Clin Neurosci. 2013;15:301–313. doi: 10.31887/DCNS.2013.15.3/puhlhaas. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, D’Onofrio SM, Luster BR, Hyde JR, Bosagno V, et al. Pedunculopontine nucleus gamma band activity- preconscious awareness, waking, and REM sleep. Frontiers in Sleep and Chronobiol. 2014;5:210. doi: 10.3389/fneur.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. 2002;39:107–140. doi: 10.1016/S0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Voss U, Holzmann R, Tuin I, Hobson JA. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep. 2009;32:1191–1200. doi: 10.1093/sleep/32.9.1191. Doi: none. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contan distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Heilman KM, Miller BD. Neglect after mesencephalic reticular formation lesions. Neurol. 1974;24:294–298. doi: 10.1212/wnl.24.3.294. Doi: none. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, et al. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-P. [DOI] [PubMed] [Google Scholar]


